Abstract
We have reported that thyroid capsular thickening with inflammation induced by an antithyroidal agent, sulfadimethoxine (SDM), might play a role in the development of invasive follicular carcinomas in rats initiated with N‐bis(2‐hydroxypropyl)nitrosamine (DHPN). Inducible nitric oxide synthase (iNOS) expressed in the inflamed capsular regions further appeared to be implicated in the tumor progression. In the present study, the effects of an iNOS inhibitor, aminoguanidine (AG), on thyroid carcinogenesis were examined. F344 male rats were treated with SDM in drinking water (0.1%) with or without concomitant dietary administration of AG (0.2%) for 4 and 10 weeks after subcutaneous injection of DHPN at 2800 mg/kg bodyweight. At week 4, thyroid capsular thickening with inflammation was observed and iNOS‐positive foci were found in the inflamed regions. In addition, single‐strand DNA‐positive inflammatory cells were scattered among neighboring follicular cells, indicating some cellular damage, at least partly in association with iNOS induction. Concurrent dietary administration of AG with SDM treatment slightly decreased the number of single‐strand DNA‐positive cells but did not alter the incidence and multiplicity of iNOS‐positive foci in the inflamed capsular regions at week 4. At week 10, however, invasive follicular carcinomas predominantly arose in the thickened capsule in the DHPN–SDM‐treated rats, and AG administration decreased (P < 0.05) their multiplicity. The carcinoma cells were partly positive for iNOS. These results thus suggested that iNOS induction in both inflammatory and tumor cells might play pivotal roles in tumor progression in this DHPN–SDM rat model. (Cancer Sci 2009; 100: 1794–1800)
Nitric oxide (NO), a signaling molecule produced by a variety of mammalian cells, mediates numerous physiological processes. It is synthesized from l‐arginine by three isoforms of the NO synthase (NOS): the constitutive endothelial and neuronal NOS types, or the inducible NOS (iNOS).( 1 ) In contrast to the constitutive isoforms that generate low levels of NO, iNOS produces high levels, and activity of iNOS is largely regulated at the levels of synthesis and stability of its mRNA and protein.( 1 ) Under some pathophysiological conditions, such as inflammation, excess NO is produced by iNOS in inflammatory and epithelial cells and this is considered to play a crucial role in carcinogenesis.( 2 , 3 ) Numerous investigations to clarify the association between iNOS and carcinogenesis in animal models and man have thus been conducted, although the results appear actually to be complex.( 1 ) For example, some studies demonstrated that iNOS is upregulated in tumor tissues in human colorectal carcinogenesis,( 4 , 5 ) whereas others showed iNOS to be expressed in normally appearing, uninflamed colonic mucosa and downregulated in tumor tissues, suggesting a possible protective influence.( 6 , 7 ) With inflammatory bowel diseases, increased expression of iNOS in stromal inflammatory cells has been demonstrated,( 7 , 8 ) but the relationship between colitis‐associated NO induction and tumor development is not completely understood. The majority of studies in both carcinogen‐induced and genetic animal models support a role for iNOS in the promotion of colon carcinogenesis, and the effects may be associated with the ability of NO to induce oxidative DNA damage, like other factors including increase in the expression or activity of the enzyme cyclooxygenese‐2.( 2 , 9 ) On the other hand, protective mechanisms by which iNOS could impact on colitis‐induced mouse carcinogenesis have also been proposed.( 10 )
In the thyroid, upregulated expression of iNOS has been reported in tumor cells in follicular adenomas, papillary carcinomas, follicular carcinomas, medullary carcinomas, and/or anaplastic carcinomas in man,( 11 , 12 ) but it is not completely understood whether iNOS expressed in the tumor cells might play a role in promotion or protection of thyroid carcinogenesis. With chronic lymphocytic thyroiditis, follicular epithelial cells surrounded by lymphoid cells become positive for iNOS immunohistochemistry,( 12 ) but again whether there is a correlation between thyroiditis‐associated NO induction and tumor development is unclear. Experimentally we have recently provided evidence that thyroid capsular thickening with inflammation induced by continuous treatment with the antithyroidal agent sulfadimethoxine (SDM) is associated with development of invasive follicular carcinomas in rats initiated with N‐bis(2‐hydroxypropyl)nitrosamine (DHPN).( 13 ) In addition, focal iNOS‐positivity in capsular inflammatory regions appeared to be implicated in the tumor progression.( 14 ) In the present study, to cast further light on the correlation between upregulation of iNOS and development of invasive carcinomas in thyroid capsules, the effects of aminoguanidine (AG), a selective iNOS inhibitor that bears some structural similarity to l‐arginine,( 15 , 16 ) on thyroid carcinogenesis were examined in the DHPN–SDM model.
