Abstract
In an earlier report, we demonstrated overexpression of a short isoform of Helios, Hel‐5, which lacks three of four N‐terminal zinc fingers, in patients with adult T‐cell leukemia/lymphoma. Here, we characterized Hel‐5 using immunoprecipitation, and gel shift and luciferase promoter assays, and found that Hel‐5 lacks the repressor function observed with a full‐length isoform of Helios. Moreover, Hel‐5 associates with the full‐length isoforms of the Ikaros gene family, Ikaros, Aiolos and Helios, and inhibits their DNA binding activity when present in excess, leading to dominant‐negative effects on the full‐length isoforms of the Ikaros gene family. Our results suggest a critical role for Helios in the mechanism of leukemogenesis. (Cancer Sci 2007; 98: 182–188)
Gene targeting studies in mice have shown that the transcription factor Ikaros plays a critical role in lymphoid development and proliferation.( 1 , 2 , 3 ) We examined the expression of Ikaros and found overexpression of a dominant‐negative isoform, Ik‐6, in patients with blast crisis of chronic myelogenous leukemia( 4 ) and B‐cell acute lymphoblastic leukemia.( 5 ) By alternative splicing,( 6 ) Ikaros encodes DNA binding proteins that dimerize both with each other( 7 ) and with the other members of the Ikaros gene family, Aiolos( 8 , 9 ) and Helios.( 10 , 11 ) Ikaros family proteins dimerize via the C‐terminal zinc fingers, whereas the N‐terminal zinc fingers mediate DNA binding. Isoforms that have less than two N‐terminal zinc fingers are reported to have a dominant‐negative effect on isoforms with more than two N‐terminal zinc fingers.( 7 ) Recently, we found overexpression of short isoforms of Helios, which lacks three of the four N‐terminal zinc fingers, in patients with T‐cell acute lymphoblastic leukemia( 12 ) and adult T‐cell leukemia/lymphoma (ATLL).( 13 ) None of the Human T‐lymphotropic Virus (HTLV‐I) carriers analyzed demonstrated overexpression of short isoforms of Helios, suggesting an important role for Helios in progression of the disease from an HTLV‐I carrier to ATLL. In the present report, we characterize Hel‐5, which is presumed to be a dominant‐negative isoform of Helios, to clarify the underlying mechanism of leukemogenesis.
Materials and Methods
Immunoprecipitation. Full‐length isoforms (HA‐Ik‐1, FLAG‐Aiolos or HA‐Hel‐1) and short isoforms (Ik‐6 or Hel‐5) were cotransfected into 293T cells using Lipofectamine Reagent (Invitrogen, Carlsbad, CA, USA). Immunoprecipitation was carried out as described previously,( 7 ) using ImmunoPure Immobilized Protein A (Pierce Biotechnology, Rockford, IL, USA) and anti‐hemagglutinin (HA) antibody (Roche, Basel, Switzerland), or anti‐FLAG M2 affinity gel (Sigma‐Aldrich, St Louis, MO, USA). Resolved proteins were transferred to a nitrocellulose filter and probed with anti‐Ikaros or anti‐Helios antibodies. Anti‐rabbit IgG, peroxidase‐linked whole antibody (Amersham Biosciences, Piscataway, NJ) and ECL Western Blotting Detection Reagents (Amersham Biosciences) were used to detect hybridizing proteins.
Electrophoretic mobility shift assay. Gel shift assays were carried out as described previously.( 14 ) Single‐stranded complementary sense and antisense oligonucleotides from the Ikaros binding site (Ik‐BS4: tcagcttttgggaatgtattccctgtca)( 6 ) were synthesized and used to generate double‐stranded oligonucleotide probes. In selected experiments, 100‐fold molar excess unlabeled double‐stranded oligonucleotide or the antibody were added to binding reactions.
