Abstract
The sentinel lymph node (SLN) is defined as the lymph node(s) first receiving lymphatic drainage from the site of the primary tumor. The histopathological status of SLN is one of the most significant predictors of recurrence and overall survival for most clinical stage I/II solid tumors. Recent progress in molecular techniques has demonstrated the presence of micrometastatic tumor cells in SLN. There is now a growing body of data to support the clinical relevance of SLN micrometastasis in a variety of solid tumors. Increasing the sensitivity of occult tumor cell detection in the SLN, using molecular‐based analysis, should enable a more accurate understanding of the clinical significance of various patterns of micrometastatic nodal disease. The establishment of metastasis to SLN might not be simply reflected by the flow dynamics of lymphatic fluid that drains from the primary site to the SLN, and the transportation of viable cancer cells. Recent studies have demonstrated that primary tumors can actively induce lymphangiogenesis and promote SLN metastasis. Moreover chemokine receptors in tumor cells may facilitate organ‐specific tumor metastasis in many human cancers and some experimental models. In contrast, recent clinical and preclinical studies regard SLN as the first lymphoid organ to respond to tumor antigenic stimulation. SLN dramatically show morphological, phenotypical and functional changes that indicate immune suppression by tumor cells. The immune suppression in SLN results in failure of prevention or eradication of tumor metastasis. The mechanism of immunomodulation remains unclear; however, several regulatory molecules produced by tumor cells and tumor‐associated macrophages or lymphocytes are likely to be responsible for inducing the immune suppression in SLN. Further studies may develop a novel immunotherapy that overcomes tumor‐induced immune suppression and can prevent or eradicate SLN metastasis. (Cancer Sci 2008; 99: 441–450)
In the history of the surgical treatment of malignant tumors, the standard procedure has been to perform complete dissection of regional lymph nodes in addition to the primary tumor. This has been believed to improve patients’ survival.( 1 ) However, the clinical significance of prophylactic lymph node dissection for patients without lymph node metastasis has been the subject of controversy over the past 10 years.( 2 , 3 , 4 )
Given this background, the concept of the sentinel lymph node (SLN), intraoperative lymphatic mapping and sentinel lymphadenectomy appeared attractive.( 5 ) The SLN is defined as the lymph node(s) first receiving lymphatic drainage from the site of a tumor ( Fig. 1).( 5 ) The pathological status of the SLN is thought to predict the status of all regional lymph nodes. If the SLN is recognizable and negative for cancer metastasis, unnecessary radical lymph node dissection could be avoided. The SLN hypothesis was advanced to specifically address those patients at high risk of having lymph node (LN) metastasis based on the characteristics of their primary tumors, but who had no evidence of clinically detectable regional metastatic disease.
Figure 1.
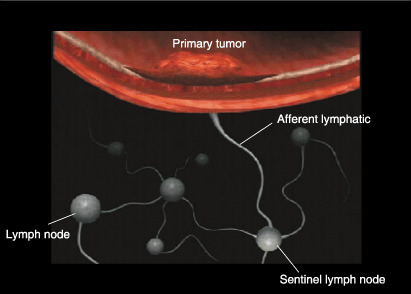
Primary tumor and sentinel lymph node (SLN). The SLN is defined as one or more lymph nodes that first receive lymphatic drainage from the site of the primary tumor. For intraoperative lymphatic mapping and sentinel lymphadenectomy, blue dye and/or radioisotope‐labeled colloid are injected intradermally (or submucosally) around the primary tumor site before surgery. Subsequently, the tracers pass through the afferent lymphatics, and blue‐stained or radioactive nodes are regarded as the SLN.
The histopathological status of tumor‐draining regional LN is one of the most significant predictors of recurrence and overall survival for most clinical stage I/II solid tumors, and is often used to justify stratification of patients for adjuvant therapy.( 6 , 7 ) More efficient and accurate diagnosis of LN metastasis and prognostic information can be obtained from a small number of LN, by intraoperative lymphatic mapping and sentinel lymphadenectomy.( 5 , 8 )
SLN mapping and biopsy was first applied to melanoma, and was subsequently extended to breast cancer and, more recently, to many other solid tumors including colorectal, gastric, esophageal, gynecologic, head and neck, thyroid, urologic, and non‐small cell lung cancers.( 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 ) The SLN concept has revolutionized the approach to the surgical staging of both melanoma and breast cancer, and these techniques can yield patient benefit by avoiding various complications due to unnecessary prophylactic complete LN dissection in cases with negative SLN for cancer metastasis.
Hematoxylin and eosin (H&E) and immunohistochemical (IHC) staining have been commonly used, in combination with thin serial sectioning of frozen and paraffin‐embedded specimens, for the detection of micrometastatic disease in the SLN/LN.( 17 , 18 , 19 ) The application of IHC has markedly improved the sensitivity of micrometastatic disease detection in the SLN/LN beyond the capability of routine H&E staining alone.( 17 , 18 , 19 ) The antibodies against tumor markers of interest must be highly specific and sensitive for detection of tumor cells, and virtually non‐reactive to the adjacent non‐tumor cells in the SLN/LN. The most commonly used IHC target for epithelial carcinomas are the cytokeratins (CK), which are ubiquitously expressed as intermediate filaments in normal eukaryotic epithelial cells.( 18 , 19 , 20 ) However, the risk of false positive results with the use of individual anti‐CK antibodies and antibody cocktails has been described.( 21 )
In comparison, the use of IHC to detect micrometastatic deposits of melanoma has been less problematic due to the high specificity of antibodies to HMB‐45 and S‐100 proteins.( 22 ) Because these antibodies also have their limitations, new antibodies such as melanoma antigens recognized by T‐cells (MART‐1) and microphthalmia‐associated transcription factor (MITF) are being investigated.( 23 ) IHC staining for detection of occult metastatic tumor cells in LN has been the gold standard. More recently, molecular techniques have provided new approaches and demonstrated undetected metastatic tumor cells.( 24 )
To date, SLN have been thought to be a preferential site of initial micrometastasis of solid tumors. In contrast, recent clinical and preclinical studies regard SLN as the first lymphoid organ to respond to tumor antigenic stimulation. However, immunologic roles of SLN in the development of tumor metastasis have not been elucidated yet. Tumor‐induced immune modulation of SLN may facilitate LN metastasis by inhibiting immune cell activities. This review also focuses on the immunoresponse in the SLN against tumor metastasis.
Molecular Diagnosis for Micrometastasis of SLN
The molecular detection of tumor cells using RNA or DNA markers with various polymerase chain reaction (PCR) techniques has evolved exponentially in the last decade. The primary approach of molecular detection of tumor cells has been focused on the mRNA of tumor markers using reverse transcription (RT)‐PCR assay. Detection of metastatic tumor cells has been clearly demonstrated in LN, organs, and body fluids. Using RT‐PCR, it is now possible to reliably detect 1–10 tumor cells within a background of 106–107 normal cells.( 20 ) The high sensitivity of the RT‐PCR assay, compared with H&E and IHC, allows the detection of the occult tumor cells among the lymphoid cells in SLN/LN. However, molecular‐based techniques require the stringent optimization of sample processing, reagents, molecular targets, RT and PCR reactions, and PCR cDNA product detection assays. Meticulous attention to techniques must be adhered to, throughout all stages of the assay, in order to ensure accurate results. One of the keys to the most efficient RT‐PCR assay is the quality of the detection marker. Finding a good marker is of the utmost importance in molecular detection.
Quantitative RT‐PCR assay is now being used more extensively to not only identify the presence of target mRNA but also to quantify the number of mRNA copies from tumor‐associated genes. Quantitative RT‐PCR analysis permits the rapid molecular analysis of multiple mRNA targets expressed in tumor cells, and these results can then be correlated to clinical outcomes in order to study the relationship between gene expression levels and outcome.( 25 ) Real‐time PCR assay, which enables rapid analysis, is currently being attempted for intraoperative molecular diagnosis. LN along the recurrent laryngeal nerves obtained from patients with esophageal cancer were assessed prospectively using intraoperative histopathologic examination and real‐time RT‐PCR assay using multiple markers (carcinoembryonic antigen [CEA], squamous cell carcinoma [SCC] antigen, and MAGE‐A3).( 26 ) The whole procedure takes only 2.5 h from the time of tissue sampling to completion of the real‐time RT‐PCR assay. Genetic diagnosis by intraoperative real‐time PCR assay can predict cervical LN metastasis and may be used to indicate subsequent cervical lymphadenectomy. Further improvements of the assay may allow the PCR‐based intraoperative diagnosis to be applicable to other cancer surgeries. Taniyama et al. reported that the newly developed one‐step nucleic acid amplification (OSNA) assay may allow rapid assessment for intraoperative diagnosis of LN metastasis.( 27 ) At the present time, however, the techniques still need further validation.
