Abstract
Somatostatin analogues ameliorated many symptoms caused by neuroendocrine tumors (NET), but their antitumor activities are limited especially in non‐functioning cases. An overactivation of signaling pathways under receptor tyrosine‐kinase (RTK) has been recently demonstrated in some NET patients, but its details have remained largely unknown. Therefore, in this study, we immunolocalized therapeutic factors and evaluated the data to study the clinical significance of the molecules in non‐functioning Japanese gastrointestinal NET. Fifty‐two NET cases were available for examination in this study and expression of somatostatin receptor (sstr) 1, 2A, 2B, 3 and 5, activated form of mammalian target of rapamycin (mTOR), eukaryotic initiation factor 4‐binding protein 1 (4EBP1), ribosomal protein s6 (S6), extracellular signal‐regulated kinase (ERK) and insulin‐like growth factor 1 receptor (IGF‐1R) was evaluated using immunohistochemistry. We then studied the correlation among the immunohistochemical results of the individual cases using hierarchical clustering analysis. Results of clustering analysis demonstrated that NET cases were basically classified into Cluster I and II. Cluster I was associated with higher expression of sstr1, 2B and 3 and Cluster II was characterized by an activation of the PI3K/Akt pathway and IGF‐1R and higher proliferative status. Cluster II was further classified into Cluster IIa and IIb. Cluster IIa was associated with higher expression of sstr1 and 5 and higher proliferative status and Cluster IIb was characterized by ERK activation. Hierarchical clustering analysis of immunoreactivity of the therapeutic factors can classify NET cases into three distinctive groups and the medical treatment may be determined according to this novel classification method for non‐functioning NET patients. (Cancer Sci 2010; 00: 000–000)
Gastroenteropancreatic endocrine tumors or neuroendocrine tumors (NET) are generally classified into two groups in terms of their localization: gastrointestinal (GI) NET or endocrine tumor and pancreatic NET.( 1 ) Both of these tumors are considered to arise from neuroendocrine cells and are histologically characterized by positive reactions to various neuroendocrine markers, including chromogranin A, neuron specific enolase (NSE), synaptophysin, and so on. In Japan, the number of GI NET patients is far more than that of pancreatic NET( 2 ) and the great majority of these cases are clinically non‐functioning NET. A GI NET is often slow‐growing and indolent, and may not become clinically apparent until the manifestation of metastatic spread,( 3 , 4 ) especially those occurring in the foregut and hindgut, the most prevalent GI NET in Japanese population.( 2 ) Therefore, it has become clinically important to manage these non‐functioning advanced NET cases using medical therapy.
We previously reported that somatostatin receptor (sstr) subtypes have been recently demonstrated in the great majority of GI NET.( 5 ) In addition, somatostatin analogues (SSA) such as octreotide that interact with sstr subtypes are the most widely used therapeutic option for NET. They are generally well tolerated and highly effective in reducing various hormone‐related symptoms caused by NET, but are not necessarily so in controlling cell proliferation of tumor cells, especially in clinically non‐functioning NET cases.( 6 ) Therefore, it has become important to examine the mechanisms related to cell proliferation, especially those related to intracellular signaling pathways, other than the somatostatin pathway in individual NET patients, in order to further explore effective antitumor agents.
Mammalian target of rapamycin (mTOR) is a serine/threonine kinase working as a central regulator of many biological processes that are essential for cell proliferation, translation and cell metabolism.( 7 , 8 , 9 ) It is regulated by upstream, phosphatidylinositol 3‐kinase (PI3K)/Akt/mTOR signaling pathway.( 10 ) An activation of this pathway has been commonly detected in various tumor types,( 11 ) and may be caused by an overactivation of insulin‐like growth factor 1 receptor (IGF‐1R) in NET cells.( 12 ) In addition, a previous study demonstrated an overexpression of activated (phosphorylated) extracellular signal‐regulated kinase (ERK) in NET cases.( 13 ) However, the correlation among these pathways and of those with sstr‐mediated pathways has not been reported at all in NET cases.
Therefore, in our present study, we first evaluated sstr subtypes, those involved in the signaling pathway under receptor tyrosine‐kinase (RTK) (PI3K/Akt/mTOR and MEK/ERK pathways) and a major therapeutic targeted RTK (IGF‐1R) in 52 clinically non‐functioning Japanese NET cases using immunohistochemical methods. We then examined the correlation among these factors above and that with the clinicopathological factors of individual patients using hierarchical clustering analysis, an established effective method for analyzing high‐volume immunohistochemical data, which was recently applied to an analysis of leiomyosarcoma and breast carcinoma cases.( 14 , 15 ) We then added the in vitro experiments in order to further evaluate the antitumor activities of rapamycin, one of the mTOR inhibitors.
Materials and Methods
Tumor tissues. Fifty‐two Japanese cases of GI NET or endocrine tumors were retrieved from the surgical pathology files at the Department of Pathology, Tohoku University Hospital and Sendai Red Cross Hospital (both in Sendai, Miyagi, Japan). Review of the charts of individual patients showed that none of the cases examined had demonstrated any clinical evidence of endocrine abnormalities prior to surgery and all tumors were diagnosed as non‐functional GI NET. The specimens had all been fixed in 10% formalin and embedded in paraffin. The clinicopathological features are summarized in Table 1. We classified the status of age (years) into <60 or ≥60 based on the reported average year of NET patients in Japan.( 2 ) Epidemiological studies in Japanese patients of GI NET demonstrated that the frequency of MEN1 genetic abnormalities was only 1%.( 2 , 16 ) However, further investigation is required for clarification. Research protocols for this study were approved by the Ethics Committee at the Tohoku University School of Medicine (2008‐122) and Sendai Red Cross Hospital (No. 32).
Table 1.
