Abstract
We previously reported Frizzled homolog 10 (FZD10), a member of the Frizzled family, to be a promising therapeutic target for synovial sarcomas. In this report, we established a murine monoclonal antibody (MAb), namely, MAb 92‐13 that had specific binding activity against native FZD10 product expressed in synovial sarcoma cell lines. Subsequent immunohistochemical analyses with the MAb 92‐13 confirmed an absence or hardly detectable level of FZD10 protein in any normal human organs. We confirmed the specific binding activity of this MAb in vivo after injection of fluorescent‐labeled MAb i.p. or i.v. into the mice carrying synovial sarcoma xenografts by the use of the in vivo fluorescent imaging system as well as radioisotopes. Moreover, MAb 92‐13 was effectively internalized into the synovial sarcoma cells after its binding to FZD10 on the cell surface. A single i.v. injection of the Yttrium‐90 (90Y)‐MAb 92‐13 drastically suppressed tumor growth of synovial sarcoma in mice without any severe toxicity. Median time to tumor progression was 58 days for mice treated with 90Y‐MAb 92‐13 and 9 days for mice treated with non‐labeled antibody control or untreated mice (difference = 49 days; P = 7 × 10−5). This result indicates that MAb 92‐13 could be utilized as the novel treatment modality for synovial sarcoma and other FZD10‐positive tumors. (Cancer Sci 2008; 99: 432–440)
Monoclonal antibodies (MAb) against cancer‐specific molecules have been proving useful in cancer treatment.( 1 ) In addition to successful examples of clinical application of the humanized or chimeric antibodies such as trastuzumab,( 2 ) rituximab( 3 ) and bevacizumab,( 4 ) a number of monoclonal antibodies against other molecular targets are in development and their antitumor activities being evaluated. These monoclonal antibodies are expected to provide hope to patients suffering tumors without any effective treatments.
Among soft tissue sarcomas, osteosarcoma, Ewing's sarcoma and rhabdomyosarcoma can be well managed by chemotherapy because of their sensitivity to it.( 5 , 6 , 7 ) On the other hand, spindle cell sarcomas are resistant to chemo‐ and radiotherapy, and the patients usually have a poor prognosis. For synovial sarcoma, surgical treatment is effective for patients at an early stage, but no effective therapeutic choice is available to those at an advanced stage. Hence, development of novel therapeutic modalities is essential to improve patients’ prognosis.
Genome‐wide gene expression analysis in tumors provides useful information in identifying promising molecular targets for development of novel anticancer drugs and tumor markers. In our previous study, we analyzed gene‐expression profiles of 47 soft‐tissue sarcomas using a genome‐wide cDNA microarray consisting of 23 040 genes and found that Frizzled homolog 10 (FZD10) was upregulated specifically in synovial sarcomas at a very high level.( 8 ) FZD10 gene product is reported to be a member of the Frizzled family and a putative Wnt signal receptor.( 9 ) We also demonstrated that FZD10 expression was absent or hardly detectable in any normal organs except the placenta, suggesting that therapeutics targeting this molecule would cause no or little adverse reaction.( 10 ) Experiments using siRNA implicated that FZD10 was significantly involved in the tumor growth of synovial sarcoma.( 10 ) Furthermore, we generated the rabbit polyclonal antibody against an extracellular domain of FZD10, and found that this polyclonal antibody had antitumor activity with antibody‐dependent cell‐mediated cytotoxicity (ADCC) in a mouse xenograft model of synovial sarcoma.( 10 ) Together, the antibody therapy against FZD10 could be expected to improve the clinical outcome of synovial sarcoma.
Here, we demonstrate generation of a murine monoclonal antibody against FZD10, MAb 92‐13, by means of cell‐immunization method and reveal its promising effect for clinical application.
Materials and Methods
Cell lines and tissue specimens. Cell lines derived from colon cancers (LoVo and SNU‐C5), and monkey kidney cells transformed with SV40 T‐antigen, COS7 cells, were purchased from were purchased from American Type Culture Collection (ATCC, Rockville, MD). YaFuSS, derived from synovial sarcoma, was kindly provided by Dr J. Toguchida (Faculty of Medicine, Kyoto University, Japan). SYO‐1 was a gift from Dr A. Kawai (National Cancer Center, Japan), HS‐SY‐II from Dr H. Sonobe (Kochi University, Japan), Fuji from Dr S. Tanaka (Hokkaido University, Japan), and 1273/99 from Dr O. Larsson (Karolinska Institute, Sweden). All cells were cultured under their respective depositors’ recommendation: Ham's F‐12 nutrient mixture (Invitrogen, Carlsbad, CA, USA) for LoVo and 1273/99, RPMI‐1640 (Sigma‐Aldrich, St. Louis, MO, USA) for SNU‐C5, Dulbecco's modified Eagle's medium (Invitrogen) for COS7, SYO‐1, YaFuSS, HS‐SY‐II and Fuji. All cell lines were grown in monolayers in appropriate media supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic/antimycotic solution, and maintained at 37°C in air containing 5% CO2. Primary synovial sarcoma samples were obtained after informed consent, and snap‐frozen in liquid nitrogen immediately after resection and stored at –80°C. All tissue samples from surgically resected synovial sarcomas were approved for our analysis by the ethics committees of the Faculty of Medicine, Kyoto University and Institute of Medical Science, University of Tokyo. Each tumor sample was fixed in 10% formalin and routinely processed for hematoxylin–eosin (HE) staining to establish a pathological diagnosis by Dr J. Toguchida.