The current definition of SLN/LN micrometastasis is a deposit of tumor cells measuring ≤2 mm. However, this definition has become somewhat arbitrary due to the high degree of sensitivity of IHC and RT‐PCR. With the advent of increasingly more sensitive detection assays for occult metastasis, the actual definition of micrometastasis may need to be reconsidered. It has been demonstrated that the metastatic potential of individual tumor cells varies and that not all embolic tumor cells are capable of progressing to functional metastatic tumors.( 28 ) There is also evidence to suggest that the number of tumor cells in the LN/SLN, as well as the location of nodal micrometastasis (i.e. single or a few occult tumor cells vs clumps of cells, and cells located within the subcapsular sinus vs the nodal parenchyma), may be pathologically relevant factors.( 23 , 29 ) Historically, the clinicopathological relevance of micrometastatic SLN/LN disease has been unclear and controversial. There is, however, growing evidence that LN/SLN micrometastasis may indeed mean a worse prognosis in many solid cancers, including breast, melanoma, colorectal, esophageal, gastric, lung, head and neck, gynecologic, and urologic cancers.
Breast cancer. A number of mRNA targets have been studied in breast cancer, including CEA, mammaglobin 1 and 2, MAGE‐A, MUC‐1, C‐MET, β1→4‐N‐acetylgalactosaminyltransferase (β1→4‐GalNAc‐T), β‐hCG, prostate specific antigen (PSA), c‐myc, prolactin inducible protein (PIP), and various CK family markers.( 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 ) However, the specificity of several of these markers for breast cancer, including CEA, MUC‐1, and CK‐19 appears to be poor, because they sometimes show positive results for RT‐PCR performed on LN and blood in healthy volunteers without breast cancer.( 38 , 39 , 40 ) MAGE‐A3 may be a promising breast cancer molecular marker, although as it appears to be expressed by approximately 50% of breast cancers, but is not expressed in normal mammary epithelium, or in the LN or blood of healthy volunteer donors.( 31 )
Although not as extensive as the studies that were performed on melanoma, there is compelling evidence to suggest a clinically relevant impact of SLN/LN micrometastasis detected using molecular assays in breast cancer (Table 1). Bostick et al. reported significant correlation between the presence of positive RT‐PCR markers β1→4‐GalNAc‐T, C‐MET, and P97) in the SLN and primary tumor estrogen receptor status and Bloom–Richardson histopathological grade, both of which are known prognostic factors.( 30 )
Table 1.
Representative SLN/LN RT‐PCR studies for breast cancer
| Author | No. of patients | Lymph node | RT‐PCR marker(s) | H&E/IHC positive (%) | RT‐PCR positive (%) | Clinical correlation of micrometastasis detected using RT‐PCR |
|---|---|---|---|---|---|---|
| Lockett (1998)( 37 ) | 35 | RLN | CK‐19, c‐myc, PIP | 0 | 40 | Primary tumor size, decreased 5‐year survival |
| Bostick (1998)( 30 ) | 41 | SLN | β1→4‐GalNAc‐T, C‐MET, p97 | 30 † | 95 † | Estrogen receptor status, histopathological grade |
| Masuda (2001)( 32 ) | 129 | RLN | CEA | 0 | 31 | Decreased 10‐year survival |
| Wascher (2001)( 41 ) | 77 | SLN | MAGE‐A3 | 45 | 53 | Infiltrating lobular carcinoma |
| Manzotti (2001)( 33 ) | 123 | SLN | Maspin, CK‐19, CEA, MUC‐1, mammaglobin | 33 | 53 † | Progesterone receptor status, peritumoral vascular invasion |
| Sakaguchi (2003)( 34 ) | 108 | SLN | CK‐19, epithelial glycoprotein 2 | 26 | 30 | No correlation with disease‐free survival |
| Ouellette (2004)( 35 ) | 42 | SLN | Mammaglobin B1, mammaglobin B2 | 40 | 52 | ND |
| Gillanders (2004)( 42 ) | 489 | RLN | CEA, mammaglobin, mammaglobin B, PIP, CK‐19, MUC‐1, PDEF | 30 | 49 | Histologic grade, St. Gallen risk category |
| Nissan (2006)( 36 ) | 28 | SLN | CK‐19, NY‐BR‐1, mammaglobin B | 27 † | 50 † | ND |
Percentage of total number of SLN found to be positive. β1→4‐GalNAc‐T, β1→4‐N‐acetylgalactosaminyltransferase; CEA, carcinoembryonic antigen; CK‐19, cytokeratin‐19; H&E, hematoxylin and eosin; IHC, immunohistochemistry; LN, lymph node; ND, not determined; PDEF, prostate‐derived Ets transcription factor; PIP, prolactin inducible protein; RLN, regional lymph node; RT‐PCR, reverse transcription‐polymerase chain reaction; SLN, sentinel lymph node.
Wascher et al. assessed MAGE‐A3 mRNA as a molecular marker for the detection of occult tumor cells in the SLN of breast cancer patients.( 41 ) Serial frozen sections of SLN (n = 121) obtained from 77 American Joint Committee on Cancer (AJCC) stage I–IIIA breast cancer patients were assessed using RT‐PCR and Southern blot analysis. Forty‐one of 77 (53%) patients were positive for MAGE‐A3. Interestingly, MAGE‐A3 mRNA expression in the SLN occurred more frequently with infiltrating lobular carcinoma than with infiltrating ductal carcinoma.
Others have studied non‐SLN axillary LN in breast cancer patients and have reported similar findings. Lockett et al. assessed 61 consecutive breast cancer patients with H&E/IHC and a multiple marker RT‐PCR assay (CK‐19, c‐myc, and PIP).( 37 ) A total of 15 of 37 (40%) patients with H&E/IHC‐negative LN were positive by RT‐PCR. An increasing number of positive RT‐PCR markers significantly correlated with both increased primary tumor size and decreased predicted 5‐year survival.
Masuda et al. evaluated 149 breast cancer patients with negative LN using both H&E and IHC evaluation.( 32 ) RT‐PCR was performed using CEA as a marker, and 40 of 129 (31%) patients were found to have RT‐PCR positive LN. Patients with RT‐PCR negative LN had a 10‐year disease‐free survival rate of 88% versus 66% for RT‐PCR positive patients (P = 0.0008) and an overall 10‐year survival rate of 94% versus 68%, respectively (P = 0.0024). On multivariate analysis, patients with RT‐PCR‐positive LN micrometastasis were found to have a hazard ratio of 3.99 for relapse and 4.29 for death due to cancer. In view of the definition of the SLN, these compelling findings in the study of axillary LN would be expected to be highly applicable with regard to the molecular status of the SLN as well.
In 2004, Gillanders et al. reported on the clinical relevance of molecular detection of micrometastasis in axillary LN in the results of a prospective multi‐institutional cohort study for 489 patients with breast cancer.( 42 ) Seven markers were used for real‐time RT‐PCR assay: CEA, mammaglobin, mammaglobin B, PIP, CK‐19, muc1, and prostate‐derived Ets transcription factor (PDEF). Patients who were histopathologically negative but PCR positive were significantly associated with traditional indicators of prognosis, including histologic grade and St Gallen risk category. They concluded that molecular markers could serve as valid surrogates for the detection of occult micrometastasis in axillary LN.
In general, the prognosis for breast cancer patients with early intervention is relatively more favorable than for other carcinomas. Therefore the prognostic significance of molecular detection of micrometastasis in SLN for breast cancer patients remains unclear. One major problem in evaluating the prognostic value of micrometastasis detection in SLN is that patients who had undergone sentinel lymphadenectomy are often treated with postoperative adjuvant therapy. At least 8 years are required for the follow up of a large number of patients to evaluate a significant number of events. Detection of occult tumor cells in the SLN has not shown clinical significance for patients with breast cancer to date.