Summary of clinicopathological findings in 52 non‐functioning neuroendocrine tumor cases examined in the present study
| No. cases (n = 52) | |
|---|---|
| Age (years) | |
| <60 | 35 (67.3%) |
| ≥60 | 17 (32.7%) |
| Mean ± SD | 51.3 ± 15.4 |
| Gender | |
| Male | 32 (61.5%) |
| Female | 20 (38.5%) |
| Localization† | |
| Foregut | 15 (28.9%) |
| Midgut | 2 (3.8%) |
| Hindgut | 35 (67.3%) |
| Lymph metastasis | |
| Presence | 3 (5.8%) |
| Absence | 49 (94.2%) |
| Vascular invasion | |
| Presence | 14 (26.9%) |
| Absence | 38 (73.1%) |
†Details of localization are as follows: foregut, 15 (lung, 5; stomach, 4; bronchus, 2; duodenum, 2; liver, 1; middle ear, 1); midgut, 2 (appendix, 2); hindgut 36 (rectum, 35; sigmoid colon, 1).
Immunohistochemistry. Tissue specimens were immunostained using a biotin‐streptavidin method with a Histofine kit (Nichirei Co. Ltd, Tokyo, Japan: phospho‐ribosomal protein [p‐S6], phospho‐eukaryotic initiation factor 4‐binding protein 1 [p‐4EBP1], p‐ERK, sstr subtypes and Ki‐67) and an EnVision method (Dako, Kyoto, Japan: p‐mTOR and p‐IGF‐1R). In the present study, we did not evaluate the immunoreactivity of sstr4, because sstr4 was rarely reported in NET cells.( 17 , 18 ) The characteristics of antibodies used in our immunostaining are summarized in Table 2. Antigen retrieval for p‐mTOR, p‐S6, p‐4EBP1, and p‐ERK analyses was performed by heating the slides in a microwave at 500 W for 15 min in citric acid buffer (2 mM citric acid and 9 mM trisodium citrate dehydrate, pH 6.0). Antigen retrieval for Ki‐67 and sstrs was performed by heating the slides in an autoclave at 121°C for 5 min in citrate acid buffer. No treatment for antigen retrieval was performed in immunostaining for p‐IGF‐1R. These slides were further incubated with primary antibodies for 36–48 h in a moist chamber at 4°C. The antigen‐antibody complex was then visualized with 3,3′‐diaminobenzidine (DAB) solution (1 mM DAB, 50 mM Tris‐HCl buffer, pH 7.6 and 0.006% H2O2) and counterstained with hematoxylin. Immunoreactivity for p‐mTOR, p‐4EBP1, p‐S6 and p‐ERK was detected in the cytoplasm of tumor cells, and classified into three groups as follows: score 0, negative; score 1, weakly positive; score 2, strongly positive (Table 3). Immunoreactivity for sstr subtypes was detected in the membrane or cytoplasm of tumor cells. An evaluation of immunoreactivity of sstrs was performed as previously reported (Table 3).( 5 ) Immunoreactivity for p‐IGF‐1R was classified into two groups as follows: score 0, negative; score 1, positive. Representative illustration of immunohistochemistry is shown in Figure 1. Ki‐67 immunoreactivity was evaluated in more than 1000 cells and the percentage of immunoreactivity (i.e. labeling index [LI]) was subsequently obtained. We scored the Ki‐67 LI followed by the histopathological grade in NET recently defined by the European Neuroendocrine Tumor Society (ENETS) as follows: score 0, <2%; score 1, 2–20%; score 2, >20% (Table 3).( 19 ) Two of the authors (S.I. and Y.M.) independently evaluated immunoreactivity and the cases of interobserver differences of more than 5% were re‐evaluated together using double‐headed light microscopy. Intraobserver differences were <5%.
Table 2.
Antibodies and their conditions of immunostaining
| Primary antibody | Dilution | Source | Antigen retrieval | Positive control |
|---|---|---|---|---|
| p‐mTOR | 1/50 | Cell Signaling Technology (Beverly, MA, USA) | MW | Rectum |
| p‐4EBP1 | 1/50 | |||
| p‐S6 | 1/100 | |||
| p‐p44/42 MAPK (p‐ERK) | 1/100 | |||
| sstr1, 2A, 2B, 3, 5 | 1/1000 | Gramsh Laboratories (Schwabhausen, Germany) Abnova (Taipei, Taiwan) | MW | Pancreas |
| p‐IGF‐1R | 1/100 | No treatment | Breast carcinoma | |
| Ki‐67 | 1/100 | Dako Cytomation (Glostrup, Denmark) | AC | Tonsil |
AC, autoclave treatment; 4EBP1, eukaryotic initiation factor 4‐binding protein 1; IGF‐1R, insulin‐like growth factor 1 receptor; MAPK, mitogen‐activated protein kinase; mTOR, mammalian target of rapamycin; MW, microwave treatment; S6, ribosomal protein s6; sstr, somatostatin receptor.
Table 3.
Summary of scoring of immunoreactivity used in the present study
| Primary antibody | Score 0 | Score 1 | Score 2 |
|---|---|---|---|
| p‐mTOR | Negative | Weakly positive | Strongly positive |
| p‐4EBP1 | |||
| p‐S6 | |||
| p‐ERK | |||
| Ki‐67 LI | <2% | 2–20% | >20% |
| sstr1, 2A, 2B, 3, 5 | Negative | Positive | |
| p‐IGF‐1R |
4EBP1, eukaryotic initiation factor 4‐binding protein 1; IGF‐1R, insulin‐like growth factor 1 receptor; LI, labeling index; mTOR, mammalian target of rapamycin; S6, ribosomal protein s6; sstr, somatostatin receptor.
Figure 1.
