Abstract
Human interacting protein X1 (PinX1) has been identified as a critical telomerase inhibitor and proposed to be a putative tumor suppressor gene. Loss of PinX1 has been found in a large variety of malignancies, but the expression status in epithelial ovarian tumors has not been investigated. In this study, immunohistochemistry for PinX1 protein was performed on a tissue microarray (TMA) of epithelial ovarian tumors (informatively containing 25 cystadenomas, 29 borderline tumors, and 157 invasive carcinomas) and 12 normal ovaries. Receiver–operator curve (ROC) analysis was used to determine cut‐off scores for tumor positivity and to evaluate patients’ survival status. The threshold for PinX1 positivity was determined to be above 60% (area under the curve = 0.856, P < 0.001) based on the area under the ROC. Positive expression of PinX1 was observed in 100% of normal ovarian tissues, in 84% of cystadenomas, in 75.9% borderline tumors, and 66.2% of ovarian carcinomas. Decreased expression of PinX1 was strongly related to patients with poor prognostic factors regarding presence of lymph node metastasis (P = 0.024), distant metastasis (P < 0.001), and late International Federation of Gynecology and Obstetrics (FIGO) stage (P < 0.001). In univariate survival analysis, a highly significant correlation between loss of PinX1 and shortened patient survival (mean, 48.2 months vs 99.2 months, P < 0.001) was displayed. Multivariate analysis demonstrated PinX1 expression (P = 0.027) was evaluated as an independent parameter. Our findings suggest that loss of PinX1 is an adverse independent molecular marker for epithelial ovarian carcinoma patients. PinX1 may be a novel target for telomerase‐based anticancer therapy due to inhibiting telomerase activity. (Cancer Sci 2010)
Ovarian cancer is the leading cause of death from a gynecological malignancy worldwide, with increasing incidence recently in Asian countries such as China and Singapore.( 1 ) Due to the lack of reliable methods of early detection and the absence of specific symptoms, the majority of ovarian cancer patients (70%) were diagnosed at late stage, and the prognosis is very poor with a 5‐year survival rate of <20%.( 2 ) Little progress has been made so far to improve long‐term survival.( 3 ) Thus, a wide variety of tumor markers are now used to aid the diagnosis and prognosis of epithelial ovarian carcinoma (EOC), as well as developing targeted therapy, predicting response to treatment, and indicating relapse. In the majority of cases, however, the role of tumor markers in patient management remains to be fully defined and numerous markers suffer from poor specificity and/or sensitivity.( 4 )
Recently, it was shown that PinX1 (interacting protein X1) was a newly cloned gene mapped to chromosome 8p23.1, consisting of seven exons in human, and a region frequently associated with loss of heterozygosity (LOH) in cancer.( 5 , 6 , 7 , 8 , 9 ) Human PinX1 was identified as a critical component in regulating telomerase activity both in vivo and in vitro. ( 10 ) Overexpression of PinX1 in tumor cells could inhibit telomerase activity, shorten telomeres, and suppress tumor growth, while depletion of endogenous PinX1 increased telomerase activity, elongated telomeres, and enhanced tumorigenicity in telomerase‐positive HT1080 cancer cells.( 10 ) Disruption of the PinX1‐dependent telomere maintenance pathway could reduce carcinogenesis, and enhance chemotherapeutic sensitivity in telomerase‐positive human cancer cells as well.( 11 ) This strongly suggests that PinX1 is an intrinsic telomerase inhibitor and a putative tumor suppressor gene in human cells.( 10 ) The correlation analysis between expression for PinX1 mRNA transcript and most human clinical cancers has been extensively studied.( 11 ) To date, despite these facts, the status of PinX1 protein in ovarian cancer tissues has not been elucidated.
The aim of our study was to evaluate whether PinX1 plays a role in the development of ovarian cancer and its prognostic significance. We examined PinX1 expression in epithelial ovarian tumors (including 25 benign cystadenomas, 29 borderline tumors, and 157 invasive carcinomas) and 12 cases of normal ovaries by immunohistochemical methods, and also assessed the relationship between PinX1 protein and clinicopathological and prognostic significance of EOC, to seek a better understanding of EOC biology and development, and to discover molecular markers of potential benefit.
Materials and Methods
Patients and tissue specimens. In this study, paraffin‐embedded tissue samples from 211 patients with epithelial ovarian tumors were obtained from the archives of Department of Pathology, the First Affiliated Hospital, Sun Yat‐Sen University, Guangzhou, China, between 1996 and 2008. The tumor cases included 157 cases with histologically confirmed invasive carcinoma, 29 borderline tumors, and 25 benign cystadenomas. Moreover, 12 normal ovaries from hysterectomy specimens resected for non‐ovarian disease were added. The cases selected were based on availability of resection tissue, follow‐up data, and had not received preoperative radiation or chemotherapy.