Materials and Methods
Animals and treatments. A total of 70 male F344 rats (Japan Charles River, Kanagawa, Japan) at 6 weeks of age were allowed access to basal diet (CRF‐1; Oriental Yeast, Tokyo, Japan) and water ad libitum and housed in polycarbonate cages with white wood chips (Sankyo Laboratory Service, Tokyo, Japan) for bedding in an airconditioned room (24 ± 1°C, 55 ± 5% relative humidity, 12 : 12 L : D cycle). DHPN was purchased from Nacalai Tesque (Kyoto, Japan). SDM and AG were from Sigma Chemical (St Louis, MO, USA). All animals were divided into four groups and initiated with a single subcutaneous injection of DHPN, diluted to 560 mg/mL in saline, at 2800 mg/kg bodyweight. One week after initiation, drinking water containing 0.1% SDM was provided ad libitum for 4 or 10 weeks to animals of groups 1 and 2. In addition, group 1 animals received AG‐containing basal diet at a concentration of 0.2% concomitantly with SDM treatment. Groups 3 and 4 were set as DHPN + AG and DHPN alone (control), respectively. General conditions were checked daily and bodyweight and water and food intake were recorded once a week. One of fifteen rats each in groups 1 and 2 died after DHPN injection and were excluded from the evaluations. Ten rats in groups 1 and 2 each and five rats in groups 3 and 4 each at week 4, and 14 rats in groups 1 and 2 each and five rats in groups 3 and 4 each at week 10 were killed under deep ether anesthesia for collection of serum and thyroid samples. The dose level of AG was selected based on previous reports.( 17 , 18 )
Hormone determinations. At necropsy, blood samples were collected from the abdominal aorta of all animals under ether anesthesia for assays of serum thyroxine (T4), triiodothyronine (T3), and thyroid stimulating hormone (TSH) levels with radioimmunoassay kits, a GammaCoat Total T4 for human kit (DiaSorin, Saluggia, Italy), a RIABEAD Kit for human T3 (Dinabot, North Chicago, IL, USA), and the Rat Thyroid Stimulating Hormone [125I] Biotrak Assay (Amersham Pharmacia Biotech, Hemel Hempstead, UK), respectively, at SRL (Tokyo, Japan).
Histopathological observations. The bilateral thyroid lobes were excised, weighed, and cut in half horizontally. They were fixed in phosphate‐buffered 10% formalin for ~24 h, routinely processed for embedding in paraffin, and serial tissue sections were prepared for staining with hematoxylin–eosin and immunohistochemistry. On histopathological evaluation, preneoplastic and neoplastic lesions of follicular epithelial cells were classified as focal hyperplasias, adenomas, and carcinomas, according to published criteria.( 19 ) In addition, follicular carcinomas were discriminated as intrathyroidal and invasive carcinomas involving the thyroid capsular and/or extrathyroidal tissues.( 14 )
Immunohistochemical staining. Anti‐rat CD3 antibody for T‐cell surface antigen (clone G4.18, diluted at 1/500) and anti‐mouse iNOS (clone 6, 1/200) were purchased from BD Biosciences (San Jose, CA, USA). Anti‐rat antibody (clone ED1, 1/100) for monocytes and tissue macrophages was from Serotec (Oxford, UK). Anti‐single‐strand DNA (ssDNA) rabbit polyclonal antibodies (1/100) and anti‐Ki‐67 antibody (clone MIB‐5, ×200) were from Dako (Glostrup, Denmark). Anti‐ssDNA and Ki‐67 antibodies were used to mark damaged cells containing ssDNA fragments and proliferating cells, respectively. Antigen retrieval was carried out in an autoclave for 40, 10, and 10 min at 121°C in 10 mM citrate buffer (pH 6.0) for CD3, iNOS, and Ki‐67, respectively. The streptavidin–biotin–peroxidase complex method (StreptABComplex/HRP; Dako) or a peroxidase‐labeled amino acid polymer method (Histofine Simple Stain Rat MAX‐PO; Nichirei Bioscience, Tokyo, Japan) were used to determine the expression and localization of each antigen, and sections were lightly counterstained with hematoxylin for microscopic examination. Negative controls without primary antibody reactions were set for each antigen using serial sections.
Statistical analysis. Statistical analysis to compare the bodyweight and thyroid weight, and serum hormone levels, as well as the multiplicity for histopathological and immunohistochemistry findings was carried out using the Student's or Welch's t‐tests following the F‐test. Histopathological capsular lesions with grading were analyzed with Mann–Whitney's U‐test. For incidences of histopathological and immunohistochemistry‐derived findings, the Fisher's exact probability test was applied. Significance was inferred at the 5, 1, and 0.1% levels.
Results
In‐life parameters and thyroid weights. No abnormalities in general condition were observed in any of the groups. Final bodyweights were lowered (P < 0.01, 0.001) by SDM treatment, but no obvious changes were observed with AG administration (Table 1). Average water or food intake values are summarized in Table 2. Although water and food consumption was decreased by SDM treatment, AG treatment was without effect, independent of SDM. There was no significant difference in SDM intakes between groups 1 (DHPN–SDM + AG) and 2 (DHPN–SDM). Due to the decreased food consumption with SDM treatment, AG intake in group 1 showed a tendency for decrease as compared to group 3 (DHPN–AG). Thyroid absolute weights and relative to bodyweight weights were increased (P < 0.001) by SDM treatment. Although no obvious difference was noted in thyroid weights between groups 3 and 4 (DHPN alone, control) at week 4, some limitations in thyroid weight increment were noted in group 1 (P < 0.01) as compared to group 2 at week 10 (Table 1).
Table 1.