Plasmid constructs. For construction of N‐terminal HA‐tagged FZD10‐expression vector, we first generated a plasmid with a signal peptide sequence (1–22 residues of FZD10 protein) and a HA epitope tag. The cDNA fragment consisting of residues 1–22 was amplified by polymerase chain reaction (PCR) using PrimeSTAR HS DNA polymerase (Takara Bio, Ohtsu, Japan) with the following set of primers: 5′‐AAGTCGACGCCAGCATGCAGCGCCCG‐3′ and 5′‐AACTCGAGGCTGATGGCGGCGCACGAG‐3′ (underlines indicate restriction enzyme sites). To generate the HA epitope tag, the following set of oligonucleotides, 5′‐GCATGTCGACTACCCATACGATGTTCCAGATTACGCT CTCGAGGCAT‐3′ and 5′‐ATGCCTCGAGAGCGTAATCTGGAACATCGTATGGGTAGTCGACATGC‐3′ (underlines indicate restriction enzyme sites) were mixed, and denatured at 94°C for 3 min and gradually cooled down to 37°C (0.1°C/s slope) for annealing. Next, to amplify the cDNA fragment consisting of residues 23–581 of FZD10, we performed PCR using the following primers: 5′‐AAGTCGACTCCATGGACATGGAGCGCCC‐3′ and 5′‐AACTCGAGTCACACGCAGGTGGGCGACT‐3′ (underlines indicate restriction enzyme sites). Each PCR fragment was digested with SalI and XhoI and ligated into a pCAGGS/neo vector, sequentially (pCAGGS/neo‐HA‐FZD10; FZD10‐2). Construction of pCAGGS/neo‐FZD10‐myc/His (FZD10‐myc/His) was conducted according to our previous report.( 10 ) DNA sequences of all constructs were confirmed by DNA sequencing (ABI3700; PE Applied Biosystems, Foster, CA, USA).
Semi‐quantitative reverse transcription (RT)‐PCR. Total RNA were extracted from each of the cell lines and primary tumor samples using TRIzol reagent (Invitrogen). The extracted RNA were treated for 30 min at room temperature with 30 units of DNase I (Roche, Basel, Switzerland) in the presence of 1 unit of RNase inhibitor (Toyobo, Osaka, Japan) to remove any contaminating genomic DNA. After inactivation at 70°C for 10 min, 3‐µg aliquots of each total RNA were reversely transcribed for single‐stranded cDNA using oligo (dT)12–18 primer and Superscript II (Invitrogen) at 42°C for 60 min. We prepared appropriate dilutions of each single‐stranded cDNA for subsequent PCR by monitoring β2‐microglobulin (β2MG) as a quantitative control. PCR amplification was performed using Ex‐taq polymerase (Takara Bio) and the cDNA as templates with the primers: 5′‐TATCGGGCTCTTCTCTGTGC‐3′ and 5′‐GACTGGGCAGGGATCTCATA‐3′ for FZD10; 5′‐TTAGCTGTGCTCGCGCTACT‐3′ and 5′‐TCACATGGTT CACACGGCAG‐3′ for β2MG. PCR were optimized for the number of cycles to ensure product intensity within the logarithmic phase of amplification. The PCR conditions were 94°C for 30 s, 58°C for 30 s and 72°C for 30 s for 21 cycles (for β2MG) and 28 cycles for FZD10, respectively. The PCR products were resolved by electrophoresis on 2.0% ethidium bromide‐stained agarose gels, and band intensity was quantified with the use of NIH Image analysis software (http://rsb.info.nih.gov/nih‐image/).
Generation of murine anti‐FZD10 monoclonal antibody (MAb) with cell immunization. Murine anti‐FZD10 MAbs were generated by immunizing four‐week‐old female BALB/c mice with COS‐7 cells transfected with pCAGGS/neo‐FZD10‐myc/His for three times every 3 days (Medical and Biological Laboratories, Nagoya, Japan). Lymph node cells from the immunized mice were harvested and fused with the myeloma cell line. The hybridomas were subcloned and assayed by cell ELISA for the ability to secrete immunoglobulin that binds to the extracellular domain of FZDIO. Fifty microliters of cells in phosphate‐buffered saline (PBS) (2.5 × 106 cells) were subcloned and assayed by cell enzyme‐linked immunosorbent assay (ELISA) for the ability to secrete immunoglobulin that binds to the extracellular domain of FZD10. For cell ELISA, COS‐7 cells expressing FZD10‐myc/His were seeded into 96‐well ELISA plates (Nalgen Nunc International, Naperville, IL, USA). Subsequently, 50 µL of the culture supernatants obtained from hybridomas were added to the plate and incubated for 30 min at room temperature. After washing the cells with PBS (–), goat antimouse immunoglobulin (Ig)G‐POD (Medical and Biological Laboratories) was added at 1:10 000 dilution, incubated for 30 min at room temperature. Bound antibodies were detected at OD450–620nm by using Ultramark Microplate Reader (Bio‐Rad, Hercules, CA, USA). Positive clones were further analyzed for specific binding activity. The MAb were affinity purified on protein G‐sepharose for further characterization.
Cell transfection. For confirmation of binding‐specificity of MAb 92‐13, SNU‐C5 cell was transiently transfected with mock vector (pCAGGS‐neo) or pCAGGS/neo‐FZD10‐myc/His construct using FuGENE 6 transfection regent (Roche) according to manufacturer's protocol. For establishment of stably overexpressing‐FZD10 cells, HA‐tagged FZD10‐expression vector (pCAGGS/neo‐HA‐FZD10) or mock vector (pCAGGS‐neo) was transfected into 1273/99 synovial sarcoma cells, in which undetectable expression of FZD10 was observed, using Lipofectamine2000 (Invitrogen) according to manufacturer's protocol. Transfected cells were incubated in the culture medium containing 0.2 mg/mL neomycin (geneticin; Invitrogen). Three weeks later, 20 individual colonies were selected by limiting dilution and screened for HA‐FZD10 stably overexpressing clones. The expression level of HA‐FZD10 was detected in each clone by western blot and immunocytochemical staining analyses using anti‐HA monoclonal antibody (Roche).
Immunohistochemical staining analyses. For immunohistochemical staining analysis, 4‐µm sections of paraffin‐embedded normal adult human tissues (lung, heart, liver, kidney, colon and placenta) (BioChain, Hayward, CA, USA) and surgical synovial sarcoma specimens were deparaffinized and processed for antigen retrieval in Target Retrieval Solution (pH 9) (DAKO Cytomation, Carpinteria, CA, USA) at 125°C for 30 s. After treatment with peroxidase blocking reagent (DAKO Cytomation) for 10 min, followed by treatment with protein blocking serum‐free (DAKO Cytomation) for 30 min, slides were incubated with 10 µg/mL MAb 92‐13. Subsequently, ENVISION Polymer Reagent (DAKO Cytomation) was added and visualized with peroxidase substrate (3,3′‐diaminobenzidine tetrahydrochloride; Sigma‐Aldrich).
Labeling antibodies with radionuclides. Iodine‐125 (125I)‐labeled MAb 92‐13 was prepared according to standard protocols of chloramine‐T method.( 11 ) 740 kBq/2 µL of Na 125I (GE Healthcare, Buckinghamshire, UK) was added to 10 µg MAb 92‐13 in 100 µL of 0.3‐M sodium phosphate buffer solution. One‐microgram of chloramine‐T in 3 µL of 0.3‐M sodium phosphate buffer was further added and incubated for 5 min at room temperature. Labeled antibody was purified using Biospin column 6 (Bio‐Rad) according to manufacture's instructions. For labeling MAb with Indium‐111 (111In), 1 µg of MAb in 100 µL of 50‐mM borate buffer (pH 8.5) was conjugated to isothiocyanatobenzyldiethylenetriaminepentaacetic acid (SCN‐BZ‐DTPA; Macrocyclics, Dallas, TX, USA) in dimethylformamide at molar ratio 1:3. After incubation at 37°C for 20 h, MAb conjugates were purified using Biospin Column 6 (Bio‐Rad). Forty microliters of 111InCl3 (Nihon Medi‐Physics, Hyogo, Japan) was incubated in 60 µL of 0.25‐M acetic acid solution (pH 5.5) and incorporated into 10 µg/mL of MAb‐DTPA conjugates for 1 h at room temperature. Labeled antibody was purified using Biospin Column 6 (Bio‐Rad) according to manufacture's instructions. For labeling MAb with Yttrium‐90 (90Y), 600 µL of 90Y solution (Chiyoda Technol, Tokyo, Japan) was incubated with 400 µL of 0.25‐M acetic acid solution (pH 5.5) for 5 min at room temperature, followed by incubation with 300 µg of MAb‐DTPA conjugates for 1 h at 45°C. It was confirmed that there was no degradation of MAb during incubation. Labeled antibody was purified using PD‐10 column (GE Healthcare) according to manufacture's instructions.
Characterization of binding activity of MAb 92‐13. We applied two methods for evaluation of the binding affinity of mouse‐monoclonal; flow cytometric analysis with fluorescent dyes and radioactive measurement using 125I. For flow cytometric analysis with indirect fluorescence, suspensions of 5 × 106 cells were incubated with 10 µg/mL of MAb 92‐13 or non‐immunized mouse IgG (Beckman Coulter, Fullerton, CA, USA) for 30 min at 4°C. After washing with PBS (–), 2 µg Alexa Fluor 488‐fluorescent goat antimouse IgG or Alexa Fluor 488‐goat antihuman IgG (Molecular Probes) were added, and the cell suspension was incubated for 30 min at 4°C for FACScan analysis (Becton Dickinson, Franklin Lakes, NJ, USA).
To evaluate the nonspecific binding activity of MAb 92‐13 against normal blood cells, 125I‐labeled MAb 92‐13 (5 ng antibody) was incubated with 100 µL of each of three healthy volunteers’ fresh blood (donors A, B and C), and was further added with a non‐labeled identical MAb 92‐13. After incubation for 1 h at room temperature, the cell suspension was centrifuged at 1000 g . The supernatant was removed and the radioactivity of each cell pellet was measured.
Immunoprecipitation (IP): western blot analysis. Cells were lyzed with lysis buffer (50 mM Tris‐HCl, 0.5% Igepal, 0.5% TritonX‐100, 2% glycerol, 150 mM NaCl, 1 mM NaF, 1 mM sodium orthovanadate, pH 7.4) including 0.2% protease inhibitor cocktail III (Calbiochem, San Diego, CA, USA). After homogenization, the cell lysates were incubated on ice for 30 min and centrifuged at 17 000 g for 15 min to separate only supernatant from cell debris. Then, the lysates were subjected to immunoprecipitation. The amount of total protein was estimated by protein assay kit (Bio‐Rad). One milligram protein was mixed with protein G‐sepharose and 1 µg antibodies as indicated. The mixtures were incubated for 1 h at 4°C and the precipitated beads were washed with lysis buffer five times. The bound proteins were eluted with sodium dodecylsulfate (SDS)‐sample buffer and subjected to SDS‐polyacrylamide gel electrophoresis. After electrophoresis, the proteins were blotted onto nitrocellulose membrane (GE Healthcare). After blocking with 4% BlockAce blocking solution (Dainippon Pharmaceutical, Osaka, Japan), membranes were incubated with the primary antibody, HA (1:3000 dilution; Anti‐HA High Affinity, clone 3F10; Roche). Finally, the membrane was incubated with HRP conjugated secondary antibody (1:30 000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and protein bands were visualized by ECL detection reagent (GE Healthcare).
Tumor xenograft models. Animal care and treatment was performed in accordance with the guidelines of animal use and animal committee of the University of Tokyo. The above committee approved all procedures. BALB/cA Jcl‐nu mice (female, 6 weeks old) were purchased from CLEA Japan (Tokyo, Japan). Mice were maintained under specific pathogen‐free conditions and provided with sterile food and water ad libitum. BALB/cA Jcl‐nu mice were injected s.c. with SYO‐1 tumor cells (5 × 106 cells) or LoVo tumor cells (1 × 107 cells), in 0.1 mL PBS, in the flanks, respectively. For biodistribution studies, mice with fully established tumors (0.5 cm3) were given 10 kBq (0.5–1 µg) of 111In‐labeled MAb 92‐13 via tail vain. Animals were killed 1, 24 and 48 h after administration, and weight and radioactivity of each tissue were measured. The distribution was expressed as percentage of injected dose/g of tissue for all samples. To examine the specificity of radiolabeled MAb 92‐13 in vivo, we examined the biodistribution of 111In‐labeled MAb 92‐13 in SYO‐1‐tumor xenografts pretreated with non‐labeled identical antibody. SYO‐1‐tumor bearing BALB/c nude mice were injected with 400 µg of non‐labeled MAb 92‐13 20 min prior to the injection of 10 kBq of 111In‐labeled MAb 92‐13. Both injections were performed i.v. They were compared with the mice that were injected with 111In‐labeled MAb 92‐13 without pretreatment of non‐labeled identical antibody. The normal organs and tumors were dissected at 48 h after the injection, and the radioactivities were measured. For optical imaging of biodistribution, the mice were subjected to the imaging study when tumors were fully established (0.2 cm3). In vivo fluorescence imaging was performed with the IVIS Imaging System 100 series (Xenogen, Alameda, CA, USA). Labeling of MAb 92‐13 with Alexa‐Fluoro 647 was carried out using a Alexa647 Monoclonal Antibody Labeling Kit (Molecular Probes) according to manufacturer's instructions. The Alexa647 reactive dye has a succinimidylester moiety that reacts with primary amines of proteins, and resulting MAb‐dye conjugates were purified by size exclusion column. An optimized Cy5.5 filter was used to acquire Alexa647‐MAb 92‐13 fluorescence in vivo. Tumor‐bearing mice were injected 20 µg Alexa647‐labeled MAb 92‐13 i.p. and subjected to fluorescent imaging at various time points. The mice were fed with food not containing alfalfa (CLEA Japan) for 4 days in prior to injecting MAb 92‐13 in order to reduce the background fluorescence. When acquiring images, mice were anesthetized with 2% of isoflurane (Abbott Laboratories, Abbott Park, IL, USA) and placed in the IVIS system. The mice were killed 5 days after injection of MAb 92‐13, the synovial sarcoma tumor and major mouse organs (liver, spleen, kidney, pancreas and colon) were dissected, and fluorescence images of each organ were obtained.