Melanoma. The molecular detection of melanoma, using RT‐PCR, is facilitated by the expression of melanogenesis‐specific genes by melanoma tumors cells, including tyrosinase, MART‐1, gp‐100/pmel‐17, MITF, and tyrosinase‐related proteins 1 and 2 (TRP‐1, TRP‐2).( 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 ) The expression of various mRNA transcripts of the human melanoma‐associated antigen (MAGE‐A) family have also been demonstrated in a variety of tumors, including melanoma and cancers of the breast and gastrointestinal tract.( 31 , 46 )
Several studies have reported the use of RT‐PCR to detect micrometastatic melanoma in the SLN, and have shown that RT‐PCR can significantly upstage patients with SLN that are negative by H&E and IHC (Table 2). In addition to the accurate detection of micrometastatic melanoma cells in the SLN using molecular assays, there is persuasive evidence that the detection of such micrometastases has prognostic significance. Regarding the SLN in particular, Shivers et al. followed 114 patients with melanoma for a mean duration of 28 months.( 44 ) Patients with SLN that were histopathologically and RT‐PCR negative had a recurrence rate of 2%, while patients with histopathologically negative but RT‐PCR positive SLN had a 13% recurrence rate (P = 0.02).
Table 2.
Representative SLN/LN RT‐PCR studies for melanoma
| Author | No. of patients | Lymph node | RT‐PCR marker(s) | H&E/IHC positive (%) | RT‐PCR positive (%) | Clinical correlation of micrometastasis detected by RT‐PCR |
|---|---|---|---|---|---|---|
| Goydos (1998)( 43 ) | 45 | SLN | Tyrosinase, MART‐1 | 22 | 29 | ND |
| Shivers (1998)( 44 ) | 114 | SLN | Tyrosinase | 20 | 61 | Decreased disease‐free and overall survival |
| Blaheta (1999)( 45 ) | 73 | SLN | Tyrosinase | 18 | 49 | Primary tumor thickness |
| Bostick (1999)( 46 ) | 72 | SLN | Tyrosinase, MART‐1, MAGE‐A3 | 24 | 50† | Increased risk of recurrence |
| Blaheta (2000)( 47 ) | 101 | SLN | Tyrosinase | 0 | 36 | Increased risk of recurrence |
| Ribuffo (2003)( 48 ) | 134 | SLN | Tyrosinase, MART‐1 | 11 | 63 | Decreased disease‐free survival |
| Goydos (2003)( 49 ) | 175 | SLN | Tyrosinase | 19 | 58 | Increased risk of recurrence |
| Kuo (2003)( 50 ) | 77 | SLN | Tyrosinase, MART‐1, TRP‐1, TRP‐2 | 48 | 55† | Decreased disease‐free and overall survival |
| Morton (2003)( 51 ) | 215 | SLN | Tyrosinase, MART‐1, TRP‐2, MITF | 25 | 47 | Decreased disease‐free and overall survival |
†Percentage of two or more markers positive. H&E, hematoxylin and eosin; IHC, immunohistochemistry; LN, lymph node; MART, melanoma antigens recognized by T‐cells; MITF, microphthalmia‐associated transcription factor; ND, not determined; RT‐PCR, reverse transcription‐polymerase chain reaction; SLN, sentinel lymph nodes; TRP, tyrosinase‐related proteins.
Bostick et al. reported on their study of the SLN of 72 patients with early stage melanoma, using a multiple marker RT‐PCR assay (tyrosinase, MART‐1, and MAGE‐3).( 46 ) Bisection and serial sectioning of SLN, prior to H&E/IHC and molecular analysis, was performed to reduce the false negative rate associated with random or limited sampling of the SLN. Twenty of 55 patients (36%) with SLN negative by H&E and IHC stains were positive for at least two of the three markers in the panel (44% of these 55 patients expressed MAGE‐3, 36% expressed MART‐1, and 29% expressed tyrosinase). By multivariate analysis, the presence of two or more RT‐PCR markers in the SLN correlated with a significantly increased risk of recurrence.
Blaheta et al. evaluated 214 SLN from 116 patients with melanoma using IHC and single marker (tyrosinase) RT‐PCR.( 47 ) Using H&E and IHC alone, 15 of 116 (13%) patients were confirmed to have SLN micrometastasis. Of the remaining 101 patients with histopathologically negative SLN, 36 (36%) SLN were positive by RT‐PCR for tyrosinase. Of the 15 patients with histopathologically detected SLN micrometastases, 10 (67%) had recurrence, compared with an overall recurrence rate of 20% among all 116 patients. During a 19‐month median follow‐up period, the recurrence rate for patients with RT‐PCR‐positive SLN was 25%, and the recurrence rate in patients with negative SLN using H&E, IHC and RT‐PCR was only 6% (P = 0.01). By multivariate analysis, histopathological and RT‐PCR SLN tumor status were the only significant predictors of disease‐free survival.
Goydos et al. studied 175 patients with stage I or II melanoma using single marker (tyrosinase) RT‐PCR.( 49 ) At a median follow up of 34 months, 17 of 34 (50%) patients with histologically positive SLN had a recurrence. Of the 141 patients with histologically negative SLN, 73 patients were negative for tyrosinase using RT‐PCR, and none of these patients had a recurrence. Of the 68 patients with histologically negative but RT‐PCR‐positive SLN, 14 patients (21%) had a recurrence.
Morton et al. assessed the paraffin‐embedded SLN of 215 patients with AJCC stage I/II melanoma using a multiple marker quantitative real‐time PCR assay.( 51 ) Tyrosinase, MART‐1, TRP‐2 and MITF were used as specific mRNA markers. Among 162 patients with histopathologically negative SLN, 49 (30%) patients showed PCR‐positive and were upstaged. These patients had a significantly increased risk of disease recurrence and death compared with both histopathology and PCR‐marker‐negative patients using multivariate analysis. These studies demonstrated the clinicopathological utility of detecting micrometastatic melanoma in SLN.
The 5‐year survival rate approaches 90% for patients with AJCC stage I malignant melanoma, and 70% for stage II melanoma, but decreases significantly to 25–50% for stage III melanoma. Therefore, accurate staging is highly important for optimal management of early stage disease. The clinicopathological relevance of micrometastatic melanoma in SLN detected using RT‐PCR assay is still controversial, because melanoma mRNA markers for RT‐PCR assay are often expressed in melanocytes or nevus cells. A recently reported large‐scale multicenter study failed to prove the clinical relevance of molecular upstaging using RT‐PCR in patients with melanoma.( 52 ) A series of previous studies that reported prognostic significance of micrometastatic melanoma in SLN detected using RT‐PCR, however, may show the clinical significance of molecular detection of micrometastasis in SLN.( 53 ) Future investigations will validate the clinicopathological importance of micrometastatic melanoma in SLN.
Colorectal cancer. The application of molecular analysis to the SLN in colorectal cancer (CRC) is currently at an early stage (Table 3). However, with the recent and successful application of the SLN concept to this disease, preliminary data from molecular‐based assays is now being generated. Molecular markers for SLN/LN analysis in CRC studied so far include CK, CEA, MAGE‐A, C‐MET, β‐hCG, MUC‐2, and matrix metalloproteinases.( 11 , 31 , 54 , 55 , 56 , 57 , 58 )
Table 3.
Representative SLN/LN RT‐PCR studies for gastrointestinal cancers
| Author | Tumor | No. of patients | Lymph node | RT‐PCR marker(s) | H&E/IHC positive (%) | RT‐PCR positive (%) | Clinical correlation of micrometastasis detected by RT‐PCR |
|---|---|---|---|---|---|---|---|
| Noguchi (1996)( 63 ) | Gastric | 12 | RLN | CK‐19 | 7 | 21 | ND |
| Ichikawa (1998)( 55 ) | Colon | 15 | RLN | MMP‐7 | 19 | 26 | ND |
| Kijima (2000)( 64 ) | Esophagus | 21 | RLN | CEA | 52 | 86 | ND |
| Bernini (2000)( 57 ) | Colorectal | 43 | RLN | MUC‐2 | 0 | 28 | Advanced T‐factor |
| Bilchik (2001)( 11 ) | Colorectal | 40 | SLN | β‐hCG, C‐MET, MAGE‐A | 35 | 60 | Advanced T‐factor |
| Yoshioka (2002)( 26 ) | Esophagus | 50 | RLN | CEA, SCC, MAGE‐A3 | 20 (intraoperative diagnosis) | 48 (intraoperative diagnosis) | Predict cervical LN metastasis |
| Noura (2002)( 58 ) | Colorectal | 64 | RLN | CEA | 55 | 30 | Decreased disease‐free and overall survival |
| Matsuda (2004)( 66 ) | GI cancers | 51 | SLN | CK‐19 | 25 | 45 | ND |
| Arigami (2006)( 65 ) | Gastric | 53 | SLN | CEA | 0 | 25 | ND |
CEA, carcinoembryonic antigen; CK‐19, cytokeratin‐19; H&E, hematoxylin and eosin; IHC, immunohistochemistry; LN, lymph node; MMP, matrix metalloproteinase; ND, not determined; RLN, regional lymph node; RT‐PCR, reverse transcription‐polymerase chain reaction; SCC, squamous cell carcinoma antigen; SLN, sentinel lymph node.