Patients whose cause of death remained unknown were excluded from our study. Ages of the 157 patients with ovarian carcinoma ranged from 19 to 84 years (mean, 51.0 years) and clinicopathological characteristics of the tumor sets are described in Table 1. The stage of tumors was assessed according to the International Federation of Gynecology and Obstetrics (FIGO). Tumors were graded according to the Silverberg grading system. All tumor cases were reevaluated for grade and histological type by the same pathologist (H‐L.R.). Histology was determined on the basis of the criteria of the World Health Organization. The Institute Research Medical Ethics Committee of Sun Yat‐Sen University granted approval for this study.
Table 1.
Association of PinX1 expression with patient’s clinicopathological features in ovarian carcinomas
| All cases | PinX1 protein | |||
|---|---|---|---|---|
| Negative expression (%) | Positive expression (%) | P‐value* | ||
| Age at surgery (years) | ||||
| ≤51.0† | 81 | 24 (29.6) | 57 (70.4) | 0.312 |
| >51.0 | 76 | 29 (38.2) | 47 (61.8) | |
| Histological type | ||||
| Serous | 106 | 40 (37.7) | 66 (62.6) | 0.067 |
| Mucinous | 20 | 8 (40.0) | 12 (60.0) | |
| Others‡ | 31 | 5 (16.1) | 26 (83.9) | |
| Histological grade (Silverberg) | ||||
| G1 | 30 | 8 (26.7) | 22 (73.3) | 0.231 |
| G2 | 89 | 28 (31.5) | 61 (68.5) | |
| G3 | 38 | 17 (44.7) | 21 (55.3) | |
| pT status | ||||
| pT1 | 43 | 10 (23.3) | 33 (76.7) | 0.230 |
| pT2 | 30 | 11 (36.7) | 19 (63.3) | |
| pT3 | 84 | 32 (38.1) | 52 (61.9) | |
| pN status | ||||
| pN0 | 79 | 20 (25.3) | 59 (74.7) | 0.024 |
| pN1 | 78 | 33 (42.3) | 45 (57.7) | |
| pM status | ||||
| pMX | 137 | 34 (24.8) | 103 (75.2) | 0.000 |
| pM1 | 20 | 19 (95.0) | 1 (5.0) | |
| FIGO stage | ||||
| I | 29 | 1 (3.4) | 28 (96.6) | 0.000 |
| II | 20 | 1 (5.0) | 19 (95.0) | |
| III | 88 | 32 (36.4) | 56 (63.6) | |
| IV | 20 | 19 (95.0) | 1 (5.0) | |
*χ2‐test; †mean age; ‡endometrioid adenocarcinoma, clear cell carcinoma or undifferentiated ovarian carcinoma. FIGO, International Federation of Gynecology and Obstetrics; PinX1, interacting protein X1.
Tissue microarrays (TMA) construction. Tissue microarray was constructed as the method described previously. (12) In brief, formalin‐fixed, paraffin‐embedded tissue blocks and the corresponding histological H&E‐stained slides were overlaid for tissue TMA sampling. The slides were reviewed by a senior pathologist (H‐L.R.) to determine and mark out representative areas of viable tumor tissue. In view of tumor heterogeneity, triplicate 0.6‐mm‐diameter cylinders of tissue were punched from selected tumor areas of individual donor tissue block and re‐embedded into a recipient paraffin block at defined position, using a tissue arraying instrument (Beecher Instruments, Silver Spring, MD, USA). The TMA block contained 249 epithelial ovarian tumors (including 30 cystadenomas, 40 borderline tumors, and 179 carcinomas). Subsequently, multiple sections (5‐μm thick) were cut from the TMA block and mounted on microscope slides. One section from the tissue array block was stained with H&E to confirm that the punches contained tumor.
Immunohistochemistry (IHC). The immunohistochemical study of PinX1 was performed using a standard two‐step technique as demonstrated previously.( 13 , 14 ) TMA slides were dried overnight at 37°C, dewaxed in xylene, rehydrated through graded alcohol, and immersed in 3% hydrogen peroxide for 20 min to block endogenous peroxidase activity. An antigen retrieval process was accomplished in a microwave oven with 10 mM citrate buffer (pH 6) for 15 min. The slides were incubated with 10% normal goat serum at room temperature for 10 min to reduce nonspecific reaction. Subsequently, the TMA slides were incubated with the rabbit polyclonal antibody against PinX1 (1:200; ProteinTech Group, Chicago, IL) and the rabbit monoclonal antibody against human telomerase reverse transcriptase (hTERT) (Abcam, Cambridge, MA, 1:100), overnight at 4°C. After rinsing five times with 0.01 mol/L phosphate‐buffered saline (PBS; pH = 7.4) for 10 min, the detection of the primary antibody was achieved with a secondary antibody (Envision; Dako, Glostrup, Denmark) for 1 h at room temperature, and stained with DAB (3,3‐diaminobenzidine) after washing in PBS again. Finally, the sections were counterstained with Mayer’s hematoxylin, dehydrated, and mounted. Phosphate‐buffered saline replaced anti‐PinX1 antibody as a negative control.
