Abstract
Ionizing radiation is a well‐known carcinogen for various human tissues and a complete carcinogen that is able to initiate and promote neoplastic progression. Studies of radiation‐induced mouse thymic lymphomas, one of the classic models in radiation carcinogenesis, demonstrated that even the unirradiated thymus is capable of developing into full malignancy when transplanted into the kidney capsule or subcutaneous tissue of irradiated mice. This suggests that radiation targets tissues other than thymocytes to allow expansion of cells with tumorigenic potential in the thymus. The idea is regarded as the ‘indirect mechanism’ for tumor development. This paper reviews the indirect mechanism and genes affecting the development of thymic lymphomas that we have analyzed. One is the Bcl11b/Rit1 tumor suppressor gene and the other is Mtf‐1 gene affecting tumor susceptibility. (Cancer Sci 2006; 97: 575–581)
Abbreviations:
- DN
double‐negative
- DSBs
double‐strand breaks
- HGF
hepatocyte growth factor
- ISP
immature single‐positive
- Mtf‐1
metal responsive transcription factor‐1
- SNPs, single‐nucleotide polymorphisms; TCR
T cell receptor
- Tgfrb2
transforming growth factor‐β type‐II receptor gene.
The multi‐step model of carcinogenesis defines cancer initiation as genomic change and promotion as the series of events leading to proliferation of initiated cells. Promoting factors can either be intrinsic or extrinsic to cells of the tumor cell lineage, and some promoting agents increase the probability that a cell will acquire additional mutations necessary for neoplastic progression. Analysis of activation of ras oncogenes after administrating a chemical carcinogen revealed that their activation is easily detectable before the onset of neoplasia, existing in apparently normal cells for long periods of time without inducing malignant transformation.( 1 , 2 ) This suggests that initiation is a frequent event and only a few initiated cells proliferate for neoplastic progression. A quantitative study of common leukemia fusion genes in humans showed a consistent result.( 3 ) It revealed that the fusion genes due to chromosome translocations, specific and stable markers for tracking leukemic clones, are detected in the blood of healthy newborns at a high rate that is significantly greater than that of the cumulative risk of the corresponding leukemia. These findings suggest that the factors that facilitate expansion of initiated cells with tumorigenic potential are important as well in determining cancer frequency.
Ionizing radiation is a well‐known carcinogen for a variety of human and mouse cells and tissues, and a complete carcinogen that is able to initiate and promote neoplastic progression.( 4 ) Radiation can induce a broad spectrum of DNA lesions including damage to nucleotide bases, cross‐linking, and DNA single‐strand breaks and DSBs. Among these lesions, DSBs are the principal lesions of importance in the induction of both chromosomal abnormalities and gene mutations. Radiation effects or consequences are modulated by various secondary factors, inducing genomic instability that is probably the initiation of carcinogenesis. Analysis of molecular and structural changes associated with radiation‐induced mutations shows that the spectrum of changes differs markedly from that for spontaneous or chemical‐induced mutations. For instance, inactivation of the p53 tumor suppressor gene has been reported in a small fraction of radiation‐induced mouse lymphomas and the pattern of inactivation significantly differs from that of lymphomas induced by chemical carcinogens.( 5 ) Mutations unique to radiation and site specificity for mutations have not yet been found in radiation‐induced tumors. However, it is well known that the induction of loss of heterozygosity at tumor suppressor loci is a general phenomenon in the initiation of radiation carcinogenesis. Knockout mice heterozygous for the p53 gene are more susceptible to radiation‐induced tumors and most of those tumors lose the wild‐type p53 allele.( 6 ) These lines of evidence indicate that cells targeted by radiation contribute to the development of cancers, and thereby support the ‘direct mechanism of radiation carcinogenesis’.
The mechanism for promotion is poorly understood, but studies of mouse thymic lymphomas provide some hints. Fractionated whole‐body irradiation causes apoptosis of thymocytes, leading to regeneration and differentiation arrest of surviving thymocytes.( 7 , 8 , 9 ) A series of transplantation experiments demonstrated that even the unirradiated thymus can develop into full malignancy when transplanted into the kidney capsule or subcutaneous tissue of irradiated mice.( 10 , 11 ) It was also shown that the lymphomagenesis is reduced by bone marrow transplantation or intravenous infusion of bone marrow cells of unirradiated mice shortly after irradiation.( 9 , 11 ) Consistently, protection of bone marrow from radiation prevented the development of thymic lymphomas (Fig. 1). These findings suggest that radiation targets not only thymocytes but also cells or tissues other than thymocytes for the development of thymic lymphomas. Thus, the major effect of radiation might be to allow expansion of cells with tumorigenic potential in the thymus. This idea is regarded as the ‘indirect mechanism’ for tumor development.
Figure 1.