CRC is being studied to evaluate the prognostic impact of micrometastatic SLN/LN disease. Liefers et al. evaluated 192 LN from 26 stage II CRC patients using nested RT‐PCR and CEA as a molecular marker.( 56 ) In this study, 14 of 26 (54%) patients had LN that were RT‐PCR positive. The 5‐year survival rate for these 14 patients was 50%, while the survival rate among the remaining 12 patients was 91% (P = 0.03).
Bernini et al. recently studied the LN of 43 CRC patients, using MUC‐2 as a molecular target for RT‐PCR.( 57 ) They found a correlation between RT‐PCR LN positivity and the size of the primary tumor. None of the 10 Tis/T1 tumors and one of six (17%) T2 tumors were shown to be LN positive using RT‐PCR, while 10 of 25 (40%) T3 tumors and one of two (50%) T4 tumors were positive using RT‐PCR. These results are of clinical significance, because primary tumor T‐stage is a known prognostic factor for CRC.
Interim results from the first multicenter phase II trial evaluating the molecular staging of the SLN in early colon cancer (clinical stage I/II) were recently reported by Bilchik et al.( 11 ) Forty patients with histopathologically negative (by H&E) SLN were assessed using IHC for CK, and using a multiple‐marker RT‐PCR panel (β‐hCG, C‐MET, and MAGE‐A3). In 10 (25%) cases, the SLN was positive by H&E. In four (10%) cases, the SLN were positive by IHC and negative by H&E. Of the remaining 26 patients with negative SLN by H&E and IHC, 12 (46%) were positive for at least two RT‐PCR markers. This study also demonstrated a correlation between the number of markers detected and the tumor T‐stage, which is, by itself, a significant prognostic factor for colon cancer. These results suggest that molecular staging can be successfully and meaningfully applied to the SLN in CRC, in addition to melanoma and breast cancer.
Noura et al. recently reported a comparative study of detection of micrometastasis using IHC and RT‐PCR assay in H&E‐negative LN of 64 AJCC stage II CRC patients.( 58 ) CEA was used for RT‐PCR assay and compared to an IHC study with anti‐CK antibody. Micrometastases were detected in 19 of 64 (30%) patients using RT‐PCR and in 35 of 64 (55%) patients using IHC. Patients who were PCR‐positive in LN showed significantly worse disease‐free and overall survival than PCR‐negative patients. However, micrometastasis in LN using IHC did not correlate with prognosis. Although a larger prospective study may be required for the validation of the assay, the results suggested the prognostic utility of molecular detection of micrometastasis in LN/SLN of CRC patients.
Other carcinomas. Gastric, esophageal, prostate, biliary, head and neck, lung and gynecologic cancers have also been upstaged following RT‐PCR analysis of LN/SLN and, in many cases, these results have correlated with known prognostic factors (Table 3).( 14 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 ) Arigami et al. reported that 13 of 53 (25%) gastric cancer patients with histopathology‐negative SLN were upstaged using RT‐PCR assay.( 65 ) They concluded that the SLN concept is applicable to patients with cT1 and cN0 gastric cancer, even when including the molecular diagnosis of micrometastasis. Other groups also suggested that nodal micrometastasis detected using RT‐PCR assay has some clinical significance in gastrointestinal cancers.( 66 ) Molecular assessment of the SLN may be a variable tool to complement histological examination for gastrointestinal cancers.
Immunoresponses in the SLN against Tumor Metastasis
The SLN are also known to be the first lymphoid organ to respond to tumor antigenic stimulation. The SLN is the site where immunoreactive lymphocytes initially encounter tumor‐specific antigens and develop antitumor immunity. The SLN may have critical roles in the development of local immunity that could reject and eradicate metastatic cancer cells. Immune dysfunction in the SLN does not directly reflect generalized immune suppression against cancer. However, there are many possible mechanisms that may explain the reasons why the SLN has limited capability to prevent cancer metastasis.
Nagata et al. have made a metastasis model in the rat mesenteric LN, and visualized the migration of cancer cells in vivo.( 67 ) Migrant cancer cells were initially arrested in the marginal sinus in the tumor‐draining LN; therefore the marginal sinus was supposed to constitute a mechanical barrier against cancer cell passage ( Fig. 2 ). The cancer cells filled the marginal sinus, and no cancer nests were found in the cortex, paracortex, or medulla before the marginal sinus was filled. Cancer cells subsequently invaded to the cortex and paracortex over the inner linings of the marginal sinus. Cytokines such as tumor necrosis factor‐α, interleukin (IL)‐1β and IL‐2 secreted by macrophages markedly increased at the early stages of metastasis, but gradually decreased according to the tumor proliferation in the LN. They suggested that parasinus macrophages may play a crucial role in the transient antimetastatic capability of the nodes, and deterioration of cytokine induction may be responsible for subsequent cancer proliferation.
Figure 2.
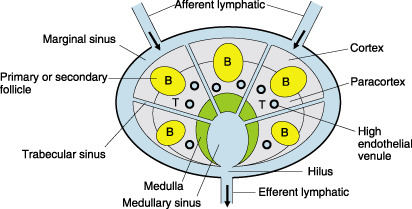
Schema of lymph node. B, B‐cell area; T, T‐cell area.
Individual LN show the heterogeneous reactivity, frequency, and density of T‐cells, dendritic cells (DC), and other lymphocytes in the paracortical area. Cytokine generation and cytotoxicity against tumor cells might vary among individual nodes. Cochran et al. have extended a series of studies to compare the cellular phenotype and physiology of metastasis‐susceptible SLN with non‐SLN from the same patient in melanoma and breast cancer.( 68 ) They demonstrated that the immunoreactivity of SLN is entirely or segmentally down‐regulated compared with non‐SLN, that is to say that SLN are likely to be immune‐modulated by tumor cells. Tumor‐induced down‐regulation of SLN immunity is certainly related to the survival of tumor cells and the development of clinically significant metastasis in SLN.
The cancer cells in the primary site produce immunomodulators that can lead to immunosuppression of the SLN affected via the direct lymphatic drainage pathway from the primary tumor ( Table 4 ). In contrast, non‐SLN in the regional basin are regarded to be less affected by the immunomodulators from the primary tumor site. The down‐regulation of immunoreactivity in SLN is particularly obvious in the density and maturity of paracortical DC (PDC) and T‐cells.( 51 ) Huang et al. reported a significant reduction in the aggregate area of the paracortex occupied by PDC, and less frequency, density and maturity of PDC in the SLN compared with non‐SLN in studies of various solid tumors, in particular breast cancer and melanoma.( 69 ) DC with long dendrites are considered to be more effective at antigen presentation; however, SLN show dendritic cells with short or no dendrites that are likely to reflect the down‐regulation of antigen presentation. Cochran et al. also found that the formation of dendritic meshworks that are related to the interaction between mature DC and T‐cells was markedly reduced in SLN compared with non‐SLN.( 68 )
Table 4.
Representative factors that relate to sentinel lymph node metastasis produced by tumor cells
| Factors | Molecules |
|---|---|
| Cytokines | IL‐6, IL‐8, IL‐10, TGF‐α, TGF‐β |
| Growth factors | VEGF‐A, VEGF‐C, VEGF‐D, basic fibroblast growth factor, platelet‐derived growth factor, placental growth factor, hepatocyte‐growth factor |
| Chemokine receptors | CCR7, CCR10, CXCR4 |
IL, interleukin; TGF, transforming growth factor; VEGF, vascular endothelial growth factor.
Huang et al. showed that T‐cell density and activation markers for T‐cells are markedly reduced within the paracortical area in the SLN compared with the non‐SLN.( 70 ) Naïve T‐cells, which arrive to the paracortical area through the endothelium of high endothelial venules (HEV), are known to encounter antigen‐presenting DC in the paracortex. Recent studies have demonstrated that HEV in SLN are fewer than in non‐SLN, and also transendothelial migration of naïve T‐cells is markedly reduced in SLN.( 71 )
Other studies also supported the concept that primary tumors enable suppression of the immune functions in the SLN and facilitate the development of LN metastasis. In SLN an increased level of IL‐10, which is secreted from the primary tumor, markedly inhibits DC maturation, migration and translocation of major histocompatibility complex (MHC)‐class II peptide complex into the plasma membrane of DC.( 72 ) This result is consistent with other reports that IL‐10 concentrations in SLN are significantly higher that those in non‐SLN.( 73 , 74 , 75 , 76 )
Torisu‐Itakura et al. investigated the nodal profile of immunoregulatory cytokines using cDNA microarray to confirm the identity of the SLN and non‐SLN.( 76 ) As a result, 57 genes were expressed at significantly different levels in SLN and non‐SLN. The expression levels of IL‐13, leptin, lymphotoxin β receptor, and macrophage inflammatory protein 1b were significantly higher for tumor‐positive SLN and compared with tumor‐negative SLN, and the expression of IL‐11Ra was significantly lower for tumor‐positive SLN. They concluded that SLN show a different immunoregulatory cytokine profile from non‐SLN. Further investigations will be needed to clarify immunomodulation by the tumor cells for the development of nodal metastasis.
New Insights into the Mechanism of SLN Metastasis
Recent advances in molecular oncology have shown that various factors such as oncogenes, tumor suppressor genes, growth factors, apoptotic factors, adhesion molecules, angiogenic factors, and cytokines are related to the development of tumor metastasis. The SLN is directly situated in the lymphatic drainage pathway from the primary tumor site. Therefore the establishment of metastasis to SLN may be simply reflected by the flow dynamics of lymphatic fluid that drains from the primary site to the SLN, and the transportation of viable cancer cells.
However, recent studies have demonstrated that primary tumors can actively induce the formation of lymphatic vessels from host vessels, and that lymphangiogenesis is correlated with enhanced (sentinel) LN metastasis in various human carcinomas and some experimental models.( 77 , 78 , 79 , 80 , 81 ) Moreover, tumor cells can induce peritumoral lymphangiogenesis and lymphatic vessel growth within SLN before and after they metastasize to the SLN, likely promoting further development of cancer metastasis.( 82 , 83 )
The functionality of intratumoral and peritumoral lymphatic vessels for cancer metastasis has been discussed in recent years. Intratumoral lymphatic vessels might not be functional with regard to lymphatic fluid transport and cancer metastasis.( 81 , 84 , 85 ) However, several studies have shown that peritumoral lymphatic vessels are more functional and important for promoting lymphatic metastasis.( 83 , 84 , 86 ) LYVE‐1, podoplanin, and PROX‐1 are useful markers for identifying lymphatic epithelial cells.( 87 , 88 , 89 ) The expression of these specific lymphatic markers in intratumoral and peritumoral lymphatic vessels has been known to vary heterogeneously, according to the maturity of lymphatic vessels and tumor progression.
Vascular endothelial growth factor (VEGF)‐C and VEGF‐D are, among the VEGF family, known as the first specific lymphangiogenetic factors.( 90 , 91 ) Many studies have shown that VEGF‐C or VEGF‐D produced by tumor cells enable not only to induce lymphangiogenesis but also to enhance lymphatic metastasis to SLN.( 77 , 78 , 84 , 92 , 93 ) VEGF receptor (VEGFR)‐3 is a lymphatic growth factor receptor of four VEGF receptors, and specifically binds to VEGF‐C and VEGF‐D, but not to VEGF‐A.( 94 ) VEGFR‐3 expression is restricted to the lymphatic epithelium in normal tissues; however, some tumor blood vessels also express VEGFR‐3.( 89 , 95 ) Activation of VEGFR‐3 is known to promote lymphatic endothelial cell proliferation, migration, and cell survival through several signal pathways such as the phosphatidylinositol 3‐kinase/AKT.( 96 ) Recent studies have reported that VEGFR‐3 is expressed in some types of cancer cells, and that generation of a paracrine loop involving VEGF‐C and VEGFR‐3 may promote cancer cell survival, lymphangiogenesis and LN metastasis.( 97 )
Several studies have revealed that lymphangiogenesis and lymphatic metastasis promoted by VEGF‐C or VEGF‐D are significantly suppressed by blocking the VEGF‐R3 signaling pathway.( 78 , 79 , 98 ) Skobe et al. reported that the human breast carcinoma cell line transfected with VEGF‐C significantly promoted peritumoral and intratumoral lymphangiogenesis (but had no effect on angiogenesis) and lymphatic metastasis.( 78 ) Other groups have also showed that another human breast carcinoma cell line, MCF‐7 transfected with VEGF‐C cDNA, was significantly correlated with lymphangiogenesis and lymphatic metastasis in SCID mice models.( 93 ) Moreover the tumor‐associated lymphangiogenesis promoted by VEGF‐C significantly inhibited VEGFR‐3 fusion protein,( 92 ) suggesting that the VEGF‐C (or VEGF‐D) and VEGFR‐3 pathway may be the therapeutic target of inhibiting tumor lymphangiogenesis.
Recent studies also suggest that binding of VEGF‐C and VEGF‐D to VEGFR‐2 may stimulate lymphangiogenesis, and VEGF‐A, which binds to VEGFR‐2, markedly promotes tumor lymphangiogenesis.( 83 , 98 ) These results suggest that VEGF‐A and/or VEGFR‐2 may be another therapeutic target of inhibiting tumor lymphangiogenesis. Other molecular markers including hepatocyte growth factor, fibroblast growth factor‐2, platelet‐derived growth factor (PDGF), angiopoietin‐1, and insulin‐like growth factors 1/2, were recently identified as potent lymphangiogenetic factors.( 94 ) However, it is still unknown whether these newly identified lymphangiogenetic factors markedly induce cancer metastasis to the SLN.
There is significant evidence that tumors of specific histology preferentially metastasize to LN.( 99 , 100 ) This preferential metastasis cannot be explained simply by the lymphatic drainage pattern from the tumor. The observation of an orderly, systematic targeting of organs by metastatic breast cancer led Paget to hypothesize the ‘seed and soil’ theory of cancer metastasis.( 101 ) In this model, organs that provide suitable environmental conditions for cancer growth are the preferential sites of cancer metastasis. Since Paget's original report more than one century ago, others have attempted to test, challenge, or supplement this theory. Recently, a novel mechanism for cancer metastasis has emerged that highlights the role of chemokines. There is evidence that antigen‐presenting cells such as DC, T‐cells, Langerhans cells, and natural killer (NK) cells bearing chemokine receptors migrate from skin to the draining LN in response to specific chemotactic factors referred to as chemokines.( 102 , 103 , 104 , 105 , 106 , 107 ) In this signaling ‘homing’ mechanism, SLN produce and release specific chemokines that attract cancer cells bearing specific corresponding receptors in primary sites. The recent demonstration of specific chemokine receptors on tumor cells and respective chemokines has provided some insight into how tumor cells may home to SLN. Chemokine receptors have been suggested to play a pivotal role in regulating the recruitment of solid tumor cells to SLN.( 108 )
Chemokines, grouped into CXC and CC subfamilies based on the arrangement of the two NH2‐terminal cysteine residues, are small secreted proteins that regulate the chemotactic response for a variety of cells.( 106 ) These ligands and receptors have been predominantly investigated on lymphoid cells. Of particular interest is CCL21/SLC, also referred to as 6Ckine or exodus, which is involved in recruiting CCR7(+) naïve T‐cells, NK, memory T‐cells, and DC.( 102 , 103 , 104 , 105 , 106 , 107 ) CCL21/SLC is constitutively expressed in the HEV of LN and lymphatic endothelial cells, Peyer's patches, thymus, spleen and mucosal tissue.( 105 , 109 ) It has a high affinity for CCR7, a member of the seven transmembrane‐spanning G protein coupled receptor family.( 110 , 111 , 112 ) CCR7 is prevalent in various subsets of T‐cells and DC.( 103 , 110 , 111 , 112 ) The release of CCL21/SLC by HEV cells recruits CCR7(+) cells to draining LN.( 103 , 107 , 109 )
The concept that chemokine receptors promote organ‐specific tumor metastasis was first experimentally addressed by Muller et al.( 113 ) They demonstrated that the chemokine receptor CXCR4 was highly expressed in human breast cancer, and its specific ligand CXCL12/SDF‐1 was expressed in a variety of tissues such as bone marrow, lung, and LN where breast cancer cells preferentially metastasize. Moreover, breast cancer cell lines enabled to show chemotactic migration to CXCL12 in vitro, and a SCID mouse model showed that experimental metastasis of a breast cancer cell line to LN is significantly inhibited by neutralizing antibodies against CXCR4.
