Abstract
Assays have been described in which duplex adeno-associated virus (AAV) DNA can be replicated in HeLa cell extracts with exogenous AAV Rep protein. These assays appear to mimic the AAV DNA replication that occurs in the cell, including the ability of extracts from adenovirus (Ad)-infected cells to replicate duplex AAV DNA templates more efficiently than extracts from uninfected cells can. We showed previously that the Ad-infected extract was able to support a more processive replication than the uninfected extract. When the Ad single-stranded DNA binding protein (Ad-DBP) was added to an uninfected extract, DNA replication became processive. Based on a strand displacement replication model, we hypothesized that the Ad-DBP was stabilizing the displaced single-stranded DNA during strand displacement replication. In this report, we show that in Ad-infected extracts most of the newly replicated duplex DNA is converted into a single-stranded form shortly after synthesis. Using the results of assays for the replication of single-stranded AAV DNA, we show that these single-stranded molecules serve as templates for additional replication. In addition, we identify a class of molecules which are likely to be intermediates of replication on single-stranded templates. We discuss a possible role for replication of single-stranded molecules in the infected cell.
Upon infecting a susceptible cell, the parvovirus adeno-associated virus (AAV) can enter into either of two pathways. In the absence of helper virus coinfection, AAV cannot productively replicate but the viral genome can become integrated site specifically into the host cell genome. In the presence of coinfection with a helper virus (typically either adenovirus [Ad] or herpesviruses), a substantial productive infection ensues (1). Within 24 h, the infected cell may contain as many as 106 AAV genome equivalents (27).
The factors which regulate or participate in productive AAV DNA replication are as yet incompletely understood. There is nevertheless a simple model that describes the replication pathway of AAV DNA (6, 17, 18, 29). There are several aspects of this model which are somewhat unclear, one of which is the potential role of single-stranded molecules in replication. The AAV genome, which is single stranded, contains 4,679 bases. At each end is a 145-base inverted terminal repeat (ITR), the outer 125 bases of which are capable of forming a hairpin. The 3′ hairpinned end is thought to serve as a primer for full-length replication of the viral genome, thereby converting the input single strand into a duplex genome. Subsequent rounds of replication are thought to proceed by a strand displacement mechanism (6, 17, 18, 29). The viral replication protein (Rep) cleaves one strand of the DNA (within the now closed hairpin) at the terminal resolution site, located 125 nucleotides from the original 3′ end (14). The newly created 3′ end allows replicative extension outward through the terminal sequences (terminal resolution) (28). The result is a blunt-ended duplex molecule. The ends are unwound in a process thought to involve the viral Rep protein (40), enabling the ITR to resume a hairpin configuration with a 3′ end. This 3′ end again serves as a primer for elongation, displacing one strand of the duplex, which becomes available for packaging into the viral capsid. Alternatively, with the distal ITR in a closed-hairpin conformation, replication of the displaced strand can lead to formation of a head-head or tail-tail dimer.
In AAV-infected cells, from 12 to 24 h postinfection there is a rapid accumulation of duplex monomer and dimer forms but only a small amount of single-stranded DNA can be detected. There is a concomitant synthesis of the four Rep proteins as well as capsid proteins (26). After 24 h, the accumulation of new protein slows and the levels of duplex DNA remain relatively constant but the levels of single-stranded DNA increase greatly (26). Chejanovsky and Carter showed that transfections of plasmids unable to produce Rep 52 and Rep 40 led to DNA replication but little accumulation of single-stranded DNA (2). It has also been shown that the detection of single-stranded DNA in infected cells was dependent upon the production of capsid protein (9, 20, 21, 31). Presumably this was a function of the sequestering of single-stranded DNA into capsids, as was first proposed for the autonomous parvoviruses (30). However, additional observations suggest that regulation of the production and accumulation of single-stranded DNA may be more complex. Holscher et al. found little single-stranded DNA in a Rep-producing cell line with AAV DNA replication and abundant Cap protein (11). The addition of a Rep-encoding plasmid led to detectable single-stranded DNA. Not only plasmids which coded for Rep 40 and Rep 52 but also those coding for Rep 68 and Rep 78 gave this effect.
It has been possible to use cell-free replication systems to gain insights into AAV DNA replication. Several such cell-free DNA replication systems which capture key aspects of the productive infection pathway have been developed (3, 23, 34). In these, exogenously produced AAV Rep protein is added to extracts made from either uninfected or Ad-infected HeLa cells, with a duplex form of the viral genome serving as the substrate for replication. Using a cell-free assay, Ni et al. identified several cellular proteins which participate in AAV DNA replication (22). It was also shown that AAV DNA replication in uninfected cell extracts was deficient in comparison to that in Ad-infected extracts in the processivity of replication (35). This processivity advantage in the Ad-infected extract was shown to be due to the Ad DNA binding protein (Ad-DBP) (36). Apparently the Ad-DBP served to stabilize the displaced strand, preventing its reannealing to the template.
One aspect of productive AAV DNA replication that has not been described in cell-free systems is the conversion of single-stranded to double-stranded DNA. In this report, we describe assays for the replication of single-stranded AAV templates. Such assays allow us to examine the fate of the Ad-DBP-stabilized displaced strand by asking whether these molecules can themselves serve as templates for replication. We show that during the replication assay the newly replicated DNA strand on a duplex is rapidly converted to a single-stranded form and that this single-stranded DNA serves as a template for further replication. In addition, we identify a class of molecules with a structure consistent with their being intermediates for replication on single-stranded templates.
MATERIALS AND METHODS
Cell extracts.
Replication extracts from uninfected HeLa cells and from HeLa cells infected with Ad were prepared as described previously (12, 33), modified from the procedure of Wobbe et al. (39).
Proteins.
Ad-DBP was made in a baculovirus expression system (19). Replication protein A (RPA) was purified from Escherichia coli expressing p11d-tRPA (8). His-TagRep 68 is the entire Rep protein with 10 histidine residues fused to the amino-terminal end. It was produced in E. coli from a pET 16b vector (New England Biolabs) and purified as specified by the manufacturer. Mung bean nuclease was purchased from Bethesda Research Laboratories. Digestion was carried out at 10 U/μl in 30 mM sodium acetate (pH 5.0)–50 mM sodium chloride–1 mM zinc acetate–50 μg of bovine serum albumin per ml. Nuclease S1 was purchased from Boehringer Mannheim. Digestion was carried out at 6 U/μl in 50 mM sodium acetate (pH 4.5)–1 mM zinc acetate–250 mM sodium chloride–50 μg of bovine serum albumin per ml.