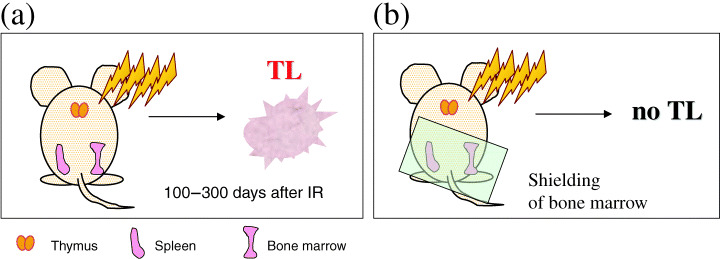
Illustration of mouse irradiation. (a) Fractionated whole‐body irradiation leading to the development of thymic lymphomas. (b) Prevention of lymphoma development by shielding the portion of bone marrow.
This review focuses on recent studies from our laboratory that address genes affecting the development of radiation‐induced mouse thymic lymphomas. One is the Bcl11b/Rit1 tumor suppressor gene and the other is the Mtf‐1 gene affecting tumor susceptibility. Mutation of Bcl11b in lymphomas has the fingerprint of DNA changes due to aberrant recombination induced not by irradiation but occurring endogenously.( 12 ) This was an unexpected result, and our further analysis revealed clues to the indirect mechanism for the development of radiogenic thymic lymphomas.
Thymic microenvironment
Thymic lymphoma cells, like normal thymocytes, reside in a complex microenvironment that includes the extracellular matrix, diffusible growth factors and cytokines, and the heterogeneous stromal cells comprising the cortex and medulla. Cortical epithelium is known to mediate the proliferation and differentiation of thymocytes and the cells that survive this developmental process migrate to the cortico‐medullary junction and medulla where further maturation takes place. In turn, the development of the thymic stromal cells that mediate these steps relies upon cross‐talk from thymocytes, hinting at a complex molecular interplay.
Fractionated whole‐body irradiation causes thymic atrophy and depletion of bone marrow cells, followed by a reduced supply of cells from bone marrow to the thymus (Fig. 2a). Cellularity of the thymus decreased to approximately 10% of its original level 3 days after γ‐irradiation and was restored to a normal level in 7 days by proliferation of large thymocytes (Fig. 2b). This injured microenvironment of thymus probably impacts on regeneration, survival and differentiation of thymocytes and might well affect the development of lymphomas. It was reported that prelymphoma cells develop in that microenvironment within 2 weeks after fractionated irradiation.( 9 ) Supply of a large number of unirradiated bone marrow cells shortly after irradiation rapidly restores the normal thymic microenvironment to prevent lymphoma development probably because of lack of proliferation of impaired T‐cell precursors with tumorigenic potential.
Figure 2.

Time‐course of the thymus and thymocytes after whole‐body irradiation. Mice were subjected to γ‐irradiation of 2.5 Gy four times at 1‐week intervals, starting at the age of 4 weeks. (a) Atrophy of thymus after irradiation and the following recovery process, as deduced from inspection and flow cytometric analysis. (b) Cellularity and the size of cells. The number of thymocytes decreased to less than 10% due to apoptosis 3 and 5 days after irradiation and was restored to a normal level in 7 days. The percentage of large thymocytes indicated above the line increased 3 and 5 days then decreased.
However, molecular interplays between the atrophic thymus and surviving thymocytes are poorly investigated. Molecules involved might include Notch ligands and receptors that play key roles in multiple steps during T cell development.( 13 ) One of their roles is the decision of a hematopoietic progenitor cell to become a T cell rather than a B cell.( 14 ) Chromosomal translocations that produce truncated forms of Notch1 are found in human T‐cell leukemias( 15 ) and those changes enhance proliferation of leukemic cells. Consistently, the transgenic expression of activated Notch alleles in mouse thymocytes lead to T‐cell leukemias.( 16 ) Notch ligands are highly expressed on thymic stromal cells and this might account, in part, for that T cell development and proliferation normally occurs efficiently only in the thymus.( 16 )
Positional cloning of tumor suppressor genes
Most of the well‐known tumor suppressor genes have been isolated by genetic analysis of humans, whereas few tumor suppressor genes have been isolated from animal models for human cancers, although the importance of those models was underlined. When we decided to perform positional cloning of novel tumor suppressor genes, we chose the model of γ‐ray‐induced mouse thymic lymphomas, one of the classic models in radiation carcinogenesis. At that time, there were studies implicating several genes in the development of mouse thymic lymphomas such as Myc, p16/p19, PTEN and p53. ( 5 , 17 , 18 , 19 ) However, all of these genes were already identified as tumor suppressor genes in humans, therefore those studies only confirmed their involvement in mouse thymic lymphomas.