For therapy studies, SYO‐1 tumors were grown in BALB/cA Jcl‐nu mice in the same way. The diameters of the tumors were measured by calipers and the tumor volumes were determined using the following formula: 0.5 × (larger diameter) × (smaller diameter)2 as described previously.( 10 ) When the tumors were fully established (0.016–1.3 cm3), animals were randomly assigned to treatment groups so that the mean tumor volume of each treatment group was approximately 0.56 cm3. Those mice received i.v. injections of the 100 µCi of 90Y‐MAb 92‐13, non‐labeled MAb 92‐13 as a control. Mice were weighed and tumor diameters were recorded.
Internalization analysis of MAb 92‐13 by confocal microscopy and flow cytometric (FACS) analyses. For microscopic analysis, cells were plated into 8‐well chamber slides (Nalgen Nunc International) at density of 5 × 104 cells/well. Cells were incubated with 30 µg/mL MAb 92‐13 for 3 h at 37°C in an air chamber containing 5% CO2. The antibodies bound to the cell surface were removed with acid stripping buffer (0.1 M glycine, 500 mM NaCl, pH 2.5) at 4°C for 10 min, and neutralized with 500 mM Tris‐Cl (pH 7.5). Cells were then fixed with 3.7% formaldehyde for 15 min at room temperature, and permeabilized by exposure to 0.2% TritonX‐100 for 10 min, followed by blocking with 3% bovine serum albumin (BSA) for 1 h at room temperature. To detect the MAb 92‐13 internalized into the cells, cells were incubated with Alexa Fluor488 goat anti rat IgG (H + L; 1:700 dilution) for 1 h at room temperature. The slides were mounted with DAPI (Vectashield, Vector Laboratories, Burlingame, CA, USA) and analyzed under Leica TCS SP1 confocal optics (Leica). For FACS analysis, SYO‐1 (5 × 106) cells were incubated in 100 µL of Dulbecco's modified Eagle medium (DMEM) containing 10% FBS with 2 µg Alexa488‐labeled MAb 92‐13 or PBS (pH 7.2) as a control. The labeled‐MAb 92‐13 bound to FZD10 on the surface of cells at 4°C for 1 h. Subsequently, cells were incubated at 37°C for 3 h to allow the internalization of labeled‐antibodies. To determine the amount of internalized anti‐FZD10 MAb into the cells, the labeled‐antibodies bound to the cell surface were removed with acidic PBS (pH 2.0) at 4°C for 10 min, and then cells were washed three times with PBS (pH 7.2), and subjected to FACS analysis. As a control, cells were kept at 4°C after stripping of the labeled antibodies that bound to the cell surface. For measurement of fully cell‐bound antibody as a positive control, cells were not treated with acidic PBS (pH 2.0) after the treatment of labeled antibodies.
Statistical analysis. Data points represent the means along with 95% confidence intervals (CI) of multiple independent experiments. The two‐sample Student's t‐test with Welch's correction was used for two‐group comparisons of tumor volumes (90Y‐MAb 92‐13 vs non‐labeled MAb 92‐13 and 90Y‐MAb 92‐13 vs 90Y‐α‐CD20) in in vivo therapeutic experiments. Time to tumor progression was defined a priori as the time when tumor volume had increased 2.5 times from the baseline value for each mouse according to a previous report.( 12 ) The Kaplan–Meier method was used to determine the median time to tumor progression. All P‐values for the xenograft studies were based on comparisons of time to tumor progression among groups by use of the log–rank test. All statistical tests were two‐sided, and P‐values less than 0.05 were considered to be statistically significant.
Results
Generation of anti‐FZD10 MAb 92‐13. We attempted to generate an anti‐FZD10 antibody for treatment of synovial sarcoma (see Materials and Methods). Balb/c mice were immunized with living COS‐7 cells transiently transfected with FZD10‐myc/His construct, and hybridomas were obtained. Among hosts of these hybridoma clones, one clone, 92‐13, was selected as a candidate to produce an antibody against FZD10 by cell ELISA assay. The MAb 92‐13 was determined to produce the IgG2a isotype by means of the IsoStrip Mouse Monoclonal antibody isotyping kit (Roche). To investigate the cell‐binding affinity of MAb 92‐13, we performed flow cytometry (FACS) experiments. MAb 92‐13 bound to cell surfaces of four FZD10‐overexpressing synovial sarcoma cell lines, SYO‐1, YaFuSS, HS‐SY‐II and Fuji, but did not bind to two cell lines, 1273/99 and LoVo, in which no transcript of FZD10 was detected (Fig. 1a,b). We found a significant correlation of relative mean fluorescent intensities (MFI) of MAb 92‐13 with the expression levels of FZD10. In addition, MAb 92‐13 also bound to the SNU‐C5 cells transfected with an FZD10‐myc/His construct, while no binding was detected with SNU‐C5 cells transfected with an empty vector (Fig. 1c), further supporting the specific binding affinity of MAb 92‐13 to FZD10. We further confirmed that this antibody could specifically immunoprecipitate FZD10 protein in HA‐tagged FZD10‐overexpressing cells (FZD10‐2), but not in mock‐transformant cells (Mock1; Fig. 1d), suggesting that MAb 92‐13 has an ability to specifically recognize the native FZD10.
Figure 1.