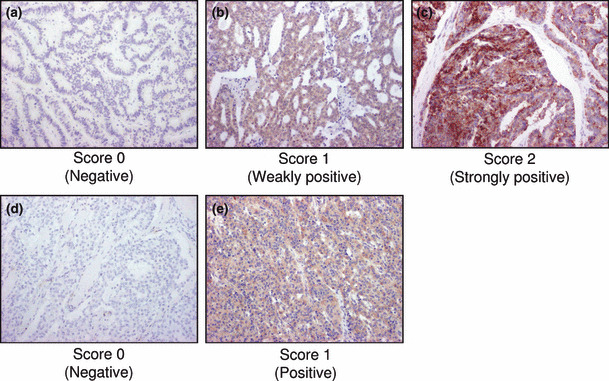
Representative illustrations of immunohistochemistry. Results of immunohistochemistry were evaluated according to Table 3. (a) p‐4EBP1 (score 0, negative); (b) p‐4EBP1 (score 1, weakly positive); (c) p‐4EBP1 (score 2, strongly positive); (d) somatostatin receptor 1 (sstr1) (score 0, negative); (e) sstr1 (score 1, positive). (a–e) Original magnification, ×100.
Cell culture and reagents. NCI‐H727 (H727), a human bronchial NET cell line, was purchased from the American Type Culture Collection (Manassas, VA, USA). COLO320‐DM (COLO), a human colon NET cell line, was purchased from The Health Science Research Resources Bank (Osaka, Japan). These cell lines were maintained in RPMI‐1640 medium (Sigma Aldrich Co., St Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS; Nichirei Co. Ltd, Tokyo, Japan). Cells were maintained at 37°C, 95% relative humidity and 5% CO2. Rapamycin was purchased from Wako (Osaka, Japan).
Cell proliferation assay. The status of cell proliferation of H727 and COLO cells was determined using WST‐8 (2‐[2‐methoxy‐4‐nitrophenyl]‐3‐[4‐nitrophenyl]‐5‐[2, 4‐disulfophenyl]‐2H‐tetrazolium monosodium salt) method (Cell Counting kit‐8; Dojindo Inc., Kumamoto, Japan). Cells were seeded in 96‐well plates at a density of 5000 cells/well. After 24 h of incubation, different concentrations of rapamycin were added into the medium. Compound was added with the exchange of medium every 3 days and measured for 3, 6 and 9 days (H727) or 1, 2 and 3 days (COLO). The medium including reagents was changed every 3 days. A volume of 10 μL of 5 mM WST‐8 was added and the plates were then incubated for 1–4 h at 37°C in 95% relative humidity and 5% CO2. The resulting optical densities (OD; 450 nm) were obtained using a Model 680 microplate reader (Bio‐Rad Laboratories, Hercules, CA, USA). The status of cell proliferation (%) was calculated according to the following equation: (cell OD value after treatment with test materials/vehicle control cell OD value) ×100.
Statistical analysis. We used hierarchical clustering analysis to sort the results of the immunohistochemistry and to further evaluate the correlation of immunohistochemical data with clinicopathological findings of individual NET cases. Hierarchical clustering analysis attempts to identify homogeneous subgroups of the cases examined are as reported by Eisen et al. ( 20 ) The correlation between individual cases and cell signaling factors is depicted graphically as a dendrogram in which branch length is determined by the distance between the results of the immunohistochemistry. Data were subjected to hierarchical clustering analysis and visualization using Cluster and TreeView, respectively (downloaded from the Eisen Lab, Barkley, CA, USA). Chi‐squared tests were used to determine which markers contributed to the formation of individual clusters. The statistical analysis on the results of cell proliferation was analyzed with Sheffe test (StatView ver. 5.0, SAS institute, Cary, NC, USA). A P‐value < 0.05 indicated the statistical significance in this study.
Results
Immunoreactivity in cell‐signaling molecules in NET cases. Results of immunohistochemical staining of sstr subtypes, activated (phosphorylated) forms of intracellular signaling factors (mTOR, 4EBP1, S6 and ERK) and IGF‐1R examined are summarized in Table 4 and 2, 3. Immunoreactivity for p‐mTOR, p‐4EBP1, p‐S6 and p‐ERK was detected in the cytoplasm of tumor cells in 33 (63.5%), 43 (82.7%), 27 (51.9%) and 18 (34.6%) of 52 NET cases examined, respectively. Immunoreactivity for sstr1, 2A, 2B, 3, 5 and p‐IGF‐1R was detected in the membrane or cytoplasm of tumor cells in 27 (51.9%), 48 (92.3%), 20 (38.5%), 29 (55.8%), 39 (75.0%) and 38 (73.1%) of 52 cases examined, respectively.
Table 4.
Summary of scoring of immunohistochemistry in the present study of neuroendocrine tumor cases
| Total (n = 52) | Score 0 | Score 1 | Score 2 | Total (n = 52) | Score 0 | Score 1 |
|---|---|---|---|---|---|---|
| p‐mTOR | 19 (36.5%) | 29 (55.8%) | 4 (7.7%) | sstr1 | 25 (48.1%) | 27 (51.9%) |
| p‐4EBP1 | 9 (17.3%) | 33 (63.5%) | 10 (19.2%) | sstr2A | 4 (7.7%) | 48 (92.3%) |
| p‐S6 | 25 (48.1%) | 24 (46.2%) | 3 (5.8%) | sstr2B | 32 (61.5%) | 20 (38.5%) |
| p‐ERK | 34 (65.4%) | 18 (34.6%) | 0 (0.0%) | sstr3 | 23 (44.2%) | 29 (55.8%) |
| Ki‐67 | 39 (75.0%) | 12 (23.1%) | 1 (1.9%) | sstr5 | 13 (25.0%) | 39 (75.0%) |
| p‐IGF‐1R | 14 (26.9%) | 38 (73.1%) | ||||
4EBP1, eukaryotic initiation factor 4‐binding protein 1; IGF‐1R, insulin‐like growth factor 1 receptor; mTOR, mammalian target of rapamycin; S6, ribosomal protein s6; sstr, somatostatin receptor.
Figure 2.
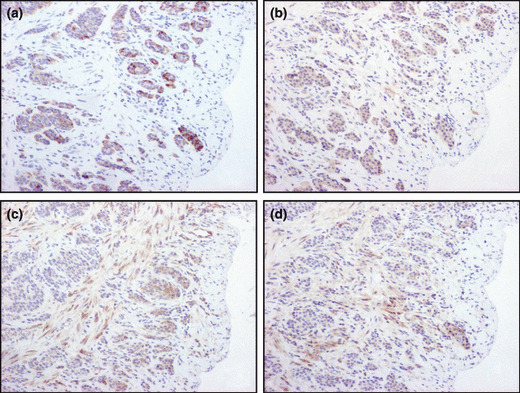
Representative illustrations of immuno‐histochemistry of (a) p‐mTOR, (b) p‐4EBP1, (c) p‐S6 and (d) p‐ERK. Immunoreactivity of all signaling factors was detected in cytoplasmic of tumor cells. (a–d) Original magnification, ×100.
Figure 3.
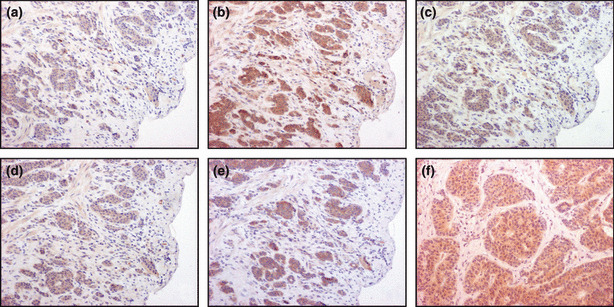
Representative illustrations of immunohistochemistry of somatostatin receptor (sstr) subtypes and p‐IGF‐1R. Immunoreactivity of all sstr subtypes was detected in the membrane or cytoplasmic of the tumor cells. (a) sstr1; (b) sstr2A; (c) sstr2B; (d) sstr3; (e) sstr5; (f) p‐IGF‐1R. (a–f) Original magnification, ×100.
Hierarchical clustering analysis of immunohistochemical results in individual clusters. Hierarchical clustering analysis was applied to results of the immunohistochemistry in NET cases and the correlation was subsequently displayed graphically using the computer program, Cluster and TreeView (downloaded from Eisen Lab; Fig. 4). The patterns of each sstr subtypes obtained were nearly identical in terms of staining patterns in the great majority of tumors, that is, co‐expressing all or none of these markers, especially in those of sstr2A and 5. In addition, there was an almost identical scoring pattern among p‐IGF‐1R, p‐mTOR and p‐4EBP1 and also between p‐ERK and p‐S6, respectively, which indicated that activation of S6 was correlated more with the MEK/ERK pathway rather than the PI3K/Akt pathway in GI NET cases.
Figure 4.
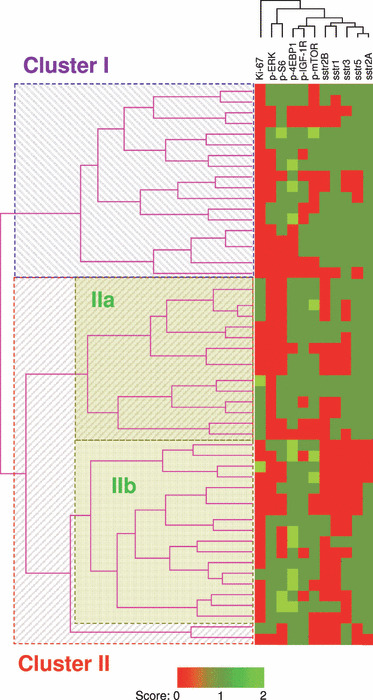
Summary of hierarchical clustering analysis of the immunohistochemical data of 52 neuroendocrine tumor cases. The branch length represents the similarity between results obtained in this system. Neuroendocrine tumor cases in the present study were classified into the following three different groups according to the results: Cluster I, 18 cases; Cluster IIa, 15 cases; Cluster IIb, 17 cases. Two cases belonging to Cluster II were excluded because of the branch length. 4EBP1, eukaryotic initiation factor 4‐binding protein 1; ERK, extracellular signal‐regulated kinase; IGF‐1R, insulin‐like growth factor 1 receptor; mTOR, mammalian target of rapamycin; S6, ribosomal protein s6; sstr, somatostatin receptor.
The results showed that the 52 NET cases examined were basically classified into two clusters, Cluster I (18 cases) and Cluster II (34 cases), and Cluster II was further sub‐classified into Cluster IIa (15 cases) and Cluster IIb (17 cases) according to the branch length, which represents the correlation of the scoring data (Fig. 4). Two cases belonging to Cluster II were eliminated because of the branch length.
Multivariate analysis of clusters of immunoreactivity and clinicopathological characteristics of NET cases examined. We performed chi‐squared tests in order to define the features of each clusters regarding the patterns of immunoreactivity of the factors examined. We first defined the features between Cluster I and Cluster II. Results of this analysis demonstrated that immunoreactivity of p‐ERK, sstr2A and sstr5 did not show any significant differences among the three groups above, but that of p‐mTOR (P = 0.038), p‐4EBP1 (P = 0.026), p‐S6 (P = 0.0066), sstr1 (P = 0.0066), sstr2B (P < 0.001), sstr5 (P = 0.0036), p‐IGF‐1R (P = 0.016) and Ki‐67 (P = 0.018) did show significant differences among the three clusters above (Table 5). These results indicated that the Cluster I cases (n = 18) were associated with expression of the sstr subtypes rather than the proteins in the intracellular signaling pathways. In contrast, the Cluster II cases (n = 34) were associated with relative abundance of p‐mTOR, p‐4EBP1 and p‐S6, compared with the sstr subtypes above and higher proliferative activities. We then studied the correlation between clinicopathological features of individual cases and the clusters above using chi‐squared tests, but there were no significant differences between the clusters of the patients examined (data not shown).
Table 5.
Summary of scoring of immunohistochemistry between Cluster I and II
| Cluster I (n = 18) | Cluster II (n = 34) | P‐value | |
|---|---|---|---|
| p‐mTOR (Score 0 vs 1, 2) | 8 | 25 | 0.038 |
| p‐4EBP1 (Score 0 vs 1, 2) | 12 | 31 | 0.026 |
| p‐S6 (Score 0 vs 1, 2) | 14 | 13 | 0.0066 |
| p‐ERK (Score 0 vs 1, 2) | 7 | 11 | 0.64 |
| sstr1, positive | 14 | 13 | 0.0066 |
| sstr2A, positive | 18 | 30 | 0.13 |
| sstr2B, positive | 13 | 7 | <0.001 |
| sstr3, positive | 15 | 14 | 0.0036 |
| sstr5, positive | 15 | 24 | 0.31 |
| p‐IGF‐1R, positive | 10 | 28 | 0.016 |
| Ki‐67 LI | |||
| <2% | 17 | 22 | 0.018 |
| ≥2% | 1 | 12 | |
4EBP1, eukaryotic initiation factor 4‐binding protein 1; ERK, extracellular signal‐regulated kinase; IGF‐1R, insulin‐like growth factor 1 receptor; LI, labeling index; mTOR, mammalian target of rapamycin; S6, ribosomal protein s6; sstr, somatostatin receptor. The bold values indicate the statistical significance.
We subsequently performed chi‐squared tests between Cluster IIa and Cluster IIb. Results showed that the Cluster IIa cases were associated with higher expression of sstr1 and 5 and higher proliferative status evaluated by Ki‐67 immunohistochemistry (Table 6; P = 0.0048, 0.0049 and 0.0014, respectively). However, the Cluster IIb cases were associated with ERK activation (P < 0.001). Therefore, we then evaluated the correlation of the results with the clinicopathological features above and the results indicated that the status of age and their localization was significantly different between these clusters (Table 7; P = 0.0078 and 0.0043, respectively).
Table 6.
Summary of scoring of immunohistochemistry between Cluster IIa and IIb
| Total (n = 32) | Cluster IIa (n = 15) | Cluster IIb (n = 17) | P‐value | |
|---|---|---|---|---|
| p‐mTOR (Score 0 vs 1, 2) | 25 (78.1%) | 12 | 13 | 0.81 |
| p‐4EBP1 (Score 0 vs 1, 2) | 30 (93.8%) | 13 | 17 | 0.12 |
| p‐S6 (Score 0 vs 1, 2) | 14 (43.8%) | 4 | 10 | 0.067 |
| p‐ERK (Score 0 vs 1, 2) | 11 (34.3%) | 0 | 11 | <0.001 |
| sstr1, positive | 13 (40.6%) | 10 | 3 | 0.0048 |
| sstr2A, positive | 29 (90.6%) | 15 | 14 | 0.087 |
| sstr2B, positive | 7 (21.9%) | 4 | 3 | 0.54 |
| sstr3, positive | 12 (37.5%) | 8 | 4 | 0.082 |
| sstr5, positive | 25 (78.1%) | 15 | 10 | 0.0049 |
| p‐IGF‐1R, positive | 26 (81.3%) | 12 | 14 | 0.84 |
| Ki‐67 LI | ||||
| <2% | 20 (62.5%) | 5 | 15 | 0.0014 |
| ≥2% | 12 (37.5%) | 10 | 2 | |
4EBP1, eukaryotic initiation factor 4‐binding protein 1; ERK, extracellular signal‐regulated kinase; IGF‐1R, insulin‐like growth factor 1 receptor; LI, labeling index; mTOR, mammalian target of rapamycin; S6, ribosomal protein s6; sstr, somatostatin receptor. The bold values indicate the statistical significance.
Table 7.
Characteristics of the clinicopathological findings of individual patients in Cluster IIa and IIb
| Total (n = 32) | Cluster IIa (n = 15) | Cluster IIb (n = 17) | P‐value | |
|---|---|---|---|---|
| Age (years) | ||||
| <60 | 23 (71.9%) | 7 | 16 | 0.0078 |
| ≥60 | 9 (28.1%) | 8 | 1 | |
| Mean ± SD | 52.7 ± 14.2 | 56.4 ± 17.4 | 49.4 ± 10.0 | |
| Gender | ||||
| Male | 22 (68.8%) | 8 | 14 | 0.077 |
| Female | 10 (31.3%) | 7 | 3 | |
| Localization | ||||
| Foregut | 9 (28.1%) | 8 | 1 | 0.0043 |
| Midgut | 1 (3.1%) | 1 | 0 | |
| Hindgut | 22 (68.8%) | 6 | 16 | |
| Lymph metastasis | ||||
| Presence | 3 (9.4%) | 3 | 0 | 0.053 |
| Absence | 29 (90.6%) | 12 | 17 | |
| Vascular invasion | ||||
| Presence | 9 (28.1%) | 6 | 3 | 0.16 |
| Absence | 23 (71.9%) | 9 | 14 | |
The bold values indicate the statistical significance.
Effects of mTOR inhibitors on the cell proliferation in NET cell lines. Because the Cluster II cases were associated with the expression of p‐mTOR and higher proliferative activities, we examined the effects of mTOR inhibitor, rapamycin, on cell proliferation using two NET cell lines, H727 and COLO. We performed a cell proliferation assay at a range of 10−9 to 10−7 M for 9 days (H727) or 3 days (COLO), and the results showed that there was a significant decrease in the cell number for 9 days in H727 and 3 days in COLO treated with rapamycin in a concentration‐dependent manner (Fig. 5).