Immunohistochemistry evaluation. Nuclear immunoreactivity for the PinX1 protein was scored by semi‐quantitative method by evaluating the number of positive tumor cells over the total number of tumor cells. Scores were assigned by using 5% increments (0%, 5%, 10% … 100%). The reproducibility of the scoring manner between pathologists has been described previously for TMAs.( 15 , 16 , 17 , 18 ) PinX1 expression was assessed by three independent pathologists (D.X., H‐L.R., and M‐Y.C.) who were blinded to clinical follow‐up data. Their conclusions were in complete agreement in 85% of the cases, which suggested that this scoring method was highly reproducible. If two or all of them agreed with the results they scored, the value was selected. If the results were completely different, then all of them would work collaboratively to confirm the score.
For the evaluation of hTERT IHC staining, a semi‐quantitative scoring criterion was used,( 19 ) in which both staining intensity and positive areas were recorded. A staining index (values 0–12), obtained as the intensity of hTERT‐positive staining (weak, 1; moderate, 2; strong, 3) and the proportion of immuno‐positive cells of interest (0%, 0; <10%, 1; 10–50%, 2; 51–80%, 3; >80%, 4) were calculated. Finally, the cases were classified into two different groups: low expression cases (score 0–6) and cases with high expression (scores 8–12).
Statistics. Statistical analysis was performed using the SPSS statistical software package (standard version 13.0; SPSS, Chicago, IL, USA). The relationship between PinX1 protein expression and ovarian carcinoma patients’ clinicopathological data was estimated with the χ2‐test. Receiver–operator curve (ROC) analysis was performed to determine the cut‐off scores for the PinX1 positivity. The association with survival and each variable was determined with the log‐rank test. Multiple Cox proportional hazards regression was carried out to identify the protein marker as an independent predictor of survival. The correlation between expression of PinX1 and hTERT was performed with the χ2‐test. Differences was considered significant if the P‐value from a two‐tailed test was <0.05.
Selection of cut‐off scores. Receiver–operator curve (ROC) analysis was also applied to this marker to determine cut‐off scores for tumor “positivity” by using the 0, 1‐criterion.( 15 ) At the PinX1 score, the sensitivity and specificity for each outcome under study was plotted, thus generating an ROC. The score closest to the point with both maximum sensitivity and specificity (i.e. the point [0.0, 1.0] on the curve) was selected as the cut‐off score. Tumors designated as “negative’ for the protein were those with scores below or equal to the threshold value, whereas positive tumors were considered those with scores above the threshold.( 15 , 20 ) In order to use ROC analysis, the clinicopathological features were dichotomized: cancer type (serous adenocarcinoma) or others (mucinous adenocarcinoma, endometrioid adenocarcinoma, clear cell carcinoma, or undifferentiated carcinoma), T stage (early [T1 + T2] or late [T3]), N stage (N0 [no lymph node involvement] or N1 [any lymph node involvement]), M stage (M0 [absence of metastasis] or M1 [presence of metastasis]), tumor grade (low [G1 + G2] or high [G3]), and survival (death due to epithelial ovarian carcinoma or censored [lost to follow‐up, alive, or death from other causes]).
Results
PinX1 expression in ovarian tissues. For PinX1 IHC staining in ovarian tumor tissues and normal ovaries, immunoreactivity was seen primarily in the nuclei within tumor cells, though occasionally yellowish brown granules could also be observed in the cytoplasm (Fig. 1). PinX1 expression could be evaluated informatively in 211 epithelial ovarian tumors (encompassing 25 cystadenomas, 29 borderline tumors, and 157 invasive carcinomas) by the TMA constructed previously and in 12 normal ovaries. The non‐informative 38 TMA samples included unrepresentative samples, samples with too few tumor cells (<300 cells per case), and lost samples. Immunoreactivity ranged from 0% to 100%. According to ROC analysis, expression percentage for PinX1 above the critical value 60% was defined as positivity. The positive expression of PinX1 was detected in 104/157 (66.2%) of invasive ovarian cancers. The decreasing frequency of PinX1 positive expression in normal ovarian tissues (100%), benign cystadenomas (84%), borderline tumours (75.9%), and ovarian carcinomas (66.2%) were statistically significant (P = 0.029, Table 2).
Figure 1.

Immunohistochemistry of PinX1 (interacting protein X1) in epithelial ovarian tumor tissue microarray and normal ovary. Positive expression of PinX1 was observed in epithelia cells of normal ovary (a), cystoadenoma (c), borderline tumor (e), and invasive carcinoma ([g], ×100). (b), (d), (f) and (h) demonstrated the higher magnification (×400) from the area of the box in (a), (c), (e), and (g), respectively, where more than 60% ovarian surface epithelial or tumor cells demonstrated immunostaining of PinX1 mainly in nuclei. Negative expression of PinX1 was detected in ovarian carcinoma ([i], ×100), with less than 60% positive staining tumor cells, higher magnification ([j], ×400) from the area of the square in (i).