Human melanoma cells have been shown to express the chemokine receptors CCR7 and CXCR4.( 114 , 115 ) The expression of these receptors is variable among melanomas, as shown by molecular analysis, both in cell lines and in microdissected tumor tissues.( 114 , 115 ) Both chemokine receptors were shown to be functional to their specific ligands, CCL21 and CXCL12/SDF‐1, respectively. To further examine the role of these chemokine receptor‐ligands in metastasis, SLN were assessed because metastasis often occurs initially at these proximal tumor‐draining LN.( 114 ) LN are known to produce the chemokines CXCL12 and CCL21. Activation of these chemokines attracts antigen‐presenting cells, such as DC and T‐cells, to help orchestrate an immune response in the nodes.( 115 , 116 ) We hypothesized that metastatic tumor cells may take advantage of chemokines activated in LN. To determine this, we examined SLN in melanoma patients with micrometastasis and those without it. Our studies demonstrated that CXCL12 and CCL21 production by SLN correlated with metastasis involvement. Interestingly, as the tumor burden increased in the SLN, chemokines were more suppressed.( 114 ) The results suggested that metastatic tumor cells or factors may suppress chemokine production through direct or indirect mechanisms. These mechanisms may be similar to inflammatory responses in LN in that, after initial activation, the nodes do not continually expand by recruiting immune cells. There appears to be a physiological mechanism of cells populating LN that regulate chemokine production.
Wiley et al. showed that the functional expression of CCR7 enhances the metastasis of B16 murine melanoma to SLN compared with control, and that the metastasis is inhibited by neutralizing antibodies against CCL21.( 117 ) Other groups have demonstrated that CCR7‐positive cancer cells significantly correlated with a high incidence of LN metastasis in gastric( 118 ) and esophageal carcinomas.( 119 )
Therapeutic Implications for SLN Metastasis
Malignant melanoma is one of the candidates for the investigation of immunotherapy because it is clinically resistant to chemotherapy and radiotherapy, and expresses many kinds of immunogenic molecules. To date, Mycobacterium bovis Bacillus Calmette‐Guérin, IL‐2, type II interferon (IFN), and granulocyte–macrophage colony‐stimulating factor (GM‐CSF) have been reported to have antitumor effects for melanoma after intratumoral injection.( 120 , 121 , 122 , 123 ) GM‐CSF has been supposed to provide an antitumor effect by acting on DC, T‐cells, and macrophages. GM‐CSF is known to cause mature DC to migrate to regional LN and increase their resistance to apoptosis,( 124 ) and also to induce T‐cell mediated antitumor immunity by activated DC. Vuylsteke et al. reported that preoperative peritumoral injections of GM‐CSF resulted in enlargement of DC and T‐cell areas in the SLN.( 125 ) These results suggest that GM‐CSF may have the potential to prevent or eradicate tumor metastasis in the SLN. Many other molecules, including IL‐13 and IFN‐α, have been reported to be candidates for immunomodulation in the SLN.( 126 , 127 ) Further studies will prove the clinical significance of these immunomodulators for the treatment of SLN metastasis.
Several studies have shown that the VEGFR‐3 and/or VEGFR‐2 pathway might be the therapeutic target of inhibiting tumor lymphangiogenesis and cancer metastasis to SLN. To date, several antibodies and molecules including anti‐VEGFR‐3 antibody, anti‐VEGF‐C antibody, anti‐VEGF‐D antibody, soluble VEGFR‐3 fusion protein, small interfering RNA to reduce VEGF‐C mRNA expression, and a number of small molecule kinase inhibitors of VEGFR‐2 have been investigated in animal models.( 79 , 92 , 94 , 98 , 128 ) Most of the studies using these antibodies or molecules have demonstrated that specific inhibition of the VEGFR‐3 and/or the VEGFR‐2 pathway markedly reduces lymphangiogenesis and lymphatic cancer metastasis, and likely also reduces the incidence of distant organ metastasis.
Chemokine receptors will be also as a target of therapeutic intervention using antibodies or small molecule inhibitors. Anti‐CXCR4 monoclonal antibody significantly inhibits the metastasis of human breast carcinoma cells to the LN of SCID mice.( 92 ) Systemic administration of the CXCR4 antagonist AMD3100, a potent blocker of HIV cell entry, inhibited the growth of intracranial glioblastoma and medulloblastoma xenografts by inducing tumor cell apoptosis.( 129 ) However, systemic inhibition of CXCL12‐CXCR4 signaling may have adverse effects on the hematopoietic stem cells, primitive germ cells and neural precursors.( 130 )
Conclusion
The development of the SLN concept has radically altered the field of diagnosis and treatment of many solid tumors. As this paradigm shift receives validation from melanoma studies, greater attention on the histopathological microstaging of the SLN formalized the concept of LN/SLN micrometastasis. The use of serial sectioning and IHC analysis, and more recently, the use of RT‐PCR, has enabled investigators to further study the potential clinical significance of micrometastatic LN/SLN disease. There is now a growing body of data to support the clinical relevance of LN/SLN micrometastasis in a variety of solid tumors. Increasing the sensitivity of occult tumor cell detection in the SLN, using molecular‐based analysis, should enable a more accurate understanding of the clinical significance of various patterns of micrometastatic nodal disease. In the future, molecular staging of SLN should benefit and improve patient management.
Lymphangiogenesis and the ‘chemokine‐chemokine receptor network’ are responsible for promoting lymphatic cancer metastasis. Metastasis to SLN might not be simply reflected only by the flow dynamics of lymphatic fluid that drains from the primary site to the SLN, but also by the diplomatic and active behavior of cancer cells. SLN dramatically show morphological, phenotypical and functional changes that indicate immune suppression by tumor cells. The immune suppression in SLN results in failure of prevention or eradication of tumor metastasis. The mechanism of immunomodulation remains unclear; however, several regulatory molecules produced by tumor cells and tumor‐associated macrophages or lymphocytes are likely to be responsible for inducing immune suppression in the SLN. Further preclinical and clinical studies may achieve the reversal of tumor‐induced immune suppression that can prevent or eradicate LN metastasis.
Acknowledgments
The authors are indebted to Professor J. Patrick Barron of the International Medical Communications Center of Tokyo Medical University for his review of this manuscript.
References
- 1. Gervasoni JE Jr, Taneja C, Chung MA, Cady B. Biologic and clinical significance of lymphadenectomy. Surg Clin North Am 2000; 80: 1631–73. [DOI] [PubMed] [Google Scholar]
- 2. Fisher B, Redmond C, Fisher ER et al . Ten‐year results of a randomized trial comparing radical mastectomy and total mastectomy with or without radiation. N Engl J Med 1985; 312: 674–81. [DOI] [PubMed] [Google Scholar]
- 3. Veronesi U, Adamus J, Bandiera DC et al . Inefficacy of immediate node dissection in stage I melanoma of the limbs. N Engl J Med 1977; 297: 627–30. [DOI] [PubMed] [Google Scholar]
- 4. Cascinelli N, Morabito A, Santinami M, MacKie RM, Belli F. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: a randomized trial. WHO Melanoma Programme. Lancet 1998; 351: 793–6. [DOI] [PubMed] [Google Scholar]
- 5. Morton DL, Wen DR, Wong JH et al . Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 1992; 127: 392–9. [DOI] [PubMed] [Google Scholar]
- 6. Eifel P, Axelson JA, Costa J et al . National Institutes of Health Consensus Development Conference Statement: adjuvant therapy for breast cancer, November 1–3, 2000. J Natl Cancer Inst 2001; 93: 979–89. [DOI] [PubMed] [Google Scholar]