Chemicals.
Camptothecin, obtained from the National Cancer Institute (no. 94600), and aphidicolin, purchased from Sigma (no. A-0781), were dissolved in dimethyl sulfoxide.
Cell-free DNA replication.
Replication assays were performed basically as described previously (34). The reaction mixture (15 μl) contained 40 mM HEPES (pH 7.7); 40 mM creatine phosphate (pH 7.7); 7 mM MgCl2; 4 mM ATP; 200 μM each CTP, GTP, and UTP; 100 μM each dATP, dGTP, and dTTP; 10 μM dCTP; 10 μCi of [α-32P] dCTP (3,000 Ci/μmol; Amersham); 2 mM dithiothreitol, 6 mM potassium glutamate; 2.0 μg of creatine phosphokinase; approximately 100 μg of HeLa cell extract protein; 0.1 μg of plasmid DNA; and 100 ng of His-TagRep 68. BglII- or BglII-XhoI-digested pAV2 (15) (the entire genome of AAV2 inserted into a modified pBR322) was used as the substrate. Reaction mixtures were preincubated at 37°C for 3 h, and the end of this period was defined as the 0.0-h time point. Incubations, with the timed addition of Rep 68, labeled dCTP, and other reagents, were carried out at 37°C for an additional 16 h unless otherwise indicated. For the pulse-chase experiments, reaction mixtures were chased with 3,000 μM dCTP. The reaction products were brought to 65 μl with digestion buffer (20 mM HEPES [pH 7.5], 10 mM KCl, 10 mM EDTA, 1.0% sodium dodecyl sulfate, 50 mM NaCl), passed over a Sephadex-50 spin colum, digested with proteinase K (10 mg/ml) for 2 h at 50°C, and analyzed by electrophoresis on 0.8% agarose gels with Tris-borate-EDTA (TBE) buffer. Reaction products that were to be enzymatically digested were also purified with Gene Clean (Bio 101) as specified by the manufacturer. The data was analyzed by PhosphorImager (Molecular Dynamics) scanning of dried gels with ImageQuant software.
Two-dimensional gel electrophoresis.
Nondenaturing gel electrophoresis was carried out in a 0.8% agarose gel with TBE buffer; for the second, denaturing dimension, lanes were excised and transferred to an 0.8% agarose gel containing 1 mM EDTA and 40 mM sodium hydroxide. The gels were equilibrated for 90 min in the denaturing buffer prior to electrophoresis.
RESULTS
Cell-free replication assays with Ad-infected extracts detect single-stranded DNA.
Previously we reported that extracts from Ad-infected cells replicated AAV DNA with greater processivity than did extracts from uninfected cells. An additional observation was that when the products of replication in Ad-infected extracts of linear duplex AAV were separated by gel electrophoresis, two full-length products were visible, the expected duplex DNA and a slightly faster-moving species (35, 36). This observation was dependent upon electrophoretic separation of the products of a reaction without prior precipitation of the DNA.
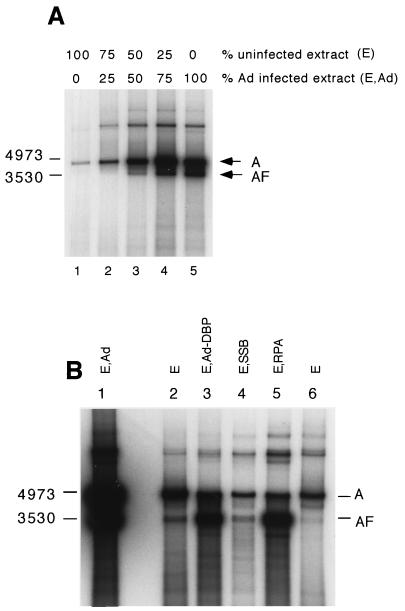
Figure 1 illustrates a set of replication assays in which extracts from uninfected and Ad-infected cells were mixed. With increasing amounts of Ad-infected extract, labeled DNA began to appear in the band labeled AF rather than solely in the band labeled A (full-length duplex AAV). Figure 1B illustrates the results of replication assays comparing an extract from Ad-infected cells with extracts from uninfected cells supplemented with either purified recombinant Ad-DBP or purified recombinant human single-stranded binding protein (RPA). Substantial amounts of AF were detected with the Ad-infected extract as well as with the uninfected extract supplemented with either Ad-DBP or RPA. AF was frequently detectable in assays with uninfected extracts but was present in much smaller amounts. Figure 1B also shows that while some AF DNA was seen when the assay mixture was supplemented with E. coli single-stranded DNA binding protein (SSB), the amount was minimal. This is in accordance with our previous results (36), which showed that E. coli SSB was not able to support increased replication in the uninfected extracts while RPA and Ad-DBP were able to do so. As seen in Fig. 1B, the product of replication assays performed with uninfected extracts, supplemented with single-stranded binding protein, was mostly form AF rather than form A (linear duplex full-length AAV). This is in contrast to replication with Ad-infected extracts, in which the products were mostly of form A.
FIG. 1.
(A) Replication reactions performed with mixtures of Ad-infected and uninfected extracts. Band A is full-length duplex AAV DNA; band AF is a faster-migrating form of full-length AAV DNA. (Band AF is a closely spaced doublet.) (B) Assays performed with extracts, unsupplemented or supplemented with single-stranded DNA binding proteins. Lanes: 1, unsupplemented Ad-infected extract; 2, unsupplemented extract from uninfected heat-shocked cells; 3, uninfected extract supplemented with the Ad-DBP; 4, uninfected extract supplemented with E. coli SSB; 5, uninfected extract supplemented with RPA; 6 uninfected, unsupplemented extract.
When forms A and AF were observed through precipitation and resuspension of the DNA, the cpm in band AF converted to the form of band A, suggesting that the material in band AF is full-length AAV, which has a different structure from that in band A (data not shown). Further studies were undertaken to demonstrate that the material in band AF is single-stranded full-length AAV. Figure 2A demonstrates that band AF is resistant to several restriction enzymes which digest AAV DNA while restriction digests of band A result in the expected band pattern; Fig. 2B demonstrates that band AF is digested by the single-strand nucleases mung bean nuclease and nuclease S1, respectively. When the products of a replication assay from either an uninfected or an Ad-infected extract were boiled prior to electrophoresis, they migrated on the gel indistinguishably from band AF in unboiled replication product (Fig. 2C). When the unreplicated substrate DNA used in the assay (BglII-digested pAV2) was boiled, it also migrated in an agarose gel indistinguishably from band AF (data not shown).
FIG. 2.