Initial and final bodyweight and thyroid weight of rats treated with sulfadimethoxine (SDM) with or without concomitant administration of aminoguanidine (AG) after N‐bis(2‐hydroxypropyl)nitrosamine (DHPN) initiation
| Group 1 DHPN–SDM + AG | Group 2 DHPN–SDM | Group 3 DHPN–AG | Group 4 DHPN alone | |
|---|---|---|---|---|
| Week 4 | ||||
| No. animals | 10 | 10 | 5 | 5 |
| Initial bodyweight (g) | 127.4 ± 6.2 | 127.2 ± 7.3 | 127.6 ± 6.7 | 127.9 ± 7.0 |
| Final bodyweight (g) | 182.9 ± 6.6*** | 185.8 ± 7.6*** | 210.4 ± 7.9 | 214.2 ± 9.3 |
| Thyroid weight | ||||
| Absolute (mg) | 90.1 ± 12.8*** | 99.1 ± 11.4*** | 11.4 ± 2.5 | 11.4 ± 2.2 |
| Relative (mg/100 g bodyweight) | 49.3 ± 7.4*** | 52.5 ± 5.1*** | 5.5 ± 1.4 | 5.3 ± 1.0 |
| Week 10 | ||||
| No. animals | 14 | 14 | 5 | 5 |
| Initial bodyweight (g) | 127.2 ± 7.2 | 127.6 ± 6.9 | 127.3 ± 6.3 | 127.5 ± 6.6 |
| Final bodyweight (g) | 239.9 ± 10.1*** | 243.5 ± 10.1** | 260.3 ± 5.9 | 263.4 ± 13.8 |
| Thyroid weight | ||||
| Absolute (mg) | 151.4 ± 10.4***, †† | 173.5 ± 20.5*** | 12.2 ± 2.8 | 11.3 ± 1.4 |
| Relative (mg/100 g bodyweight) | 63.1 ± 3.6***, †† | 71.2 ± 7.3*** | 4.7 ± 1.1 | 4.3 ± 0.5 |
Data are mean ± SD values.
P < 0.01,
P < 0.001 vs group 4 (control);
P < 0.01 vs group 2.
Table 2.
Water and food consumption and chemical intake of rats treated with sulfadimethoxine (SDM) with or without concomitant administration of aminoguanidine (AG) after N‐bis(2‐hydroxypropyl)nitrosamine (DHPN) initiation
| Group 1 DHPN–SDM + AG | Group 2 DHPN–SDM | Group 3 DHPN–AG | Group 4 DHPN alone | |
|---|---|---|---|---|
| Water consumption (g/rat/day) | 21.1 | 21.3 | 27.5 | 28.1 |
| SDM intake (mg/kg bodyweight/day) | 109.8 | 109.5 | 0 | 0 |
| Food consumption (g/rat/day) | 10.6 | 10.8 | 13.4 | 13.7 |
| AG intake (mg/kg bodyweight/day) | 109.4 | 0 | 123.3 | 0 |
Serum hormone levels. Serum T4 levels were significantly (P < 0.001) decreased and the T3 levels showed a tendency for decrease on SDM treatment, but no obvious changes in either were observed with AG administration (Table 3). TSH levels were prominently increased by SDM treatment (P < 0.001), but no obvious changes were detected again by AG administration, independent of SDM (Table 3).
Table 3.
Serum thyroid hormones and thyroid stimulating hormone (TSH) levels in rats treated with sulfadimethoxine (SDM) with or without concomitant administration of aminoguanidine (AG) after N‐bis(2‐hydroxypropyl)nitrosamine (DHPN) initiation
| Group 1 DHPN–SDM + AG | Group 2 DHPN–SDM | Group 3 DHPN–AG | Group 4 DHPN alone | |
|---|---|---|---|---|
| Week 4 | ||||
| No. animals | 10 | 10 | 5 | 5 |
| Thyroxine (ng/mL) | 17.9 ± 1.2*** | 16.8 ± 2.0*** | 36.8 ± 4.4 | 38.8 ± 1.5 |
| Triiodothyronine (ng/mL) | <0.5 | <0.5 | 0.7 ± 0.1 | 0.8 ± 0.1 |
| TSH (ng/mL) | 137.5 ± 19.9*** | 128.4 ± 9.9*** | 6.9 ± 0.5 | 7.3 ± 1.5 |
| Week 10 | ||||
| No. animals | 14 | 14 | 5 | 5 |
| Thyroxine (ng/mL) | 22.9 ± 2.9*** | 22.6 ± 3.0*** | 52.0 ± 7.4 | 47.2 ± 5.5 |
| Triiodothyronine (ng/mL) | <0.5 | <0.5 | 0.8 ± 0.1 | 0.8 ± 0.2 |
| TSH (ng/mL) | 141.8 ± 33.8*** | 133.0 ± 40.0*** | 7.3 ± 1.3 | 7.3 ± 0.9 |
Data are mean ± SD values.
P < 0.001 vs group 4 (control).
Histopathology and immunohistochemistry. At week 4, diffuse follicular cell hyperplasia, so‐called goiter, was obviously induced in all SDM‐treated rats of groups 1 and 2, together with slight inflammatory cell infiltration in the thyroid capsular region (Fig. 1a). A more advanced lesion, thyroid capsular thickening with inflammation, was partly or extensively observed in 4 and 6 of 10 rats each in groups 1 and 2, respectively (Fig. 2a). Grading of the advanced capsular lesions based on the spread of inflammation was carried out, and the results are summarized in Table 4. There was no significant difference in the degree of capsular inflammatory changes between the groups. In the inflamed capsular regions, disrupted follicles and diffuse and/or multifocal migration of follicular epithelium were noted in both groups 1 and 2 (Fig. 1a). For follicular preneoplastic and neoplastic lesions, values for incidence and multiplicity are summarized in Table 5. At week 4, focal hyperplasias and adenomas were observed in both groups 1 and 2, and their incidences and multiplicities were comparable. At week 10, capsular inflammation had almost disappeared, but severe fibrous thickening without inflammation remained in all rats of groups 1 and 2 (Fig. 3a–c). As follicular neoplastic lesions, intrathyroidal and invasive carcinomas were evident at this time point (Fig. 4a). Although the incidences and multiplicities of intrathyroidal carcinomas and the incidence of invasive carcinomas were comparable between groups 1 and 2, the multiplicity of invasive carcinomas was lower (P < 0.05) in group 1 (Table 5). No histopathological abnormalities were detected in thyroids of rats in groups 3 and 4 at weeks 4 or 10.
Figure 1.
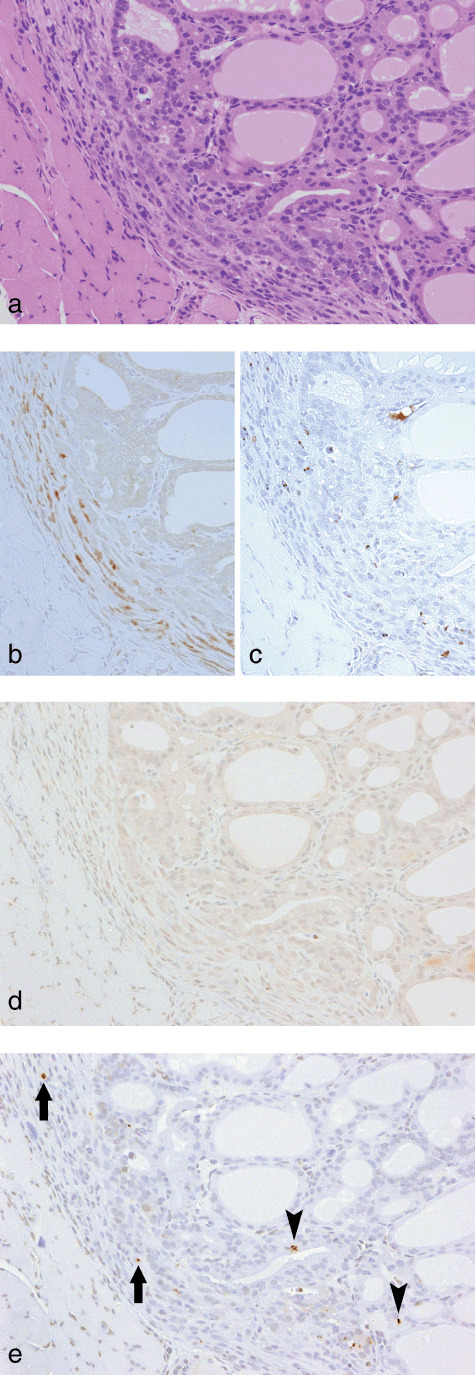
Slight inflammatory cell infiltration with focal migration of epithelial cells from disrupted follicles into the thyroid capsular region at week 4 in a rat treated with sulfadimethoxine following N‐bis(2‐hydroxypropyl)nitrosamine‐initiation. (a) H&E. Original magnification ×180. (b) A serial section of (a). CD3 immunohistochemistry. Some inflammatory cells are positive for CD3, indicative of a T‐cell nature. (c) A serial section of (a). ED1 immunohistochemistry. A few inflammatory cells are positive for ED1, indicative of a macrophage nature. (d) A serial section of (a). inducible nitric oxide synthase immunohistochemistry. No positive cells were found. (e) A serial section of (a). Single‐strand DNA (ssDNA) immunohistochemistry. A few ssDNA‐positive dot signals, demonstrating fragmented nuclei, are evident in some inflammatory and/or interstitial cells (arrows) and follicular epithelial cells (arrowheads).
Figure 2.
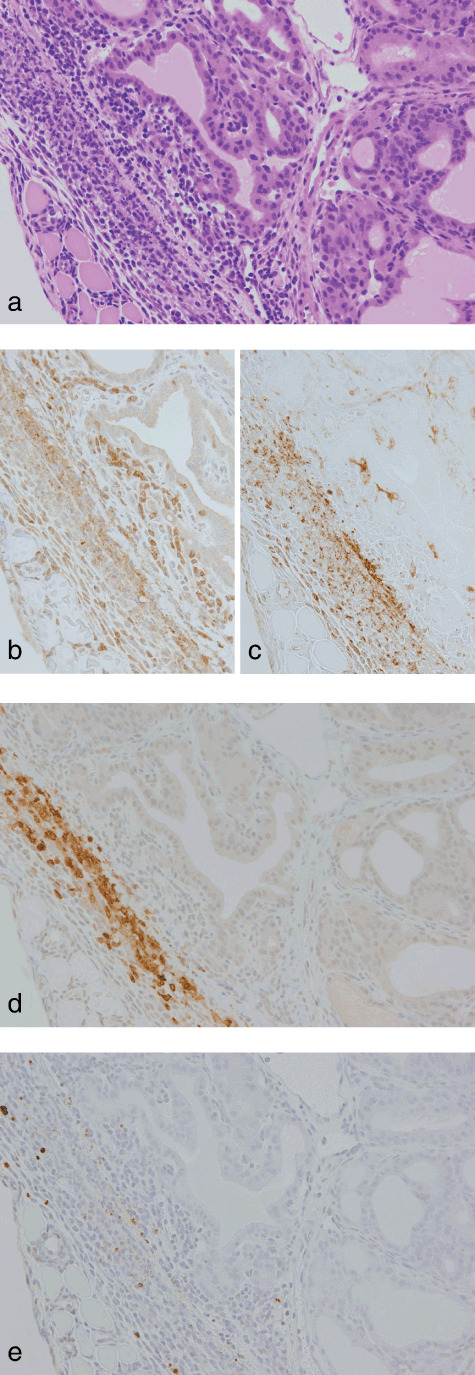
Thyroid capsular thickening with inflammation at week 4 in a rat treated with sulfadimethoxine following N‐bis(2‐hydroxypropyl)nitrosamine‐initiation. (a) H&E. Original magnification ×180. (b) A serial section of (a). CD3 immunohistochemistry. Some inflammatory cells scattered beside follicles are positive for CD3, indicative of a T‐cell nature. (c) ED1 immunohistochemistry. The majority of capsular inflammatory cells are positive for ED1, indicative of a macrophage nature. (d) A serial section of (a). Inducible nitric oxide synthase (iNOS)‐immunohistochemistry. Note an iNOS‐positive focus consisting mainly of macrophages. (e) Single‐strand DNA (ssDNA)‐immunohistochemistry. ssDNA‐positive dot signals are present in some inflammatory and/or interstitial cells.
Table 4.
Histopathological findings at week 4 in the thyroid capsular region of rats treated with sulfadimethoxine (SDM) with or without concomitant administration of aminoguanidine (AG) after N‐bis(2‐hydroxypropyl)nitrosamine (DHPN) initiation (N = 10)
| Grade | Group 1 DHPN–SDM + AG | Group 2 DHPN–SDM | |
|---|---|---|---|
| Inflammatory cell infiltration | + | 6 (60) | 4 (40) |
| Capsular thickening with inflammation | + | 3 (30) | 3 (30) |
| ++ | 1 (10) | 3 (30) |
Numbers in parentheses are percentage values. +, regional and/or unilateral; ++, diffuse and bilateral changes.
Table 5.
Incidence and multiplicity of follicular preneoplastic and neoplastic lesions in rats treated with sulfadimethoxine (SDM) with or without concomitant administration of aminoguanidine (AG) after N‐bis(2‐hydroxypropyl)nitrosamine (DHPN) initiation
| Group 1 DHPN–SDM + AG | Group 2 DHPN–SDM | |
|---|---|---|
| Week 4 | ||
| No. animals | 10 | 10 |
| Focal hyperplasia | 10 (100) † | 10 (100) |
| 10.4 ± 2.6 ‡ | 10.6 ± 3.3 | |
| Adenoma | 8 (80) | 7 (70) |
| 1.3 ± 1.1 | 1.1 ± 1.0 | |
| Intrathyroidal carcinoma | 0 | 0 |
| Invasive carcinoma | 0 | 0 |
| Week 10 | ||
| No. animals | 14 | 14 |
| Focal hyperplasia | 14 (100) | 14 (100) |
| 17.6 ± 4.2 | 17.4 ± 4.0 | |
| Adenoma | 14 (100) | 14 (100) |
| 4.4 ± 2.4 | 5.1 ± 2.5 | |
| Intrathyroidal carcinoma | 3 (21) | 4 (29) |
| 0.2 ± 0.4 | 0.3 ± 0.5 | |
| Invasive carcinoma | 10 (71) | 12 (86) |
| 1.6 ± 1.3* | 3.1 ± 2.2 | |
Incidence (%),
‡ multiplicity.
P < 0.05 vs group 2.
Figure 3.
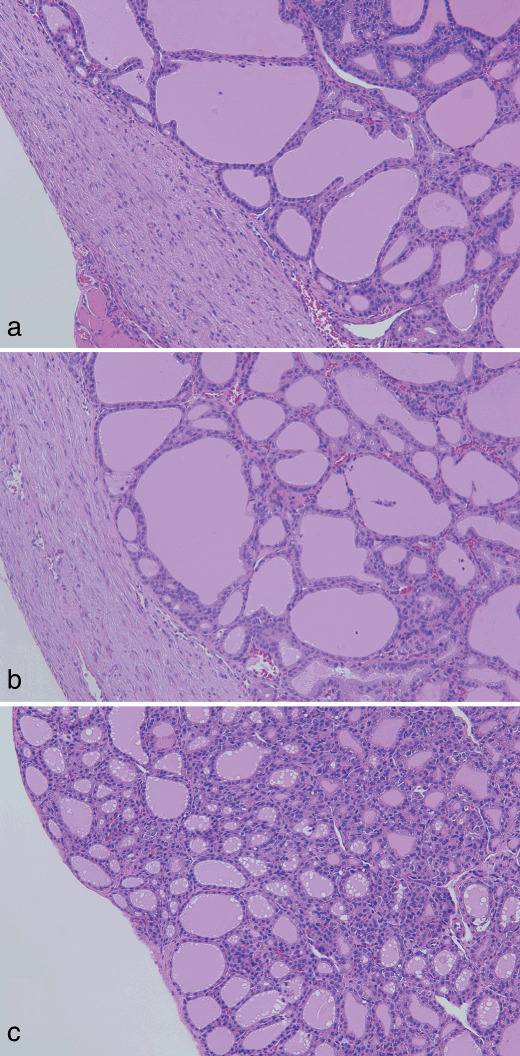
(a) Capsular fibrous thickening without inflammation at week 10 in a rat treated with sulfadimethoxine and aminoguanidine following N‐bis(2‐hydroxypropyl)nitrosamine‐initiation. (b) Similar lesion as (a) at week 10 in a rat treated with sulfadimethoxine following N‐bis(2‐hydroxypropyl)nitrosamine‐initiation. (c) Normally appearing thyroid capsule at week 10 in a non‐treated control rat. H&E. Original magnification ×90.
Figure 4.
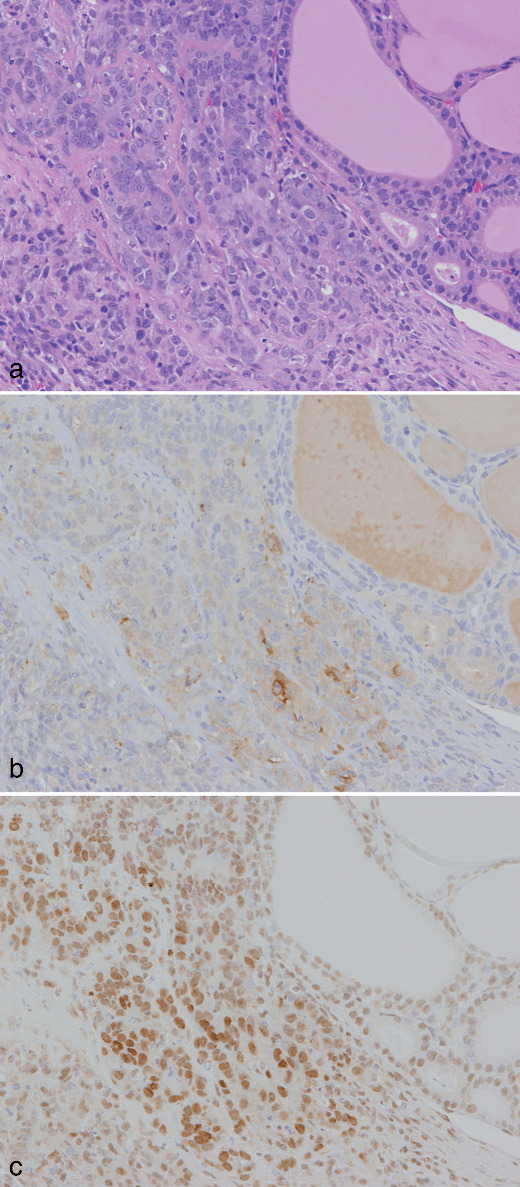
A carcinoma invading a capsule at week 10 in a rat treated with sulfadimethoxine following N‐bis(2‐hydroxypropyl)nitrosamine‐initiation. (a) H&E. Original magnification ×180. (b) A serial section of (a). Inducible nitric oxide synthase immunohistochemistry. Some carcinoma cells are weakly positive. (c) A serial section of (a). Single‐strand DNA (ssDNA) immunohistochemistry. Note the diffuse nuclear positivity for ssDNA in carcinoma cells.
Ki‐67‐positive ratios in follicular cells surrounding the preneoplastic and neoplastic lesions in groups 1 and 2 at week 4 were 10.3 ± 4.6 and 10.0 ± 4.9%, respectively, and no significant difference was found in the cell proliferative activity between the groups. Immunohistochemistry for CD3 and ED1 revealed that the inflammatory cells in inflamed capsular regions of groups 1 and 2 at week 4 were mainly T cells and macrophages (1, 2). Although iNOS‐positive cells were not found in capsular regions with slight inflammatory cell infiltration (Fig. 1d), iNOS‐positive foci, consisting predominantly of ED1‐positive macrophages, were sporadically found in advanced thickened capsular lesions (Fig. 2d). There were no obvious differences in the incidence and multiplicity of the iNOS‐positive foci between the two groups (Table 6). Some inflammatory and/or interstitial cells and neighboring follicular epithelial cells exhibited various sizes of ssDNA‐positive dot signals, probably due to fragmented nuclei. The ssDNA‐positive cells were mainly localized in iNOS‐positive foci but some were scattered also in the other inflamed regions (1, 2). To compare intensities of tissue damage in the capsular regions between groups 1 and 2, ssDNA‐positive cells were counted, and the multiplicity in group 1 showed a tendency for decrease as compared to group 2 (Table 6). At week 10, cytoplasmic expression of iNOS was seen in subsets of carcinoma cells in 4 of 13 (31%) and 2 of 21 (10%) invasive carcinomas examined in groups 1 and 2, respectively (Fig. 4b). There was variation in the staining intensity and proportion of iNOS among the carcinomas, but no obvious difference was found between the groups. In all invasive carcinomas and some intrathyroidal carcinomas, diffuse nuclear positivity for ssDNA in carcinoma cells was observed, but fragmentation of the nuclei was limited (Fig. 4c). In groups 3 and 4, no obvious iNOS‐ and/or ssDNA‐positive cells were detected in the thyroid capsular regions.
Table 6.
Incidence and multiplicity of inducible nitric oxide synthase (iNOS)‐positive foci and intensity of tissue damage in inflamed thyroid capsular regions at week 4 in rats treated with sulfadimethoxine (SDM) with or without concomitant administration of aminoguanidine (AG) after N‐bis(2‐hydroxypropyl)nitrosamine (DHPN) initiation (N = 10)
| Group 1 DHPN–SDM + AG | Group 2 DHPN–SDM | ||
|---|---|---|---|
| iNOS‐positive foci | Incidence (%) | 3 (30) | 5 (50) |
| Multiplicity (no./rat) | 0.4 ± 0.7 | 0.6 ± 0.7 | |
| Single‐strand DNA‐positive cells | Incidence (%) | 10 (100) | 10 (100) |
| Multiplicity (no./rat) | 24.3 ± 14.8 | 43.0 ± 32.4 |
Discussion
In the present study, thyroid capsular thickening with inflammation and iNOS‐positive foci were observed with SDM, and subsequently invasive follicular carcinomas arose in the capsular region. Their multiplicity was decreased by concomitant AG administration, suggesting that iNOS induction in inflamed capsular regions observed in the early stage of the experiment was at least partly associated with tumor promotion and/or progression in this model. SDM is a potent inhibitor of thyroid peroxidase and shows strong goitrogenic activity via suppression of serum thyroid hormone levels followed by increment in TSH production. Elevated TSH is considered to directly contribute to goiter development and thus thyroid carcinogenesis in rats.( 20 , 21 ) In the present study, absolute and relative thyroid weights were actually increased by SDM treatment, but they were lowered by AG administration. On the other hand, serum TSH levels were prominently increased by SDM treatment, and no obvious changes were detected with AG administration with or without SDM. In addition, cell proliferative activity in follicular cells surrounding the preneoplastic and neoplastic lesions was not altered by AG administration. Therefore, although the reason for lowering of thyroid weights by AG was not clear, inhibitory effects on induction of invasive follicular carcinomas may be associated with its effects or iNOS directly, rather than indirectly through SDM.