Table 2.
The expression of PinX1 in normal ovaries and in a series of epithelial ovarian tumors*
| PinX1 protein | |||
|---|---|---|---|
| All cases | Negative expression (%) | Positive expression (%) | |
| Normal ovaries | 12 | 0 (0) | 12 (100) |
| Cystadenomas | 25 | 4 (16) | 21 (84) |
| Borderline tumors | 29 | 7 (24.1) | 22 (75.9) |
| Invasive carcinomas | 157 | 53 (33.8) | 104 (66.2) |
*Values are n (%). A significant decreasing frequency of positive expression of PinX1 (interacting protein X1) was detected in cystadenomas, borderline tumors, and invasive carcinomas (P = 0.029, χ2‐test).
Selection of PinX1 cut‐off scores. The ROC for each clinicopathological parameter (Fig. 2) clearly show the point on the curve closest to (0.0, 1.0) which maximizes both sensitivity and specificity for the outcome. The analysis of ROC for each clinicopathological feature and PinX1 expression (area under the curve [AUC] = 0.666, P < 0.001) is carried out to evaluate the patients’ survival status (Fig. 3). Tumors with scores above the obtained cut‐off values were considered positive for the expression of PinX1 protein leading to the greatest number of tumors correctly classified as having or not having the clinical outcome. The corresponding AUCs (95% confidence interval [CI]) are listed in Table 3. The cut‐off score was determined to be above 60% for PinX1 positive expression.
Figure 2.

Receiver–operator curves (ROC) were used to determine the cut‐off score for positive expression of PinX1 (interacting protein X1) protein. The sensitivity and specificity for each outcome were plotted: survival status (a), pT stage (b), pN stage (c), pM stage (d), tumor grade (e), and histological type (f).
Figure 3.
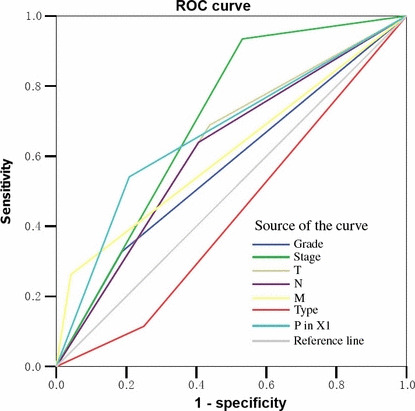
Receiver–operator curve (ROC) analysis for different clinicopathological parameters and PinX1 (interacting protein X1) expression was performed to evaluate the survival status. International Federation of Gynecology and Obstetrics (FIGO) stage (area under the curve [AUC] = 0.702, P < 0.001), PinX1 expression (AUC = 0.666, P < 0.001), T stage (AUC = 0.626, P = 0.008), N stage (AUC = 0.617, P < 0.001), and M stage (AUC = 0.610, P < 0.001) implied significant statistical associations with the survival.
Table 3.
Area under the receiver–operator curve for each clinico‐pathological feature
| Feature | AUC (95% CI) | P‐value |
|---|---|---|
| Survival | 0.717 (0.635–0.798) | 0.000 |
| T stage | 0.652 (0.561–0.742) | 0.001 |
| N stage | 0.632 (0.543–0.721) | 0.004 |
| M stage | 0.856 (0.762–0.950) | 0.000 |
| Histological grade | 0.635 (0.533–0.737) | 0.020 |
| Histological type | 0.629 (0.524–0.733) | 0.027 |
AUC, area under the curve; CI, confidence interval.
Association of PinX1 protein expression with clinico‐pathological parameters. The expression rates of PinX1 in ovarian carcinomas with respect to several standard clinicopathological features are presented in Table 1. The PinX1 expression rate was higher in patients with negative lymph node (P = 0.024) and no metastasis (P < 0.001), and in patients with earlier FIGO stage (P < 0.001). There was no significant difference in PinX1 expression rate and other clinicopathological features, such as patient age (≤51.0 years vs >51.0 years), pT status, histological grade, and tumor type (P > 0.05, Table 1).
Relationship between clinicopathologic variables, PinX1 expression, and ovarian carcinoma patient survival: Univariate survival analysis. In univariate Cox regression, Kaplan–Meier survival curves and the P‐values for these curves were determined by log‐rank method. Above all, to confirm the representativeness of the ovarian carcinomas in our study, we analyzed established prognostic factors of patient survival. Kaplan–Meier analysis demonstrated a significant impact of well‐known clinicopathological prognostic parameters, such as pT status (P = 0.004), pN status (P < 0.001), pM status (P < 0.001), and FIGO stage (P < 0.001) on patient survival (Table 4). Assessment of survival in all specimens demonstrated that high expression rate of PinX1 was associated with better disease‐specific survival (P < 0.001, Fig. 4a), and the mean survival time for patients with tumors having PinX1 expression was 99.2 months compared to 48.2 months for patients with tumors no having PinX1 expression (Table 4).