(A) Restriction digest of the replication products generated with Ad-infected extracts. Lanes: 1, undigested; 2, NarI digested; 3, PstI digested; 4, SacII digested; 5, ScaI digested. (B) Nuclease digestions of the replication products with Ad-infected extracts. Lanes: C, no enzyme; MBN, digestion with 10 U of mung bean nuclease per μl; S1, digestion with 6 U of nuclease S1 per μl. Incubation times are indicated in minutes. (C) Products of assays either not boiled or boiled for 5 min immediately prior to gel electrophoresis.
The detection of abundant single-stranded DNA in assay mixtures in which a single-stranded DNA binding protein is present (Ad-DBP or RPA) is consistent with the notion that the single-stranded DNA binding protein stabilizes the displaced strand. However, the question arises whether single-stranded DNA is merely the product of strand displacement replication or can itself actively serve as a replication substrate.
In Ad-infected extracts, most of the newly synthesized DNA is converted to a single-stranded form.
In Fig. 3A a time course replication assay with Ad-infected extracts is shown. Aliquots were withdrawn from the reaction mixture at the indicated times. At early and later times, the proportion of labeled material that was in a single-stranded form was lower than at an intermediate time. To better understand the relationship of the double- and single-stranded forms, a pulse-chase reaction with Ad-infected extracts was performed (Fig. 3B). The reaction was allowed to proceed for 2.0 h in the presence of Rep but in the absence of radioactively labeled nucleotides. Radioactively labeled dCTP was added and after 20 min was chased with a 300-fold excess of unlabeled dCTP. At various time points, aliquots were withdrawn and processed for gel electrophoresis. Figure 3B shows the results of this assay, with the single-stranded/double-stranded DNA ratio given under each lane. Whether synthesis occurred by strand displacement or extension of the hairpinned ITR on a single-stranded template, all newly labeled molecules must be double stranded. Immediately after the 20-min pulse, some of the newly synthesized DNA was single stranded and must therefore have been converted from a double-stranded to a single-stranded form during the 20-min labeling period. Most of the material synthesized in the 20-min pulse was rapidly converted into a single-stranded form and, as demonstrated by the 16-h time point, subsequently converted back to a double-stranded form. The assay of Fig. 3B would seem to represent a minimal estimate for the percentage of replicated material that is converted to the single-stranded form, because it is likely that single-stranded DNA is converted back to double-stranded DNA continuously. The equivalent amounts of single-stranded and double-stranded forms at the 3-h time point in Fig. 3A, coupled with the data in Fig. 3B, suggest that conversion between single- and double-stranded forms may be a steady-state process.
FIG. 3.
(A) Replication in Ad-infected extracts in which aliquots were withdrawn at the times shown (in hours). (B) Pulse-chase experiment with Ad-infected extracts. At 2.5 h after the addition of Rep, the reaction mixture was given a pulse of [32P]dCTP, followed 20 min later by 300-fold chase of cold dCTP. Aliquots were withdrawn after the chase at the times indicated above each lane. Single-stranded/double-stranded DNA ratios (as determined by PhosphorImager analysis) are given below each lane. (C) Pulse-chase experiment with uninfected extract. At 2.5 h after the addition of Rep, the reaction mixtures were given a pulse of [32P]dCTP, followed 20 min later by a 300-fold chase of cold dCTP. Aliquots were withdrawn at the indicated times after the chase.
Figure 3C shows the same assay performed with an extract from uninfected cells. After the 20-min pulse, all label was in the double-stranded form and there was no evidence that it was ever converted to a single-stranded form.
Conversion of single-stranded molecules to double-stranded molecules does not occur by reannealing.
To determine whether conversion of the single-stranded DNA to the double-stranded form occurs through replication or reannealing, two additional assays were performed. Assays with parallel reactions were allowed to proceed for 2.5 h in the presence of labeled dCTP. At this point, the reaction products were chased with a 300-fold excess of cold dCTP followed immediately by various amounts of aphidicolin. (Aphidicolin is an inhibitor of human pol alpha, delta, and epsilon, whose presence leads to chain termination [13, 16]. In an Ad-infected extract, the presence of 0.25, 0.5, and 1.0 μM aphidicolin inhibits total AAV DNA replication by 68, 83, and 93%, respectively [data not shown].) One reaction in each set was stopped, and the products were processed for gel electrophoresis. The remaining reactions were allowed to continue in the presence of aphidicolin for either 2.0 or 16 h. The chase with cold dCTP stopped the synthesis of radioactively labeled new product and allowed us to determine the fate of molecules synthesized during the first 2.5 h. If the single-stranded form was merely the product of a displacement reaction on a previously labeled substrate and was a dead end for replication, we would expect that increasing amounts of aphidicolin would reduce the production of new single-stranded molecules from the previously labeled double-stranded molecules while allowing the reannealing of previously labeled single-stranded molecules to continue unimpeded. The result would be that the greater the concentration of aphidicolin, the lower the ratio of single-stranded to double-stranded DNA. If, however, the single-stranded molecules were converting to double-stranded molecules by replication, then increasing concentrations of aphidicolin might not correlate with lower ratios of single-stranded to double-stranded molecules. The latter result was obtained. Figure 4A gives the single-stranded/double-stranded ratios in the presence of various amounts of aphidicolin for these reactions. For the four levels of aphidicolin tested, higher levels of the inhibitor correlated with a greater proportion of DNA in the single-stranded form. As diagrammed in Fig. 4B, this result suggests that in the cycling between double-stranded and single-stranded DNA, aphidicolin introduces its most effective block in the conversion of single-stranded molecules to double-stranded molecules. The implication is that single-stranded molecules are becoming double stranded not through reannealing but, rather, through replication.
FIG. 4.
(A) Replication assay performed with Ad-infected extracts. At 2 h after addition of [32P]dCTP, the reaction mixtures were chased with a 300-fold excess of unlabeled dCTP and brought to the indicated concentrations of aphidicolin. The reactions were allowed to continue for either 2 or 16 h, except that in both cases the reactions to which no aphidicolin was added were stopped immediately after the chase. Bars show the final ratios of single-stranded to double-stranded labeled DNA as determined by PhosphorImager analysis. (B) Schematic showing the cycle of conversion between double-stranded and single-stranded forms of AAV. The data in panel A indicate that the predominant aphidicolin block is occurring as shown.