Luciferase assay. For promoter assays, pGL3 luciferase reporter vectors (Promega, Madison, WI, USA) were modified. Four copies of the Ikaros binding site (IkBS2: tcagcttttgggaatctcctgtca)( 6 ) were introduced upstream of the TATA box of the pGL3‐Enhancer Vector (4xIkBS2‐TATA‐Luc). The TATA box was then substituted for SV40 promoter (4xIkBS2‐SV40‐Luc) or TK promoter (4xIkBS2‐TK‐Luc). Into 293T cells, 1 µg of reporter vector and 0.1 µg of control vector, pRL‐TK, were cotransfected, and promoter activity was analyzed using the Dual‐Luciferase Reporter Assay System (Promega). Promoter activity was calculated as the firefly luciferase activity of the reporter vector divided by the renilla luciferase activity of the control vector. Dominant‐negative effect of the short isoform was shown as percentage inhibition against repressor function of the full‐length isoform.
Results
Homo‐ and hetero‐dimerization of the Ikaros gene family. The carboxy‐terminal zinc fingers of the Ikaros gene family members, Ikaros, Aiolos and Helios, which mediate their homodimerization and heterodimerization, are highly conserved.( 7 , 8 , 10 , 11 ) In 293T cell lysates cotransfected with HA‐Ik‐1 and Ik‐6 (Fig. 1a), both Ikaros isoforms were found in complexes immunoprecipitated with anti‐HA antibody (Fig. 2a, lane 3). Moreover, either Ik‐1 or Ik‐6 coprecipitated with HA‐Hel‐1 (Fig. 2a, lanes 1 and 2) and FLAG‐Aiolos (Fig. 2a, lanes 4 and 5). Similarly, in 293T cells cotransfected with HA‐Hel‐1 and Hel‐5 (Fig. 1b), both Helios isoforms were found in complexes immunoprecipitated with anti‐HA antibody (Fig. 2b, lane 3), and either Hel‐1 or Hel‐5 coprecipitated with HA‐Ik‐1 (Fig. 2b, lanes 1 and 2) and FLAG‐Aiolos (Fig. 2b, lanes 4 and 5). These results suggest that the short isoforms of the Ikaros gene family, Ik‐6 and Hel‐5, dimerize with the full‐length isoforms, Ik‐1, Aiolos or Hel‐1.
Figure 1.
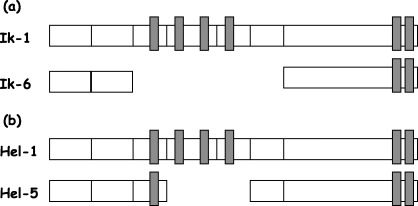
Diagrammatic representation of (a) Ikaros and (b) Helios isoforms. The four N‐terminal zinc fingers and the two C‐terminal zinc fingers are shown as solid perpendicular boxes.
Figure 2.
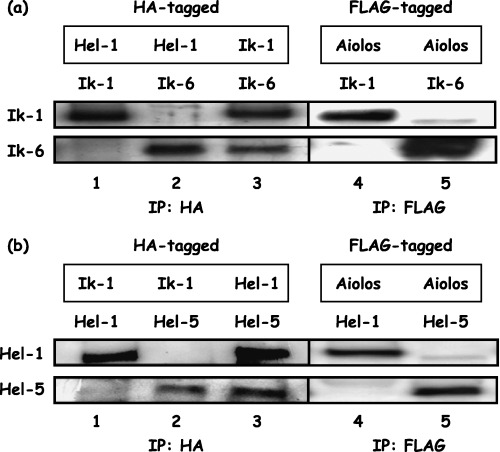
Homo‐ and heterodimerization of the Ikaros gene family. Constructs containing genes encoding tagged full‐length isoforms of Ikaros, Aiolos or Helios (Ik‐1, Aiolos or Hel‐1, respectively), which are shown in the box, and the short isoforms of Ikaros or Helios (Ik‐6 or Hel‐5) were expressed in 293T cells. Heterodimerization of (a) Hel‐1/Ik‐1 or Ik‐6 (lanes 1 and 2) and (b) Ik‐1/Hel‐1 or Hel‐5 (lanes 1 and 2) were demonstrated by precipitation with anti‐hemagglutinin (HA) antibody. Homodimerization of Ikaros or Helios proteins were also demonstrated by precipitation with anti‐HA antibody: (a) lane 3, (b) lane 3. Heterodimerization of (a) Ikaros/Aiolos (lanes 4 and 5) and (b) Aiolos/Helios (lanes 4 and 5) were demonstrated by precipitation with anti‐FLAG antibody. Anti‐Ikaros or ‐Helios antibody was used to detect (a) Ik‐1 and Ik‐6 or (b) Hel‐1 and Hel‐5 in precipitated complexes.