Table 4.
Clinical pathological parameters and expression of PinX1 for prognosis of 157 patients with ovarian carcinoma by univariate survival analysis (log‐rank test)
| Variable | All cases | Mean survival (months) | Median survival (months) | P‐value |
|---|---|---|---|---|
| Age at surgery (years) | ||||
| ≤51.0* | 81 | 74.1 | NR | 0.423 |
| >51.0 | 76 | 78.8 | 62.0 | |
| Histological type | ||||
| Serous | 106 | 67.9 | 62.0 | 0.168 |
| Mucinous | 20 | 66.8 | 45.0 | |
| Others | 31 | 109.1 | NR | |
| Histological grade (Silverberg) | ||||
| G1 | 30 | 104.7 | NR | 0.066 |
| G2 | 89 | 75.5 | 64.0 | |
| G3 | 38 | 51.7 | 34.0 | |
| pT status | ||||
| pT1 | 43 | 107.8 | NR | 0.004 |
| pT2 | 30 | 89.7 | NR | |
| pT3 | 84 | 60.8 | 36.0 | |
| pN status | ||||
| pN0 | 79 | 98.6 | NR | <0.001 |
| pN1 | 78 | 53.0 | 15.8 | |
| pM status | ||||
| pMX | 137 | 91.4 | NR | <0.001 |
| pM1 | 20 | 21.5 | 9.0 | |
| FIGO stage | ||||
| I | 33 | 134.2 | NR | <0.001 |
| II | 21 | 115.0 | NR | |
| III | 99 | 71.5 | 37.0 | |
| IV | 26 | 21.5 | 9.0 | |
| PinX1 expression | ||||
| Negative | 53 | 48.2 | 24 | <0.001 |
| Positive | 104 | 99.2 | NR | |
*Mean age. FIGO, International Federation of Gynecology and Obstetrics; NR, not reached; PinX1, interacting protein X1.
Figure 4.
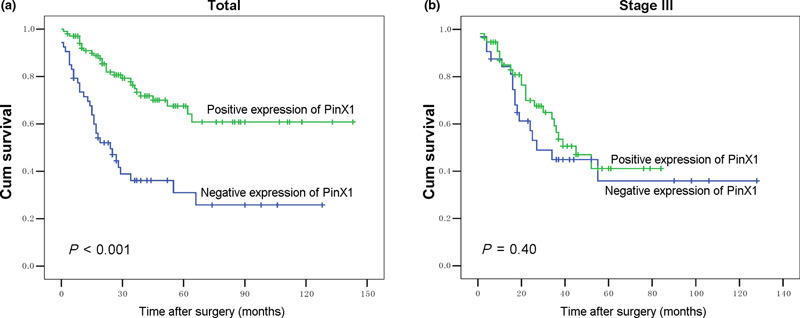
Kaplan–Meier survival analysis of PinX1 (interacting protein X1) expression in total patients and in the subset of stage III patients with invasive ovarian carcinoma (log‐rank test). Total, probability of survival of all patients with ovarian carcinoma: positive expression, n = 104; negative expression, n = 53 ([a], P < 0.001). Stage III, probability of survival of stage III patients with ovarian carcinoma: positive expression, n = 56; negative expression, n = 32 ([b], P = 0.40).
Independent prognostic factors of epithelial ovarian carci‐noma: Multivariate Cox regression analysis. A multivariate progression analysis based on the Cox proportional hazard model was applied to test the independent value of each parameter predicting overall survival (Table 5). Expressions of PinX1 as well as other clinicopathological features that were significant by univariate analysis (pT stage, pN stage, and pM stage) were included in multivariate analysis (Table 5). The expression of PinX1 was found to be an independent prognostic factor for favorable overall survival (relative risk, 0.503; 95% CI, 0.273–0.924, P = 0.027). Of the other parameters, pT stage (P = 0.004), pN stage (P < 0.001), and pM stage (P < 0.001) were demonstrated as well an independent prognostic factor for overall survival.
Table 5.
Multivariate analysis on overall survival (Cox regression model)
| Variable | β | Relative risk | 95% Confidence interval | P‐value |
|---|---|---|---|---|
| PinX1* | −0.688 | 0.503 | 0.273–0.924 | 0.027 |
| pT stage† | 0.603 | 1.828 | 1.211–2.759 | 0.004 |
| pN stage‡ | 1.020 | 2.772 | 1.566–4.906 | 0.000 |
| pM stage§ | 1.472 | 4.359 | 2.078–9.145 | 0.000 |
*Negative expression vs positive expression; †pT1 versus pT2 versus pT3; ‡pN0 versus pN1; §pMX versus pM1. PinX1, interacting protein X1.