It is notable that aphidicolin is more effective at blocking the conversion of single-stranded to double-stranded molecules than of double-stranded to single-stranded molecules. It might be that replication from a single-stranded template is more aphidicolin sensitive than replication from a double-stranded template (i.e., strand displacement replication). This explanation seems insufficient in that at the higher concentrations, aphidicolin lowers total synthesis very substantially. A more attractive possibility is that the production of single-stranded molecules from double-stranded molecules can also occur in a replication-independent manner, i.e., by helicase activity.
Preincubation with the Rep protein allows camptothecin-resistant replication in Ad-infected extracts.
A second assay suggesting that single-stranded molecules are substrates for replication makes use of the topoisomerase I inhibitor camptothecin (10). Topoisomerase activity is needed to assist in the unwinding of the double helix during replication of a double-stranded template, and thus camptothecin can inhibit the processivity of DNA replication. In our camptothecin assay, the reactions were allowed to proceed for 2.5 h in the absence of label. At this point, radioactively labeled dCTP was added and the reactions were continued for an additional 16 h, at which point the products were processed for gel electrophoresis. The Rep proteins were added to the individual reaction mixtures at either the zero time point or 2.5 h, as indicated in Fig. 5. In addition, camptothecin was added to several reaction mixtures at either the zero- or the 2.5-h time point.
FIG. 5.
Camptothecin inhibition of replication in Ad-infected extracts. Shown above each lane are the addition of R (Rep 68) and/or C (camptothecin) at either the 0.0- or the 2.5-h time point. Radioactive label was added just after the 2.5-h time point; the reactions were allowed to proceed for an additional 13.5 h. The relative incorporation into the full-length product is shown underneath each lane.
The results shown in Fig. 5 are in accord with previous observations on the effect of camptothecin in that its addition to the replication reaction mixture in the Ad-infected extract, either simultaneously or prior to the addition of Rep, substantially inhibits DNA synthesis (P. Ward and K. I. Berns, unpublished observation). However, in the reaction in which Rep was added prior to camptothecin, a fairly robust replicative activity occurred after the addition of camptothecin, as measured by the incorporation of labeled nucleotides. The conclusion is that Rep created a replication substrate that was camptothecin resistant. This result is consistent with the notion that the unlabeled single-stranded molecules, which we have shown will be present in the reaction after a 2.5-h incubation with Rep, are serving as replication templates. It seems likely that replication from a single-stranded template would not be dependent on topoisomerase I. A comparison of the amount of DNA synthesized in the reaction in which Rep was added prior to camptothecin with the amount synthesized in the reactions which did not receive camptothecin suggests that replication from single-stranded molecules can play a prominent role in the total replicative activity.
Some single-stranded replication templates are produced by a mechanism other than strand displacement replication.
The previous assays suggested the possibility that some single-stranded molecules serving as replication templates were produced by a mechanism other than strand displacement replication. To test this notion, an assay similar to the camptothecin assay of Fig. 5 was used. In this latter assay we used two substrates in each reaction. One substrate, serving as a control, was the standard full-length duplex AAV (A), and the other was an XhoI digest of duplex AAV (AX); XhoI divides AAV into two fragment of equal length, each containing one ITR. In the shorter (i.e., one-ITR) substrate, the single strands made by strand displacement replication will necessarily have the potential to form only a 5′ hairpin and therefore should be unable to replicate. Figure 6 is an autoradiogram of the products of four parallel reactions. V, linearized vector DNA, present in equimolar amounts to the sum of A and AX, served as a nonreplicating control to ensure that incorporation of radiolabeled nucleotides was specific. When a preincubation with Rep was performed before the addition of camptothecin and radioactive label, there was a 6.4-fold increase in radioactive label in band A and a 3.9-fold increase in band AX (the average of three repetitions each) compared to the levels in the reaction in which camptothecin was added just before the addition of Rep at the 2.5 h time point. The nonspecific incorporation of label into vector molecules, which is at the limit of detection, was the same in all four reactions. Incorporation of label suggests that some of AX like A, was converted to single-stranded DNA with 3′ hairpins. The most likely explanation is that conversion occurred by helicase activity on the duplex molecule. The increase in synthesis of AX should be 50% the increase in A. This is because after XhoI digestion, only half of the strands had 3′ ITRs. That the increase of AX was more than 50% that of A indicates that the shorter molecules may be at some advantage. Perhaps the shorter molecules are more likely to be replicated for their full length or are more easily separated into single strands by helicase activity.
FIG. 6.
Camptothecin inhibition of replication in Ad-infected extracts of a one-ITR substrate. A designates duplex full-length AAV; V designates linear, duplex pBR; AX designates duplex AAV digested with XhoI; and AF designates single-stranded AAV DNA. Shown above each lane are the addition of R (Rep 68) and/or C (camptothecin) at either the 0.0- or the 2.5-h time point. Radioactive label was added just after the 2.5-h time point; the reactions were allowed to proceed for an additional 13.5 h. Shown below is a diagram of the substrates.
There are species that migrate at positions intermediate between full-length double-stranded and full-length single-stranded molecules.
Figure 1A shows that radioactively labeled material migrates between the positions of full-length single-stranded and full-length double-stranded DNA. Figure 7 is a PhosphorImager tracing giving a quantitative sense of the amount of labeled material that migrates between these two species.
FIG. 7.
PhosphorImager scan of lane 5 of Fig. 1A, i.e., a replication assay performed with Ad-infected extracts. Duplex AAV DNA is band A. Single-stranded AAV DNA is band AF. Shown is a population of labeled molecules which have a mobility intermediate to fully duplex or fully single-stranded DNA (band I).
Intermediately migrating species have a structure consistent with second-strand synthesis on a single-stranded template.
Figure 8 shows an autoradiogram of a two-dimensional gel (first dimension, native; second dimension, denaturing) of the products of a replication reaction with full-length duplex AAV DNA as substrate (top). Also shown is a map identifying the species present on this gel (bottom). The main replication products are the double-stranded full-length AAV molecules with open ends (A), double-stranded full-length AAV molecules with one hairpinned end (HA), and single-stranded full length molecules (the doublet AF). On a diagonal to the right of AF is a population of smaller single-stranded molecules (b-AF), which most probably represent molecules which were simply broken during processing. Of note is a faint arc (H-arc), which extends from the full-length single-stranded position (AF) to the full-length double-stranded hairpinned position (HA). The molecules found along the arc therefore represent a population of molecules with one full-length strand and a second strand of (moving from right to left) progressively longer length. We propose that the H-arc species represent single-stranded molecules in which the ITR has folded over and served as a primer for extension. Upon completion of this synthesis, the result is a full-length duplex with one end in the hairpin conformation. The molecules of H-arc were apparently interrupted at various points during the replicative process and therefore are partly single stranded and partly double stranded.