DNA binding properties of the Ikaros gene family. Next, we investigated the DNA‐binding affinity of the Ikaros gene family. Whole‐cell lysates from 293T cells transfected with Ik‐1, Aiolos or Hel‐1 demonstrated major shifted bands (Fig. 3, lanes 1, 6 and 11, respectively), which were inhibited with 100‐fold molar excess unlabeled double‐stranded oligonucleotide and confirmed to be supershifted by anti‐Ikaros, ‐Aiolos or ‐Helios antibodies (data not shown). Interestingly, the mobilities of members of the Ikaros gene family were different from each other, although they bind the same probe.( 6 ) As expected from a previous report,( 7 ) the major shifted bands were not observed with whole‐cell lysates from 293T cells transfected with Ik‐6 or Hel‐5 (data not shown). Mixing a consistent amount of Ik‐1 with increasing amounts of Ik‐6 showed a significant reduction of the DNA‐binding activity of Ik‐1 (Fig. 3, lanes 2 and 3). Similarly, mixing a consistent amount of Hel‐1 with increasing amounts of Hel‐5 showed a marked reduction of the DNA‐binding activity of Hel‐1 (Fig. 3, lanes 14 and 15). Moreover, blocking the DNA‐binding activity of full‐length isoforms of other Ikaros gene family members, Aiolos and Helios with Ik‐6 (Fig. 3, lanes 7–8 and 12–13, respectively) and Ikaros and Aiolos with Hel‐5 (Fig. 3, lanes 4–5 and 9–10, respectively), was also observed.
Figure 3.
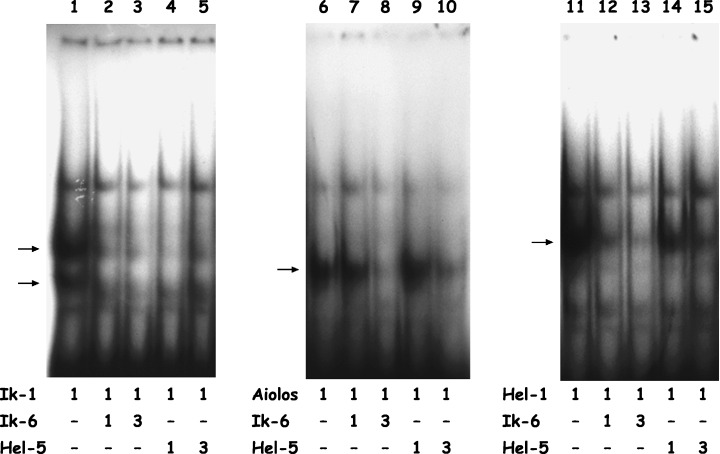
DNA binding properties of the Ikaros gene family. Ikaros gene family proteins bind the same DNA sequence (Ik‐BS4: tcagcttttgggaatgtattccctgtca) with high affinity. The shifted bands are indicated by arrows. Consistent amounts of full‐length isoforms of Ikaros, Aiolos or Helios (Ik‐1, Aiolos or Hel‐1, respectively) and increasing amounts of the short isoforms of Ikaros or Helios (Ik‐6 or Hel‐5, respectively) were expressed in 293T cells, and whole‐cell lysates were extracted. The molar ratios of full‐length and short isoforms are shown.