Excess NO is considered to be directly cytotoxic and also to react with superoxide anions, resulting in production of peroxynitrites that may cause severe tissue damage.( 22 , 23 ) ssDNA‐positive cells indicative of tissue damage in the inflamed capsular regions appeared to be associated with the iNOS induction and their numbers showed a tendency for decrease with AG administration, whereas AG did not alter the incidence and multiplicity of iNOS‐positive foci. This seeming discrepancy should be resolved by considering the mechanisms of iNOS‐inhibitory action of AG, which shows structural similarity to the iNOS substrate l‐arginine and antagonistic binding potencies to iNOS.( 15 , 16 ) NO and peroxynitrite have been reported to induce not only DNA and tissue damage but also other various biological alterations, such as activation of cyclooxygenase‐2( 2 , 9 ) and matrix metalloproteinases( 24 ) leading to tumor angiogenesis and/or metastasis. Thus further studies are needed to clarify how these factors are involved in DHPN–SDM‐induced rat thyroid carcinogenesis. In addition, as ssDNA‐positive cells were scattered not only in the iNOS‐positive foci but also in the other inflamed regions, active oxygen species other than NO produced by inflammatory cells might also be involved in DNA and tissue damage leading to tumor promotion or progression.
Migration of follicular epithelium from disrupted follicles into capsules was demonstrated at 4–6 weeks after the start of SDM treatment in rats initiated with DHPN in our previous experiments conducted under the same protocol as the present one, but their biological significance is uncertain.( 13 , 14 , 25 ) Again in the present study, migrating cells were frequently observed, but they appeared to lack iNOS expression. On the other hand, immunoreactivity for iNOS was observed in carcinoma cells in invasive carcinomas, in which diffuse nuclear positivity for ssDNA was also demonstrated. Therefore active nitrogen and/or oxygen species generated not only by inflammatory cells but also by tumor cells might be associated with neoplastic promotion or progression.
In humans, an association between Hashimoto's autoimmune thyroiditis and thyroid carcinomas has been reported,( 26 , 27 , 28 ) along with iNOS expression in both inflammatory cells in thyroiditis and carcinoma cells.( 29 , 30 ) Therefore, the present DHPN–SDM rat thyroid carcinogenesis model appears to resemble human thyroiditis cases, except for the localization of the inflammation; regional capsulitis is characteristic but intrathyroidal diffuse inflammation is not evident in this model. In conclusion, the present results suggest that iNOS induction in both inflammatory and tumor cells might play pivotal roles in tumor progression in this DHPN–SDM rat model.
Disclosure Statement
This work was supported in part by Health and Labour Sciences Research Grants from the Ministry of Health, Labour, and Welfare of Japan.
Acknowledgments
We thank Dr Malcolm A. Moore for revision of the scientific English language and Ms Ayako Kaneko for expert technical assistance.
References
- 1. Crowell JA, Steele VE, Sigman CC et al . Is inducible nitric oxide synthase a target for chemoprevention? Mol Cancer Ther 2003; 2: 815–23. [PubMed] [Google Scholar]
- 2. Rao CV. Nitric oxide signaling in colon cancer chemoprevention. Mutat Res 2004; 555: 107–19. [DOI] [PubMed] [Google Scholar]
- 3. Kawanishi S, Hiraku Y, Pinlaor S et al . Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation‐related carcinogenesis. Biol Chem 2006; 387: 365–72. [DOI] [PubMed] [Google Scholar]
- 4. Ohta T, Takahashi M, Ochiai A. Increased protein expression of both inducible nitric oxide synthase and cyclooxygenase‐2 in human colon cancers. Cancer Lett 2006; 239: 246–53. [DOI] [PubMed] [Google Scholar]
- 5. Ambs S, Bennett WP, Merriam WG et al . Relationship between p53 mutations and inducible nitric oxide synthase expression in human colorectal cancer. J Natl Cancer Inst 1999; 91: 86–8. [DOI] [PubMed] [Google Scholar]
- 6. Hao XP, Pretlow TG, Rao JS et al . Inducible nitric oxide synthase (iNOS) is expressed similarly in multiple aberrant crypt foci and colorectal tumors from the same patients. Cancer Res 2001; 61: 419–22. [PubMed] [Google Scholar]
- 7. Roberts PJ, Riley GP, Morgan K et al . The physiological expression of inducible nitric oxide synthase (iNOS) in the human colon. J Clin Pathol 2001; 54: 293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moochhala S, Chhatwal VJ, Chan ST et al . Nitric oxide synthase activity and expression in human colorectal cancer. Carcinogenesis 1996; 17: 1171–4. [DOI] [PubMed] [Google Scholar]
- 9. Takahashi M, Wakabayashi K. Gene mutations and altered gene expression in azoxymethane‐induced colon carcinogenesis in rodents. Cancer Sci 2004; 95: 475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang R, Ma A, Urbanski SJ et al . Induction of inducible nitric oxide synthase: a protective mechanism in colitis‐induced adenocarcinoma. Carcinogenesis 2007; 28: 1122–30. [DOI] [PubMed] [Google Scholar]