FIG. 8.
Two-dimensional gel analysis of the products of an assay performed with uninfected extracts supplemented with RPA. The diagram denotes the origin of species seen on the gel, as described in the text. HA and A are full-length duplex hairpinned and nonhairpinned AAV, respectively. AF is full-length single-stranded AAV. H-arc is a collection of AAV molecules with an ITR in the closed-hairpin conformation containing one complete AAV strand and various lengths of the second strand. N-arc is a collection of incomplete second strands, which are derived from molecules with an ITR which has been nicked and which contained one complete AAV strand and various lengths of the second strand.
Found lower on the gel is a similar arc of molecules, designated N-arc, which would represent the same population of molecules as seen in H-arc, except that they had been nicked at their ITR after synthesis had commenced. In the first dimension of electrophoresis, they migrated at a position intermediate between those of completely single-stranded and completely double-stranded molecules, depending on the extent of their second-strand synthesis (i.e., the same as the H-arc material). In the second (denaturing) dimension, because of this nick, they separated into a full-length strand and a shorter piece of DNA. The shorter pieces of DNA, which are derived from molecules which in the first dimension migrated near the single-stranded species, are as expected, quite short; the pieces of DNA that originated from molecules which migrated near the duplex were almost full length. These arcs were observed on all two-dimensional gels of the products of assays with Ad-infected extracts or uninfected extracts supplemented with either the Ad-DBP or the human RPA but not on two-dimensional gels of the products of assays with unsupplemented uninfected extracts.
Interruption of replication in Ad-infected extracts leads to an increase in intermediates between double-stranded and single-stranded molecules.
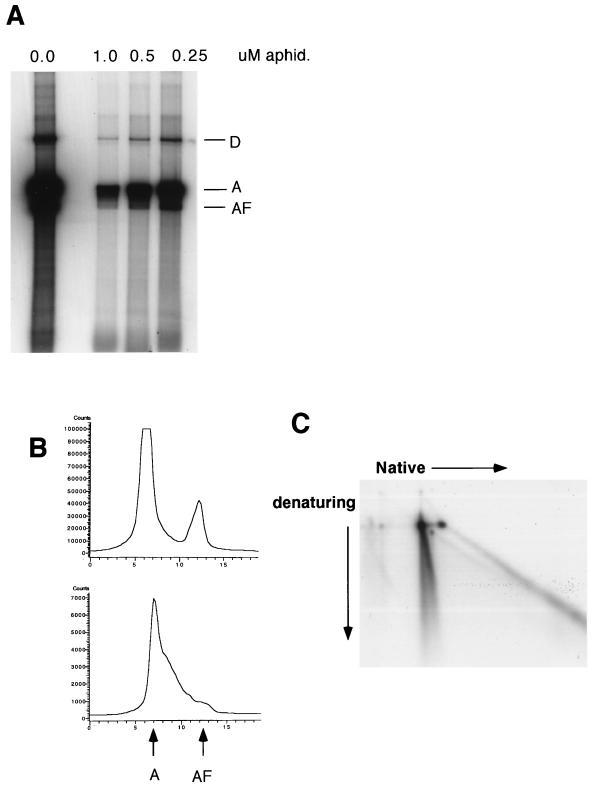
Figure 9A shows the results of assays performed in the presence of various amounts of aphidicolin. We reasoned that the addition of aphidicolin would trap molecules in the replication process, thereby increasing the proportion of replication intermediates compared with other species. As seen in Fig. 9A, with the addition of aphidicolin, there is a comparatively larger amount of material at positions intermediate to full-length single-stranded and full length double-stranded molecules. Figure 9B shows PhosphorImager tracings of the results of two of the assays in Fig. 9A, demonstrating an increased proportion of intermediates with aphidicolin. Figure 9C shows products from the 1.0 μM aphidicolin assay separated on a two-dimensional gel, demonstrating that the intermediates are found on the N-arc (Fig. 8). These molecules, which are partially single stranded and partially double stranded, are explained most simply as prematurely terminated replications on a single-stranded template.
FIG. 9.
(A) Aphidicolin inhibition of replication in an Ad-infected extract. Shown are the results of assays to which various concentrations of aphidicolin were added, as indicated above each lane. Aphidicolin was added simultaneously with Rep and radioactive label. D indicates an AAV dimer, formed by either replication or ligation in the extract. (B) PhosphorImager tracing of the products of two replication assays shown in panel A, demonstrating an aphidicolin-associated relative increase in the amount of labeled DNA migrating at positions intermediate to A and AF. The top panel is with 0.0 μM aphidicolin, and the bottom panel is with 1.0 μM aphidicolin. (C) Two-dimensional gel of the results of a replication assay of Ad-infected extract with 1.0 μM aphidicolin (see Fig. 8).
An alternative explanation for the molecules along the arc is that they might derive from the premature termination of strand displacement replication on a double-stranded substrate. If strand displacement is continued to completion by a helicase activity upon the premature termination of replication, the starting substrate molecule would now also consist of a full-length strand and a second strand of less than full length.
To rule out this possibility, the following assay was performed. At 2 h after the addition of Rep and [32P]dCTP, the reaction mixture was given a 300-fold chase with cold dCTP and brought to 0.25 μM aphidicolin. The reaction was allowed to continue for an additional 16 h. At longer times in the presence of 0.25 μM aphidicolin (which inhibits at 63%), there will be detectable replicative activity. Figure 10 shows a PhosphorImager tracing of the results of this assay. The left panel shows results with material withdrawn 2 h after the chase with cold dCTP and separated into double- and single-stranded forms by gel electrophoresis. The right panel shows results with the remainder of the reaction mixture taken at 16 h after the chase with cold dCTP, demonstrating a considerable quantity of intermediates. A large quantity of intermediate structures is detected due to a termination of replication because of the presence of aphidicolin. The question is whether the intermediates were created by replication of a single-stranded or a double-stranded substrate. In this assay, we were examining the fate of previously replicated DNA because of the chase of cold dCTP. If intermediates are derived from incomplete replication of double-stranded templates, a newly created full-length displaced strand must accompany each new intermediate molecule. Both the displaced strand and the full-length strand of the newly created intermediate would be equally likely to be labeled. Therefore, an increase in the quantity of labeled intermediates means an equivalent increase in the amount of labeled single-stranded DNA, with the consequence that the absolute difference in the amounts of radioactive label between the single-stranded and intermediate forms cannot decrease. This is not seen in Fig. 10. At 16 h, the absolute difference in the amounts of label in the two forms had decreased to the extent that there was approximately as much label in the intermediate forms as in the single-stranded forms. Between 2 and 16 h, the increase in the quantity of intermediates seemed to occur at the expense of the single-stranded forms, supporting the notion that the intermediates are derived from a single-stranded to double-stranded replication pathway.