Our genome‐wide analysis of allelic loss( 20 ) and subsequent sequencing allowed us to identify Ikaros on mouse chromosome 11( 21 ) and to clone a novel gene, Bcl11b/Rit1, on mouse chromosome 12.( 22 , 23 ) Ikaros encodes a zinc finger transcription factor detected in pluripotent hematopoietic stem cells and its expression is maintained at high levels in mature lymphocytes.( 24 ) An Ikaros knockout mouse strain carrying a deletion of the Ikaros DNA‐binding domain displays dominant negative effects on transcription through interaction at the C‐terminal domain with the Ikaros isoforms.( 25 ) Homozygous mice lack all fetal and adult lymphoid lineages, whereas heterozygous mice exhibit defects in the T cell lineage, which lead to an abnormal accumulation of CD4/CD8 double‐positive thymocytes and ultimately result in T‐cell leukemia and lymphomas. This suggests an implication of Ikaros in lymphomagenesis. Ikaros has a proapoptotic function and inactivation of Ikaros can contribute to resistance for apoptosis.( 26 ) This can account for Ikaros as a tumor suppressor. Our analysis of radiogenic thymic lymphomas revealed point mutations in the N‐terminal zinc finger and activation domains of Ikaros, and frameshift or nonsense mutations in various regions that resulted in truncation of Ikaros protein. These results demonstrate a direct role for Ikaros in the development of mouse thymic lymphomas. As for humans, Ikaros mutations were reported in lymphoid malignancies.( 27 , 28 )
Bcl11b/Rit1
Bcl11b/Rit1, also called CTIP2, is a tumor suppressor gene that encodes zinc finger transcription factors,( 29 , 30 ) homologous to a proto‐oncogene Bcl11a/Evi9. ( 31 , 32 ) Bcl11b‐knockout (Bcl11b –/– ) mice die soon after birth and exhibit some developmental anomalies. One of the anomalies is a developmental arrest of αβ T cells and another is a defect in the function of motor neurons.( 33 ) The developmental arrest occurs at immature CD4/CD8 DN and ISP stages accompanied by apoptosis (Fig. 3), but not involving cells of the γδ T or B cell lineages. Therefore, cellularity of thymocytes in Bcl11b –/– mice is reduced to approximately 10% in wild‐type mice. As Bcl11b –/– thymocytes fail to express the pre‐TCR on the cell surface, the arrest at the DN stage and low cellularity might be ascribed to the lack of this expression. However, the low cellularity and apoptosis in Bcl11b –/– thymocytes are unexpected findings because cell survival due to the reduction of apoptosis might be expected if a tumor suppressor gene is lost.
Figure 3.

Developmental arrests of αβ T cells in Bcl11b –/– and p53 –/– Bcl11b –/– mice. Flow cytometric analysis is shown of thymocytes in SCID mice that were reconstituted with E18.5 fetal liver cells of p53 +/+ Bcl11b +/+ , p53 +/+ Bcl11b –/– and p53 –/– Bcl11b –/– mice. The upper panel shows expression of CD8 and CD4, and the lower panel displays expression of CD25 and CD44 that was analyzed within the CD8−CD4− DN thymocyte subset. Cellularity was as follows: 0.94 × 106 cells for p53 +/+ Bcl11b –/– (n = 4) and 3.1 × 106 cells for p53 –/– Bcl11b –/– (n = 3).
A failure of the pre‐TCR signaling, as in scid, rag‐1−/– or rag‐2−/– mice that cannot rearrange TCR genes,( 34 ) results in an arrest at the DN stage of development, accompanied by apoptosis. As expected, introduction of the functional TCRβ chain into those mice can rescue the transition from DN to CD4/CD8 double‐positive stage and prevent apoptosis.( 35 ) Apoptosis due to the absence of pre‐TCR signaling appears to involve the tumor suppressor p53, since the deficiency of p53 in pre‐TCR‐deficient thymocytes can restore the development and survival.( 36 ) Accordingly, we introduced p53 deficiency into Bcl11b –/– mice. As Bcl11b –/– mice die soon after birth, fetal liver cells were transferred into SCID mice, and the ability of p53 deficiency to rescue the development and survival of T cells was examined 8 weeks after transfer. As shown in Fig. 3, it restored thymocyte differentiation from the DN to ISP, but not the double‐positive stage and increased cellularity to a certain level.( 37 ) In contrast, the expression of TCRβ in Bcl11b –/– mice failed to promote the transition from the DN to the ISP stage of development, indicating that the pre‐TCR signal cannot compensate for the deficiency of Bcl11b.( 38 ) These results suggest that Bcl11b –/– thymocytes have defects in not only the pre‐TCR signaling but also some other signaling required for development of αβ T cells.
Bcl11b +/– heterozygous mice, however, are viable and do not develop tumors spontaneously. But when those heterozygous mice were irradiated at the age of 1 month, they showed higher susceptibility to thymic lymphomas than wild‐type mice (Ohi et al., unpublished data). This clearly demonstrates the tumor suppressive property of Bcl11b/Rit1 and indicates that loss‐of‐function mutations of Bcl11b/Rit1 contribute to mouse lymphomagenesis and possibly to human cancer development.
Recent studies indicate that the human Bcl11b locus is recurrently involved in chromosomal aberrations in hematopoietic malignancies, mostly of T cell origin,( 39 , 40 ) and have characterized DNA rearrangements between Bcl11b and TCR gene loci in two T‐cell acute lymphoblastic leukemias.( 41 ) However, the majority of these leukemias express Bcl11b from an undisrupted allele, therefore it has not been concluded whether Bcl11b acts as a tumor suppressor gene or as an oncogene. Our recent findings show that Bcl11b heterozygous state in mice of p53 +/– background can contribute to lymphomagenesis even retaining the expression in lymphomas (Kamimura et al., unpublished data). This indicates the relevance of Bcl11b aberration to some human hematopoietic malignancies.
Irradiation and intragenic deletions of Bcl11b/Rit1
Bcl11b/Rit1 underwent biallelic changes in thymic lymphomas at a high frequency.( 20 , 22 ) In most cases one allele of Bcl11b was inactivated by intragenic deletions encompassing exon 2 and the other allele by allelic losses.( 12 ) Deletions might be a result of genomic instability induced by γ‐radiation or could be generated by illegitimate V(D)J recombinase activity as exemplified by p16 and Notch1 genes in lymphomas/leukemias.( 42 ) Lymphocyte precursors undergo the developmentally regulated process of V(D)J recombination that produces functional immunoglobulin and TCR genes.( 43 ) The V(D)J recombination involves site‐specific DNA cleavages mediated by the RAG1/2 recombinase and this reaction can also operate on any DNA regions comprising the RAG1/2‐recognition sequences or cryptic sequences.( 44 , 45 ) We thus examined the sites of breakage and rejoining of the intragenic deletions by polymerase chain reaction mapping and sequencing. The sites of deletions were highly clustered in the vicinities of potential V(D)J recombination motifs, suggesting that cleavages were provided by the RAG1/2 recombinase.( 12 ) This implicates an illegitimate V(D)J recombinase activity in the genesis of Bcl11b/Rit1 intragenic deletions.
If this is the case, one might expect such intragenic deletions of Bcl11b/Rit1 in the thymocytes of unirradiated mice and the question of concern is whether or not the number of such thymocytes increases after γ‐irradiation. Hence we performed nested polymerase chain reaction analysis of unirradiated and irradiated thymuses.( 12 ) Intragenic deletions were found in unirradiated thymocytes and rough estimates indicated that there were as many as 103−104 thymocytes with the deletions, assuming the residence of 108 thymocytes in the mouse thymus (Table 1). Of importance is that irradiation did not increase the detection efficiency of deletions 1–4 days and probably 14 days after irradiation. As direct consequences conferred by radiation disappear within a few hours, this suggests that the target of radiation is not for creating the intragenic deletions at Bcl11b/Rit1. As mentioned above, cellularity of thymocytes decreases to less than 10% due to apoptosis 3 days after irradiation and is restored to a normal level by 7 days. Therefore, the result also suggests that thymocyte populations with Bcl11b/Rit1 deletions do not grow selectively by 4 days (and possibly 14 days) after. The thymocytes having Bcl11b/Rit1 deletion at one allele are initiated cells with a potential to grow, but the growth would begin at a later stage(s) to contribute to the development of thymic lymphomas.
Table 1.
Detection of Rit1 intragenic deletions in thymus of 4‐week‐old mice
| Estimated prevalence (per 104 cells) | |
|---|---|
| Unirradiated | |
| BALB/c | 0.5 (0.18–1.3) † |
| RAG2‐KO | 0.0 |
| After irradiation | |
| 4 h | 0.6 |
| 24 h | 0.2 |
| 4 days | 0.2 |
| 14 days | 1.0 |
The prevalence of cells bearing Rit1 intragenic deletions were estimated assuming a Poisson distribution. Cell equivalents were calculated assuming 600 pg DNA per 105 cells. †95% confidence interval.
Toward clues of an indirect mechanism for radiation carcinogenesis
The importance of tissue microenvironment in radiation carcinogenesis is now well recognized. This issue is noted in breast tumors as well as thymic lymphomas. In mice, when non‐transformed mammary epithelial cells were transplanted into fat pads containing irradiated fibroblasts, the incidence of breast tumors increased compared to transplant of similar epithelial cells into fat pads with unirradiated fibroblasts.( 46 ) This demonstrates that changes in fibroblasts can contribute to epithelial transformation. Apart from radiation, evidence was recently provided for involvement of adjacent stromal fibroblasts in the development of carcinomas. Mice were generated in which the Tgfrb2 gene was inactivated specifically in stromal fibroblasts using Cre‐loxP technology. The mice showed prostatic intraepithelial neoplasia and squamous cell carcinomas of the forestomach by 6 weeks of age.( 47 , 48 ) Tgfrb2‐deficient fibroblasts overexpressed HGF, and the increase in activating phosphorylation of the cognate HGF receptor, c‐Met, was found in forestomach carcinoma cells. This suggests that activation of paracrine HGF signaling is one possible mechanism for the stimulation of epithelial cell proliferation. As the transforming growth factor‐β signaling pathway can indirectly inhibit epithelial proliferation when acting in adjacent stromal fibroblasts in vivo, loss of this pathway in fibroblasts results in increased epithelial proliferation and might promote invasive carcinoma in some tissues.