- 7. Yoshino I, Nakanishi R, Osaki T et al . Unfavorable prognosis of patients with stage II non‐small cell lung cancer associated with macroscopic nodal metastases. Chest 1999; 116: 144–9. [DOI] [PubMed] [Google Scholar]
- 8. Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg 1994; 220: 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morton DL, Thompson JF, Essner R, Elashoff R, Stern SL, Nieweg OE. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early‐stage melanoma: a multicenter trial. Multicenter Selective Lymphadenectomy Trial Group. Ann Surg 1999; 230: 453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krag D, Weaver D, Ashikaga T et al . The sentinel node in breast cancer – a multicenter validation study. N Engl J Med 1998; 339: 941–6. [DOI] [PubMed] [Google Scholar]
- 11. Bilchik AJ, Saha S, Wiese D et al . Molecular staging of early colon cancer on the basis of sentinel node analysis: a multicenter phase II trial. J Clin Oncol 2001; 19: 1128–36. [DOI] [PubMed] [Google Scholar]
- 12. Pelizzo MR, Boschin IM, Toniato A et al . The sentinel node procedure with Patent Blue V dye in the surgical treatment of papillary thyroid carcinoma. Acta Otolaryngol 2001; 121: 421–4. [DOI] [PubMed] [Google Scholar]
- 13. Akduman B, Fleshner NE, Ehrlich L, Klotz L. Early experience in intermediate‐risk penile cancer with sentinel node identification using the gamma probe. Urology 2001; 58: 65–8. [DOI] [PubMed] [Google Scholar]
- 14. Van Trappen PO, Gyselman VG, Lowe DG et al . Molecular quantification and mapping of lymph‐node micrometastases in cervical cancer. Lancet 2001; 357: 15–20. [DOI] [PubMed] [Google Scholar]
- 15. Liptay MJ, Masters GA, Winchester DJ et al . Intraoperative radioisotope sentinel lymph node mapping in non‐small cell lung cancer. Ann Thorac Surg 2000; 70: 384–9. [DOI] [PubMed] [Google Scholar]
- 16. Kitagawa Y, Fujii H, Mukai M et al . The role of the sentinel lymph node in gastrointestinal cancer. Surg Clin North Am 2000; 80: 1799–809. [DOI] [PubMed] [Google Scholar]
- 17. Cote RJ, Peterson HF, Chaiwun B et al . Role of immunohistochemical detection of lymph‐node metastases in management of breast cancer. International Breast Cancer Study Group. Lancet 1999; 354: 896–900. [DOI] [PubMed] [Google Scholar]
- 18. Czerniecki BJ, Scheff AM, Callans LS et al . Immunohistochemistry with pancytokeratins improves the sensitivity of sentinel lymph node biopsy in patients with breast carcinoma. Cancer 1999; 85: 1098–103. [PubMed] [Google Scholar]
- 19. Turner RR, Ollila DW, Krasne DL, Giuliano AE. Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma. Ann Surg 1997; 226: 271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pantel K, Cote RJ, Fodstad O. Detection and clinical importance of micrometastatic disease. J Natl Cancer Inst 1999; 91: 1113–24. [DOI] [PubMed] [Google Scholar]
- 21. Xu X, Roberts SA, Pasha TL, Zhang PJ. Undesirable cytokeratin immunoreactivity of native nonepithelial cells in sentinel lymph nodes from patients with breast carcinoma. Arch Pathol Laboratory Med 2000; 124: 1310–3. [DOI] [PubMed] [Google Scholar]
- 22. Cochran AJ, Balda BR, Starz H et al . The Augsburg Consensus. Techniques of lymphatic mapping, sentinel lymphadenectomy, and completion lymphadenectomy in cutaneous malignancies. Cancer 2000; 89: 236–41. [PubMed] [Google Scholar]
- 23. King R, Weibaecher KN, McGill G, Cooley E, Mihm M, Fisher DE. Microphthalmia transcription factor. A sensitive and specific melanocyte marker for Melanoma Diagnosis. Am J Pathol 1999; 155: 731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takeuchi H, Wascher RA, Kuo C, Turner RR, Hoon DS. Molecular diagnosis of micrometastasis in the sentinel lymph node. Cancer Treat Res 2005; 127: 221–52. [DOI] [PubMed] [Google Scholar]
- 25. Takeuchi H, Kuo C, Morton DL, Wang HJ, Hoon DS. Expression of differentiation melanoma‐associated antigen gene is associated with favorable disease outcome in advanced‐stage melanomas. Cancer Res 2003; 15: 441–8. [PubMed] [Google Scholar]
- 26. Yoshioka S, Fujiwara Y, Sugita Y et al . Real‐time rapid reverse transcriptase‐polymerase chain reaction for intraoperative diagnosis of lymph node micrometastasis: clinical application for cervical lymph node dissection in esophageal cancers. Surgery 2002; 132: 34–40. [DOI] [PubMed] [Google Scholar]
- 27. Taniyama K, Motoshita J, Sakane J et al . Combination analysis of a whole lymph node by one‐step nucleic acid amplification and history for intraoperative detection of micrometastasis. Pathobiology 2006; 73: 183–91. [DOI] [PubMed] [Google Scholar]
- 28. Nasser IA, Lee AK, Bosari S, Saganich R, Heatley G, Silverman ML. Occult axillary lymph node metastases in ‘node‐negative’ breast carcinoma. Hum Pathol 1993; 24: 950–7. [DOI] [PubMed] [Google Scholar]
- 29. Kamath VJ, Giuliano R, Dauway EL et al . Characteristics of the sentinel lymph node in breast cancer predict further involvement of higher‐echelon nodes in the axilla: a study to evaluate the need for complete axillary lymph node dissection. Arch Surg 2001; 136: 688–92. [DOI] [PubMed] [Google Scholar]
- 30. Bostick PJ, Huynh KT, Sarantou T et al . Detection of metastases in sentinel lymph nodes of breast cancer patients by multiple‐marker RT‐PCR. Int J Cancer 1998; 79: 645–51. [DOI] [PubMed] [Google Scholar]
- 31. Miyashiro I, Kuo C, Huynh K et al . Molecular strategy for detecting metastatic cancers with use of multiple tumor‐specific MAGE‐A genes. Clin Chem 2001; 47: 505–12. [PubMed] [Google Scholar]
- 32. Masuda N, Tamaki Y, Sakita I et al . Clinical significance of micrometastases in axillary lymph nodes assessed by reverse transcription‐polymerase chain reaction in breast cancer patients. Clin Cancer Res 2000; 6: 4176–85. [PubMed] [Google Scholar]
- 33. Manzotti M, Dell’Orto P, Maisonneuve P, Zurrida S, Mazzarol G, Viale G. Reverse transcription‐polymerase chain reaction assay for multiple mRNA markers in the detection of breast cancer metastases in sentinel lymph nodes. Int J Cnacer 2001; 95: 307–12. [DOI] [PubMed] [Google Scholar]
- 34. Sakaguchi M, Virmani A, Dudak MW et al . Clinical relevance of reverse transcriptase‐polymerase chain reaction for the detection of axillary lymph node metastases in breast cancer. Ann Surg Oncol 2003; 10: 117–25. [DOI] [PubMed] [Google Scholar]
- 35. Ouellette RJ, Richard D, Maïcas E. RT‐PCR for mammaglobin genes, MGB1 and MGB2, identifies breast cancer micrometastases in sentinel lymph nodes. Am J Clin Pathol 2004; 121: 637–43. [DOI] [PubMed] [Google Scholar]
- 36. Nissan A, Jager D, Roystacher M et al . Multimarker‐RT‐PCR assay for the detection of minimal residual disease in sentinel lymph nodes of breast cancer patients. Br J Cancer 2006; 94: 681–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lockett MA, Baron PL, O’Brien PH et al . Detection of occult breast cancer micrometastases in axillary lymph nodes using a multimarker reverse transcriptase‐polymerase chain reaction panel. J Am Coll Surg 1998; 187: 9–16. [DOI] [PubMed] [Google Scholar]
- 38. Yun K, Gunn J, Merrie AE, Phillips LV, McCall JL. Keratin 19 mRNA is detectable by RT‐PCR in lymph nodes of patients without breast cancer. Br J Cancer 1997; 76: 1112–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Merrie AE, Yun K, Gunn J, Phillips LV, McCall JL. Analysis of potential markers for detection of submicroscopic lymph node metastases in breast cancer. Br J Cancer 1999; 80: 2019–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bostick PJ, Chatterjee S, Chi DD et al . Limitations of specific reverse‐transcriptase polymerase chain reaction markers in the detection of metastases in the lymph nodes and blood of breast cancer patients. J Clin Oncol 1998; 16: 2632–40. [DOI] [PubMed] [Google Scholar]