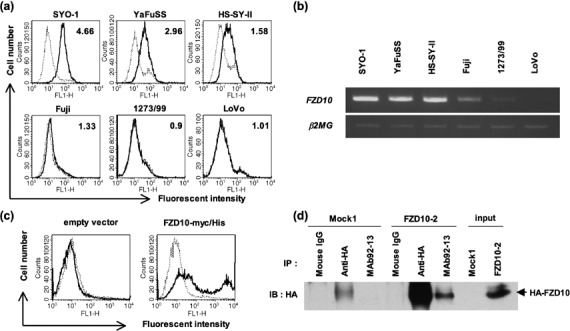
Characterization of binding specificity for anti‐FZD10 monoclonal antibody (MAb) 92‐13. (a) Flow cytometric analysis of MAb 92‐13 using five synovial sarcoma cell lines (SYO‐1, YaFuSS, HS‐SY‐II, Fuji and 1273/99) and one colon cancer cell line (LoVo). Solid lines show the fluorescent intensity detected by MAb 92‐13; broken lines depict the fluorescent intensities of cells incubated with non‐immunized mouse immunoglobulin (Ig)G as a negative control. The number within each panel indicates mean fluorescent intensities (MFI) of MAb 92‐13 against each cell line. (b) Semiquantitative reverse transcription polymerase chain reaction (RT‐PCR) of FZD10 transcript in the same cell lines as shown in (a). β2MG served as an internal control. (c) Flow cytometric analysis of MAb 92‐13 against exogenous FZD10. Colon cancer cell line, SNU‐C5, was transfected with pCAGGS/neo empty vector (left panel) or pCAGGS‐FZD10‐myc/His (right panel), and analyzed at 48 h after the transfection. Solid lines show the fluorescent intensity detected by MAb 92‐13; broken lines depict the fluorescent intensities of cells incubated with non‐immunized mouse IgG as a negative control. (d) Specificity of MAb 92‐13. Immunoprecipitate (IP)‐western blot analysis of FZD10 protein in 1273/99 cells in which HA‐tagged FZD10 was stably expressed (FZD10‐2) or those transfected with mock vector (Mock1) was performed. Mouse IgG is control for IP experiments.
We also performed immunohistochemical staining analysis using MAb 92‐13 in order to confirm FZD10 expression at protein level in human normal tissues and synovial sarcoma tissues (Fig. 2a,b). In concordance with the transcript level,( 10 ) we detected a high level of FZD10 protein in two synovial sarcoma specimens examined, and an absence or a hardly detectable level of FZD10 protein in normal human colon, heart, lung, liver and kidney except placenta tissue (Fig. 2c–h).
Figure 2.

Immunohistochemical staining analyses of synovial sarcoma and normal human tissue sections using MAb 92‐13; (a,b) synovial sarcoma tissue sections (c) colon, (d) heart, (e) lung, (f) liver, (g) kidney and (h) placenta. Original magnification ×100. Bar, 50 µm.
In vivo distributions of anti‐FZD10 MAb in mice xenograft model. In vivo distribution of MAb 92‐13 was examined by means of two independent methods, fluorescent imaging and radionuclide activity, using mice in which synovial sarcoma xenograft was implanted. For in vivo imaging using fluorescence, 20 µg Alexa647‐labeled MAb 92‐13 was injected i.p. into the SYO‐1‐bearing mice, and distribution of the MAb was visualized using the IVIS system (see Materials and Methods). Obvious accumulation of the fluorescent signal was detected at the location of tumor 24 h after the injection (Fig. 3a, upper panels). The fluorescent signal reached maximum level at approximately 48 h, and could be detectable at 96 h after the injection. Then, we killed these mice at a 120‐h time point, and measured the fluorescence intensity in the tumor as well as some normal organs (liver, spleen, kidney, pancreas and colon). A very strong fluorescence signal was observed in the dissected tumor, whereas no fluorescence signal was detected in any normal organs examined (Fig. 3a, lower panels). As a control, we also generated xenografts of the FZD10‐negative LoVo cell line in nude mice and performed fluorescent imaging analysis after injection of Alexa647‐labeled MAb 92‐13. In these mice, the fluorescent signal was detected neither at the location of the tumor (Fig. 3b, upper panels), in the dissected tumor nor in any other organs examined (liver, spleen, kidney, pancreas and colon) (Fig. 3b, lower panels).
Figure 3.
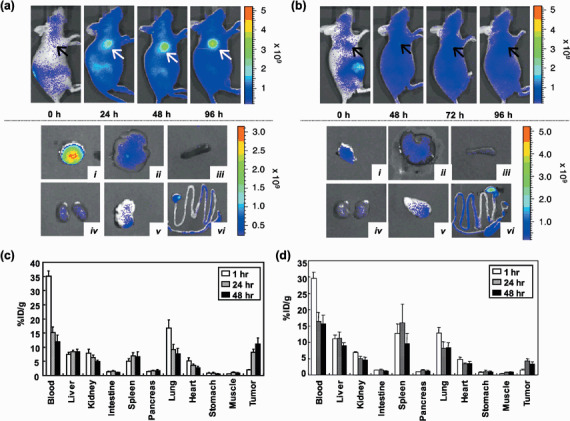
Biodistribution of MAb 92‐13 in vivo. (a, upper panels) In vivo fluorescence imaging of SYO‐1‐tumor bearing mice after injection of Alexa647‐labeled MAb 92‐13. Fluorescence‐labeled antibody was administered at a dose of 20 µg per mouse i.p. All fluorescence images were acquired with a 60‐s exposure time (f/stop = 2) immediately after the injection (0 h) and at 24, 48 and 96 h after the injection. The arrows indicate the position of the tumor. Fluorescence signal from Alexa647 was pseudo‐colored according to the color bar indicated on right. (Lower panels). Representative images of dissected organs and tumors from mice shown in upper panels; (i) SYO‐1 tumor; (ii) liver; (iii) spleen; (iv) kidney; (v) pancreas; and (vi) colon. (b, Upper panels) In vivo fluorescence imaging of LoVo‐tumor bearing mice after the injection of Alexa647‐labeled MAb 92‐13. Images were obtained at 0, 48, 72 and 96 h after the injection of fluorescent‐labeled antibody as described in upper panels. (Lower panels) Representative images of dissected organs and tumors from mice shown in upper panels; (i) LoVo tumor; (ii) liver; (iii) spleen; (iv) kidney; (v) pancreas; and (vi) colon. (c) Biodistribution of 111In‐labeled MAb 92‐13 in SYO‐1 xenografts. Ten kBq of 111In‐labeled 92‐13 was injected i.v. into SYO‐1‐tumor bearing BALB/c nude mice. The normal organs and tumor were dissected at 1 h (open bar), 24 h (hatched bar) and 48 h (closed bar) after the injection, and the radioactivities were measured. The data shown are the representative data in two independent experiments. (d) Biodistribution of 111In‐labeled MAb 92‐13 LoVo in tumor xenografts. Ten kBq of 111In‐labeled MAb 92‐13 was injected i.v. into LoVo‐tumor bearing BALB/c nude mice. The normal organs and tumors were dissected at 1 h (open bar), 24 h (hatched bar) and 48 h (closed bar) after the injection, and the radioactivities were measured. The data shown are the representative data in two independent experiments.