No strong strategy for identifying genes controlling the microenvironment has been proposed. It might be worth searching genes that are expressed in the stromal cells and influence proliferation or apoptosis of thymocytes. Otherwise, because Notch ligands are such genes, it might be useful to generate mice in which one of the Notch ligands is activated specifically in thymic stromal cells and test whether or not it can promote the development of thymic lymphomas. We conducted a genetic strategy, however, that elucidates complex traits of mouse thymic lymphoma susceptibility, because there are several findings suggesting that susceptibility genes control the microenvironment.
Susceptibility to lymphomas and radiation effect on the thymus and bone marrow cells differ among mouse strains. For instance, BALB/c and C57BL/10(B10) strains develop thymic lymphomas at high frequencies after γ‐irradiation whereas MSM and C3H strains do not.( 49 , 50 ) Interestingly, bone marrow transplantation from C3H into B10, but not B10 into C3H, can suppress γ‐ray induction of thymic lymphomas, suggesting that susceptibility of B10 mice relative to C3H mice is ascribed to bone marrow‐derived cells.( 49 , 51 ) This indicates that susceptibility genes might control the reconstitution of the thymic microenvironment after irradiation. Susceptibility seems to be a complex property, however, because the suppression by bone marrow transplantation does not take place for induction of thymic lymphomas by a chemical carcinogen, dimethylbenz(a)anthracene.( 52 ) The difference might be that radiation has an ability to penetrate cells and to deposit energy within them in a random fashion whereas chemical agents are affected by cellular barriers and modifying enzymes.
Our study of susceptibility used backcross and congenic mice between BALB/c and MSM and mapped susceptibility/resistance loci of lymphomas near D4Mit12 on chromosome 4 and D5Mit7 on chromosome 5.( 50 ) N‐methyl‐N‐nitrosourea given to mice induces thymic lymphomas at a high frequency. The chemical carcinogen gives base changes of DNA whereas ionizing radiation mainly induces DNA DSBs. Our study of the N‐methyl‐N‐nitrosourea‐induced thymic lymphomas showed that susceptibility was affected by the D4Mit12 but not by the D5Mit7 locus.( 53 , 54 ) This suggests that the two loci harbor different types of susceptibility genes.
Candidate susceptibility gene: Mtf‐1
Fine mapping and sequencing of the susceptibility locus near D4Mit12 allowed us to identify Mtf‐1 as a candidate susceptibility gene to mouse thymic lymphomas.( 55 ) The Mtf‐1 gene encodes a transcription factor regulating responses to heavy metals and γ‐irradiation.( 56 ) Exposure of cells to γ‐radiation activates cytoplasmic signaling pathways including that of the MTF‐1 through the generation of reactive oxygen species( 57 ) and this modulates apoptosis and repair processes. Activated MTF‐1 in the cytoplasm enters the nucleus and activates transcription of target genes such as MT1 and PlGF. MT1 and MT2 are efficient scavengers of hydroxyl radicals, and yeast and mammalian MTs can functionally substitute for superoxide dismutase in protecting yeast from oxidative stress.( 58 ) PlGF is a survival factor for macrophages and vascular cells and inhibits apoptosis in vitro. ( 59 )
Sequence analysis of the coding region of Mtf‐1 revealed that one of the eight SNPs identified alters the corresponding amino acid at position 424 from a serine in BALB/c to proline in MSM. This polymorphism might be relevant for the different susceptibility, because mouse MTF‐1 possessing serine displays a reduced metal response compared to human MTF‐1 with proline at the corresponding position when transfected transiently.( 60 ) We produced a mouse Mtf‐1 clone encoding proline in the place of serine and asked whether or not this change confers a higher metal responsiveness. Transfection assay indeed showed a higher inducibility of the proline‐type Mtf‐1 upon zinc administration. Furthermore, congenic mice of BALB/c background substituting the D4Mit12 locus for the MSM genome showed higher inducibilities of MT1 and PlGF after irradiation than the parental BALB/c mice.( 55 ) These results strongly suggest that this Mtf‐1 polymorphism is responsible for the strain difference in the transcriptional activation of MTF‐1 downstream genes. Thus, we interpret that the Mtf‐1 allele inherited from resistant strains shows higher radiation inducibility of target genes, hence highly inducible strains are more resistant to cancer development by being refractory to radiation effects.
Radiation exposure to mice induces apoptosis leading to reduced cellularity of thymocytes, then residual thymocytes start to proliferate up to a normal level. Our preliminary analysis showed that this recovery process of thymus differs between susceptible and resistant strains. The number of thymocytes decreased to between 1% and 10% of normal levels within 3 days of γ‐irradiation, depending on the dose of radiation. Cellularity differed after 5 or 7 days between the susceptible BALB/c strain and the congenic BALB/c strain with the substituted D4Mit4 locus (Maruyama et al., unpublished data). Radiation‐induced apoptosis of thymocytes might be a key factor contributing to lymphomagesis as described in the second paragraph, therefore this difference might be an important property conferred by susceptibility genes. However, it has yet to be tested vigorously whether or not the Mtf‐1 polymorphism is relevant for this different susceptibility.