FIG. 10.
PhosphorImager tracing of a chase experiment with Ad-infected extract. At 2.5 h after the addition of Rep and [32P]dCTP, the reaction mixtures were given a 300-fold chase of cold dCTP. The left panel shows an aliquot withdrawn 2.0 h later; the right panel shows the remainder of the reaction mixture (a larger volume) at 16 h, when the reaction was terminated. Double-stranded (A), single-stranded (AF), and intermediate (I) forms are indicated.
DISCUSSION
To investigate AAV DNA replication, we and others have established cell-free systems. We have been unable to establish a cell-free replication system which has as a starting substrate single-stranded DNA (the substrate that the virus particle delivers to the cell), due to degradation by single-stranded nucleases. To circumvent this problem, we have used full-length duplex DNA (equivalent to the product of first-round synthesis followed by terminal resolution). Single-stranded DNA subsequently produced in the Ad-infected extract is resistant to nuclease degradation, apparently due to the systematic loading of a single-stranded binding protein as the single strands are produced. Since our double-stranded substrate is produced by digestion of a plasmid (pAV2), it differs from viral DNA by possessing a partial BglII recognition sequence at each end. While it might be thought that these sequences would interfere with replication of this substrate because foldover of the hairpin terminus would no longer be perfect, the observation is that they do replicate. An additional problem with the extract was that it nonspecifically “repair labeled” DNA. We avoid this with a preincubation in the absence of both labeled nucleotide and Rep protein. Success was monitored by including in the assay mixture a linear vector molecule which would, in the absence of “repair” synthesis, remain unlabeled. Except for the “repair” labeling, there was no qualitative difference between reactions performed with or without the 3-h preincubation. However, with the preincubation, there was a substantial decrease in the total amount of incorporated labeled nucleotides, presumably because enzymatic activity decreased with time.
A hallmark of AAV biology has been the requirement for a coinfection with Ad or herpesviruses for productive replication of AAV (1). Cell-free DNA replication systems have been established which capture the need for factors induced or provided by Ad in order to see extensive viral DNA synthesis in extracts of HeLa cells (3, 23, 34, 35). One component of the helper effect was an increase in the processivity of replication, which could be attributed to the Ad-DBP (36). This report describes an additional aspect of cell-free AAV DNA replication likely to involve the Ad-DBP. In our previous report (36), we discussed the likely relationship between cell-free results with respect to the Ad-DBP and the in vivo work of numerous investigators who used Ad-DBP mutants. In some cases (1a), these in vivo results showed a less dramatic dependence on the Ad-DBP than our cell-free assay results did. The reasons for this are unclear but might involve in part a substitution of RPA for the Ad-DBP.
We show that Rep-mediated replication of AAV DNA in the Ad-infected extract generates significant quantities of single-stranded DNA and that most single-stranded molecules are subsequently converted to double-stranded molecules. That this conversion is aphidicolin sensitive and that assays in which single-stranded molecules have been allowed to accumulate can undergo DNA synthesis in the presence of camptothecin implies that the pathway of conversion of single-stranded to double-stranded DNA is by replication. In support of this conclusion, we found a population of molecules which result from incomplete synthesis on single-stranded templates. In this assay, the Ad-DBP-stabilized single-stranded DNA not only was a product of replication but also furnished a template for additional replication, setting up a cycle between double-stranded and single-stranded forms.
An additional observation was that substrates with only one ITR could furnish single-stranded templates (Fig. 6). In that assay, single-stranded molecules created by single-strand displacement replication would necessarily have the potential for forming only a 5′ hairpin. Therefore one component of the single-stranded, double-stranded cycle in the Ad-infected extract must be the creation of single strands through strand separation of duplex molecules. The efficiency with which the one-ITR substrates replicated compared to the two-ITR substrates (which could give single-stranded templates through both helicase activity and strand displacement during replication) raises the possibility that a considerable fraction of single-stranded templates are created by helicase activity.
This report suggests that if single strands are created in the cell by strand displacement, they are likely to be converted to duplex DNA by replication. AAV DNA replication in the cell occurs in divergent foci in which Rep, AAV DNA, and the Ad-DBP colocalize (37). In the early stages of infection, replication centers and capsid proteins are found in separate cellular compartments (38). It remains unclear whether the later commingling of DNA replication and capsids is sufficient to explain the rising levels of single-stranded DNA or whether other signals must be provided late in infection to ensure that single-stranded DNA is not immediately converted to the duplex form by replication. Presumably, capsids can preserve AAV in the single-stranded form by taking up single strands shortly after or simultaneously with their production. It has been noted that single-stranded DNA is not detected in infected cells in the absence of capsid protein (9, 20, 21, 31). This last observation suggests that single strands may be converted to the double-stranded form fairly rapidly after synthesis if not sequestered by capsid protein and that the presence of capsid protein may be sufficient to prevent this conversion.
Are single-stranded forms used as replication templates in the cell during productive AAV replication? Productive AAV replication requires an enormous amplification of input AAV DNA, necessitating that newly synthesized DNA serve as a template for further replication. The strand displacement model for AAV DNA replication allows for amplification without the displacement of monomer single strands (6, 7, 29). When a replication complex reaches the end of its template strand, the newly formed strand folds on itself and resumes replicating. If the strand now being displaced is still attached to the template strand by the hairpin at the distal end, then rather than being released from the duplex, it will also be replicated, resulting in the formation of a dimer. In support of this model, duplex dimers are commonly observed in vivo. In our cell-free assays, the formation of dimers is extremely inefficient, presumably due to the efficiency with which Rep 68 transforms dimers into monomers (23). In vivo, however, all four Rep proteins are present during productive infection. Of the Rep proteins required for DNA replication (Rep 68 and Rep 78), Rep 78 is the more abundant and is detected earlier (26). It has been noted, in cell-free assays, that Rep 78 is less efficient at processing dimers to monomers than is Rep 68 (22, 23).
However, the larger portion of duplex DNA in the infected cell seems to be in the monomer state. The manner in which duplex concatemers are processed to monomers in the infected cell is unclear. If concatemers are processed by Rep nicking on both strands, many monomers will have both ends in an open configuration. In this case, replication by strand displacement will produce free single strands. Abundant single-stranded DNA is not detected in the early stages of in vivo AAV DNA replication, when the amounts of duplex forms are increasing greatly (26). However, if single strands are rapidly converted to duplex monomers, their concentration in the cell might be relatively low prior to sequestration by capsids. If, on the other hand, concatemers are processed by the initiation of internal replication, i.e., nicking on one strand only, followed by a displacement replication, there will be no displaced single strands. The latter mechanism, however, is likely to create trimers, which are not readily detected in infected cells. It is difficult to formulate a productive replication pathway that avoids the production of single-stranded molecules.