Figure 5.
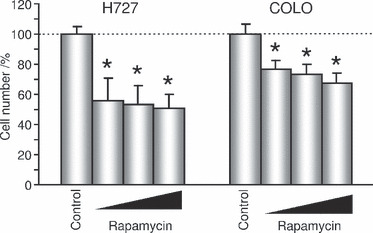
Antitumor effects of rapamycin in neuroendocrine tumor cell lines in a concentration‐dependent manner. Rapamycin, 10−9, 10−8, 10−7 M; H727, NCI‐H727; COLO, COLO320‐DM. All data are shown as mean (n = 6) ±SD. *P < 0.001 (vs Control).
Discussion
It is true that the main therapy of NET is surgical excision. Neuroendocrine tumor patients are generally considered resistant to traditional cytotoxic agents when they are in an advanced clinical stage.( 21 ) In particular, the majority of NET cases arising in the foregut and hindgut, which were the predominant NET cases in Japan,( 2 ) do not manifest clinically detectable endocrine manifestations and may be first detected at advanced clinical stages.( 22 ) Somatostatin receptor subtypes have been demonstrated in the great majority of NET cases, including those arising in the foregut and hindgut, even at advanced clinical stages.( 5 ) Octreotide is well known to inhibit the release of hormones and subsequently control symptoms in NET patients. Recently, a newly developed SOM230 (pasireotide; Novartis, St Louis, MO, USA), which could react with wider sstr subtypes, has been reported to be more effective in controlling cell proliferation and symptoms in preclinical studies.( 23 ) In addition, some groups, including our laboratories, showed the antitumor effects of SSA in preclinical and clinical study.( 5 , 24 , 25 ) However, its clinically effective antitumor activity has not necessarily been detected with octreotide alone, because the tumor response rate for octreotide represents <10%,( 4 , 26 ) and thus, the antitumor activities of SSA have been controversial. Therefore, other modes of medical therapy have been in demand clinically, particularly for controlling tumor cell proliferation of non‐functioning NET cases including those arising in the foregut and hindgut.
Other modes of intracellular signaling pathways have been reported to be involved in NET cases and among these pathways, in particular, mTOR activities have been shown to increase in NET cells, as a result of mutations of the tumor suppressor genes in the PI3K/Akt/mTOR pathway, rather than the genes encoding mTOR. For instance, the loss of heterozygosity of the NF1 gene led to constitutive mTOR activation.( 27 ) Neurofibromatosis type 1 (NF‐1) is an autosomal dominant disorder clinically characterized by the presence of cutaneous and subcutaneous neurofibromas, café‐au‐lait spots and Lisch nodules. Neurofibromatosis type 1 appears to play a role as a tumor suppressor gene to function the Ras pathway.( 28 ) Tumors associated with NF‐1 include not only neurogenic neoplasms such as neurofibromas and neurofibrosarcomas, but also pheochromocytomas and NET, suggesting a broader role for NF‐1 as a tumor suppressor gene. However, the GI NET harboring NF‐1 genetic abnormalities often occurs in duodenal, ampullary NET and somatostatinomas. In addition, the presence of NF‐1 mutations in NET was reported in only 1–2% of cases.( 16 , 29 ) However, it also awaits further investigations to clarify the possible involvement of NF‐1 genetic abnormalities in patients with NET. The overactivation of IGF‐1R is also reported to be correlated with activation of the PI3K/Akt/mTOR pathway in NET cells.( 13 , 30 ) von Wichert et al. ( 12 ) demonstrated that low‐grade NET co‐expressed IGF‐1 and IGF‐1R, and BON, a human pancreatic NET cell line, expressed functionally active IGF‐1R and secreted IGF‐1, which all suggest an autocrine action of this growth factor in NET. In addition, a Phase II clinical trial in which the IGF‐1R monoclonal antibody is used for NET patients is in progress.( 31 ) However, the immunohistochemical study of p‐IGF‐1R in human NET cases has not been previously reported. In addition, correlation of the sstr subtypes with the IGF‐1R signaling pathway has also not been reported.
Therefore, in this study, we evaluated sstr subtypes, key factors in major signaling pathways under RTK and a potential therapeutic targeted RTK in NET cases using immunohistochemistry combined with hierarchical clustering analysis. Neuroendocrine tumors have been reported to be associated with specific patterns of sstr expression and sstr2 and sstr5 were predominant subtypes reported in Japanese NET patients.( 5 ) Somatostatin receptor 1 and sstr3 are expressed less frequently and sstr4 is rarely expressed in NET as described above.( 17 , 18 ) Results of our present immunohistochemical study were also consistent with those reported previously, and in particular, sstr2A and sstr5 were the most frequently detected sstr subtypes in these GI NET.( 2 , 5 ) In addition, results of our present study also showed that the NET cases were basically classified into two different groups, Cluster I and II, and Cluster II was then further sub‐classified into Cluster IIa and IIb. Between Cluster I and II, Cluster I was associated with a higher expression of the sstr subtypes, but there were no significant differences between these two clusters in the expression of sstr2A and sstr5. In addition, all Cluster IIa cases expressed sstr2A, but not Cluster IIb. Therefore, the status of the proliferative activity and lymph node metastasis was indeed associated with that of sstr2A and sstr5 expressions regardless of the status of p‐IGF‐1R immunoreactivity in the cases examined.
Shah et al. ( 14 ) also demonstrated a relative high abundance of p‐endothelial growth factor receptor (p‐EGFR) and p‐ERK in NET cases using immunohistochemistry. In our present study, phosphorylated factors in the PI3K/Akt/mTOR pathway were also detected in many of the NET cases examined, but the cases associated with activated ERK were relatively low in number. Possible reasons for the discrepancy between the report of Shah et al. and our present study might be due to differences of the sensitivities of the primary antibodies, or the majority of the localization (midgut vs hindgut) of the cases examined. In addition, results of our present study demonstrated that the cases belonging to Cluster IIb were associated with PI3K/Akt/mTOR and MEK/ERK pathways related to IGF‐1R. These cases were associated with a relatively low proliferative status but may be treated with mTOR inhibitors/IGF‐1R antagonists combined with MEK inhibitors, but further investigation is required for clarification.
Mammalian target of rapamycin inhibitors are macrolide antibiotics with potent immunosuppressive and antitumor activities. These agents bind immunophilin FK506‐binding protein 12 (FKBP12), and this complex subsequently binds to mTOR, which inhibits downstream signaling pathways.( 32 , 33 ) Recently, the antitumor activities of mTOR inhibitors have been extensively studied, and treatment of the mTOR inhibitors such as temsirolimus (CCI‐779; Wyeth, Philadelphia, PA, USA) and everolimus (RAD001; Novartis, Basel, Switzerland) for advanced renal cell carcinoma after vascular endothelial growth factor receptor (VEGFR)‐targeted therapy have been approved in Europe, the USA and Japan.( 34 , 35 ) In NET patients, the effects of mTOR inhibitors have also been evaluated in both preclinical and clinical studies.( 36 , 37 ) In the present study, we performed an in vitro study using NET cell lines in order to evaluate whether this classification has any relationship with sensitivity to the molecular target therapy. We examined the antitumor effects of rapamycin in NET cell lines, in which the PI3K/Akt/mTOR pathway was shown to be activated,( 38 , 39 ) which suggests that the cases associated with overexpression of p‐mTOR may be treated with mTOR inhibitors.
We subjected the results of the immunohistochemistry into hierarchical clustering analysis. This analysis is one of the multivariative statistical methods that identifies groups of samples that behave similarly or show similar characteristics.( 20 ) Therefore, hierarchical clustering analysis following immunohistochemistry of different molecules may contribute to a potential new classification method according to biological features. Results of the present study revealed that NET cases were basically classified into the “sstr subtypes expressing predominant” group (Cluster I) and the “activating signaling pathways predominant” group (Cluster II), and the latter group was further sub‐classified into the “sstr expression with higher proliferative status predominant” group, or Cluster IIa, and the “activating ERK cascade predominant” group, or Cluster IIb.
In conclusion, we are first to demonstrate the application of a novel classification method for non‐functioning NET patients using hierarchical clustering analysis based on the immunohistochemical data of sstr subtypes, factors of a major signaling pathway under RTK and major RTK and clinicopathological factors of individual patients. It will be important to evaluate which group the cases with non‐functioning NET belong to, and to determine the treatment of adequate drugs for individual NET patients.
Acknowledgments
The authors appreciate Dr Fumiaki Tezuka (Department of Pathology, Sendai Red Cross Hospital) for providing NET samples.
References
- 1. Kloppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann NY Acad Sci 2004; 1014: 13–27. [DOI] [PubMed] [Google Scholar]
- 2. Ito T, Sasano H, Tanaka M et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol 2010; 45: 234–43. [DOI] [PubMed] [Google Scholar]
- 3. Robertson RG, Geiger WJ, Davis NB. Carcinoid tumors. Am Fam Physician 2006; 74: 429–34. [PubMed] [Google Scholar]
- 4. Schnirer II, Yao JC, Ajani JA. Carcinoid – a comprehensive review. Acta Oncol 2003; 42: 672–92. [DOI] [PubMed] [Google Scholar]
- 5. Ono K, Suzuki T, Miki Y et al. Somatostatin receptor subtypes in human non‐functioning neuroendocrine tumors and effects of somatostatin analogues SOM230 on cell proliferation in cell line NCI‐H727. Anticancer Res 2007; 27: 2231–40. [PubMed] [Google Scholar]
- 6. Arnold R, Simon B, Wied M. Treatment of neuroendocrine GEP tumours with somatostatin analogues. Digestion 2000; 62: 84–91. [DOI] [PubMed] [Google Scholar]
- 7. Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer 2004; 4: 335–48. [DOI] [PubMed] [Google Scholar]
- 8. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006; 124: 471–84. [DOI] [PubMed] [Google Scholar]
- 9. Pouysségur J, Dayan F, Mazure N. Hypoxia signaling in cancer and approaches to enforce tumour regression. Nature 2006; 441: 437–43. [DOI] [PubMed] [Google Scholar]
- 10. Manning BD, Cantley LC. United at last: the tuberous sclerosis complex gene products connect the phosphoinositide 3‐kinase/Akt pathway to mammalian target of rapamycin (mTOR) signaling. Biochem Soc Trans 2003; 31: 573–8. [DOI] [PubMed] [Google Scholar]
- 11. Osaki M, Oshimura M, Ito H. PI3K‐Akt pathway: its functions and alterations in human cancer. Apoptosis 2004; 9: 667–76. [DOI] [PubMed] [Google Scholar]
- 12. Von Wichert G, Jehle PM, Hoeflich A et al. Insulin‐like growth factor‐I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumors cells. Cancer Res 2000; 60: 4573–81. [PubMed] [Google Scholar]