We further confirmed accumulation of MAb 92‐13 using Indium‐111 (111In) label; the radioactivity in the blood dropped from 35% of the injected‐dose per gram (%ID/g) at 1 h to 12% at 48 h after the injection (Fig. 3c). Radioactivities in blood, liver, kidney, intestine, spleen, pancreas, lung, heart, stomach and muscle remained constantly or decreased gradually throughout the observation, whereas radioactivity in the tumors accumulated throughout the experiment from 2%ID/g at 1 h after the injection to 11%ID/g after 48 h. On the other hand, 111In‐labeled MAb 92‐13 (111In‐MAb 92‐13) did not accumulate to tumor in LoVo‐tumor bearing mice (Fig. 3d). Furthermore, when SYO‐1‐bearing mice were injected with an excess amount of non‐labeled MAb 92‐13 i.v. prior to injection of 111In‐MAb 92‐13, accumulation of radiolabeled antibody to tumor was completely blocked (Appendix I, Fig. S1). Because we previously reported that LoVo cells express all molecules of the FZD family (FZD1‐FZD9) except FZD10,( 10 ) 111In‐labeled MAb 92‐13 specifically bound to the FZD10 molecule on the cell surface of synovial sarcoma in vivo.
Internalization of MAb 92‐13 into synovial sarcoma cells. As described above, the observation by in vivo imaging IVIS system indicated accumulation of MAb 92‐13 at the tumor region at 5 days after the injection. Hence, we examined a level of internalization of MAb 92‐13 into the cells by confocal microscopy using Alexa488‐labeled goat antimouse IgG antibody. By the method described above, we observed effective incorporation of MAb 92‐13 into the cytosol of SYO‐1 and YaFuSS cells at 3 h after the incubation of the MAb 92‐13 (Fig. 4ai,ii) compared to PBS treatment as a control (Fig. 4aiv,v). On the other hand, its fluorescence signal was hardly detectable in LoVo cells in which the FZD10 expression was absent (Fig. 4aiii,vi), indicating the effective internalization of the MAb 92‐13 into FZD10‐overexpressing synovial sarcoma cells. Furthermore, we performed FACS analysis to validate the internalization of MAb92‐13. We also observed the incorporation of this antibody into the SYO‐1 cells at 3 h after the incubation of the MAb 92‐13 according to the method described above (Fig. 4b). Taken together, our results suggest that MAb 92‐13 was internalized into the cell in an FZD10‐specific manner.
Figure 4.

Internalization of MAb 92‐13 into FZD10‐expressing cells. (a) Internalized antibody was assessed by confocal microscopy. Cells were treated with 30 µg/mL of MAb 92‐13 (i–iii) or phosphate‐buffered saline (PBS) (iv–vi) for 3 h at 37°C. Antibodies bound to the cell surface were acid‐stripped with 0.1 M glycine buffer (pH 2.5). Cells were fixed, permeabilized and then blocked with 3% bovine serum albumin (BSA). Intracellular antibodies were detected with goat antimouse IgG‐Alexa488 and nucleus was stained with DAPI. (i,iv) SYO‐1; (ii,v) YaFuSS; (iii,vi) LoVo. Bar, 20 µm. (b) Detection of the internalized antibody by FACS analysis. The Alexa488 labeled‐MAb92‐13 antibodies were bound to the surface of SYO‐1 cells at 4°C, followed by incubation at either 4°C (broken line histgram) or 37°C (gray histgram and black histgram) for 3 h. Cells were also incubated with PBS (pH 7.2) as a control (solid line histgram). To remove the antibodies that bound to the cell surface, cells were treated with (broken line histgram and gray histgram) or without acidic‐PBS (black histgram).
Effect of 90Y‐labeled MAb 92‐13 on tumor growth. We then applied the radioimmunotherapy using 90Y‐labeled MAb 92‐13 (90Y‐MAb 92‐13) for treatment of synovial sarcoma tumors. Balb/c nude mice were injected s.c. in their flanks with SYO‐1 cells. When tumors were fully established (0.016–1.3 cm3; mean volume = 0.56 cm3/group), mice were administrated i.v. with 100 µCi of 90Y‐MAb 92‐13, 100 µCi of 90Y‐α‐CD20, non‐labeled MAb 92‐13, or were not treated. Tumors in mice treated with non‐labeled MAb 92‐13 grew very rapidly and exceeded 3 cm3 in 12 days and those of untreated mice became more than 3 cm3 at day 16 (Fig. 5a). The sizes of tumors in mice treated with 90Y‐α‐CD20 were transiently reduced in the first 14 days after the treatment, but the tumors regrew very rapidly and exceeded 3 cm3 in 33 days. On the other hand, in all of the mice that received 100 µCi of 90Y‐MAb 92‐13, tumor volumes were markedly reduced immediately after the treatment. The mean tumor volume of 90Y‐MAb 92‐13‐treated mice was decreased to approximately 0.1 cm3 at day 9 after the injection. Median time to tumor progression, as defined by tumors reaching 2.5‐times baseline size in accordance with a previous report,( 12 ) was 58 days for mice injected with 90Y‐MAb 92‐13, 23 days for mice injected with 90Y‐α‐CD20 and 9 days for mice injected with non‐labeled 92‐13 or left untreated (90Y‐MAb 92‐13 vs 90Y‐α‐CD20; P = 2 × 10−5, 90Y‐MAb 92‐13 vs non‐labeled MAb 92‐13; P = 7 × 10−5, 90Y‐MAb 92‐13 vs no treatment; P = 7 × 10−5) (Fig. 5a). The tumors in seven of the 11 mice were less than 0.02 cm3 at day 33. However, the tumor volume in seven of the 11 mice increased after day 33. Importantly, in four mice, regrowth of the tumors was not observed even at day 54 after the treatment (Fig. 5b). The mice that received 90Y‐MAb 92‐13 and 90Y‐α‐CD20 showed temporary decrease of the bodyweight (10–15%, Appendix I, Fig. S2), but they recovered within 1 week, and no visible toxic sign was observed until 30 days after injection (Fig. 5c). Our results indicate that a single injection of 90Y‐MAb 92‐13 has specific and strong antitumor effect against synovial sarcoma with FZD10 expression.