Acknowledgments
This work was supported by grants‐in‐aid for cancer research by the Ministry of Education, Culture, Sports, Science and Technology of Japan, and also from the Ministry of Health, Labour and Welfare of Japan.
References
- 1. Kumar R, Sukumar S, Barbacid M. Activation of ras oncogenes preceding the onset of neoplasia. Science 1990; 248: 1101–4. [DOI] [PubMed] [Google Scholar]
- 2. Lu SJ, Archer MC. Ha‐ras oncogene activation in mammary glands of N‐methyl‐N‐nitrosourea‐treated rats genetically resistant to mammary adenocarcinogenesis. Proc Natl Acad Sci USA 1992; 89: 1001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mori H, Colman SM, Xiao Z et al. Chromosome translocations and covert leukemic clones are generated during normal fetal development. Proc Natl Acad Sci USA 2002; 99: 8242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Little JB. Radiation carcinogenesis. Carcinogenesis 2000; 1: 397–404. [DOI] [PubMed] [Google Scholar]
- 5. Brathwaite O, Bayona W, Newcomb EW. p53 mutations in C57BL/6J murine thymic lymphomas induced by γ‐irradiation and N‐methylnitrosourea. Cancer Res 1992; 52: 3791–5. [PubMed] [Google Scholar]
- 6. Kemp CJ, Wheldon T, Balmain A. p53‐deficient mice are extremely susceptible to radiation‐induced tumorigenesis. Nat Genet 1994; 8: 66–9. [DOI] [PubMed] [Google Scholar]
- 7. Kaplan HS. The role of radiation in experimental leukemogenesis. Natl Cancer Inst Monogr 1964; 14: 207–17. [PubMed] [Google Scholar]
- 8. Ludwig FC, Elashoff RM, Wellington JS. Murine radiation leukemia and the preleukemic state. Lab Invest 1968; 19: 240–51. [Google Scholar]
- 9. Sado T, Kamisaku H, Kubo E. Bone marrow–thymus interactions during thymic lymphomagenesis induced by fractionated radiation exposure in B10 mice: analysis using bone marrow transplantation between Thy 1 congenic mice. J Radiat Res 1991; 32: 168–80. [DOI] [PubMed] [Google Scholar]
- 10. Kaplan HS, Carnes WH, Brown MB et al. Indirect induction of lymphomas in irradiated mice. I. Tumor incidence and morphology in mice bearing nonirradiated thymic grafts. Cancer Res 1956; 16: 422–5. [PubMed] [Google Scholar]
- 11. Humblet C, Defresne MP, Greimers R et al. Further studies on the mechanism of radiation induced thymic lymphoma prevention by bone marrow transplantation in C57BL mice. Leukemia 1989; 3: 813–18. [PubMed] [Google Scholar]
- 12. Sakata J, Inoue J, Ohi H et al. Involvement of V(D)J recombinase in the generation of intragenic deletions in the Rit1/Bcl11b tumor suppressor gene in gamma‐ray‐induced thymic lymphomas and in normal thymus of the mouse. Carcinogenesis 2004; 25: 1069–75. [DOI] [PubMed] [Google Scholar]
- 13. Gray DH, Ueno T, Chidgey AP et al. Controlling the thymic microenvironment. Curr Opin Immunol 2005; 17: 137–43. [DOI] [PubMed] [Google Scholar]
- 14. Robey EA, Bluestone JA. Notch signaling in lymphocyte development and function. Curr Opin Immunol 2004; 16: 360–6. [DOI] [PubMed] [Google Scholar]
- 15. Ellisen LW, Bird J, Sklar J et al. TAN‐1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 1991; 66: 649–61. [DOI] [PubMed] [Google Scholar]
- 16. Pear WS, Soffer B, Baltimore D et al. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J Exp Med 1996; 183: 2283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Graham M, Adams JM, Cory S. Murine T lymphomas with retroviral inserts in the chromosomal 15 locus for plasmacytoma variant translocations. Nature 1985; 314: 740–3. [DOI] [PubMed] [Google Scholar]
- 18. Zhuang SM, Schippert A, Haugen‐Strano A et al. Inactivations of p16INK4a‐alpha, p16INK4a‐beta and p15INK4b genes in 2′,3′‐dideoxycytidine‐ and 1,3‐butadiene‐induced murine lymphomas. Oncogene 1998; 16: 803–8. [DOI] [PubMed] [Google Scholar]
- 19. Mao HJ, Wu D, Perez‐Losada J et al. Genetic interactions between Pten and p53 in radiation‐induced lymphoma development. Oncogene 2003; 2: 8379–85. [DOI] [PubMed] [Google Scholar]
- 20. Matsumoto Y, Kosugi S, Shinbo T et al. Allelic loss analysis of gamma‐ray‐induced mouse thymic lymphomas: two candidate tumor suppressor gene loci on chromosomes 12 and 16. Oncogene 1998; 6: 2747–54. [DOI] [PubMed] [Google Scholar]