In this study, the stabilized single-stranded molecules present in the assay mixture with Ad-infected extracts served as replication templates. This makes possible a cycling between double- and single-stranded forms such that all products of replication can serve as templates for further replication. By analogy, this single-stranded, double-stranded replication cycle would provide a very efficient mechanism by which viral DNA in the infected cell might be amplified.
We previously showed that adding either Ad-DBP or RPA to uninfected extracts substantially increased the amount of full-length product, apparently by overcoming a deficiency in the processivity of replication in uninfected extracts (36). Nevertheless, replication in an uninfected extract supplemented with optimal amounts of single-stranded DNA binding proteins has still been 5- to 10-fold less efficient with respect to total DNA synthesis than replication in Ad-infected extracts. When replication assays are performed with uninfected extracts supplemented with either Ad-DBP or RPA, the products consist mostly of single-stranded DNA (as seen in Fig. 1), suggesting that the uninfected extract, although supplemented with single-stranded DNA binding protein, is less efficient than the Ad-infected extract at using single-stranded DNA as a template for further replication. This difference in the ability to replicate a single-stranded molecule might in part explain the remaining 5- to 10-fold difference in replication efficiencies between the supplemented uninfected extract and the Ad-infected extract.
It has been suggested previously that Ad infection may provide a factor needed for or relieve an inhibitor of replication of single-stranded molecules (4, 5). The latter possibility has been pursued by Srivastava and colleagues, who report a single-stranded D-region binding protein that binds the D-region of single-stranded AAV DNA. Binding of this protein is hypothesized to prevent the elongation which converts incoming viral DNA to the duplex form (24, 25, 32). This activity is relieved by dephosphorylation of the D-region binding protein consequent to Ad infection of the cells. If their model is correct, this activity presumably would impede replication from single-stranded AAV not only at the point of infection but also continually thereafter. The above in vivo observations might account for the poor replication of single-stranded templates by the uninfected extract even when supplemented with a single-stranded DNA binding protein. The data in this report suggest that efficient DNA replication upon transfection of AAV-derived plasmids into cells would also require that this inhibitory activity be blocked.
ACKNOWLEDGMENTS
We thank Ron Hay for his gift of Ad-DBP and Frank Dean and Michael O'Donnell for their gift of RPA. We thank Christopher Burrow and William Holloman for helpful comments.
This work was supported in part by NIH grant DK55609 (R.M.L.).
REFERENCES
- 1.Berns K I. Parvovirus replication. Microbiol Rev. 1990;54:316–329. doi: 10.1128/mr.54.3.316-329.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Carter B J, Antoni B A, Klessig D F. Adenovirus containing a deletion of the early region 2A gene allows growth of adeno-associated virus with decreased efficiency. Virology. 1992;191:473–476. doi: 10.1016/0042-6822(92)90213-9. [DOI] [PubMed] [Google Scholar]
- 2.Chejanovsky N, Carter B J. Mutagenesis of an AUG codon in the adenoassociated virus rep gene: effects on viral DNA replication. Virology. 1989;173:120–128. doi: 10.1016/0042-6822(89)90227-4. [DOI] [PubMed] [Google Scholar]
- 3.Chiorini J A, Weitzman M D, Owens R A, Urcelay E, Safer B, Kotin R M. Biologically active Rep proteins of adeno-associated virus type 2 produced as fusion proteins in Escherichia coli. J Virol. 1994;68:797–804. doi: 10.1128/jvi.68.2.797-804.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari F K, Samulski T, Shenk T, Samulski R J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer K J, Gao G-P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauswirth W W, Berns K I. Adeno-associated virus DNA replication: nonunit-length molecules. Virology. 1979;93:57–68. doi: 10.1016/0042-6822(79)90275-7. [DOI] [PubMed] [Google Scholar]
- 7.Hauswirth W W, Berns K I. Origin and termination of adeno-associated virus DNA replication. Virology. 1977;78:488–499. doi: 10.1016/0042-6822(77)90125-8. [DOI] [PubMed] [Google Scholar]
- 8.Henricksen L A, Umbricht C B, Wold M S. Recombinant replication protein A: expression, complex formation, and characterization, J. Biol Chem. 1994;269:11121–11132. [PubMed] [Google Scholar]
- 9.Hermonat P L, Labow M A, Wright R, Berns K I, Muzyczka N. Genetics of adeno-associated virus: isolation and preliminary characterization of adeno-associated virus type 2 mutants. J Virol. 1984;51:329–339. doi: 10.1128/jvi.51.2.329-339.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hertzberg R P, Caranza M J, Hecht S M. On the mechanism of topoisomerase I inhibition by camptothecin: evidence for binding to an enzyme-DNA complex. Biochemistry. 1989;28:4629–4638. doi: 10.1021/bi00437a018. [DOI] [PubMed] [Google Scholar]
- 11.Holscher C, Horer M, Kleinschmidt J A, Zentgraf H, Burkle A, Heilbronn R. Cell lines inducibly expressing the adeno-associated virus (AAV) Rep gene: requirements for productive replication of Rep-negative AAV mutants. J Virol. 1994;68:7169–7177. doi: 10.1128/jvi.68.11.7169-7177.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong G, Ward P, Berns K I. In vitro replication of adeno-associated virus DNA. Proc Natl Acad Sci USA. 1992;89:4673–4677. doi: 10.1073/pnas.89.10.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikegami, S., T. Taguchi, M. Ohashi, M. Oguro, H. Nagano, and Y. Mano. 1978. Aphidicolin prevents mitotic cell division by interfering with Nature 275:458–460. [DOI] [PubMed]
- 14.Im D-S, Muzyczka N. The AAV origin binding protein Rep68 is an ATP-dependent site-specific endonuclease with DNA helicase activity. Cell. 1990;61:447–457. doi: 10.1016/0092-8674(90)90526-k. [DOI] [PubMed] [Google Scholar]
- 15.Laughlin C A, Tratschin J D, Coon H, Carter B J. Cloning of infectious adeno-associated virus genomes in bacterial plasmids. Gene. 1983;23:65–73. doi: 10.1016/0378-1119(83)90217-2. [DOI] [PubMed] [Google Scholar]