Loss of repressor function of short isoforms of the Ikaros gene family. As reported previously,( 15 ) Ikaros gene family members, Ikaros, Aiolos and Helios, could not activate a minimal promoter that consists of only a TATA box that is under the control of four copies of the Ikaros binding site (4xIkBS2‐TATA‐Luc, data not shown). However, in contrast to a previous report that demonstrated activation by the Ikaros gene family,( 7 , 8 , 15 , 16 ) full‐length isoforms, Ik‐1, Aiolos and Hel‐5, repressed the reporter in which TK or SV40 promoters were substituted for the TATA box (4xIkBS2‐TK‐Luc or 4xIkBS2‐SV40‐Luc, respectively). These repressor functions of the Ikaros gene family appeared dose dependent (Fig. 4a–c). Ik‐1 repressed TK promoter activity from 14.4 down to 5.6, 4.0, 3.0 and 2.2 with increasing amounts (Fig. 4a; n = 5). Aiolos repressed TK promoter activity from 16.1 down to 12.3, 9.4, 7.4 and 5.5 with increasing amounts (Fig. 4b; n = 5), and Hel‐1 repressed TK promoter activity from 10.4 down to 6.0, 4.7, 3.9 and 3.2 with increasing amounts (Fig. 4c; n = 5). Similar results were obtained with the SV40 promoter (Fig. 4a–c; n = 5). However, Ik‐6 and Hel‐5, short isoforms of the Ikaros gene family, could not demonstrate the repressor function observed with the full‐length isoforms (Fig. 5a,b). Ik‐1 repressed TK promoter activity from 15.6 down to 3.1; however, Ik‐6 could not show repressor activity at 13.1, and the difference was statistically significant (Fig. 5a; n = 5, P = 0.01). Hel‐1 repressed TK promoter activity from 11.9 down to 4.1, in contrast Hel‐5 could not demonstrate repressor activity at 9.7; again the difference was statistically significant (Fig. 5b; n = 5, P = 0.0001). Similar results were obtained with the SV40 promoter (Fig. 5a,b; n = 5, P < 0.001).
Figure 4.
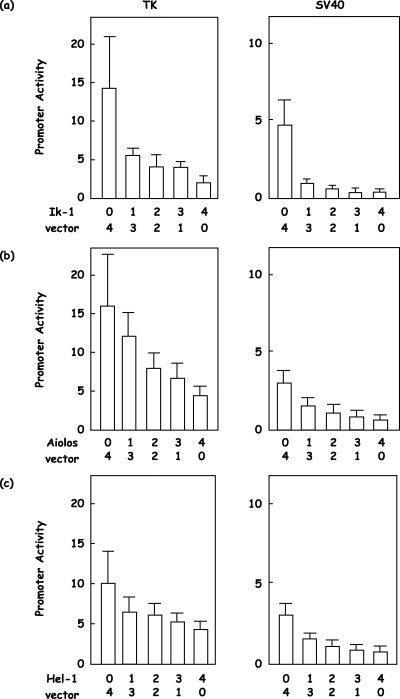
Repressor functions of the Ikaros gene family. Full‐length isoforms of (a) Ikaros, (b) Aiolos or (c) Helios (Ik‐1, Aiolos or Hel‐1, respectively) were cotransfected with a reporter vector, 4xIkBS2‐TK‐Luc (TK) or 4xIkBS2‐SV40‐Luc (SV40), and an internal control vector. The empty vector was used to supplement the total amounts of transfected expression vector, and the molar ratios are shown. Promoter activity was calculated as the firefly luciferase activity of the reporter vector divided by the renilla luciferase activity of the control vector.
Figure 5.
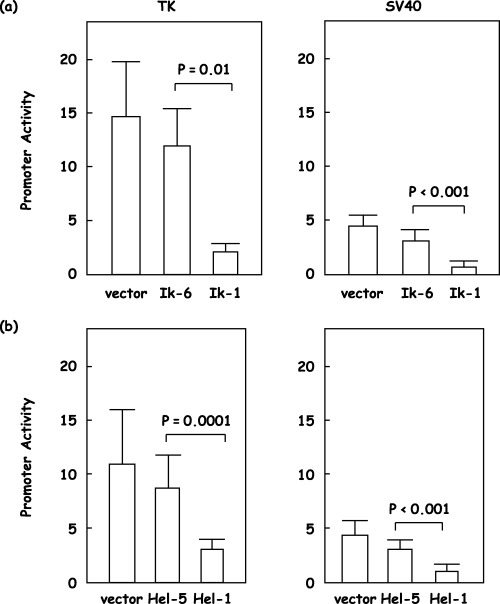
Loss of repressor function of short isoforms of the Ikaros gene family. The same amounts of the empty vector, the short isoform of the Ikaros gene family, (a) Ik‐6 or (b) Hel‐5, or the full‐length isoform of the Ikaros gene family, (a) Ik‐1 or (b) Hel‐1, were cotransfected with a reporter vector, 4xIkBS2‐TK‐Luc (TK) or 4xIkBS2‐SV40‐Luc (SV40), and an internal control vector. Promoter activity was calculated as the firefly luciferase activity of the reporter vector divided by the renilla luciferase activity of the control vector. Statistical analysis was carried out using Student's t‐test (n = 5).
Functional interactions between full‐length isoforms and short isoforms of the Ikaros gene family. Finally, we examined promoter activity mixing consistent amounts of full‐length isoforms with increasing amounts of short isoforms, as Ik‐6 was reported to be a dominant‐negative isoform.( 7 , 8 ) We found that there was an interfering activity of Ik‐6 on the repressor function of Ik‐1 (Fig. 6a). TK promoter activity at 12.5 was repressed down to 5.3 by Ik‐1, and the addition of Ik‐6 in increasing amounts canceled the repressor function of Ik‐1, which went up to 8.4, 9.3 and 9.1 (56% inhibition; Fig. 6a, n = 5). SV40 promoter activity at 4.4 was repressed down to 0.7 by Ik‐1, and the addition of Ik‐6 in increasing amounts blocked the repressor function of Ik‐1, which went up to 1.5, 2.1 and 2.4 (46% inhibition; Fig. 6a, n = 5). In contrast to a previous report,( 8 ) similar results could not be obtained with other Ikaros gene family members, Aiolos and Hel‐1 (Fig. 6b–c). However, Hel‐5 demonstrated its presumed dominant‐negative effect on the repressor function of the full‐length isoforms of the Ikaros gene family, although the effect appeared to be variable (Fig. 7a–c). SV40 promoter activity at 5.1 was repressed down to 0.7 by Ik‐1, and the addition of increasing amounts of Hel‐5 could cancel the repressor function of Ik‐1, which went up to 1.4, 1.8 and 1.9 (27% inhibititon; Fig. 7a; n = 5). SV40 promoter activity at 4.2 was repressed down to 1.5 by Hel‐1, and the addition of increasing amounts of Hel‐5 could effectively block the repressor function of Hel‐1, which went up to 1.8, 2.2 and 2.5 (37% inhibition; Fig. 7c; n = 5).
Figure 6.
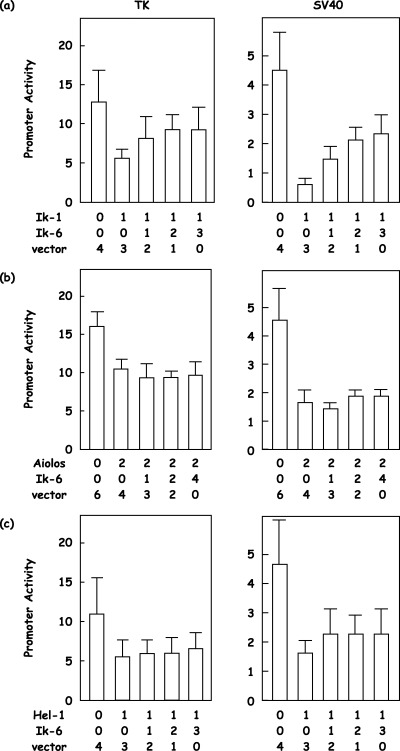
Functional interactions between full‐length isoforms of the Ikaros gene family and a short isoform, Ik‐6. Full‐length isoforms of (a) Ikaros, (b) Aiolos or (c) Helios (Ik‐1, Aiolos or Hel‐1, respectively) and a short isoform of Ikaros (Ik‐6) were cotransfected with a reporter vector, 4xIkBS2‐TK‐Luc (TK) or 4xIkBS2‐SV40‐Luc (SV40), and an internal control vector. The empty vector was used to supplement the total amounts of transfected expression vector, and the molar ratios are shown. Promoter activity was calculated as the firefly luciferase activity of the reporter vector divided by the renilla luciferase activity of the control vector.
Figure 7.
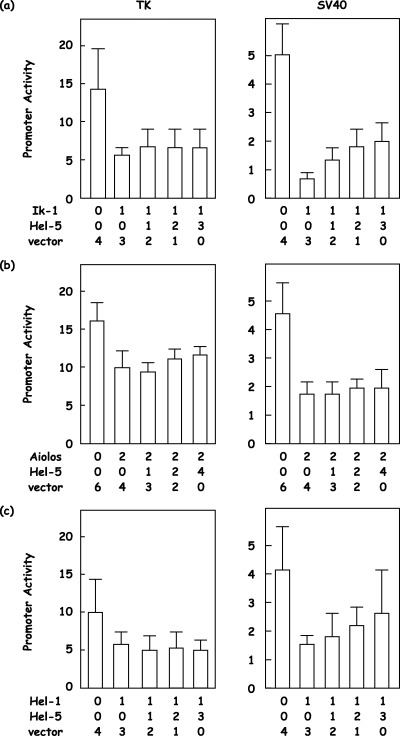
Functional interactions between full‐length isoforms of the Ikaros gene family and a short isoform, Hel‐5. Full‐length isoforms of (a) Ikaros, (b) Aiolos or (c) Helios (Ik‐1, Aiolos or Hel‐1, respectively) and a short isoform of Helios (Hel‐5) were cotransfected with a reporter vector, 4xIkBS2‐TK‐Luc (TK) or 4xIkBS2‐SV40‐Luc (SV40), and an internal control vector. The empty vector was used to supplement the total amounts of transfected expression vector, and the molar ratios are shown. Promoter activity was calculated as the firefly luciferase activity of the reporter vector divided by the renilla luciferase activity of the control vector.
Discussion
This report presents the first analysis of the function of a short isoform of Helios, Hel‐5, which was found to be overexpressed with high frequency in ATLL patients.( 13 ) Our results suggest that the full‐length isoform of Helios acts as a repressor and that the short isoform of Helios lacks the repressor function. Moreover, the short isoform of Helios associates with full‐length isoforms of the Ikaros gene family and inhibits their DNA binding activity, leading to a dominant‐negative effect for Hel‐5 on full‐length isoforms of the Ikaros gene family.
Whereas Ikaros family members are important transcription factors in the lymphoid system, previously published experiments were all carried out in 293T cells because of their easy transfectability. However, our results contradict previous reports,( 7 , 8 ) and the dominant‐negative effect of short isoforms appear to be variable depending on the conditions examined. In detail, we could not observe promoter activation with the full‐length isoform of Ikaros gene family members.( 7 , 8 ) Sun et al. reported that Ik‐1 stimulates expression of the reporter under the control of four copies of an Ikaros recognition site (4xIkBS1: tcagcttttgggaataccctgtca‐tk‐CAT( 6 )) in NIH 3T3 cells.( 7 ) Using exactly the same system, Morgan et al. reported that Aiolos is a more potent transcriptional activator than Ikaros.( 8 ) We used a quite similar system, and the differences were minimal: DNA‐binding sequences of Ikaros (IkBS1 vs IkBS2), expression vector (CDM8 vs pGL3), reporter gene (CAT vs luciferase) and cell line (NIH 3T3 vs 293T). Moreover, the dominant‐negative effect of Ik‐6 was not as dramatic as reported previously.( 7 , 8 ) Sun et al. reported that coexpression of Ik‐1 with excess amounts of Ik‐6 strongly interfered with its ability to activate transcription.( 7 ) Morgan et al. reported similar results with Aiolos, demonstrating the dominant‐negative effect of Ik‐6 on other members of the Ikaros gene family.( 8 ) At present, the reasons for these discrepancies are not clear, and further attempts to establish a lymphoid system for the Ikaros gene family are necessary.