- 21. Okano H, Saito Y, Miyazawa T et al. Homozygous deletions and point mutations of the Ikaros gene in gamma‐ray‐induced mouse thymic lymphomas. Oncogene 1999; 18: 6677–83. [DOI] [PubMed] [Google Scholar]
- 22. Shinbo T, Matsuki A, Matsumoto Y et al. Allelic loss mapping and physical delineation of a region harboring a putative thymic lymphoma suppressor gene on chromosome 12. Oncogene 1999; 18: 4131–6. [DOI] [PubMed] [Google Scholar]
- 23. Wakabayashi Y, Inoue J, Takahashi Y et al. Homozygous deletions and point mutations of the Rit1/Bcl11b gene in gamma‐ray induced mouse thymic lymphomas. Biochem Biophy Res Comm 2003; 301: 598–603. [DOI] [PubMed] [Google Scholar]
- 24. Georgopoulos K, Moore DD, Derfler B. Ikaros, an early lymphoid‐specific transcription factor and a putative mediator for T cell commitment. Science 1992; 258: 808–12. [DOI] [PubMed] [Google Scholar]
- 25. Winandy S, Wu P, Georgopoulos K. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell 1995; 83: 289–99. [DOI] [PubMed] [Google Scholar]
- 26. Yagi T, Hibi S, Takanashi M et al. High frequency of Ikaros isoform 6 expression in acute myelomonocytic and monocytic leukemias: implications for up‐regulation of the antiapoptotic protein Bcl‐xL in leukemogenesis. Blood 2002; 99: 1350–5. [DOI] [PubMed] [Google Scholar]
- 27. Sun L, Heerema N, Crotty L et al. Expression of dominant‐negative and mutant isoforms of the antileukemic transcription factor Ikaros in infant acute lymphoblastic leukemia. Proc Nat Acad Sci USA 1999; 96: 680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ruiz A, Jiang J, Kempski H et al. Overexpression of the Ikaros 6 isoform is restricted to t(4;11) acute lymphoblastic leukaemia in children and infants and has a role in B‐cell survival. Br J Haematol 2004; 125: 31–7. [DOI] [PubMed] [Google Scholar]
- 29. Wakabayashi Y, Watanabe H, Inoue J et al. Bcl11b is required for differentiation and survival of αβ T lymphocytes. Nat Immunol 2003; 4: 533–9. [DOI] [PubMed] [Google Scholar]
- 30. Avram D. Isolation of a novel family of C2H2 zinc finger proteins implicated in transcriptional repression mediated by chicken ovalbumin upstream promoter transcription factor (COUP‐TF) orphan nuclear receptors. J Biol Chem 2000; 275: 10315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakamura T, Largaespada DA, Shaughnessy et al. Cooperative activation of Hoxa and Pbx1‐related genes in murine myeloid leukaemias. Nat Genet 1996; 12: 149–53. [DOI] [PubMed] [Google Scholar]
- 32. Liu P, Keller RJ, Ortiz M et al. Bcl11a is essential for normal lymphoid development. Nat Immunol 2003; 4: 525–32. [DOI] [PubMed] [Google Scholar]
- 33. Arlotta P, Molyneaux BJ, Chen J et al. Neuronal subtype‐specific genes that control corticospinal motor neuron development in vivo . Neuron 2005; 45: 207–21. [DOI] [PubMed] [Google Scholar]
- 34. Habu S, Kimura M, Nomura T et al. Correlation of T cell receptor gene rearrangements to T cell surface antigen expression and to serum immunoglobulin level in scid mice. Eur J Immunol 1987; 17: 1467. [DOI] [PubMed] [Google Scholar]
- 35. Shinkai Y, Koyasu S, Alt FW et al. Restoration of T cell development in RAG‐2‐deficient mice by functional TCR transgenes. Science 1993; 259: 822. [DOI] [PubMed] [Google Scholar]
- 36. Haks MC, Krimpenfort P, Kruisbeek AM et al. Pre‐TCR signaling and inactivation of p53 induces crucial cell survival pathways in pre‐T cells. Immunity 1999; 11: 91. [DOI] [PubMed] [Google Scholar]
- 37. Okazuka K, Wakabayashi Y, Kashihara M et al. p53 prevents maturation of T cell development to the immature CD4−CD8+ stage in Bcl11b −/– mice. Biochem Biophys Res Commun 2005; 328: 545–9. [DOI] [PubMed] [Google Scholar]
- 38. Inoue J, Kanefuji T, Okazuka K et al. Expression of TCRαβ partly rescues developmental arrest and apoptosis of αβ T cells in Bcl11b −/– mice. J Immunology 2006. [DOI] [PubMed]
- 39. Itoyama T, Chaganti RS, Yamada Y et al. Cytogenetic analysis and clinical significance in adult T‐cell leukemia/lymphoma: a study of 50 cases from the human T‐cell leukemia virus type‐1 endemic area, Nagasaki. Blood 2001; 97: 3612–20. [DOI] [PubMed] [Google Scholar]
- 40. Bernard OA, Busson‐LeConiat M, Ballerini P et al. A new recurrent and specific cryptic translocation, t(5;14)(q35;q32), is associated with expression of the Hox11L2 gene in T acute lymphoblastic leukemia. Leukemia 2001; 15: 1495–504. [DOI] [PubMed] [Google Scholar]