- 11. Choe W, Kim S, Hwang TS et al . Expression of inducible nitric oxide synthase in thyroid neoplasms: immunohistochemical and molecular analysis. Pathol Int 2003; 53: 434–9. [DOI] [PubMed] [Google Scholar]
- 12. Nose F, Ichikawa T, Fujiwara M et al . Upregulation of cyclooxygenase‐2 expression in lymphocytic thyroiditis and thyroid tumors: significant correlation with inducible nitric oxide synthase. Am J Clin Pathol 2002; 117: 546–51. [DOI] [PubMed] [Google Scholar]
- 13. Imai T, Onose J, Hasumura M et al . Sequential analysis of development of invasive thyroid follicular cell carcinomas in inflamed capsular regions of rats treated with sulfadimethoxine after N‐bis (2‐hydroxypropyl) nitrosamine‐initiation. Toxicol Pathol 2004; 32: 229–36. [DOI] [PubMed] [Google Scholar]
- 14. Imai T, Hasumura M, Cho YM et al . Depression of T cell‐mediated immunity reduces sulfadimethoxine‐induced capsular inflammation and inhibits associated development of invasive thyroid follicular cell carcinomas in rats. Cancer Sci 2007; 98: 294–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stuhlmiller DF, Boje KM. Characterization of 1‐arginine and aminoguanidine uptake into isolated rat choroid plexus: differences in uptake mechanisms and inhibition by nitric oxide synthase inhibitors. J Neurochem 1995; 65: 68–74. [DOI] [PubMed] [Google Scholar]
- 16. Boer R, Ulrich WR, Klein T et al . The inhibitory potency and selectivity of arginine substrate site nitric‐oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol Pharmacol 2000; 58: 1026–34. [PubMed] [Google Scholar]
- 17. Rao CV, Indranie C, Simi B et al . Chemopreventive properties of a selective inducible nitric oxide synthase inhibitor in colon carcinogenesis, administered alone or in combination with celecoxib, a selective cyclooxygenase‐2 inhibitor. Cancer Res 2002; 62: 165–70. [PubMed] [Google Scholar]
- 18. Fujihara CK, Mattar AL, Vieira JM Jr. et al . Evidence for the existence of two distinct functions for the inducible NO synthase in the rat kidney: effect of aminoguanidine in rats with 5/6 ablation. J Am Soc Nephrol 2002; 13: 2278–87. [DOI] [PubMed] [Google Scholar]
- 19. Hardisty JF, Boorman GA. Thyroid gland. In: Boorman GA, Eustis SL, Elwell MR, Montgomery CA Jr, MacKenzie WF, eds. Pathology of the Fischer Rat. San Diego: Academic Press, 1990; 519–36. [Google Scholar]
- 20. Mitsumori K, Onodera H, Takahashi M et al . Effect of thyroid stimulating hormone on the development and progression of rat thyroid follicular cell tumors. Cancer Lett 1995; 92: 193–202. [DOI] [PubMed] [Google Scholar]
- 21. Capen CC. Thyroid gland (follicular cells). In: Klaassen CD, ed. Casarett and Doull's Toxicology, the Basic Science of Poisons, 6th edn. New York: McGraw‐Hill, 2001; 723–34. [Google Scholar]
- 22. Beckman JS, Beckman TW, Chen J et al . Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A 1990; 87: 1620–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kimura H, Hokari R, Miura S et al . Increased expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in colonic mucosa of patients with active ulcerative colitis. Gut 1998; 42: 180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu J, Akaike T, Hayashida K et al . Enhanced vascular permeability in solid tumor involving peroxynitrite and matrix metalloproteinases. Jpn J Cancer Res 2001; 92: 439–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Imai T, Hasumura M, Onose J et al . Development of invasive follicular cell carcinomas in a rat thyroid carcinogenesis model: biological impact of capsular inflammation and reduced cyclooxygenase‐2 expression. Cancer Sci 2005; 96: 31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Di Pasquale M, Rothstein JL, Palazzo JP. Pathologic features of Hashimoto's‐associated papillary thyroid carcinomas. Hum Pathol 2001; 32: 24–30. [DOI] [PubMed] [Google Scholar]
- 27. Pisanu A, Piu S, Cois A et al . Coexisting Hashimoto's thyroiditis with differentiated thyroid cancer and benign thyroid diseases: indications for thyroidectomy. Chir Ital 2003; 55: 365–72. [PubMed] [Google Scholar]
- 28. Larson SD, Jackson LN, Riall TS et al . Increased incidence of well‐differentiated thyroid cancer associated with Hashimoto thyroiditis and the role of the PI3k/Akt pathway. J Am Coll Surg 2007; 204: 764–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Donckier JE, Michel L, Delos M et al . Interrelated overexpression of endothelial and inducible nitric oxide synthases, endothelin‐1 and angiogenic factors in human papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2006; 64: 703–10. [DOI] [PubMed] [Google Scholar]
- 30. Patel A, Fenton C, Terrell R et al . Nitrotyrosine, inducible nitric oxide synthase (iNOS), and endothelial nitric oxide synthase (eNOS) are increased in thyroid tumors from children and adolescents. J Endocrinol Invest 2002; 25: 675–83. [DOI] [PubMed] [Google Scholar]