Correlation between the expression of PinX1 and hTERT in ovarian carcinomas. In our IHC study, among the total 249 ovarian tumor TMA, in 201 samples (including 22 cystadenomas, 26 borderline tumors, and 153 invasive carcinomas), PinX1 and hTERT IHC was detected successfully and simultaneously. By utilizing the criterion of a semi‐quantitative scale as previously described,( 19 ) a high expression of hTERT was observed in 0/22 (0%) of cystadenomas, in 10/26 (38.5%) of borderline tumors, and 72/153 (47.1%) of ovarian carcinomas. Further correlation analysis demonstrated a significant inverse correlation between expression of PinX1 and hTERT in our ovarian carcinoma cohorts (P = 0.006, Fisher’s exact test, Table 6 ). The frequency of cases with high expression of hTERT was significantly larger in ovarian carcinomas with negative expression of PinX1 (32/51 cases, 62.7%) than in those cases with positive expression of PinX1 (40/102, 39.2%).
Table 6.
Correlation between expression of PinX1 and hTERT in 153 cases of ovarian carcinoma*
| PinX1 protein | hTERT protein | ||
|---|---|---|---|
| All cases | Low expression (%) | High expression (%) | |
| Negative expression | 51 | 19 (37.3) | 32 (62.7) |
| Positive expression | 102 | 62 (60.8) | 40 (39.2) |
*Spearman correlation analysis indicated that PinX1 (interacting protein X1) and hTERT (human telomerase reverse transcriptase) expression levels were inversely correlated (P = 0.006, Fisher’s exact test).
Discussion
Maintenance and protection of telomere homeostasis by the telomere‐associated proteins is pivotal in dominating the balance between cellular senescence and cancer progress. However, it is still not clear how these proteins interact with telomerase to regulate telomere lengths. Recently, PinX1 has been identified as a critical component in regulating telomerase activity through its communication with one significant shelterin, TRF1 (telomeric repeat binding factor 1), and proposed to be a putative tumor suppressor.( 10 ) In human, ectopic overexpression of PinX1 leads to decrease of both telomerase activity and tumorigenicity of cancer cells, whereas suppression of PinX1 expression results in an increase in both telomerase activity and cancer cell tumorigenicity.( 10 ) Although the relationship between the PinX1 gene and human tumors has been studied widely, such as in medulloblastoma, hepatocelllular carcinoma, prostate cancer, and gastric cancer,( 14 , 21 , 22 , 23 , 24 ) the expression of PinX1 protein has not been investigated in ovarian cancer tissue. In addition, the prognostic value of PinX1 protein has not yet been established in ovarian carcinoma.
In the present study, immunohistochemistry for PinX1 was performed on a large cohort of epithelial ovarian tumor samples (211 cases) with complete clinicopathological and follow‐up data. PinX1 immunoreactivity was assessed using a scoring system based on the percentage of positive tumor cells.( 15 ) This assessment method is reproducible, and resulted in a more complete evaluation of the prognostic or predictive value of several markers in colorectal cancer.( 18 , 25 ) The reliability of this scoring system for PinX1 was assessed by three pathologists and was again found to be highly reproducible. In order to avoid the use of predetermined and often arbitrarily set cut‐off values, and the selection of IHC cut‐off scores for PinX1 positivity, ROC analysis was carried out for each of the clinicopathological parameters, including tumor type, histological grade, pT stage, pN stage, pM stage, and survival.( 15 ) Receiver–operator curve analysis for different clinicopathological features and PinX1 expression was also used to evaluate the survival status. PinX1 expression (AUC = 0.666, P < 0.001) demonstrated significant statistical associations with the survival status. This cut‐off score is consistent with the literature demonstrating PinX1 as a putative tumor suppressor gene.( 10 )
The immunostaining results showed that PinX1 expression was in 100% of normal ovarian tissues, 84% of benign cystadenomas, 75.9% of borderline tumors, and 66.2% of ovarian carcinoma studied by IHC. The PinX1 expression rate was significantly higher in patients with favorable prognostic factors with regard to absence of lymph node metastasis (P = 0.024), distant metastasis (P < 0.001), and earlier FIGO stage (P < 0.001). In univariate study, negative PinX1 expression in EOC was associated with the shortened survival time (mean, 48.2 months vs 99.2 months; P < 0.001). Furthermore, we also found that decreased expression of PinX1 in EOC was an independent predictor of shorter overall survival by Kaplan–Meier curves and multivariable Cox proportional hazards regression analysis. These results suggest that loss of PinX1 protein in ovarian carcinoma may facilitate cancer cell invasion and/or metastasis. In contrast, patients retaining expression of PinX1 had a significantly favorable prognosis than those with loss of expression of the protein. These findings raise the question of a potentially important role of PinX1 as an underlying biological mechanism in the development and/or growth of human cancers.