Figure 5.
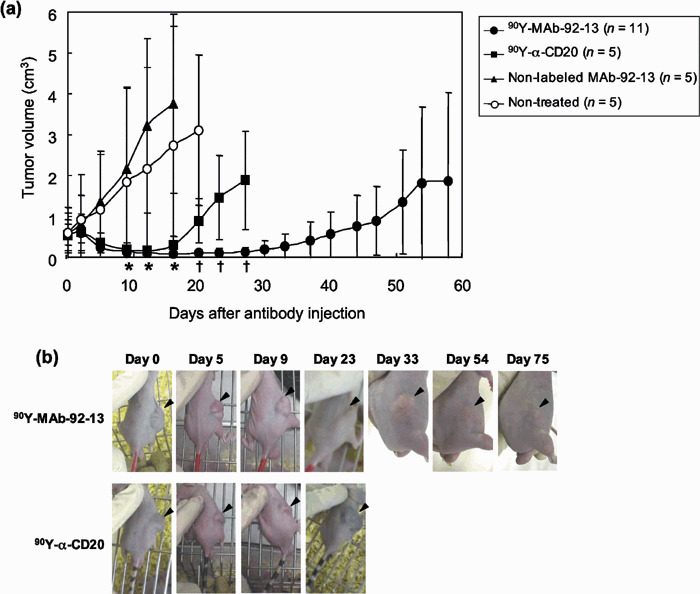
Radioimmunotherapy in SYO‐1‐tumor bearing nude mice. (a) Balb/c nude mice bearing fully established (mean tumor volume = 0.56 cm3) SYO‐1 tumors were treated with Yttrium‐90 (90Y)‐MAb 92‐13 (n = 11), 90Y‐α‐CD20 (n = 5), non‐labeled MAb 92‐13 (n = 5) or untreated (non‐treated) (n = 5). Mice were given a single injection at day 0. The mean tumor volumes were plotted against days after the antibody injection (error bars are 95% confidence interval). Closed circle, 90Y‐MAb 92‐13; closed square, 90Y‐α‐CD20; closed triangle, non‐labeled MAb 92‐13; open circle, non‐treated. Welch's two‐sided Student's t‐test was used for comparison with control mice, and statistically significant points (P < 0.05) are noted: *, vs non‐labeled MAb 92‐13; †, vs 90Y‐α‐CD20. (b) Representative images of 90Y‐MAb 92‐13‐treated mice. The photographs of 90Y‐MAb 92‐13‐treated mice (upper panels) and 90Y‐α‐CD20‐treated mice (lower panels) were taken at the days indicated. Arrowheads indicate the tumor. Note that the tumor size was reduced in a mouse treated with 90Y‐α‐CD20 at day 5, but was increased again at day 9, while the tumor in that with 90Y‐MAb 92‐13 diminished completely at day 33, and showed no regrowth at day 75.
Discussion
We previously reported that FZD10, a member of the Frizzled family, was significantly transactivated in synovial sarcoma, but not expressed in normal human organs except placenta,( 8 , 10 ) and involved in cancer cell growth or survival by RNAi experiments.( 10 ) Furthermore, because we demonstrated that anti‐FZD10 polyclonal antibody showed antitumor activity with ADCC in vitro and in vivo,( 10 ) we have been attempting to generate a murine monoclonal antibody against FZD10 for antibody‐based synovial sarcoma therapy. Due to formation of homo‐oligomerization of FZD10 as described previously,( 10 ) it had been very difficult to generate specific monoclonal antibodies against FZD10. After failure of multiple attempts to generate anti‐FZD10 monoclonal antibodies that could recognize a native form of FZD10 by the use of full‐length or partial recombinant FZD10 proteins encoding the N‐terminal extracellular region, we finally attempted immunize Balb/c mice by injection of living COS‐7 cells in which FZD10 was exogenously introduced and overexpressed. We successfully obtained a clone of hybridoma, namely 92‐13 (MAb 92‐13), producing specific a murine monoclonal antibody against FZD10. To investigate the cross‐reactivity of MAb 92‐13 with other members of the FZD family, we demonstrated that MAb 92‐13 did not bind to cell surfaces of LoVo and SNU‐C5, colon cancer cell lines (Fig. 1a,c), in which all known members of the FZD family except FZD10 were expressed as described previously,( 10 ) strongly supporting the specific binding of MAb 92‐13 FZD10, but not to other FZD family members.
When antibodies are applied for cancer therapy, cross‐reaction of the antibody to normal organs or blood cells there is a concern that they will cause severe adverse reactions. To investigate the in vivo distribution of MAb 92‐13, we applied two methods; one based on the radionuclide modalities using 111In‐labeled antibody, and the other based on the fluorescence imaging using near‐infrared‐labeled antibodies. Near‐infrared fluorescence is now widely used in the in vivo imaging for diagnostic purpose because the light of this wavelength penetrates living tissue efficiently.( 13 ) The results obtained by two approaches were very concordant and indicated that MAb 92‐13 bound and accumulated to SYO‐1 tumor cells, but not to any other tissues. Moreover, we confirmed that MAb 92‐13 has no immunoreactivity against normal human blood cells (Appendix I, Fig. S3), strongly implying clinical applicability of this antibody with a very minimum risk of adverse effects to patients. In addition, our in vitro experiments using confocal microscopy reveled that MAb 92‐13 was internalized into the cell in an FZD10‐specific manner. Recently, because it has been reported that Frizzled 4, a member of FZD family, was internalized through clathrin‐mediated endocytosis on Wnt stimulation,( 14 , 15 ) FZD10 may be also internalized with unknown ligands as well as MAb 92‐13 although further studies will be required.
Zevalin (anti‐CD20 antibody conjugated with 90Y) has been proven to provide a new means of cancer treatment called radioimmunotherapy and, in fact, has been highly effective against malignant lymphoma.( 16 ) We confirmed that MAb 92‐13 had no antitumor activity in vitro when treated to FZD10‐overexressing cells, SYO‐1 (data not shown). However, because we observed the effective internalization and accumulation of MAb 92‐13 into the synovial sarcoma cells as shown in 3, 4, we, in this study, designed the radioimmunotherapy using 90Y‐conjugated MAb 92‐13 for treatment of synovial sarcomas. In the mouse xenograft model, tumors rapidly diminished after the single treatment of 90Y‐MAb 92‐13 and no toxicity was observed. Because MAb 92‐13 was internalized effectively into antigen‐positive cells as described above, conjugation of anticancer drugs to MAb 92‐13 may also be expected to exert the high anticancer effect to synovial sarcoma cell as well as 90Y‐MAb 92‐13.
In conclusion, we successfully generated a monoclonal antibody, which specifically bound to FZD10 in vitro and in vivo, and was internalized into and accumulated in FZD10‐expressing cells. A single i.v. injection of radioisotope‐labeled MAb 92‐13 showed strong antitumor activity in vivo. Taken together, we are confident that anti‐FZD10 antibody MAb 92‐13 has a great potential for development of a novel treatment of synovial sarcoma as well as other tumors that show overexpression of FZD10. Interestingly, our cDNA microarray data and immunohistochemical staining analysis showed the upregulation of FZD10 in a subset of colon cancer, lung cancer and gastric cancer (data not shown). Hence, this antibody treatment might be applied to a wide range of cancer types.
Acknowledgments
We thank Chiyoda Technol for providing 90Yttrium solution, Dr Ryo Takata for statistical analysis, and Ms Kie Naito, Ms Yoshiko Fujisawa, Ms Kyoko Kijima, Ms Akiko Konuma and Ms Aya Sasaki for excellent technical assistance. All authors have contributed significantly, and are in agreement with the content of this manuscript.
References
- 1. Harris M. Monoclonal antibodies as therapeutic agents for cancer. Lancet Oncol 2004; 5: 292–302. [DOI] [PubMed] [Google Scholar]
- 2. Baselga J. Herceptin® alone or in combination with chemotherapy in the treatment of HER2‐positive metastatic breast cancer: pivotal trials. Oncology 2001; 61 (Suppl 2): 14–21. [DOI] [PubMed] [Google Scholar]
- 3. Maloney DG, Grillo‐López AJ, White CA et al . IDEC‐C2B8 (Rituximab) Anti‐CD20 monoclonal antibody therapy in patients with relapsed low‐grade non‐Hodgkin's lymphoma. Blood 1997; 90: 2188–95. [PubMed] [Google Scholar]
- 4. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti‐VEGF antibody for treating cancer. Nat Rev Drug Discov 2004; 3: 391–400. [DOI] [PubMed] [Google Scholar]
- 5. Crist WM, Anderson JR, Meza JL et al . Intergroup rhabdomyosarcoma study‐IV. results for patients with nonmetastatic disease. J Clin Oncol 2001; 19: 3091–102. [DOI] [PubMed] [Google Scholar]
- 6. Ferguson WS, Goorin AM. Current treatment of osteosarcoma. Cancer Invest 2001; 19: 292–315. [DOI] [PubMed] [Google Scholar]
- 7. Wunder JS, Paulian G, Huvos AG, Heller G, Meyers PA, Healey JH. The histological response to chemotherapy as a predictor of the oncological outcome of operative treatment of Ewing sarcoma. J Bone Joint Surg Am 1998; 80: 1020–33. [DOI] [PubMed] [Google Scholar]
- 8. Nagayama S, Katagiri T, Tsunoda T et al . Genome‐wide analysis of gene expression in synovial sarcomas using a cDNA Microarray. Cancer Res 2002; 62: 5859–66. [PubMed] [Google Scholar]
- 9. Koike J, Takagi A, Miwa T, Hirai M, Terada M, Katoh M. Molecular cloning of Frizzled‐10, a novel member of the Frizzled gene family. Biochem Biophys Res Commun 1999; 262: 39–43. [DOI] [PubMed] [Google Scholar]
- 10. Nagayama S, Fukukawa C, Katagiri T et al . Therapeutic potential of antibodies against FZD10, a cell‐surface protein, for synovial sarcomas. Oncogene 2005; 24: 6201–12. [DOI] [PubMed] [Google Scholar]
- 11. Arano Y, Fujioka Y, Akizawa H et al . Chemical design of radiolabeled antibody fragments for low renal radioactivity levels. Cancer Res 1999; 59: 128–34. [PubMed] [Google Scholar]
- 12. Arpino G, Gutierrez C, Weiss H et al . Treatment of human epidermal growth factor receptor 2‐overexpressing breast cancer xenografts with multiagent HER‐targeted therapy. J Natl Cancer Inst 2007; 99: 694–705. [DOI] [PubMed] [Google Scholar]
- 13. Chen X, Conti PS, Moats R. In vivo near‐infrared fluorescence imaging of integrin αvβ3 in brain tumor xenografts. Cancer Res 2004; 64: 8009–14. [DOI] [PubMed] [Google Scholar]
- 14. Chen W, Ten Berge D, Brown J et al . Dishevelled 2 recruits β‐arrestin 2 to mediate Wnt5A‐stimulated endocytosis of Frizzled 4. Science 2003; 301: 1391–4. [DOI] [PubMed] [Google Scholar]
- 15. Bryja V, Cajánek L, Grahn A, Schulte G. Inhibition of endocytosis blocks Wnt signaling to β‐catenin by promoting disheveled degradation. Acta Physiol 2007; 190: 55–61. [DOI] [PubMed] [Google Scholar]
- 16. Wiseman GA, Witzig TE. Yttrium‐90 (90Y) ibritumomab tiuxetan (Zevalin) induces long‐term durable responses in patients with relapsed or refractory B‐cell non‐Hodgkin's lymphoma. Cancer Biother Radiopharm 2005; 20: 185–8. [DOI] [PubMed] [Google Scholar]