- 13. Shah T, Hochhauser D, Frow R, Quaglia A, Dhillon P, Caplin ME. Epidermal growth factor receptor expression and activation in neuroendocrine tumours. J Neuroendocrinol 2006; 18: 355–60. [DOI] [PubMed] [Google Scholar]
- 14. Meijinen P, Perterse JL, Antonini N, Rutgers EJTh, Van De Vijver MJ. Immunohistochemical categorization of ductal carcinoma in situ of the breast. Br J Cancer 2008; 98: 137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carvalho JC, Thomas DG, Lucas DR. Cluster analysis of immunohistochemical markers in leimyosarcoma delineates specific anatomic and gender subgroups. Cancer 2009; 115: 4186–95. [DOI] [PubMed] [Google Scholar]
- 16. Toumpanakis CG, Caplin ME. Molecular genetics of gastroenteropancreatic neuroendocrine tumors. Am J Gastroenterol 2008; 103: 729–32. [DOI] [PubMed] [Google Scholar]
- 17. Papotti M, Bongiovanni M, Valante E et al. Expression of somatostatin receptor types 1–5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse‐transcriptase polymerase chain reaction analysis. Virchows Arch 2002; 440: 461–75. [DOI] [PubMed] [Google Scholar]
- 18. Jais P, Terris B, Ruszniewski P et al. Somatostatin receptor subtype gene expression in human endocrine gastroentero‐pancreatic tumours. Eur J Clin Invest 1997; 27: 639–44. [DOI] [PubMed] [Google Scholar]
- 19. Rindi G, Klöppel G, Alhman H et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 2006; 449: 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome‐wide expression patterns. Proc Natl Acad Sci USA 1998; 95: 14863–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gardner N. Targeting the mTOR pathway in neuroendocrine tumors. Clin J Oncol Nurs 2009; 13: 558–63. [DOI] [PubMed] [Google Scholar]
- 22. Yao JC. Molecular targeted therapy for carcinoid and islet‐cell carcinoma. Clin Endocrinol Metab 2007; 21: 163–72. [DOI] [PubMed] [Google Scholar]
- 23. Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov 2003; 2: 999–1017. [DOI] [PubMed] [Google Scholar]
- 24. Rinke A, Muller HH, Schade‐Brittinger C et al. Placebo‐controlled, double‐blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group. J Clin Oncol 2009; 27: 4656–63. [DOI] [PubMed] [Google Scholar]
- 25. Grozinsky‐Glasberg S, Franchi G, Teng M et al. Octreotide and the mTOR inhibitor RAD001 (everolimus) block proliferation and interact with the Akt‐mTOR‐p70S6K pathway in a neuro‐endocrine tumor cell line. Neuroendocrinology 2008; 87: 168–81. [DOI] [PubMed] [Google Scholar]
- 26. Arnold R, Trautmann ME, Creutzfeldt ME et al. Somatostatin analogue octreotide and inhibition of tumour growth in metastatic endocrine gastroenteropancreatic tumours. Gut 1996; 38: 430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verhoef S, Van Diemen‐Steenvoorde R, Akkersdijk WI et al. Malignant pancreatic tumour within the spectrum of tuberous sclerosis complex in childhood. Eur J Pediatr 1999; 158: 284–7. [DOI] [PubMed] [Google Scholar]
- 28. Basu TN, Gutmann DH, Fletcher JA et al. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature 1992; 356: 713–5. [DOI] [PubMed] [Google Scholar]
- 29. Gutmann DH, Aylsworth A, Carey JC et al. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA 1997; 278: 51–7. [PubMed] [Google Scholar]
- 30. Hopfner M, Baradari V, Huether A et al. The insulin‐like growth factor receptor 1 is a promising target for novel treatment approaches in neuroendocrine gastrointestinal tumours. Endocr Relat Cancer 2006; 13: 135–49. [DOI] [PubMed] [Google Scholar]
- 31. Tolcher AW, Rothenberg ML, Rodon J et al. A phase I pharmacokinetic and pharmacodynamic study of AMG 479, a fully human monoclonal antibody against insulin‐like growth factor type 1 receptor (IGF‐1R), in advanced solid tumors. J Clin Oncol 2007; 25: 3002. [DOI] [PubMed] [Google Scholar]
- 32. Jayaraman T, Marks AR. Rapamycin‐FKBP12 blocks proliferation, induces differentiation, and inhibits cdc2 kinase activity in a myogenic cell line. J Biol Chem 1993; 268: 25385–8. [PubMed] [Google Scholar]
- 33. Vilella‐Bach M, Nuzzi P, Fang Y et al. The FKBP12‐rapamycin‐binding domain is required for FKBP12‐rapamycin‐associated protein kinase activity and G1 progression. J Biol Chem 1999; 274: 4266–72. [DOI] [PubMed] [Google Scholar]
- 34. Motzer RJ, Escudier B, Oudard S et al. Efficacy of everolimus in advanced renal cell carcinoma: a double‐blind, randomized, placebo‐controlled phase III trial. The Lancet 2008; 372: 449–56. [DOI] [PubMed] [Google Scholar]
- 35. Hudes G, Carducci M, Tomczak P et al. A phase 3, randomized, 3‐arm study of temsirolimus (TEMSR) or interferon‐alpha (IFN) or the combination of TEMSR + IFR in the treatment of first‐line, poor‐risk patients with advanced renal cell carcinoma (adv RCC). J Clin Oncol 2006; 24: 2006. [Google Scholar]
- 36. Zitzmann K, De Toni EN, Brand S et al. The novel mTOR inhibitor RAD001 (everolimus) induces antiproliferative effects in human pancreatic neuroendocrine tumor cells. Neuroendocrinology 2007; 85: 54–60. [DOI] [PubMed] [Google Scholar]
- 37. Duran I, Kortmansky J, Singh D et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer 2006; 95: 1148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moreno A, Akcakanat A, Munsell MF et al. Antitumor activity of rapamycin and octreotide as single agents or in combination in neuroendocrine tumors. Endocr Relat Cancer 2008; 15: 257–66. [DOI] [PubMed] [Google Scholar]
- 39. Endo H, Murata K, Mukai M et al. Activation of insulin‐like growth factor signaling induces apoptotic cell death under prolonged hypoxia by enhancing endoplasmic reticulum stress response. Cancer Res 2007; 67: 8095–103. [DOI] [PubMed] [Google Scholar]