- 41. Bezrookove V, Van Zelderen‐Bhola LS et al. A novel t(6;14)(q25–q27;q32) in acute myelocytic leukemia involves the BCL11B gene. Cancer Genet Cytogenet 2004; 149: 72–6. [DOI] [PubMed] [Google Scholar]
- 42. Cayuela JM, Gardie B, Sigaux F. Disruption of the multiple tumor suppressor gene MTS1/p16INK4a/CDKN2 by illegitimate V(D)J recombinase activity in T‐cell acute lymphoblastic leukemias. Blood 1997; 90: 3720–6. [PubMed] [Google Scholar]
- 43. Ferguson DO, Sekiguchi JM, Chang S et al. The nonhomologous end‐joining pathway of DNA repair is required for genomic stability and the suppression of translocations. Proc Natl Acad Sci USA 2000; 97: 6630–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell 2002; 109: 45–55. [DOI] [PubMed] [Google Scholar]
- 45. Aplan PD, Lombardi DP, Ginsberg AM et al. Disruption of the human SCL locus by ‘illegitimate’ V‐(D)‐J recombinase activity. Science 1990; 250: 1426–9. [DOI] [PubMed] [Google Scholar]
- 46. Kuperwasser C, Chavarria T, Wu M et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci USA 2004; 101: 4966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bhowmick NA, Chytil A, Plieth D et al. TGF‐beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 2004; 303: 848–51. [DOI] [PubMed] [Google Scholar]
- 48. Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature 2004; 432: 332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kamisaku H, Aizawa S, Tanaka K et al. Different cellular basis for the resistance of C3H and STS strain mice to the development of thymic lymphomas following fractionated whole‐body irradiation: analysis using radiation bone marrow chimeras. Int J Radiat Biol 2000; 76: 1105–11. [DOI] [PubMed] [Google Scholar]
- 50. Saito Y, Ochiai Y, Kodama Y et al. Genetic loci controlling susceptibility to gamma‐ray‐induced thymic lymphoma. Oncogene 2001; 20: 5243–7. [DOI] [PubMed] [Google Scholar]
- 51. Kamisaku H, Aizawa S, Kitagawa M et al. Limiting dilution analysis of T‐cell progenitors in the bone marrow of thymic lymphoma‐susceptible B10 and ‐resistant C3H mice after fractionated whole‐body X‐irradiation. Int J Radiat Biol 1997; 72: 191–9. [DOI] [PubMed] [Google Scholar]
- 52. Chen L. Further studies of the effect of bone marrow cells on chemically induced lymphoma in C57BL‐6 mice. Br J Cancer 1970; 24: 554–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kodama Y, Yoshikai Y, Tamura Y et al. The D5Mit7 locus on mouse chromosome 5 provides resistance to gamma‐ray‐induced but not N‐methyl‐N‐nitrosourea‐induced thymic lymphomas. Carcinogenesis 2004; 25: 143–8. [DOI] [PubMed] [Google Scholar]
- 54. Sato H, Tamura Y, Ochiai Y et al. The D4Mit12 locus on mouse chromosome 4 provides susceptibility to both gamma‐ray induced and N‐methyl‐N‐nitrosourea‐induced thymic lymphomas. Cancer Sci 2003; 94: 668–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tamura Y, Maruyama M, Mishima Y et al. Predisposition to mouse thymic lymphomas in response to ionizing radiation depends on variant alleles encoding metal responsive transcription factor‐1 (Mtf‐1). Oncogene 2005; 24: 399–406. [DOI] [PubMed] [Google Scholar]
- 56. Lichtlen P, Schaffner W. Putting its fingers on stressful situations: the heavy metal‐regulatory transcription factor MTF‐1. Bioessays 2001; 23: 1010–17. [DOI] [PubMed] [Google Scholar]
- 57. Criswell T, Leskov K, Miyamoto S et al. Transcription factors activated in mammalian cells after clinically relevant doses of ionizing radiation. Oncogene 2003; 22: 5813–27. [DOI] [PubMed] [Google Scholar]
- 58. Tamai KT, Gralla EB, Ellerby LM et al. Yeast and mammalian metallothioneins functionally substitute for yeast copper‐zinc superoxide dismutase. Proc Natl Acad Sci USA 1993; 90: 8013–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Adini A, Kornaga T, Firoozbakht F et al. Placental growth factor is a survival factor for tumor endothelial cells and macrophages. Cancer Res 2002; 62: 2749–52. [PubMed] [Google Scholar]
- 60. Radtke F, Georgiev O, Muller HP et al. Functional domains of the heavy metal‐responsive transcription regulator MTF‐1. Nucleic Acids Res 1995; 23: 2277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]