Nevertheless, Hel‐5 obviously lost the repressor function that would normally suppress the targeted genes, suggesting Helios as a candidate tumor suppressor. Further analyses of the short isoforms of the Ikaros gene family overexpressed in patients with hematological malignancies are warranted.
Acknowledgments
We appreciate the generous gifts from Dr Smale (HA‐Ik‐1, HA‐Hel‐1, anti‐Ikaros antibody and anti‐Helios antibody) and Dr Georgopoulos (FLAG‐Aiolos and anti‐Aiolos antibody).
References
- 1. Georgopoulos K, Bigby M, Wang J‐H et al. The Ikaros gene is required for the development of all lymphoid lineages. Cell 1994; 79: 143 – 56. [DOI] [PubMed] [Google Scholar]
- 2. Winandy S, Wu P, Georgopoulos K. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell 1995; 83: 289 – 99. [DOI] [PubMed] [Google Scholar]
- 3. Wang J‐H, Nichogiannopoulou A, Wu L et al. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity 1996; 5: 537 – 49. [DOI] [PubMed] [Google Scholar]
- 4. Nakayama H, Ishimaru F, Avitahl N et al. Decreases in Ikaros activity correlate with blast crisis in patients with chronic myelogenous leukemia. Cancer Res 1999; 59: 3931 – 4. [PubMed] [Google Scholar]
- 5. Nakase K, Ishimaru F, Avitahl N et al. Dominant‐negative isoform of Ikaros gene in patients with adult B‐cell acute lymphoblastic leukemia. Cancer Res 2000; 60: 4062 – 5. [PubMed] [Google Scholar]
- 6. Molnar A, Georgopoulos K. The Ikaros gene encodes a family of functionally diverse zinc finger DNA binding proteins. Mol Cell Biol 1994; 14: 8292 – 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun L, Liu A, Georgopoulos K. Zinc finger‐mediated protein interactions modulate Ikaros activity, a molecular control of lymphocyte development. EMBO J 1996; 15: 5358 – 69. [PMC free article] [PubMed] [Google Scholar]
- 8. Morgan B, Sun L, Avitahl N et al. Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation. EMBO J 1997; 16: 2004 – 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang J‐H, Avitahl N, Cariappa A et al. Aiolos regulates B cell activation and maturation to effector state. Immunity 1998; 9: 543 – 53. [DOI] [PubMed] [Google Scholar]
- 10. Hahm K, Cobb BS, McCarty AS et al. Helios, a T cell‐restricted Ikaros family member that quantitatively associates with Ikaros at centromeric heterochromatin. Genes Dev 1998; 12: 782 – 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kelley CM, Ikeda T, Koipally J et al. Helios, a novel dimerization partner of Ikaros expressed in the earliest hematopoietic progenitors. Curr Biol 1998; 8: 508 – 15. [DOI] [PubMed] [Google Scholar]
- 12. Nakase K, Ishimaru F, Fujii K et al. Overexpression of novel short isoforms of Helios in a patient with T‐cell acute lymphoblastic leukemia. Exp Hematol 2002; 30: 313 – 17. [DOI] [PubMed] [Google Scholar]
- 13. Fujii K, Ishimaru F, Nakase K et al. Over‐expression of short isoforms of Helios in patients with adult T‐cell leukaemia/lymphoma. Br J Haematol 2003; 120: 986 – 9. [DOI] [PubMed] [Google Scholar]
- 14. Ishimaru F, Mari B, Shipp MA. The type 2 CD10/neutral endopeptidase 24.11 promoter: functional characterization and tissue‐specific regulation by CBF/NF‐Y isoforms. Blood 1997; 89: 4136 – 45. [PubMed] [Google Scholar]
- 15. Koipally J, Heller EJ, Seavitt JR, Georgopoulos K. Unconventional potentiation of gene expression by Ikaros. J Biol Chem 2002; 277: 13007 – 15. [DOI] [PubMed] [Google Scholar]
- 16. Koipally J, Georgopoulos K. Ikaros interactions with CtBP reveal a repression mechanism that is independent of histone deacetylase activity. J Biol Chem 2000; 275: 19594 – 602. [DOI] [PubMed] [Google Scholar]


