Abstract
Lung cancer is one of the leading causes of cancer death in the world and is notoriously difficult to treat effectively. In the present study, male Swiss albino mice were divided into five groups of six animals each: group I animals received corn oil orally and served as a control; group II cancer‐induced animals received benzo(a)pyrene (50 mg/kg bodyweight dissolved in corn oil, orally) twice weekly for four successive weeks; group III cancer‐bearing animals (after 12 weeks of induction) were treated with paclitaxel (33 mg/kg bodyweight, i.p.) once weekly for 4 weeks; group IV cancer‐bearing animals were treated with paclitaxel along with Withania somnifera (400 mg/kg bodyweight) orally once weekly for 4 weeks; and group V animals constituted the drug control treated with paclitaxel along with W. somnifera. The serum, lung and liver were investigated biochemically for aryl hydrocarbon hydroxylase, γ‐glutamyl transpeptidase, 5′‐nucleotidase, lactate dehydrogenase and protein‐bound carbohydrate components (hexose, hexosamine and sialic acid). These enzyme activities were increased significantly in cancer‐bearing animals compared with control animals. The elevation of these in cancer‐bearing animals was indicative of the persistent deteriorating effect of benzo(a)pyrene in cancer‐bearing animals. Our data suggest that paclitaxel, administered with W. somnifera, may extend its chemotherapeutic effect through modulating protein‐bound carbohydrate levels and marker enzymes, as they are indicators of cancer. The combination of paclitaxel with W. somnifera could effectively treat the benzo(a)pyrene‐induced lung cancer in mice by offering protection from reactive oxygen species damage and also by suppressing cell proliferation. (Cancer Sci 2006; 97: 658–664)
Lung cancer is a major cause of morbidity and mortality worldwide both in men and women, accounting for 29% of all cancers. The incidence of lung cancer remains very high. The World Health Organization estimates that lung cancer is the most frequent cancer in the world today, and the global incidence of lung cancer is increasing at a rate of 0.5% per year due to the fact that the smoking epidemic continuous to spread to developing countries. Lung cancer is strongly related to smoking. We know that 85–95% of lung cancer patients are smokers. In many developed countries smoking prevalence is approximately the same among women and men.( 1 )
Polycyclic aromatic hydrocarbons (PAH), many of which are carcinogenic, are widespread environmental contaminants produced by incomplete combustion. Mammalian metabolism of carcinogenic PAH mediated by cytochromes P450 and epoxide hydrolase generates, among other metabolites, bay region diol epoxides. The tumorigenic potential of these metabolites most likely results from their reaction with cellular DNA to form miscoding adducts.( 2 ) The radical cationic forms of benzo(a)pyrene (B[a]P) may be involved in both the mechanism and metabolic activation leading to the formation of DNA adducts, which are key components of the tumor initiation process.( 3 )
Chemotherapy is often the first choice for treatment of many cancers, and chemotherapeutic drugs work by disrupting the growth of cancer cells.( 4 ) Paclitaxel was first isolated from the bark of the Western yew tree Taxus brevifolia ( 5 ) and exhibits unusual promise as an antitumor agent.( 6 ) Paclitaxel belongs to the general group of chemotherapeutic drugs known as taxanes. As an antimitotic, antitumor drug, paclitaxel has undergone extensive clinical development as a result of its efficacy in the treatment of lung and other cancers. The cellular target for paclitaxel has been identified as the microtubule system, which plays a significant role in mitosis, intracellular transport, cell motility and maintenance of cell shape. It promotes the assembly of stable microtubules from α‐tubulin and β‐tubulin.( 7 ) Though paclitaxel is an effective drug for the treatment of cancer, it has several important side‐effects, particularly neutropenia, peripheral neuropathy and hypersensitivity reactions.( 8 ) However, that is only part of the solution. On the latest research front, scientists are studying methods to actually prevent the unwanted side‐effects produced by chemotherapy by combining it with natural antioxidants.
The present study was conducted to delineate the protective role of Withania somnifera on the biochemical changes that take place during paclitaxel chemotherapy and also to evaluate the extent of its potency. W. somnifera (Solanaceae) is an ayurvedic medicinal plant. It is an evergreen tomentose shrub, grown wild and also cultivated for medicinal use in many parts of India, that is popular as a home remedy for several diseases and human requirements.( 9 ) The chemical composition, and pharamacological and therapeutic efficacies of W. somnifera have been established.( 10 ) These reports suggest that W. somnifera is a rich source of bioactive compounds. The roots of W. somnifera contains several alkaloids, withanolides, a few flavanoids and reducing sugars.( 11 )
Materials and Methods
Animals
Healthy male Swiss albino mice (6–8 weeks old) weighing 20–25 g were used throughout the study. The animals were purchased from the King Institute of Preventive Medicine (Chennai, India) and maintained in a controlled environment for temperature and humidity on a 12 : 12 h L : D cycle. This research work on Swiss albino mice was sanctioned and approved by the Institutional Animal Ethical Committee (IAEC No. 02/019/02).
Experimental set‐up
The animals were divided into five groups, with each group consisting of six animals. The treatments were as follows: group I, control animals treated with corn oil (vehicle) orally; group II, cancer‐induced animals treated with B(a)P (50 mg/kg bodyweight dissolved in corn oil, orally) twice weekly for four successive weeks; group III, cancer‐bearing animals (after 12 weeks of induction) treated with paclitaxel (33 mg/kg bodyweight, i.p.) for 4 weeks; group IV, cancer‐bearing animals treated with paclitaxel along with W. somnifera (400 mg/kg bodyweight) orally for 4 weeks; and group V, control animals treated with paclitaxel along with W. somnifera. At the end of the experimental period, the animals were fasted overnight and killed by cervical decapitation.
Plant materials
Withania somnifera root powder was purchased from Indian Medical Practitioners Co‐operative Pharmacy and Stores (Chennai, India). Plant material powder (1 kg) was allowed to soak in 95% alcohol for 4 days at room temperature with occasional shaking. The alcoholic solvent extract was then filtered through Whatman filter paper #4 and evaporated in a rotary evaporator under reduced pressure at 60°C, before being stored at 4°C until further use. The dose level selected for the present study was non‐toxic and safe.
Statistical analysis
The effects of the various treatments on the biological outcomes were evaluated statistically using an analysis of variance the student's t‐test. The levels of significance were evaluated using P‐values.
Marker enzymes
The aryl hydrocarbon hydroxylase (AHH) assay was modified from the method of Mildred et al.( 12 ) The activity of γ‐glutamyl transpeptidase was estimated according to the method of Orlowski and Meister.( 13 ) 5′‐Nucleotidase was assayed using the method of Luly et al.( 14 ) The activity of lactate dehydrogenase (LDH) was assayed using the method of King.( 15 )
Acid hydrolysis for estimation of hexose and hexosamine levels
To the weighed amount of defatted tissues, 2 mL of 4 M HCl was added and the mixture was refluxed at 100°C for 4 h in a test tube with suitable marble lids. The hydrolysate was neutralized with sodium hydroxide. Aliquots of the neutralized samples were taken for the analysis. The hexose level was estimated using the method of Niebes.( 16 ) Hexosamine content was estimated using the method of Wagner.( 17 ) The weighed amount of defatted tissues was hydrolyzed with 1.0 mL of 0.1 M sulfuric acid at 80°C for 60 min to release sialic acid bound to the proteins. The solution was neutralized with sodium hydroxide. The sialic acid level was determined using the method of Warren.( 18 )
Polyamines
The levels of polyamines were assayed using the method of Endo.( 19 )
Preparation of samples for CM‐cellulose column chromatography
Mice tissues (lung and liver; 100 mg) were homogenized in ice‐cold 0.4 M HClO4 containing 2 mM ethylenediaminetetracetic acid. The homogenate was centrifuged at 503 g for 5 min. The neutralized supernatant was applied to a CM‐cellulose column. Each sample solution (0.5–3 mL) was applied to a CM‐cellulose column (0.6 × 10 cm) equilibrated with 0.01 M phosphate buffer (pH 6.2). After the column was washed with 15 mL of 0.01 M phosphate buffer (pH 6.2) and 15 mL of 0.03 M phosphate buffer (pH 6.2), histamine, putrescine, spermidine and spermine were eluted out from the column with borate buffer without NaCl (30 mL), borate buffer containing 0.03 M NaCl (20 mL), borate buffer containing 0.075 M NaCl (20 mL), and borate buffer containing 0.15 M NaCl (20 mL), respectively. Fractions (3 mL) were collected at a flow rate of approximately 3 mL/min. Trinitrobenzenesulphonate (TNBS) reagent (1 mL) was added to the elute (3 mL) from the CM‐cellulose column. The reaction was carried out at 50°C for 10 min and terminated by cooling the reaction mixture in water. Absorbance at 420 nm was measured within 20 min.
Results
Tumor weight and bodyweight
1, 2 represents the effect of paclitaxel with W. somnifera on the bodyweight and lung weight of control and experimental animals. A significant decrease (P < 0.001) in bodyweight and an increase in lung weight was found in the cancer‐bearing animals (group II) compared with control animals (group I). After treatment with paclitaxel (group III), a significant (P < 0.05) increase in bodyweight and a significant (P < 0.01) decrease in lung weight was found compared with the cancer‐induced group (group II). Combined administration of paclitaxel and W. somnifera (group IV) also caused a significant (P < 0.01) increase in bodyweight and a decrease in lung weight of the animals compared with the cancer‐bearing animals. However, no significant difference was found in the bodyweights and lung weights of control (group I) animals and the control group treated with a combination of paclitaxel and W. somnifera (group V), as well as in cancer‐bearing animals treated with paclitaxel alone (group III) and in combination with W. somnifera (group IV).
Figure 1.
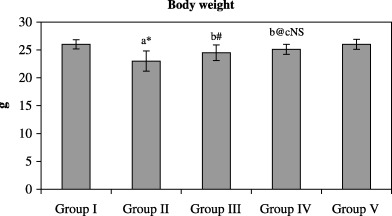
Effect of paclitaxel in combination with Withania somnifera on the bodyweight of control and experimental animals.
Figure 2.
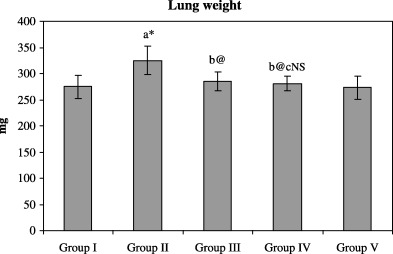
Effect of paclitaxel in combination with Withania somnifera on lung weight of control and experimental animals.
Marker enzymes
Figure 3 depicts the effect of paclitaxel with W. somnifera on the activities of some of the marker enzymes in the serum of control and experimental animals. The activities of the marker enzymes AHH, γ‐glutamyl transpeptidase, 5′‐nucleotidase and LDH were found to be significantly (P < 0.001) increased in cancer‐bearing animals (group II) compared with the control group (group I). Paclitaxel treatment (group III) caused a significant (P < 0.05; P < 0.001) decrease in these enzyme activities compared with the cancer‐bearing group. Combination treatment with paclitaxel and W. somnifera (group IV) also caused a significant (P < 0.001) decrease in their activities compared with the cancer‐bearing animals (group II). Paclitaxel‐treated (group III) and combination‐treated (group IV) animals also showed a significant (P < 0.05) decrease in the activities of the marker enzymes AHH and LDH when compared with each other. However, no significant difference in the activities of these marker enzymes between the control animals (group I) and the control animals treated with a combination of paclitaxel and W. somnifera (group V) was observed.
Figure 3.
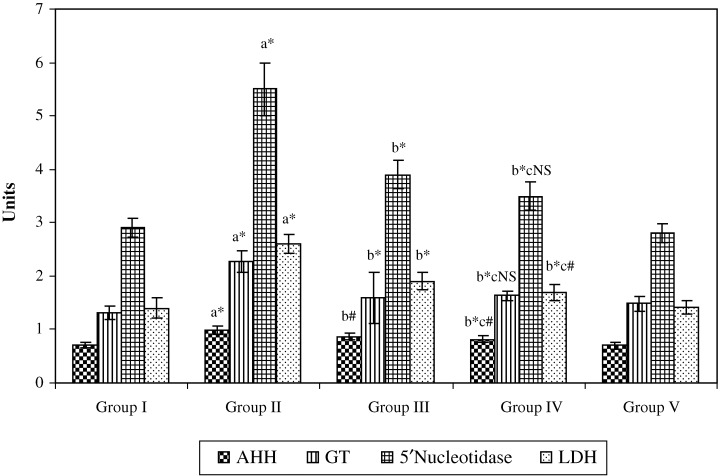
Effect of paclitaxel in combination with Withania somnifera on the activity of some of the marker enzymes in the serum of control and experimental animals.
Table 1 shows the effect of paclitaxel with W. somnifera on the activities of the marker enzymes AHH, γ‐glutamyl transpeptidase, 5′‐nucleotidase and LDH in the lung of control and experimental animals. The activities of these marker enzymes were found to be significantly (P < 0.001) increased in carcinogen‐treated animals (group II) when compared with the control animals (group I). After treatment with paclitaxel (group III) there was found to be a significant (P < 0.001; P < 0.05) decrease in the activities of these marker enzymes compared with the cancer‐bearing group. Combination treatment with paclitaxel and W. somnifera (group IV) caused a significant (P < 0.001) decrease in their activities compared with the cancer‐bearing group II animals. Paclitaxel‐treated (group III) and combination‐treated (group IV) animals also showed a significant (P < 0.01; P < 0.05; P < 0.001) increase in the activities of the marker enzymes when compared with each other. However, no significant difference was found in the activities of these marker enzymes between the control animals (group I) and the control animals treated with paclitaxel and W. somnifera (group V).
Table 1.
Effect of paclitaxel in combination with Withania somnifera on the activity of marker enzymes in the lung of control and experimental animals
| Enzyme | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| AHH | 0.51 ± 0.04 | 0.83 ± 0.07 † , * | 0.68 ± 0.06 ‡ , * | 0.57 ± 0.05 ‡ , § , *, ** | 4.49 ± 0.03 |
| γ‐GT | 1.12 ± 0.08 | 1.87 ± 0.02 † , * | 1.42 ± 0.16 ‡ , * | 1.26 ± 0.15 ‡ , § , *, *** | 1.10 ± 0.08 |
| 5′‐ND | 1.75 ± 0.13 | 2.63 ± 0.21 † , * | 2.52 ± 0.31 ‡ , *** | 1.92 ± 0.18 ‡ , § , * | 1.77 ± 0.12 |
| LDH | 1.06 ± 0.09 | 1.85 ± 0.18 † , * | 1.46 ± 0.17 ‡ , * | 1.24 ± 0.14 ‡ , § , *, *** | 1.07 ± 0.07 |
Group II compared with group I;
‡ group II compared with groups III and IV;
§ group III compared with group IV;
P < 0.001;
P < 0.01;
P < 0.05. Each value is expressed as mean ± SD for six mice in each group. Units: aryl hydrocarbon hydroxylase (AHH), moles of fluorescent phenolic metabolites formed/min/mg protein; γ‐glutamyl transpeptidase (γ‐GT), nmoles of p‐nitroaniline formed/min/mg protein; 5′‐nucleotidase (5′‐ND), nmoles of Pi liberated/min/mg protein; lactate dehydrogenase (LDH), moles of pyruvate liberated/min/mg protein.
Glycoproteins
Table 2 shows the effects of paclitaxel along with W. somnifera on the levels of glycoproteins in the lung of control and experimental animals. The levels of all three glycoproteins (hexose, hexosamine and sialic acid) were found to be significantly (P < 0.001) increased in cancer‐bearing animals (group II) compared with the control group (group I). Treatment with paclitaxel (group III) caused a significant (P < 0.001) decrease in the levels of these glycoproteins compared with cancer‐bearing animals (group II). However, treatment with paclitaxel and W. somnifera (group IV) caused a significant (P < 0.001) decrease in their levels compared with the cancer‐induced group (group II). When comparisons were made between the paclitaxel‐treated (group III) and combination‐treated (group IV) animals, a significant (P < 0.001; P < 0.01) decrease in the levels of hexosamine and sialic acid was observed. There was no significant difference in the glycoprotein levels between the control animals (group I) and the control animals treated with the combination of paclitaxel and W. somnifera (group V).
Table 2.
Effect of paclitaxel in combination with Withania somnifera on the level of glycoproteins in the lung of control and experimental animals
| Glycoprotein | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| Hexose | 1.26 ± 0.09 | 3.37 ± 0.33 † , * | 1.85 ± 0.13 ‡ , * | 1.37 ± 0.17 ‡ , § , *,NS | 1.23 ± 0.16 |
| Hexosamine | 0.44 ± 0.05 | 0.94 ± 0.07 † , * | 0.75 ± 0.06 ‡ , * | 0.55 ± 0.06 ‡ , § , * | 0.45 ± 0.05 |
| Sialic acid | 0.21 ± 0.04 | 0.48 ± 0.04 † , * | 0.33 ± 0.01 ‡ , * | 0.24 ± 0.03 ‡ , § , *, ** | 0.21 ± 0.05 |
Group II compared with group I;
‡ group II compared with groups III and IV;
§ group III compared with group IV;
P < 0.001;
P < 0.01;
P < 0.05; NS, not significant. Each value is expressed as mean ± SD for six mice in each group. Units: mg/g of defatted tissue.
Polyamines
Figure 4 shows the effect of paclitaxel with W. somnifera on the levels of polyamines in the lung of control and experimental animals. The levels polyamines were found to be significantly increased in carcinogen‐treated animals (group II) compared with the control animals (group I). After treatment with paclitaxel (group III) a significant decrease in the levels of polyamines was found, compared with the cancer‐bearing group. Combination treatment with paclitaxel and W. somnifera (group IV) caused a significant decrease in the levels of polyamines compared with the cancer‐bearing group II animals. However, no significant difference in the levels of polyamines was found between the control animals (group I) and the control animals treated with paclitaxel and W. somnifera (group V).
Figure 4.
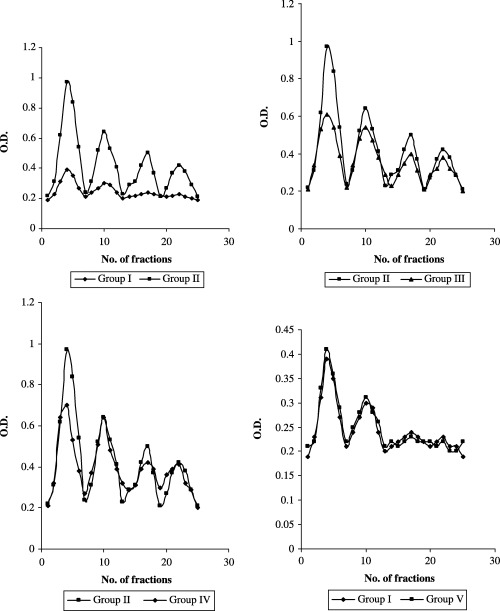
Effect of paclitaxel in combination with Withania somnifera on the level of polyamines in lung of control and experimental animals.
Discussion
There is great hope offered by new therapies targeted to biological abnormalities in lung cancer. Paclitaxel is one of the most promising anticancer agents for lung cancer. Drugs used in combination are more effective than drugs used individually. In combination, each drug has a different mechanism of action. It can be given to the individual according to their maximum tolerated dose.( 20 ) We conducted our study to examine changes in bodyweight due to lung cancer, and found that cancer‐bearing animals showed a significant change in their bodyweight and lung weights. After treatment with W. somnifera in combination with paclitaxel, these changes were brought back to normal levels, thus showing the protective effect of the drug against cancer development.
Tumor markers are biological or biochemical substances produced by tumors that are secreted into blood or body tissues in higher than normal amounts. The first tumor marker introduced into medical science was a proteinurea used by Dr Bence‐Jones from a patient with multiple myeloma. The majority of tumor markers in use are circulating substances detected in blood or organs of tumor patients.( 21 ) Analysis of tumor marker enzymes serves as an indicator of cancer response to therapy. Marker enzymes, such as AHH, γ‐glutamyl transpeptidase, 5′‐nucleotidase and LDH, are specific indicators of lung damage.( 22 , 23 , 24 ) Chen and Liu reported that AHH is a useful biomarker in the early diagnosis of lung cancer.( 25 ) In the present study, AHH activity was increased in lung cancer‐bearing animals. A reduction in the activity of AHH was found in animals treated with paclitaxel in combination with W. somnifera. Paclitaxel and W. somnifera have strongly proved to inhibit AHH activity.
γ‐Glutamyl transpeptidase catalyzes the transfer of the γ‐glutamyl moiety from peptides, amino acids or water. This enzyme is bound primarily to the plasma membranes of cells that show high secretory or absorptive capacity, such as hepatocytes, and cleaves extracellular glutathione, thereby providing the components for increased intracellular glutathione synthesis.( 26 )γ‐Glutamyl transpeptidase activity serves as a specific marker for the prognosis of carcinogenic events. Increased levels of γ‐glutamyl transpeptidase have been observed in cancerous cells.( 27 ) Moderate smoking causes a 10% increase in γ‐glutamyl transpeptidase activity, whereas heavy smoking produces a 20% rise in values.( 28 ) Chemical carcinogens that enter the liver may initiate some systemic effects that induce γ‐glutamyl transpeptidase synthesis. The paclitaxel and W. somnifera‐treated animals showed a decreased level of γ‐glutamyl transpeptidase activity compared with the cancer‐bearing (group II) animals.
5′‐Nucleotidase has been reported to be altered in the sera of patients with solid tumors. Dao et al.( 29 ) have reported that an increase in the activity of 5′‐nucleotidase seems to originate from proliferating tumor cells. In the human lymphoid system, 5′‐nucleotidase is anchored to the plasma membrane and has been described as an important enzyme for differentiation of B‐lymphocytes.( 30 ) The 5′‐nucleotidase enzyme hydrolyzes nucleotides with a phosphate group on the carbon 5 atom of ribose. A fast‐moving 5′ nucleotide phosphodiesterase was found to be elevated in tumors that had metastasized from the liver to the lung and brain.( 31 ) In the present study, elevated 5′‐nucelotidase activity was observed in cancer‐bearing (group II) animals. However, after treatment with paclitaxel and W. somnifera, the 5′‐nucleotidase activity was close to normal values, indicating the antitumor activity of this combination treatment.
Lactate dehydrogenase is a cytoplasmic enzyme that catalyzes the oxidation of lactate to pyruvate and vice versa. Elevated LDH activity has been reported in the serum of lung cancer patients.( 32 ) A possible reason for this elevated LDH activity in cancer‐bearing animals may be enhanced glycolysis during tumor growth.( 33 ) Alternately, it may be due to overproduction by tumor cells,( 34 ) or the release of isoenzymes from destroyed tissues. LDH activity was found to be increased in the malignant lung cell line NC1‐H82 compared with normal cells.( 35 ) In the present study, combined treatment with paclitaxel and W. somnifera reduced the activities of these isoenzymes to near‐normal levels, which may indicate the antiproliferative effects of the drug.
Glycoproteins are common components of animal cell surfaces, are constituents of lysosomes, and are among the products exported by the cell. Cell surface glycoproteins have been found to play an important role in tumorigenesis, acting as mediators of immunological specificity.( 36 ) Carbohydrate moieties of glycoproteins have been implicated in the transport of metabolites across cell membranes, and a direct relationship between glycoproteins and tumorigenesis has been observed. Many chemical changes in glycoproteins are detectable before the onset of secondary physiological and nutritional changes that may be associated with the condition of tumor‐bearing animals.( 37 ) In the present study, the levels of hexose, hexosamine and sialic acid were increased in the lung cancer‐bearing group II animals. However, the levels of these glycoproteins were near‐normal in the paclitaxel and W. somnifera‐treated animals. This is due to the antioxidant properties of W. somnifera, the direct reaction of paclitaxel and W. somnifera with free radicals, and the toxic reactive intermediates of B(a)P. This reduction in the levels of glycoprotein components indicate that the drug has the ability to suppress malignancy by modulating cell transformation by controlling cell proliferation.
Polyamine production is associated with cell division. An increase in the intracellular levels of polyamines, particularly putrescine and spermidine in the early phases of both normal and neoplastic cell proliferation, are well known.( 38 , 39 ) Red blood cells (RBC) carries a substantial portion of circulating polyamines. Because the enzyme systems necessary to synthesize polyamines are not found in RBC, it is theorized that RBC act as carriers of polyamines from the site of production to sites of conjugation and excretion.( 40 ) It is evident that polyamines are associated intimately with growth processes. Earlier reports have shown increased levels of polyamine content in various cancer patients.( 41 , 42 ) It was explained that high levels of polyamines are generally associated with carcinomatous growth at high rates of cell multiplication.( 43 ) Previous experiments with different carcinoma tissues have demonstrated a marked increase in the levels of polyamines and ornithine decarboxylase (ODC) activity, confirming their correlation with neoplastic growth and high rates of cell proliferation.( 44 , 45 ) In the present study, the levels of polyamines were found to be increased in cancer‐bearing group II animals. Paclitaxel and W. somnifera combination treatment reduced the level of polyamine synthesis in cancer‐bearing animals. This may be due to the inhibition of ODC activity and reduced cell proliferation in lung cancer‐bearing animals. The reduction in polyamine synthesis induced during chemotherapy is an important event for the effective treatment of lung cancer.
Based on the above biochemical studies it may be suggested that paclitaxel can be used along with W. somnifera as a promising chemotherapeutic agent. From these results and the results of our previous studies, it is concluded that paclitaxel along with W. somnifera exerts a synergetic effect in the treatment of cancer. Further studies are in progress to identify the compound responsible for this effect and to confirm the anticancer potential of W. somnifera in combination with paclitaxel as a chemotherapeutic agent in the treatment of lung cancer. Treatment with these drugs will be important in the development of new anticancer drugs.
References
- 1. Haugan A. Women who smoke: are women more susceptible to tobacco induced lung cancer? Carcinogenesis 2002; 23: 227–9. [DOI] [PubMed] [Google Scholar]
- 2. Graziewicz MA, Sayer JM, Jerina DM, Copeland WC. Nucleotide incorporation by human DNA polymerase γ opposite benzo[a]pyrene and benzo[c]phenanthrene diol epoxide adducts of deoxyguanosine and deoxyadenosine. Nucl Acids Res 2004; 32 (1): 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hucks RM. Pathological and biochemical aspects of tumor promotion. Carcinogenesis 1983; 4: 1209–14. [DOI] [PubMed] [Google Scholar]
- 4. Conklin KA. Dietary antioxidants during cancer chemotherapy: Impact on chemotherapeutic effectiveness and development of side effects. Nutr Cancer 2000; 37: 1–18. [DOI] [PubMed] [Google Scholar]
- 5. Wani MC, Taylor HL, Wall ME, Coggon P, MacPhail AT. Plant antitumor agents VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia . J Am Chem Soc 1971; 93: 2325–7. [DOI] [PubMed] [Google Scholar]
- 6. Shen Y‐C, Chen C‐Y, Chen Y‐J, Duh C‐Y, Hung M‐C. Cytotoxicity of taxoids isolated from the seeds of Taxus mairei and T. chinensis against human tumor. Chinese Pharmceut J 2000; 52: 219–26. [Google Scholar]
- 7. Torres K, Horwitz SB. Mechanisms of taxol‐induced cell death are concentration dependent. Cancer Res 1998; 58: 3620–6. [PubMed] [Google Scholar]
- 8. Gupta YK, Sharma SS, Rai K, Katiyar CK. Reversal of paclitaxel induced neutropenia by Withania somnifera in mice. Indian J Physiol Pharmacol 2001; 45 (2): 253–7. [PubMed] [Google Scholar]
- 9. Umadevi P. Withania somnifera dunal (Ashwagandha): potential plant source of promising drug for cancer chemotherapy and radiosensitization. Indian J Exp Biol 1996; 34: 927–32. [PubMed] [Google Scholar]
- 10. Ziauddin M, Phansalkar N, Patki P, Diwanay S, Patwarthan B. Studies on the immunomodulatory effects of Ashwagandha. J Ethnopharmacol 1996; 50: 69–6. [DOI] [PubMed] [Google Scholar]
- 11. Ganzera M, Choudhary MI, Khan IA. Quantitative HPLC analysis of withanolides in Withania somnifera . Fitoterapia 2003; 74: 68–6. [DOI] [PubMed] [Google Scholar]
- 12. Mildred K, Richerd L, Joseph G, Alexander W, Conney A. Activation and inhibition of benzo(a)pyrene and aflatoxin B1 metabolism in human liver microsomes by naturally accruing flavonoids. Cancer Res 1981; 41: 67–2. [PubMed] [Google Scholar]
- 13. Orlowski M, Meister A. Isolation of γ‐glutamyl transpeptidase from hog kidney. J Biol Chem 1965; 240: 338–47. [PubMed] [Google Scholar]
- 14. Luly P, Barnabei O, Tria E. Hormonal control of in vitro plasma membrane bound Na+/K+ ATPase of rat liver. Biochem Biophys Acta 1972; 282: 447–52. [DOI] [PubMed] [Google Scholar]
- 15. King J. In: Van D, ed. Practical Clinical Enzymology. London: Nostrand, 1965; 83–93. [Google Scholar]
- 16. Niebes P. Determination of enzyme and degradation products of GAG metabolism in the serum of healthy and varicose subjects. Clin Chim Acta 1972; 42: 399–408. [Google Scholar]
- 17. Wagner WD. More sensitive assay discriminating galactosamine and glucosamine in mixtures. Anal Biochem 1974; 94: 394–7. [DOI] [PubMed] [Google Scholar]
- 18. Warren L. The thiobarbituric acid assay of sialic acid. J Biol Chem 1954; 234: 1971–5. [PubMed] [Google Scholar]
- 19. Endo Y. A simple and sensitive method of analysis of histamine, putrescine and polyamines without the use of an amino acid analyser. Anal Biochem 1978; 89: 235–46. [DOI] [PubMed] [Google Scholar]
- 20. Kalant H, Roschlau WHE. Principles of Medical Pharmacology. New York: Oxford University Press, 1998. [Google Scholar]
- 21. Fleischhacker M, Beinert T, Possinger K. Molecular genetic characteristics of lung cancer useful as ‘real’ tumor markers? Lung Cancer 1999; 25: 7–24. [DOI] [PubMed] [Google Scholar]
- 22. Durak I, Umitisik CA, Canbolt O, Akyol O, Kavutcu M. Adenosine deaminase, 5′−nucleotidase, xanthine oxidase, SOD, CAT activities in cancerous and noncancerous human laryngeal tissues. Free Rad Biol Med 1993; 15: 681–4. [DOI] [PubMed] [Google Scholar]
- 23. Ferrigno D, Buccheri G, Biggi A. Serum tumour markers in lung cancer: history, biology and clinical applications. Eur Respir 1994; 7: 186–97. [DOI] [PubMed] [Google Scholar]
- 24. Yildrim Z, Hasanoglu C, Omer Okyol HJ, Gokirmak M, Koksai N. Serum adenosine deaminase activities in lung cancer and mesothelioma. Clin Biochem 1999; 32: 283–5. [DOI] [PubMed] [Google Scholar]
- 25. Chen L, Liu Y. Application of aryl hydrocarbon hydroxylase in diagnosis of lung cancer. Zhongua Jie He Xi Zhi 2000; 23: 151–4. [PubMed] [Google Scholar]
- 26. Durham JR, Frierson FH, Hannigan HM. Gamma glutamyl transpeptidase immunoreactivity in benign and malignant tissues. Breast Cancer Res Treat 1997; 45: 55–62. [DOI] [PubMed] [Google Scholar]
- 27. Komlosh A, Volohonsky G, Porat N, Taby C, Oesch F, Stork AA. γ‐Glutamyl transpeptidase and glutathione biosynthesis in non‐tumorigenic and tumorigenic rat liver oral cell lines. Carcinogenesis 2002; 23: 671–6. [DOI] [PubMed] [Google Scholar]
- 28. Nilssen O, Forde OH, Brenn T. The tromso study. Distribution and population determinants of gamma‐glutamyl transferase. Am J Epidemiol 1990; 132 (2): 318–26. [DOI] [PubMed] [Google Scholar]
- 29. Dao TL, Ip C, Pater J. Serum sialyl transferase and 5′‐nucleotidase as reliable biomarkers in cancer. J Natl Cancer Inst 1980; 65: 529–34. [PubMed] [Google Scholar]
- 30. Thompson LF, Ruedi JM, O’Connon RD, Bastian JF. Ecto5′‐nucleotidase expression during human B cell development. An explanation for the heterogenicite in B‐lymphocyte aceto 5′‐nucleotidase activity in patients with hypogammaglobulinemia. J Immunol 1986; 137: 2459–500. [PubMed] [Google Scholar]
- 31. Davis CW, Kuo JF. Ontogenetic changes in levels of phosphodiesterase for adenosine 3′:5′‐monophosphate and glucosine 3′:5′‐monophosphate in the lung, brain and heart from guinea pigs. Biochim Biophys Acta 1976; 444: 554–62. [DOI] [PubMed] [Google Scholar]
- 32. Engan T, Hannisdal E. Blood analysis as prognostic factor in primary lung cancer. Acta Oncol 1990; 29: 151–4. [DOI] [PubMed] [Google Scholar]
- 33. Nakashimhan S, Schachter H, Rajalakshmi S. Expression of N‐acetyl‐glucosaminyltransferase III in hepatic nodules during rat liver carcinogenesis promoted by orotic acid. J Biol Chem 1998; 263: 1273–81. [PubMed] [Google Scholar]
- 34. Helmes MH, Modia A, Moneim EL, Moustafae MS, Bale EL, Safinoz MEL. Clinical values of serum LDH, ceruloplasmin and lipid bound sialic acid in monitoring patients with malignant lymphomas. Med Sci Res 1998; 26: 613–17. [Google Scholar]
- 35. Vanisree AJ, Shyamaladevi CS. Effect of therapeutic strategy established by N‐acetyl cysteine and vitamin C on the activities of tumour marker enzymes in vitro . Indian J Pharmacol 1998; 31: 275–8. [Google Scholar]
- 36. Herzfeld A, Greengard O. The effect of lymphoma and other neoplasms on hepatic and plasma enzymes of the host rat. Cancer Res 1997; 37: 231–8. [PubMed] [Google Scholar]
- 37. Thirunavukkarasu C, Sakthisekaran D. Influence of sodium selenite on glycoprotein contents in normal and N‐nitrosodiethylamine initiated and phenobarbital promoted rat liver tumors. Pharmacol Res 2003; 48: 167–73. [DOI] [PubMed] [Google Scholar]
- 38. Janne J, Poso H, Rina A. Polyamines in rabbit growth and cancer. Biochim Biophys Acta 1978; 473: 241–7. [DOI] [PubMed] [Google Scholar]
- 39. Thomas T, Thomas TJ. Polyamines in cell growth and cell death, molecular mechanisms and therapeutic applications. Cellular Mol Life Sci 2001; 58: 244–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takami H, Nishoka K. The effect of glycosylation inhibitors on fc receptor mediated phagocytosis and carbohydrate incorporation by mouse peritoneal macrophages. Fed Proc 1980; 37: 1685–91. [Google Scholar]
- 41. Cipolla B, Guille F, Moulinoux JP et al. Polyamine and prostatic carcinoma: Clinical and therapeutic implications. Eur Urol 1993; 24: 124–31. 7689970 [Google Scholar]
- 42. Chanda R, Ganguly AK. Diamine oxidase activity and tissue histamine content of human skin, breast and rectal carcinoma. Cancer Let 1987; 34: 207–12. [DOI] [PubMed] [Google Scholar]
- 43. Selvendiran K, Banu SM, Sakthisekaran D. Protective effect of piperine on benzo(a)pyrene‐induced lung carcinogenesis in Swiss albino mice. Clin Chim Acta 2004; 350: 73–8. [DOI] [PubMed] [Google Scholar]
- 44. Bandyopadhyay M, Ganguly A. Serum and urine concentrations of diamine and polyamines in patients with uterine, ovarian, breast or rectal cancer. Med Sci Res 1999; 27: 645–7. [Google Scholar]
- 45. Farriol M, Segovia ST, Venero Y, Orta X. Role of polyamines in cell proliferation in a colon carcinoma cell line. Nutrition 2001; 17: 934–8. [DOI] [PubMed] [Google Scholar]