- 16.Liu P K, Chang C C, Trosko J E, Dube D K, Martin G M, Loeb L A. Mammalian mutator mutant with an aphidicolin-sensitive DNA polymerase alpha. Proc Natl Acad Sci USA. 1983;80:797–801. doi: 10.1073/pnas.80.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lusby E, Bohenzky R, Berns K I. Inverted terminal repetition in adeno-associated virus DNA: independence of the orientation at either end of the genome. J Virol. 1981;37:1083–1086. doi: 10.1128/jvi.37.3.1083-1086.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lusby E, Fife K H, Berns K I. Nucleotide sequence of the inverted terminal repetition in adeno-associated virus DNA. J Virol. 1980;34:402–409. doi: 10.1128/jvi.34.2.402-409.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monaghan A, Webster A, Hay R T. Adenovirus DNA binding protein: helix destabilising properties. Nucleic Acids Res. 1994;22:742–748. doi: 10.1093/nar/22.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers M W, Carter B J. Assembly of adeno-associated virus. Virology. 1980;102:71–82. doi: 10.1016/0042-6822(80)90071-9. [DOI] [PubMed] [Google Scholar]
- 21.Myers M W, Carter B J. Adeno-associated virus replication. The effects of l-canavanine on a helper virus mutation on accumulation of viral capsids and progeny single-stranded DNA. J Biol Chem. 1981;256:567–570. [PubMed] [Google Scholar]
- 22.Ni T H, McDonald W F, Zolotukhin I, Melendy T, Waga S, Stillman B, Muzyczka N. Cellular proteins required for adeno-associated virus DNA replication in the absence of adenovirus coinfection. J Virol. 1998;72:2777–2787. doi: 10.1128/jvi.72.4.2777-2787.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ni T H, Zhou X, McCarty D M, Zolotukhin I, Muzyczka N. In vitro replication of adeno-associated virus DNA. J Virol. 1994;68:1128–1138. doi: 10.1128/jvi.68.2.1128-1138.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qing K, Khuntirat B, Mah C, Kube D M, Wang X S, Ponnazhagan S, Zhou S, Dwarki V J, Yoder M C, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: correlation of tyrosine phosphorylation of the cellular single-stranded D sequence-binding protein with transgene expression in human cells in vitro and murine tissues in vivo. J Virol. 1998;72:1593–1599. doi: 10.1128/jvi.72.2.1593-1599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qing K, Wang X S, Kube D M, Ponnazhagan S, Bajpai A, Srivastava A. Role of tyrosine phosphorylation of a cellular protein in adeno-associated virus 2-mediated transgene expression. Proc Natl Acad Sci USA. 1997;94:10879–10884. doi: 10.1073/pnas.94.20.10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redemann B E, Mendelson E, Carter B J. Adeno-associated virus Rep protein synthesis during productive infection. J Virol. 1989;63:873–882. doi: 10.1128/jvi.63.2.873-882.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose J A, Koczot F J. Adeno-associated virus multiplication. VII. Helper requirement for viral deoxyribonucleic acid and ribonucleic acid synthesis. J Virol. 1972;10:1–8. doi: 10.1128/jvi.10.1.1-8.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snyder R O, Samulski R J, Muzyczka N. In vitro resolution of covalently joined AAV chromosome ends. Cell. 1990;60:105–113. doi: 10.1016/0092-8674(90)90720-y. [DOI] [PubMed] [Google Scholar]
- 29.Straus S E, Sebring E D, Rose J A. Concatemers of alternating plus and minus strands are intermediates in adenovirus-associated virus DNA synthesis. Proc Natl Acad Sci USA. 1976;73:742–746. doi: 10.1073/pnas.73.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tatersall P, Ward D C. Rolling hairpin model for replication of parvovirus and linear chromosome DNA. Nature. 1976;263:106–109. doi: 10.1038/263106a0. [DOI] [PubMed] [Google Scholar]
- 31.Tratschin J D, Miller I L, Carter B J. Genetic analysis of adeno-associated virus: properties of deletion mutants constructed in vitro and evidence for an adeno-associated virus replication function. J Virol. 1984;51:611–619. doi: 10.1128/jvi.51.3.611-619.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X S, Ponnazhagan S, Srivastava A. Rescue and replication of adeno-associated virus type 2 as well as vector DNA sequences from recombinant plasmids containing deletions in the viral inverted terminal repeats: selective encapsidation of viral genomes in progeny virions. J Virol. 1996;70:1668–1677. doi: 10.1128/jvi.70.3.1668-1677.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward P, Berns K I. In vitro rescue of an integrated hybrid adeno-associated/simian virus 40 genome. J Mol Biol. 1991;218:791–804. doi: 10.1016/0022-2836(91)90267-a. [DOI] [PubMed] [Google Scholar]
- 34.Ward P, Urcelay E, Kotin R, Safer B, Berns K I. Adeno-associated virus DNA replication in vitro: activation by a maltose binding protein/Rep 68 fusion protein. J Virol. 1994;68:6029–6037. doi: 10.1128/jvi.68.9.6029-6037.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward P, Berns K I. In vitro replication of adeno-associated virus DNA: enhancement by extracts from adenovirus-infected HeLa cells. J Virol. 1996;70:4495–4501. doi: 10.1128/jvi.70.7.4495-4501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward P, Dean F B, O'Donnell M E, Berns K I. Role of the adenovirus DNA-binding protein in in vitro-associated virus DNA replication. J Virol. 1998;72:420–427. doi: 10.1128/jvi.72.1.420-427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weitzman M D, Fisher K J, Wilson J M. Recruitment of wild-type and recombinant adeno-associated virus into adenovirus replication centers. J Virol. 1996;70:1845–1854. doi: 10.1128/jvi.70.3.1845-1854.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wistuba A, Kern A, Weger S, Grimm D, Kleinschmidt J A. Subcellular compartmentalization of adeno-associated virus type 2 assembly. J Virol. 1997;71:1341–1352. doi: 10.1128/jvi.71.2.1341-1352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wobbe C R, Dean F, Weissbach L, Hurwitz J. In vitro replication of duplex circular DNA containing the simian virus 40 DNA origin site. Proc Natl Acad Sci USA. 1985;81:5710–5714. doi: 10.1073/pnas.82.17.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou X, Zolotukhin I, Im D-S, Muzyczka N. Biochemical characterization of adeno-associated virus Rep68 DNA helicase and ATPase activities. J Virol. 1999;73:1580–1590. doi: 10.1128/jvi.73.2.1580-1590.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]