- 41. Wascher RA, Bostick PJ, Huynh KT et al . Detection of MAGE‐A3 in breast cancer patients’ sentinel lymph nodes. Br J Cancer 2001; 85: 1340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gillanders WE, Mikhitarian K, Hebert R et al . Molecular detection of micrometastatic breast cancer in histopathology negative axillary lymph nodes correlates with traditional predictors of prognosis. An interim analysis of prospective multi‐institutional cohort study. Ann Surg 2004; 239: 828–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goydos JS, Ravikumar TS, Germino FJ, Yudd A, Bancila E. Minimally invasive staging of patients with melanoma. sentinel lymphadenectomy and detection of the melanoma‐specific proteins MART‐1 and tyrosinase by reverse transcriptase polymerase chain reaction. J Am Coll Surg 1998; 187: 182–8. [DOI] [PubMed] [Google Scholar]
- 44. Shivers SC, Wang X, Li W et al . Molecular staging of malignant melanoma. JAMA 1998; 280: 1410–15. [DOI] [PubMed] [Google Scholar]
- 45. Blaheta HJ, Schittek B, Breuninger H et al . Detection of melanoma micrometastasis in sentinel nodes by reverse transcription‐polymerase chain reaction correlates with tumor thickness and is predictive of micrometastatic disease in the lymph node basin. Am J Surg Pathol 1999; 23: 822–8. [DOI] [PubMed] [Google Scholar]
- 46. Bostick PJ, Morton DL, Turner RR et al . Prognostic significance of occult metastases detected by sentinel lymphadenectomy and reverse transcriptase‐polymerase chain reaction in early‐stage melanoma patients. J Clin Oncol 1999; 17: 3238–44. [DOI] [PubMed] [Google Scholar]
- 47. Blaheta HJ, Ellwanger U, Schittek B et al . Examination of regional lymph nodes by sentinel node biopsy and molecular analysis provides new staging facilities in primary cutaneous melanoma. J Invest Dermatol 2000; 114: 637–42. [DOI] [PubMed] [Google Scholar]
- 48. Ribuffo D, Gradilone A, Vonella M et al . Prognostic significance of reverse transcriptase‐polymerase chain reaction‐negative sentinel nodes in malignant melanoma. Ann Surg Oncol 2003; 10: 396–402. [DOI] [PubMed] [Google Scholar]
- 49. Goydos JS, Patel KN, Shih WJ et al . Patterns of recurrence in patients with melanoma and histologically negative but RT‐PCR‐positive sentinel lymph nodes. J Am Coll Surg 2003; 196: 196–204. [DOI] [PubMed] [Google Scholar]
- 50. Kuo CT, Hoon DS, Takeuchi H et al . Prediction of disease outcome in melanoma patients by molecular analysis of paraffin‐embedded sentinel lymph nodes. J Clin Oncol 2003; 21: 3566–72. [DOI] [PubMed] [Google Scholar]
- 51. Morton DL, Hoon DS, Cochran AJ et al . Lymphatic mapping and sentinel lymphadenectomy for early‐stage melanoma: therapeutic utility and implications of nodal microanatomy and molecular staging for improving the accuracy of detection of nodal micrometastases. Ann Surg 2003; 238: 538–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Scoggins CR, Ross MI, Reintgen DS et al . Prospective multi‐institutional study of reverse transcriptase polymerase chain reaction for molecular staging of melanoma. J Clin Oncol 2006; 24: 2849–57. [DOI] [PubMed] [Google Scholar]
- 53. Mocellin S, Hoon DS, Pilati P, Rossi CR, Nitti D. Sentinel lymph node molecular ultrastaging in patients with melanoma: a systematic review and meta‐analysis of prognosis. J Clin Oncol 2007; 25: 1588–95. [DOI] [PubMed] [Google Scholar]
- 54. Weitz J, Kienle P, Magener A et al . Detection of disseminated colorectal cancer cells in lymph nodes, blood and bone marrow. Clin Cancer Res 1999; 5: 1830–6. [PubMed] [Google Scholar]
- 55. Ichikawa Y, Ishikawa T, Momiyama N et al . Detection of regional lymph node metastases in colon cancer by using RT‐PCR for matrix metalloproteinase 7, matrilysin. Clin Exp Metastasis 1998; 16: 3–8. [DOI] [PubMed] [Google Scholar]
- 56. Liefers GJ, Cleton‐Jansen AM, Van De Velde CJ et al . Micrometastases and survival in stage II colorectal cancer. N Engl J Med 1998; 339: 223–8. [DOI] [PubMed] [Google Scholar]
- 57. Bernini A, Spencer M, Frizelle S et al . Evidence for colorectal cancer micrometastases using reverse transcriptase‐polymerase chain reaction analysis of MUC2 in lymph nodes. Cancer Detect Prev 2000; 24: 72–9. [PubMed] [Google Scholar]
- 58. Noura S, Yamamoto H, Ohnishi T et al . Comparative detection of lymph node micrometastases of stage II colorectal cancer by reverse transcriptase polymerase chain reaction and immunohistochemistry. J Clin Oncol 2002; 20: 4232–41. [DOI] [PubMed] [Google Scholar]
- 59. Mori M, Mimori K, Inoue H et al . Detection of cancer micrometastases in lymph nodes by reverse transcriptase‐polymerase chain reaction. Cancer Res 1995; 55: 3417–20. [PubMed] [Google Scholar]
- 60. Cortesina G, Martone T, Galeazzi E et al . Staging of head and neck squamous cell carcinoma using the MET oncogene product as marker of tumor cells in lymph node metastases. Int J Cancer 2000; 89: 286–92. [PubMed] [Google Scholar]
- 61. Salerno CT, Frizelle S, Niehans GA et al . Detection of occult micrometastases in non‐small cell lung carcinoma by reverse transcriptase‐polymerase chain reaction. Chest 1998; 113: 1526–32. [DOI] [PubMed] [Google Scholar]
- 62. Ferrari AC, Stone NN, Eyler JN et al . Prospective analysis of prostate‐specific markers in pelvic lymph nodes of patients with high‐risk prostate cancer. J Natl Cancer Inst 1997; 89: 1498–504. [DOI] [PubMed] [Google Scholar]
- 63. Noguchi S, Hiratsuka M, Furukawa H et al . Detection of gastric cancer micrometastases in lymph nodes by amplification of keratin 19 mRNA with reverse transcriptase‐polymerase chain reaction. Jpn J Cancer Res 1996; 87: 650–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kijima F, Natsugoe S, Takao S et al . Detection and clinical significance of lymph node micrometastasis determined by reverse transcription‐polymerase chain reaction in patients with esophageal carcinoma. Oncology 2000; 58: 38–44. [DOI] [PubMed] [Google Scholar]
- 65. Arigami T, Natsugoe S, Uenosono Y et al . Evaluation of sentinel node concept in gastric cancer based on lymph node micrometastasis determined by reverse transcription‐polymerase chain reaction. Ann Surg 2006; 243: 341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Matsuda J, Kitagawa Y, Fujii H et al . Significance of metastasis detected by molecular techniques in sentinel nodes of patients with gastrointestinal cancers. Ann Surg Oncol 2004; 11: 250–4S. [DOI] [PubMed] [Google Scholar]
- 67. Nagata H, Arai T, Soejima Y, Suzuki H, Ishii H, Hibi T. Limited capacity of regional lymph nodes to eradicate metastatic cancer cells. Cancer Res 2004; 64: 8239–48. [DOI] [PubMed] [Google Scholar]
- 68. Cochran AJ, Huang RR, Lee J, Itakura E, Leong SP, Essner R. Tumor‐induced immune modulation of sentinel lymph nodes. Nature Rev Immunol 2006; 6: 659–70. [DOI] [PubMed] [Google Scholar]
- 69. Huang RR, Wang HJ, Lin LL, Wen DR, Itakura E, Cochran AJ. MHC‐Class II molecules expression by dendritic cells correlates with activated OPD4 + T cell in of sentinel and non‐sentinel nodes from melanoma patients. Mod Pathol 2004; 17: 382. [Google Scholar]
- 70. Huang RR, Paul E, Wang HJ et al . Sentinel lymph nodes are immunosuppressed whether or not they contain metastatic melanoma. Mod Pathol 2005; 18: 379. [Google Scholar]
- 71. Shu S, Cochran AJ, Huang RR, Morton DL, Maecher HT. Immune responses in the draining lymph nodes against cancer: Implications for immunotherapy. Cancer Metastasis Rev 2006; 25: 233–42. [DOI] [PubMed] [Google Scholar]
- 72. Hoon DS, Korn EL, Cochran AJ. Variation in functional immunocompetence of individual tumor‐draining lymph nodes in humans. Cancer Res 1987; 47: 1740–4. [PubMed] [Google Scholar]
- 73. Lee JH, Torisu‐Itakura H, Cochran AJ et al . Quantitative analysis of melanoma‐induced cytokine‐mediated immunosuppression in melanoma sentinel nodes. Clin Cancer Res 2005; 11: 107–12. [PubMed] [Google Scholar]
- 74. Botella‐Estrada R, Dasi F, Ramos D et al . Cytokine expression and dendritic cell density in melanoma sentinel nodes. Melanoma Res 2005; 15: 99–106. [DOI] [PubMed] [Google Scholar]
- 75. Polak ME, Borthwick NJ, Gabriel FG et al . Mechanisms of local immunosuppression in cutaneous melanoma. Br J Cancer 2007; 96: 1879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Torisu‐Itakura H, Lee JH, Scheri RP et al . