Abstract
We assessed the antitumor efficacy of KRN951, a novel tyrosine kinase inhibitor of vascular endothelial growth factor receptors, using a rat colon cancer RCN‐9 syngeneic model in which the tumor cells are transplanted into the peritoneal cavity of F344 rats. KRN951 treatments that commenced 4 days after tumor transplantation (day 4) significantly inhibited tumor‐induced angiogenesis, the formation of tumor nodules in the mesenteric windows, and the accumulation of malignant ascites. Moreover, KRN951 treatments initiated on day 14, by which time angiogenesis and malignant ascites have already been well established, resulted in the regression of newly formed tumor vasculatures with aberrant structures and also in the apparent loss of malignant ascites by the end of the study period. Quantitative analysis of the vessel architecture on mesenteric windows revealed that KRN951 not only regressed, but also normalized the tumor‐induced neovasculature. Continuous daily treatments with KRN951 significantly prolonged the survival of rats bearing both early stage and more advanced‐stage tumors, compared with the vehicle‐treated animals. The results of our current study thus show that KRN951 inhibits colon carcinoma progression in the peritoneal cavity by blocking tumor angiogenesis, ascites formation, and tumor spread, thereby prolonging survival. Moreover, these studies clearly demonstrate the therapeutic effects of KRN951 against established tumors in the peritoneal cavity, including the regression and normalization of the tumor neovasculature. Our findings therefore suggest that KRN951 has significant potential as a future therapeutic agent in the treatment of peritoneal cancers with ascites. (Cancer Sci 2008; 99: 623–630)
Abbreviations:
- EBM
endothelial cell basal medium
- ELISA
enzyme‐linked immunosorbent assay
- FACS
fluorescence‐activated cell sorting
- FDA
Federal Drug Administration, USA
- HUVEC
human umbilical vein endothelial cells
- IC50
50% inhibitory concentration
- KRN951
N‐(2‐chloro‐4‐[{6,7‐dimethoxy‐4‐quinolyl}oxy] phenyl)‐N′‐(5‐methyl‐3‐isoxazolyl) urea hydrochloride monohydrate
- MST
median survival time
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
Vascular endothelial growth factor and VEGFR are promising targets for antitumor therapies as they play major roles during tumor angiogenesis, which is an essential process for tumor growth and metastasis. A number of studies have now demonstrated a clear correlation between VEGF expression, microvessel density, and a poorer prognosis.( 1 , 2 , 3 , 4 ) VEGF mediates angiogenic signaling to the endothelium via two receptor tyrosine kinases, VEGFR‐1 and VEGFR‐2. In particular, VEGFR‐2 in vascular endothelial cells is the main transmitter of the activation signals that induce the proliferation and migration of endothelial cells upon their binding of VEGF.( 5 ) In addition, it seems that the signaling pathways mediated via VEGFR‐1 and ‐2 are also involved in the VEGF‐induced hyperpermeability of tumor vasculatures.( 6 )
As a result of these previous findings, there is now great interest in developing blocking antibodies and small molecules that target the VEGF and VEGFR pathway as such agents are good candidate anticancer therapeutics. In this regard, several agents that target angiogenesis through VEGF function, including those targeting VEGF itself or the VEGFR, are now in advanced stages of development.( 7 , 8 , 9 , 10 , 11 , 12 , 13 ) Bevacizumab, a VEGF‐neutralizing monoclonal antibody, is the first antiangiogenic agent to be approved by the FDA as an anticancer agent for the treatment of both colorectal and non‐small‐cell lung cancer.( 14 , 15 ) Many clinical studies have also demonstrated clear clinical benefits of bevacizumab treatments( 14 , 15 , 16 , 17 ) and have thus reaffirmed the potential for using antiangiogenic agents in the treatment of cancer.
The overexpression of VEGFR is associated with the development of metastases in colon cancer,( 18 ) and the level of angiogenic activity in such colorectal tumors has been shown also to be a important determinant of survival.( 16 , 17 , 19 ) Colon cancers metastasize to the liver, lymph nodes, and peritoneal cavity, resulting in peritoneal carcinomatosis. Peritoneal carcinomatosis is also sometimes associated with the formation of ascites, which is part of the process of malignancy that signifies end‐stage disease.( 20 ) The progression of such cancers to an ascites‐formation stage limits both the quality of life and survival outcome.( 21 ) Peritoneal carcinomatosis and malignant ascites formation are also sometimes observed in stomach, ovarian, and pancreatic cancers.( 21 ) Despite recent advances in cancer therapies, peritoneal carcinomatoses remain difficult to treat in patients with recurrent disease.
We have previously reported that KRN951, a novel quinoline‐urea derivative, inhibited the tyrosine kinase activity of VEGFR‐1, VEGFR‐2, and VEGFR‐3 with subnanomolar IC50 values.( 22 ) KRN951 also blocked the VEGF‐driven proliferation of human endothelial cells (IC50, 0.67 nM), but did not affect the proliferation of various cancer cells in vitro at concentrations <1 µM.( 22 ) In addition, we found that KRN951 inhibited tumor angiogenesis in vivo and displayed a broad spectrum of antitumor activities against tumor xenografts that had been implanted subcutaneously into mice and rats.( 22 )
In our present study, we used a syngeneic peritoneal disseminated tumor model system in the rat to further study and elucidate the effects of KRN951. In this model, a VEGF‐secreting cell line, RCN‐9, which was established from a colon carcinoma,( 23 , 24 ) is inoculated into the peritoneal cavity of the rat, and the cells then adhere to the transparent mesenteric windows. These cells then form multiple tumor nodules accompanied by tumor‐induced angiogenesis within the mesenteric windows( 25 ) and progress with ascites accumulation. As the rodent mesenteric window is natively avascular, and newly formed neovasculatures can be readily detected, the rat model that we used in our present analyses has often been used for the study of various aspects of tumor angiogenesis.( 23 , 25 , 26 ) Moreover, the thinness and two‐dimensional structures of the tumor‐induced vessels on the mesenteric windows enable the quantitative architectural analysis of these vessels and also an assessment of the effects of KRN951 upon these structures. This is not possible using standard immunohistochemistry in a subcutaneous tumor model.
Because of the characteristics of this rat model, we predicted that it would be a suitable system to investigate the efficacy of angiogenesis inhibitors on intraperitoneal tumor development and tumor angiogenesis. We therefore used this system to evaluate the effects of KRN951 on colon cancer in terms of tumor angiogenesis, already established tumor neovasculatures, ascites formation, and survival outcomes.
Materials and Methods
Cells. The RCN‐9 tumor cell line, which was established from Fischer 344 rat colon carcinoma cells,( 24 ) was obtained from the Riken Cell Bank (Tsukuba, Japan). The cells were cultured in RPMI‐1640 medium (Gibco BRL, Grand Island, NY, USA) supplemented with 10% heat‐inactivated fetal bovine serum at 37°C in 5% CO2. HUVEC were purchased from Cambrex (Walkersville, MD, USA) and cultured in Cambrex endothelial cell growth medium‐2.
Animals. Male Fisher 344 rats were obtained from Charles River Japan (Kanagawa, Japan) and maintained under specific pathogen‐free conditions throughout this study. These animals were used at 7 weeks of age. All animal experiments were reviewed and approved by the Kirin Institutional Ethics Committee for Animal Experiments.
Peritoneal disseminated tumor model. To establish the peritoneal disseminated tumor model, RCN‐9 colon cancer cells were inoculated intraperitoneally into rats according to the method established by Yanagi and Ohshima.( 25 ) Briefly, 1 × 107 cells in 1 mL phosphate‐buffered saline were injected into the peritoneal cavity of each rat using a 26‐gauge needle under sterile conditions.
Preparation of the KRN951 solution. KRN951 was synthesized by the Production Department, Research and Development Center, Kirin Pharma (Tokyo, Japan). KRN951 was suspended in vehicle (0.5% methyl cellulose in distilled water) and stored at 4°C. Fresh solutions were prepared weekly.
Experimental design. Rats inoculated with RCN‐9 cells were assigned randomly to three groups and given daily oral doses of KRN951 (1 or 3 mg/kg) or a 0.5% methylcellulose vehicle control. These treatments commenced at 4 or 14 days after tumor transplantation, and continued for 10 or 11 days, respectively. At the end of the treatment periods, the rats were killed and tumor progression was evaluated. Ascites were also collected and their volumes were measured. Each transparent window in the mesentery surrounded by fatty tissue was observed microscopically. The percentages of the mesenteric windows with a vasculature and the number of tumor nodules with or without a vasculature on the mesenteric windows were then counted.
In a subsequent survival study, rats inoculated with RCN‐9 cells were assigned randomly to vehicle‐treated or 1 mg/kg KRN951‐treated groups (n = 10 per group). Separate treatments then commenced from the day of tumor inoculation or at 14 days after this transplantation. The results were plotted using Kaplan–Meier methods and the differences in survival were analyzed by log‐rank test. A P‐value of <0.05 was considered statistically significant.
Tumor vessel imaging. RCN‐9 cell‐inoculated rats with and without KR951 treatment were anesthetized and injected intravenously with fluorescein isothiocyanate‐labeled dextran (molecular weight 200 000; Sigma, St Louis, MO, USA). After the animals had been killed, the mesenteries were fixed with 4% paraformaldehyde and placed on glass slides. The vasculature associated with each mesenteric window was then photographed microscopically. The number of vessel joints and paths (as vessel bifurcation characteristics), the areas and lengths of vessels (as the angiogenesis density), and the tortuosity of the vasculatures were recorded objectively and evaluated quantitatively using an angiogenesis image analyzer (Kurabo, Osaka, Japan). Four rats were used in these experiments from each group and 12–15 different fields from each animal were analyzed.
Enzyme‐linked immunosorbent assay. The VEGF levels in ascites were measured using a commercial rat VEGF‐specific ELISA Kit (Quantkine M; R & D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions (detection limit, 7.8 pg/mL). Ascites were harvested as described above and centrifuged, and the supernatant was frozen until analysis.
Permeability assay and detection of VEGFR‐2 phosphorylation. The effects of malignant ascites on endothelial cell permeability and VEGFR‐2 phosphorylation, and the inhibitory activity of KRN951 on these effects, were evaluated. Ascites samples taken from a vehicle‐treated peritoneal disseminated tumor model on day 25 were pooled and the resulting supernatant was used in these assays. We examined propidium iodide uptake as a measure of permeability in an in vitro assay.( 20 ) VEGFR‐2 phosphorylation was determined by western blotting. For the permeability assay, HUVEC at 90% confluence in culture were serum starved overnight in a basic medium (EBM‐2) containing 0.5% fetal bovine serum. These cells were then washed with phosphate‐buffered saline, and malignant ascites and a 10‐nM concentration of KRN951 were added to the culture plates. Medium alone or 50 ng/mL VEGF (PeproTech EC, London, UK) without ascites served as the internal controls. After 7 h incubation, the cells were harvested and treated with propidium iodide (1 µg/mL), and subjected to FACS analysis. Permeability was assessed by measuring the uptake of propidium iodide by the HUVEC. For western blotting, HUVEC were treated in the same way except that the stimulation time with ascites of 10 min. Following cell lysis, VEGFR proteins were immunoprecipitated with an anti‐VEGFR‐2 antibody (A‐3; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and then subjected to immunoblotting with an antiphosphotyrosine antibody (4G10; Upstate Biotechnology, Lake Placid, NY, USA), as described previously.( 27 )
Results
Suppression of both tumor‐induced angiogenesis and tumor nodule formation by KRN951. In vehicle‐treated rats, a number of tumor nodules accompanied by newly formed blood vessels in the mesenteric windows were observed at day 14 (Fig. 1Aa). KRN951 treatment at a dose of 1 mg/kg, however, efficiently blocked angiogenesis on the mesenteric windows and also prevented the formation of tumor nodules (Fig. 1Ac). In addition, almost no neovasculature or visible nodules could be observed in rats treated with 3 mg/kg KRN951 (Fig. 1Ae). At day 25, the tumors in the vehicle‐treated rats were found to be far more advanced and most of these animals showed tissue adherence as a result of the progression of these cancers. One of the few observable mesenteries that was free from tissue adherence is shown in Fig. 1Ab. KRN951 treatment efficiently inhibited the progression of these tumors, even when treatment was initiated from day 14. The tumor nodules that did form were also smaller in size compared with the vehicle‐treated rats and were found to be quite avascular in the KRN951‐treated rats at this stage (Fig. 1Ad,f), suggesting that treatment with KRN951 caused a regression in neovascularity.
Figure 1.
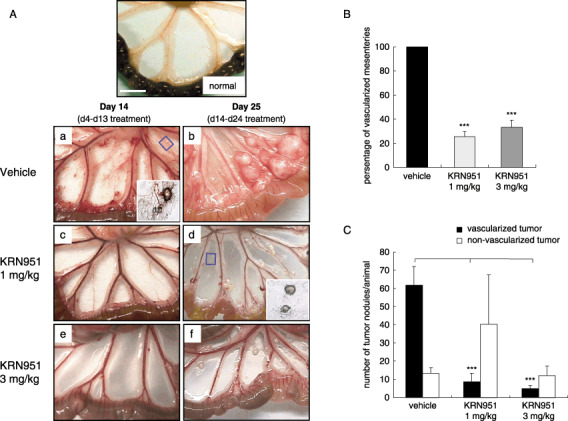
(A) Representative examples of angiogenesis and tumor growth in the mesenteries of rats treated with (a,d) vehicle alone, and either (b,e) 1 or (c,f) 3 mg/kg/day KRN951 over the indicated schedules (days 4–13 or days 14–24). RCN‐9 colon cancer cells were inoculated into the peritoneal cavities of the rats and KRN951 or vehicle preparations were administrated orally for 10 days (days 4–13) or 11 days (days 14–24). On the last treatment days (days 14 and 25), four animals per group were killed to evaluate angiogenesis and tumor progression. Representative examples of vascularized and non‐vascularized tumors are shown in the insets in (a) and (d), respectively. Scale bar = 1 cm. (B) The number of vascularized mesenteries at day 14. (C) The number of tumor nodules in the mesenteric windows at day 14. Tumor nodules were counted according to their vascularization and the data shown represent the mean values and standard errors. P‐values were calculated by comparison with the vehicle‐treated group using the Dunnett's test. ***P < 0.001.
The status of the angiogenic response and the number of tumor nodules on the mesenteric windows were further evaluated at day 14. These evaluations were not possible at day 25 because of the extent of the tumor progression in the vehicle‐treated rats as described above. The majority of the mesenteries (including those with no tumor nodules) had clearly become vascularized in the vehicle‐treated rats but KRN951‐treated animals showed significantly reduced vascularized mesenteries (Fig. 1B).
The total numbers of tumor nodules on the mesenteries were also found to be lower in the KRN951‐treated groups compared with the vehicle‐treated group (Fig. 1C). These numbers were reduced by 67.7% (P < 0.01) in the 3 mg/kg KRN951 group and 34.7% (not significant) in the 1 mg/kg KRN951 group, compared with the control group. We subdivided these tumors into vascularized and non‐vascularized groups and representative images are shown in the insets in Fig. 1Aa–e. A greater number of vascularized tumors was evident in the vehicle‐treated rats compared with the KRN951‐treated animals that had a significantly decreased number of such lesions at both the 1 mg/kg and 3 mg/kg dose levels (Fig. 1C; P < 0.001).
Effects of KRN951 treatments on the tumor vascular architecture. To investigate whether KRN951, in addition to inhibiting tumor angiogenesis, had any impact also on the tumor‐vessel architecture in our rat model, the mesenteric windows were evaluated. Numerous irregular and tortuous vessels that showed multiple branchings and irregular loops were evident on the mesenteric windows in vehicle‐treated rats at day 14 (Fig. 2Aa). There was also evidence of vascular leakage in this vehicle‐treated group. In contrast, the vessels in both the 1 and 3 mg/kg KRN951‐treated rats at the same timepoint showed architectures that were similar to normal rats (Fig. 2Ac,e). The vessel architectures in the vehicle‐treated group at day 25 could not be evaluated except in one case (Fig. 2Ab) due to tissue adherence and increased vascular leakage.
Figure 2.
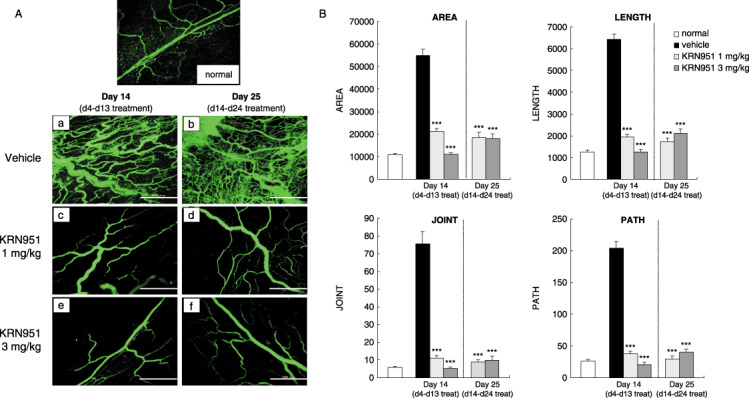
The inhibitory and normalizing effects of KRN951 on the tumor‐induced neovasculature in a rat model. (A) Representative examples of vessel architectures on the mesenteric windows in RCN‐9‐bearing rats treated with (a,d) vehicle alone, and either (b,e) 1 or (c,f) 3 mg/kg/day KRN951 over the indicated schedules (days 4–13 or days 14–24). The vessel lumen was then detected following an intravenous injection of fluorescein isothiocyanate‐labeled dextran. Scale bars = 500 µm. (B) Quantitative analysis of vessel architectures. The areas, lengths, paths, and joints of the vasculatures as indicated were measured using an angiogenesis image analyzer. Four rats from each group were used in these experiments and 12–15 different fields were analyzed for each animal. The results shown are the mean values and standard errors. P‐values were calculated by comparisons with vehicle‐treated animals at day 14 using the Dunnett's test. ***P < 0.001.
We carried out further quantitative analyses to confirm the effects of KRN951 on the tumor architecture (Fig. 2b). KRN951 treatments from day 4 were found to inhibit the formation of a tumor‐induced aberrant vascular architecture in the mesenteric‐windows, for example, there was increased tortuosity (length and area) and a greater number of paths and joints were not evident. Interestingly, KRN951 treatments from day 14 were found to not only inhibit progression of the tumor vasculature but also to revert the tumor‐induced aberrant vessel structures that could be observed at day 14 to a normal architecture by day 25 (Fig. 2b).
Effects of KRN951 treatment on the accumulation of ascites and VEGF levels. The vehicle‐treated rats showed abdominal swelling and accumulation of bloody ascites at day 14 (Fig. 3a). In contrast, the mean ascites volumes in rats treated with 1 or 3 mg/kg KRN951 was markedly reduced by 90.2 and 97.9%, respectively, compared with vehicle‐treated rats (Fig. 3a). By day 25, a massive build up of ascites was observed in vehicle‐treated rats at day 25 (Fig. 3b), with a mean volume that was almost 10‐fold greater than that measured at day 14. In addition, tumor metastases were observed in several intraperitoneal organs (data not shown). KRN951 treatments from day 14 demonstrated significant inhibitory activities against such a massive ascites accumulation, and almost no ascites could in fact be detected in most of the 3 mg/kg KRN951‐treated rats (Fig. 3b). The total VEGF levels in the peritoneal cavity in each animal were calculated from the VEGF concentration and ascites volumes. On day 14, the total amount of VEGF did not differ between the vehicle‐ and KRN951‐treated groups (Fig. 3c). In contrast, by day 25, the VEGF amounts in the peritoneal cavity were significantly lower in both KRN951‐treated groups (Fig. 3d; P < 0.05 and 0.01, respectively).
Figure 3.
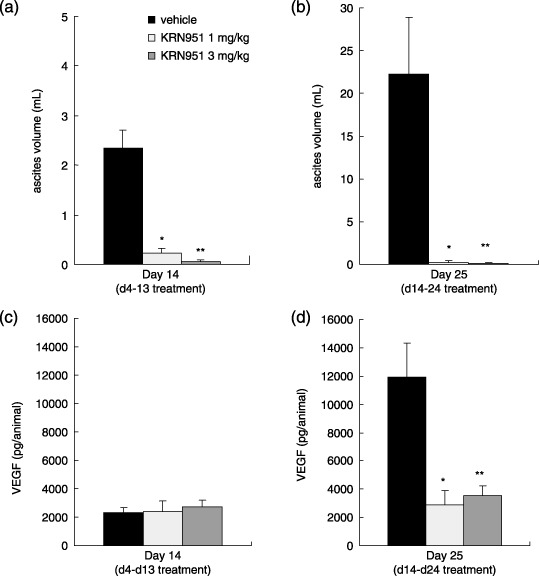
The effects of KRN951 on ascites formation and total amount of peritoneal vascular endothelial growth factor (VEGF). Rats injected with RCN‐9 colon cancer cells were treated daily with KRN951 (1 or 3 mg/kg p.o) or vehicle for 10 days (days 4–13) or 11 days (days 14–24). (a) Ascites volume at day 14 (day 4–13 treatments). (b) Ascites volume at day 25 (day14–24 treatments). (c) Peritoneal VEGF at day 14. (d) Peritoneal VEGF at day 25. Six or seven assessable examples from each group were analyzed and the data shown are the mean values for these rats with standard errors. P‐values were calculated by comparisons with the vehicle‐treated group using the Dunnett's test. *P < 0.05, **P < 0.01.
In the vehicle‐treated rats, the serum VEGF levels were below the detection limit of ELISA throughout the experimental period. Conversely, 10‐day treatment with 1 or 3 mg/kg KRN951 from day 4 resulted in a dose‐dependent increase in the serum VEGF levels (31 ± 6 and 249 ± 114 pg/mL, respectively). KRN951 treatments from days 14 to 24 also increased the serum VEGF levels in a dose‐dependent manner (17 ± 9 and 224 ± 55 pg/mL, respectively).
Effects of KRN951 on the ascites‐induced phosphorylation of VEGFR‐2 and cell hyperpermeability. Using cultured endothelial cells, we next investigated the ability of malignant ascites to induce the phosphorylation of VEGFR‐2 and cause cell hyperpermeability, and then determined the efficacy of KRN951 against these ascites‐induced effects. Ascites taken from the rat peritoneal disseminated tumor model were found to markedly induce the phosphorylation of VEGFR‐2 and increase the permeability of HUVEC in culture (Fig. 4). KRN951 efficiently inhibited this phosphorylation of VEGFR‐2 (Fig. 4a) and also the elevated permeability of the cultured HUVEC (Fig. 4b).
Figure 4.
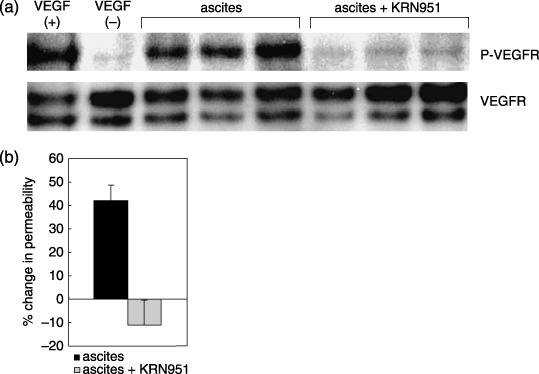
The effects of KRN951 on the ascites‐induced phosphorylation of vascular endothelial growth factor receptor (VEGFR)‐2 and hyperpermeability of cultured endothelial cells. Cultured human umbilical vein endothelial cells were treated with ascites harvested from RCN‐9‐bearing rats with or without KRN951. After incubation, the phosphorylation of (a) VEGFR‐2 and (b) the cell permeability levels were investigated. The permeability data shown represent the mean values and standard deviation from three different cultures.
Effects of KRN951 on survival outcome in the rat peritoneal disseminated tumor model. To determine whether the suppressive effects of KRN951 on tumor growth and malignant ascites formation also resulted in improved overall survival outcomes in the animal model we used, KRN951 was administrated continuously to the rats bearing RCN‐9 cells either from day 0, or at 14 days after tumor inoculation. We then measured the overall survival times in each animal group. Treatment with 1 mg/kg/day KRN951 from the day of tumor inoculation significantly prolonged the survival of the tumor‐bearing rats (Fig. 5a, P < 0.001), and the MST were in fact almost doubled by treatment with KRN951 (MST = 53.5 days), compared with the vehicle control (27.5 days). The efficacy of the KRN951 treatments initiated at 14 days after tumor inoculation, by which time the tumors are well established and ascites have begun to accumulate in this model, was also investigated. KRN951 treatments were also found to be effective at this stage, resulting again in a significantly prolonged survival time (Fig. 5b; P < 0.001). The MST of the vehicle‐treated rats and KRN951‐treated rats were 22.5 and 34.5 days, respectively, in this experiment. There was also no apparent bodyweight loss associated with any of the KRN951‐treated animals in these analyses.
Figure 5.
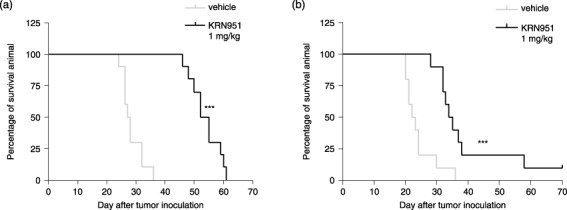
The effects of KRN951 therapy on overall survival. After the intraperitoneal injection of 1 × 107 RCN‐9 cells into the rats (day 0), 1 mg/kg/day KRN951 or vehicle treatments were continuously administered orally to the animals, and survival outcomes were compared between the KRN951‐treated and control groups (n = 10). The treatment schedules were as follows: (a) KRN951 was administrated from 1 day after the intraperitoneal inoculation of RCN‐9 cells. P < 0.001 versus vehicle. (b) KRN951 treatment was initiated 14 days after tumor inoculation. P < 0.001 versus vehicle. Differences in survival were analyzed using the log‐rank test.
Discussion
We show in our current study using a rat peritoneal disseminated tumor model that KRN951 markedly inhibits tumor‐induced angiogenesis, the growth of peritoneal carcinomatoses, and ascites formation. Moreover, continuous treatment with KRN951 lead to significantly prolonged survival times, even when initiated after the tumor nodules and ascites had been established. Blockade of VEGF using monoclonal antibodies or low molecular weight inhibitors has been shown previously to dramatically reduce ascites formation and tumor burden in peritoneal tumor models.( 28 , 29 , 30 , 31 , 32 , 33 , 34 ) However, differences have been reported in the outcomes following the use of these agents. Some were found to produce survival benefits coupled with ascites inhibition.( 29 , 30 , 31 ) In contrast, other treatments were reported to have limited antitumor activity( 32 , 33 ) or no survival benefits as a result of monotherapy, despite the almost complete inhibition of ascites.( 34 ) Our present study in a rat tumor model has revealed, however, that KRN951 is significantly beneficial against tumor growth and ascites formation, and had a positive impact on the overall survival outcome.
The antiangiogenic activity of KRN951 in vivo was clearly demonstrated by the reduced number of vascularized mesenteries and vascularized tumors. In addition, the small avascular tumors observed mainly in KRN951‐treated rats accord with Folkman's theory that tumors cannot grow beyond a few cubic millimeters without promoting the growth of blood vessels.( 35 ) This finding also indicates that KRN951 restricts their growth ability through the inhibition of new blood vessel formation.
We further demonstrated the efficacy of KRN951 as a VEGF signaling inhibitor of tumor‐induced ascites accumulation ex vivo. In these analyses, we have shown that tumor‐induced ascites promote VEGFR phosphorylation and increase the permeability of endothelial cells, both of which are blocked by KRN951. These data also help to explain the fact that although the levels of peritoneal VEGF did not differ between the vehicle and KRN951‐treated rats at day 14, angiogenesis and ascites formation were clearly blocked in the KRN951‐treated animals. It is likely therefore that unlike VEGF neutralizing antibody, KRN951 does not act directly on VEGF itself in the ascites but blocks their function by inhibiting the phosphorylation of VEGFR. VEGF levels have been found to be elevated in malignant ascites from ovarian, gastric, and colon cancer patients.( 20 ) There is also now substantial evidence to show that VEGF plays a role in the formation of ascites.( 33 , 36 ) Hence, the efficacy of KRN951 against ascites accumulation that we observed in the present study is potentially applicable to those VEGF‐dependent ascites accumulations.
The vehicle‐treated rats in this model did not show detectable serum VEGF regardless of tumor progression. In contrast, KRN951 treatments clearly increased the blood VEGF levels in a dose‐dependent manner, suggesting that they may serve as a biomarker for KRN951 treatment in accordance with other clinical reports of VEGF signaling inhibitors.( 37 , 38 ) However, the mechanism underlying VEGF induction by KRN951 in rats and whether it can be applied in a clinical setting is not yet well understood and needs further investigation.
One of the most remarkable effects of KRN951 demonstrated in this study was the regression and normalization of the established tumor‐induced neovasculatures in the rat model we used. Tumor vessels are structurally and functionally abnormal, are characterized by irregular tortuosity and bifurcation, and show high permeability due to their structural deficiencies. By quantitative vessel architecture analysis, we revealed in our current experiments that KRN951 not only inhibits tumor angiogenesis and growth, but also normalizes the tumor‐induced aberrant vessel architecture. These normalization effects of KRN951 are probably due to immature vessel regression as a result of the inhibition of VEGF signaling, which is a critical survival pathway for the endothelial cells of immature vessels.( 39 ) Part of the antitumor effects of KRN951 may thus be explained by these vessel normalizing effects. This suggests that by the inhibition or regression of immature tumor vessels, KRN951 decreases vascular permeability and blocks a number of serum‐derived growth factors that promote tumor cell growth. This normalization effect of KRN951 may also contribute in part to the inhibition of ascites accumulation, which is likely to be the result of an elimination of highly permeable tumor‐induced immature blood vessels.( 40 )
Continuous treatments with KRN951 resulted in a statistically significant survival benefit in the peritoneal disseminated tumor model used in this study. Survival benefits were even seen when KRN951 treatments were initiated after the establishment of tumors and ascites had begun to build up, suggesting the therapeutic potential of this agent. Importantly, the long‐term continuous administration of KRN951 did not cause any visible side effects, including significant bodyweight reductions. These results thus confirm that there is a significant survival benefit associated with this drug and that there is a tolerability for continuous treatment with this agent against both early stage and advanced‐stage tumors.
The results of our survival analysis in the present study may suggest a further benefit of using KRN951 in a combination treatment strategy. At the end of our survival analyses, the KRN951‐treated rats eventually did succumb to disease, even though the therapeutic efficacy of KRN951 had been demonstrated. This is likely to reflect the complexities of tumor biology. Because VEGF is unlikely to be the only growth factor involved in the maintenance and expansion of intraperitoneal carcinomatoses, and as KRN951 is a relatively selective inhibitor of VEGFR, combination therapies with other specific targeting or chemotherapeutic agents might improve its antitumor effects. It should also be possible to achieve this without causing any additional toxicity as a result of the unfavorable non‐specific inhibition of other kinases. The absence of any visible cytotoxic effects of KRN951( 22 ) also suggest the potential of using this drug in a combination therapy from a safety viewpoint. Moreover, the tumor vessel normalizing effects of KRN951 demonstrated herein also emphasize the usefulness of combination therapies to improve the delivery and effectiveness of treatments.( 17 , 34 , 41 , 42 )
In summary, our current data demonstrate that KRN951 has potent antitumor properties and thus significant therapeutic benefits in vivo, including tumor vessel normalizing effects and the suppression of ascites accumulation, against intraperitoneal tumor progression. Our findings also show that KRN951 improves the survival outcomes for both early stage and advanced‐stage tumors in a rat model. There are currently no established clinical protocols for the treatment or prevention of peritoneal carcinomatosis. However, the results of our current study suggest that antiangiogenesis therapy with KRN951 in a clinical setting may well be a novel and effective treatment for both early stage and advanced‐stage peritoneal carcinomatosis, particularly those accompanied by the formation of malignant ascites.
Acknowledgments
The authors would like to thank Dr Wada (Kirin Pharma) for his valuable discussions. We are grateful to Kazumi Takahashi for her technical assistance.
References
- 1. Inoue K, Ozeki Y, Suganuma T, Sugiura Y, Tanaka S. Vascular endothelial growth factor expression in primary esophageal squamous cell carcinoma. Association with angiogenesis and tumor progression. Cancer 1997; 79: 206–13. [DOI] [PubMed] [Google Scholar]
- 2. Hartenbach EM, Olson TA, Goswitz JJ et al . Vascular endothelial growth factor (VEGF) expression and survival in human epithelial ovarian carcinomas. Cancer Lett 1997; 121: 169–75. [DOI] [PubMed] [Google Scholar]
- 3. Ishigami S‐I, Arii S, Furutani M et al . Predictive value of vascular endothelial growth factor (VEGF) in metastasis and prognosis of human colorectal cancer. Br J Cancer 1998; 78: 1379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takahashi Y, Tucker SL, Kitadai Y et al . Vessel counts and expression of vascular endothelial growth factor as prognostic factors in nondenegative colon cancer. Arch Surg 1997; 132: 541–6. [DOI] [PubMed] [Google Scholar]
- 5. Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 2002; 20: 4368–80. [DOI] [PubMed] [Google Scholar]
- 6. Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989; 246: 1306–9. [DOI] [PubMed] [Google Scholar]
- 7. Morabito A, De Maio E, Di Maio M, Normanno N, Perrone F. Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: current status and future directions. Oncologist 2006; 11: 753–64. [DOI] [PubMed] [Google Scholar]
- 8. Hahn O, Stadler W. Sorafenib. Curr Opin Oncol 2006; 18: 615–21. [DOI] [PubMed] [Google Scholar]
- 9. Drevs J, Zirrgiebel U, Schmidt‐Gersbach CI et al . Soluble markers for the assessment of biological activity with PTK787/ZK 222584 (PTK/ZK), a vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor in patients with advanced colorectal cancer from two phase I trials. Ann Oncol 2005; 16: 558–65. [DOI] [PubMed] [Google Scholar]
- 10. George DJ. Phase 2 studies of sunitinib and AG013736 in patients with cytokine‐refractory renal cell carcinoma. Clin Cancer Res 2007; 13: 753S–7S. [DOI] [PubMed] [Google Scholar]
- 11. Fury MG, Zahalsky A, Wong R et al . A phase II study of SU5416 in patients with advanced or recurrent head and neck cancers. Invest New Drug 2007; 25: 165–72. [DOI] [PubMed] [Google Scholar]
- 12. Baka S, Clamp AR, Jayson GC. A review of the latest clinical compounds to inhibit VEGF in pathological angiogenesis. Expert Opin Ther Targets 2006; 10: 867–76. [DOI] [PubMed] [Google Scholar]
- 13. Hoff PM, Wolff RA, Bogaard K, Waldrum S, Abbruzzese JL. A phase I study of escalating doses of the tyrosine kinase inhibitor semaxanib (SU5416) in combination with irinotecan in patients with advanced colorectal carcinoma. Jpn J Clin Oncol 2006; 36: 100–3. [DOI] [PubMed] [Google Scholar]
- 14. Hurwitz H, Fehrenbacher L, Novotny W et al . Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–42. [DOI] [PubMed] [Google Scholar]
- 15. Sandler A, Gray R, Perry MC et al . Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. N Engl J Med 2006; 355: 2542–50. Erratum in: N Engl J Med 2007; 356: 318. [DOI] [PubMed] [Google Scholar]
- 16. Kabbinavar FF, Hambleton J, Mass RD, Hurwitz HI, Bergsland E, Sarkar S. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol 2005; 23: 3706–12. [DOI] [PubMed] [Google Scholar]
- 17. Mulcahy MF, Benson AB 3rd. Bevacizumab in the treatment of colorectal cancer. Expert Opin Biol Ther 2005; 5: 997–1005. [DOI] [PubMed] [Google Scholar]
- 18. Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res 1995; 55: 3964–8. [PubMed] [Google Scholar]
- 19. O'Dwyer PJ. The present and future of angiogenesis‐directed treatments of colorectal cancer. Oncologist 2006; 11: 992–8. [DOI] [PubMed] [Google Scholar]
- 20. Zebrowski BK, Liu W, Ramirez K, Akagi Y, Mills GB, Ellis LM. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann Surg Oncol 1999; 6: 373–8. [DOI] [PubMed] [Google Scholar]
- 21. Garrison RN, Kaelin LD, Galloway RH, Heuser LS. Malignant ascites. Clinical and experimental observations. Ann Surg 1986; 203: 644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakamura K, Taguchi E, Miura T et al . KRN951, a highly potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, has antitumor activities and affects functional vascular properties. Cancer Res 2006; 66: 9134–42. [DOI] [PubMed] [Google Scholar]
- 23. Miyoshi C, Ohshima N. Vascular endothelial growth factor (VEGF) expression regulates angiogenesis accompanying tumor growth in a peritoneal disseminated tumor model. In Vivo 2001; 15: 233–8. [PubMed] [Google Scholar]
- 24. Inoue Y, Kashima Y, Aizawa K, Hatakeyama K. A new rat colon cancer cell line metastasizes spontaneously: biologic characteristics and chemotherapeutic response. Jpn J Cancer Res 1991; 82: 90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yanagi K, Ohshima N. Angiogenic vascular growth in the rat peritoneal disseminated tumor model. Microvasc Res 1996; 51: 15–28. [DOI] [PubMed] [Google Scholar]
- 26. Norrby K. In vivo models of angiogenesis. Cell Mol Med 2006; 10: 588–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakamura K, Yamamoto A, Kamishohara M et al . KRN633: A selective inhibitor of vascular endothelial growth factor receptor‐2 tyrosine kinase that suppresses tumor angiogenesis and growth. Mol Cancer Ther 2004; 3: 1639–49. [PubMed] [Google Scholar]
- 28. Shaheen RM, Ahmad SA, Liu W et al . Inhibited growth of colon cancer carcinomatosis by antibodies to vascular endothelial and epidermal growth factor receptors. Br J Cancer 2001; 85: 584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yoshiji H, Kuriyama S, Hicklin DJ et al . The vascular endothelial growth factor receptor KDR/Flk‐1 is a major regulator of malignant ascites formation in the mouse hepatocellular carcinoma model. Hepatology 2001; 33: 841–7. [DOI] [PubMed] [Google Scholar]
- 30. Machida S, Saga Y, Takei Y et al . Inhibition of peritoneal dissemination of ovarian cancer by tyrosine kinase receptor inhibitor SU6668 (TSU‐68). Int J Cancer 2005; 114: 224–9. [DOI] [PubMed] [Google Scholar]
- 31. Garofalo A, Naumova E, Manenti L et al . The combination of the tyrosine kinase receptor inhibitor SU6668 with paclitaxel affects ascites formation and tumor spread in ovarian carcinoma xenografts growing orthotopically. Clin Cancer Res 2003; 9: 3476–85. [PubMed] [Google Scholar]
- 32. Hu L, Hofmann J, Zaloudek C, Ferrara N, Hamilton T, Jaffe RB. Vascular endothelial growth factor immunoneutralization plus Paclitaxel markedly reduces tumor burden and ascites in athymic mouse model of ovarian cancer. Am J Pathol 2002; 161: 1917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mesiano S, Ferrara N, Jaffe RB. Role of vascular endothelial growth factor in ovarian cancer: inhibition of ascites formation by immunoneutralization. Am J Pathol 1998; 153: 1249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hu L, Hofmann J, Holash J, Yancopoulos GD, Sood AK, Jaffe RB. Vascular endothelial growth factor trap combined with paclitaxel strikingly inhibits tumor and ascites, prolonging survival in a human ovarian cancer model. Clin Cancer Res 2005; 11: 6966–71. [DOI] [PubMed] [Google Scholar]
- 35. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285: 1182–6. [DOI] [PubMed] [Google Scholar]
- 36. Luo JC, Yamaguchi S, Shinkai A, Shitara K, Shibuya M. Significant expression of vascular endothelial growth factor/vascular permeability factor in mouse ascites tumors. Cancer Res 1998; 58: 2652–60. [PubMed] [Google Scholar]
- 37. Norden‐Zfoni A, Desai J, Manola J et al . Blood‐based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib‐resistant gastrointestinal stromal tumor. Clin Cancer Res 2007; 13: 2643–50. [DOI] [PubMed] [Google Scholar]
- 38. Willett CG, Boucher Y, Duda DG et al . Surrogate markers for antiangiogenic therapy and dose‐limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol 2005; 23: 8136–9. [DOI] [PubMed] [Google Scholar]
- 39. Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest 1999; 103: 157–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dvorak HF, Sioussat TM, Brown LF et al . Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J Exp Med 1991; 174: 1275–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jain RK. Normalizing tumor vasculature with anti‐angiogenic therapy: a new paradigm for combination therapy. Nat Med 2001; 7: 987–9. [DOI] [PubMed] [Google Scholar]
- 42. Winkler F, Kozin SV, Tong RT et al . Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin‐1, and matrix metalloproteinases. Cancer Cell 2004; 6: 553–63. [DOI] [PubMed] [Google Scholar]