Our results are in line with the findings in malignant tumors that identified loss of PinX1 as a key feature in tumor development,( 14 , 21 , 22 , 23 , 24 ) and LOH was statistically correlated with reduced expression of PinX1 in the cancer cases.( 26 ) It also showed that the IHC expression of PinX1 was significantly associated with the differentiation and lymphatic metastasis in carcinoma of the large intestine.( 25 ) Moreover, PinX1 expression in the gastric carcinoma specimens revealed a correlation with the prognosis of the cancer. An increased expression of PinX1 could contribute towards better prognosis in gastric cancer.( 14 ) So far, there have been no reports of a prognostic significance of PinX1 expression in ovarian tumors. To our knowledge, this study demonstrates for the first time the highly significant prognostic value of PinX1 expression in EOC. Further studies are needed to verify our results to establish PinX1 as a molecular prognostic marker in ovarian cancer. This might aid the clinician to select a suitable therapy for the individual patient, for example favoring a more aggressive regimen in tumors with negative expression of PinX1. Moreover, disruption of the PinX1‐dependent telomere maintenance pathway could compromise tumorigenicity as well as increase chemotherapeutic sensitivity in telomerase‐positive human cancer cells.( 11 ) Telomerase represents a promising target for patients currently undergoing telomerase‐based anticancer therapy. It has been recommended as a plausible anticancer target due to its critical role in cancer cells. Most telomerase‐based therapies rely on the inhibition of telomerase activity and require extensive telomere shortening.( 27 , 28 , 29 ) In the present study, a significant inverse correlation of PinX1 expression and telomerase activity was evaluated in our ovarian carcinoma cohorts, that is a low expression of hTERT was more likely to be observed in ovarian carcinomas with positive expression of PinX1. This result provides evidence of a telomerase‐inhibited function of PinX1 in ovarian carcinomas, suggesting that PinX1 may be a new target for telomerase‐based anticancer therapy as it interrupts telomere maintenance.
In summary, we find that PinX1 is commonly expressed in ovarian cancer and that PinX1 expression is an independent molecular marker of prognosis in this cancer. Decreased PinX1 expression is a marker of adverse outcomes in EOC, and appears to affect survival time independently of known prognostic indicators. PinX1 may become a new avenue for telomerase‐based anticancer therapy as an inhibitor of telomerase activity.
Acknowledgments
This study was supported by grants from the Major State Basic Research Program of China (2006CB910104), the Nature Science Foundation of China (no. 30772334 and 30901769), and the 863 Project of China (2007AA021901).
References
- 1. Lynch HT, Casey MJ, Lynch J, White TE, Godwin AK. Genetics and ovarian carcinoma. Semin Oncol 1998; 25: 265–80. [PubMed] [Google Scholar]
- 2. Kosary CL. FIGO stage, histology, histologic grade, age and race as prognostic factors in determining survival for cancers of the female gynecological system: an analysis of 1973–87 SEER cases of cancers of the endometrium, cervix, ovary, vulva, and vagina. Semin Surg Oncol 1994; 10: 31–46. [DOI] [PubMed] [Google Scholar]
- 3. Engel J, Eckel R, Schubert‐Fritschle G et al. Moderate progress for ovarian cancer in the last 20 years: prolongation of survival, but no improvement in the cure rate. Eur J Cancer 2002; 38: 2435–45. [DOI] [PubMed] [Google Scholar]
- 4. Meyer T, Rustin GJ. Role of tumour markers in monitoring epithelial ovarian cancer. Br J Cancer 2000; 82: 1535–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baffa R, Santoro R, Bullrich F, Mandes B, Ishii H, Croce CM. Definition and refinement of chromosome 8p regions of loss of heterozygosity in gastric cancer. Clin Cancer Res 2000; 6: 1372–7. [PubMed] [Google Scholar]
- 6. Bova GS, Carter BS, Bussemakers MJ et al. Homozygous deletion and frequent allelic loss of chromosome 8p22 loci in human prostate cancer. Cancer Res 1993; 53: 3869–73. [PubMed] [Google Scholar]
- 7. Bova GS, MacGrogan D, Levy A, Pin SS, Bookstein R, Isaacs WB. Physical mapping of chromosome 8p22 markers and their homozygous deletion in a metastatic prostate cancer. Genomics 1996; 35: 46–54. [DOI] [PubMed] [Google Scholar]
- 8. Kishimoto Y, Shiota G, Wada K et al. Frequent loss in chromosome 8p loci in liver cirrhosis accompanying hepatocellular carcinoma. J Cancer Res Clin Oncol 1996; 122: 585–9. [DOI] [PubMed] [Google Scholar]
- 9. MacGrogan D, Levy A, Bova GS, Isaacs WB, Bookstein R. Structure and methylation‐associated silencing of a gene within a homozygously deleted region of human chromosome band 8p22. Genomics 1996; 35: 55–65. [DOI] [PubMed] [Google Scholar]
- 10. Zhou XZ, Lu KP. The Pin2/TRF1‐interacting protein PinX1 is a potent telomerase inhibitor. Cell 2001; 107: 347–59. [DOI] [PubMed] [Google Scholar]
- 11. Zhang B, Bai YX, Ma HH et al. Silencing PinX1 compromises telomere length maintenance as well as tumorigenicity in telomerase‐positive human cancer cells. Cancer Res 2009; 69: 75–83. [DOI] [PubMed] [Google Scholar]
- 12. Xie D, Sham JS, Zeng WF et al. Heterogeneous expression and association of beta‐catenin, p16 and c‐myc in multistage colorectal tumorigenesis and progression detected by tissue microarray. Int J Cancer 2003; 107: 896–902. [DOI] [PubMed] [Google Scholar]
- 13. Xie D, Zeng YX, Wang HJ et al. Expression of cytoplasmic and nuclear Survivin in primary and secondary human glioblastoma. Br J Cancer 2006; 94: 108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ma Y, Wu L, Liu C, Xu L, Li D, Li JC. The correlation of genetic instability of PINX1 gene to clinico‐pathological features of gastric cancer in the Chinese population. J Cancer Res Clin Oncol 2009; 135: 431–7. [DOI] [PubMed] [Google Scholar]
- 15. Zlobec I, Steele R, Terracciano L, Jass JR, Lugli A. Selecting immunohistochemical cut‐off scores for novel biomarkers of progression and survival in colorectal cancer. J Clin Pathol 2007; 60: 1112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zlobec I, Minoo P, Baker K et al. Loss of APAF‐1 expression is associated with tumour progression and adverse prognosis in colorectal cancer. Eur J Cancer 2007; 43: 1101–7. [DOI] [PubMed] [Google Scholar]
- 17. Zlobec I, Vuong T, Hayashi S et al. A simple and reproducible scoring system for EGFR in colorectal cancer: application to prognosis and prediction of response to preoperative brachytherapy. Br J Cancer 2007; 96: 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zlobec I, Steele R, Michel RP, Compton CC, Lugli A, Jass JR. Scoring of p53, VEGF, Bcl‐2 and APAF‐1 immunohistochemistry and interobserver reliability in colorectal cancer. Mod Pathol 2006; 19: 1236–42. [DOI] [PubMed] [Google Scholar]
- 19. Brustmann H. Immunohistochemical detection of human telomerase reverse transcriptase (hTERT) and c‐kit in serous ovarian carcinoma: a clinicopathologic study. Gynecol Oncol 2005; 98: 396–402. [DOI] [PubMed] [Google Scholar]
- 20. Hanley JA. Receiver operating characteristic (ROC) methodology: the state of the art. Crit Rev Diagn Imaging 1989; 29: 307–35. [PubMed] [Google Scholar]
- 21. Chang Q, Pang JC, Li J, Hu L, Kong X, Ng HK. Molecular analysis of PinX1 in medulloblastomas. Int J Cancer 2004; 109: 309–14. [DOI] [PubMed] [Google Scholar]
- 22. Liao C, Zhao MJ, Zhao J et al. Mutation analysis of novel human liver‐related putative tumor suppressor gene in hepatocellular carcinoma. World J Gastroenterol 2003; 9: 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park WS, Lee JH, Park JY et al. Genetic analysis of the liver putative tumor suppressor (LPTS) gene in hepatocellular carcinomas. Cancer Lett 2002; 178: 199–207. [DOI] [PubMed] [Google Scholar]
- 24. Hawkins GA, Chang BL, Zheng SL et al. Mutational analysis of PINX1 in hereditary prostate cancer. Prostate 2004; 60: 298–302. [DOI] [PubMed] [Google Scholar]
- 25. Lugli A, Zlobec I, Gunthert U et al. Overexpression of the receptor for hyaluronic acid mediated motility is an independent adverse prognostic factor in colorectal cancer. Mod Pathol 2006; 19: 1302–9. [DOI] [PubMed] [Google Scholar]
- 26. Kondo T, Oue N, Mitani Y et al. Loss of heterozygosity and histone hypoacetylation of the PINX1 gene are associated with reduced expression in gastric carcinoma. Oncogene 2005; 24: 157–64. [DOI] [PubMed] [Google Scholar]
- 27. Gellert GC, Dikmen ZG, Wright WE, Gryaznov S, Shay JW. Effects of a novel telomerase inhibitor, GRN163L, in human breast cancer. Breast Cancer Res Treat 2006; 96: 73–81. [DOI] [PubMed] [Google Scholar]
- 28. Shay JW, Wright WE. Mechanism‐based combination telomerase inhibition therapy. Cancer Cell 2005; 7: 1–2. [DOI] [PubMed] [Google Scholar]
- 29. Saretzki G. Telomerase inhibition as cancer therapy. Cancer Lett 2003; 194: 209–19. [DOI] [PubMed] [Google Scholar]


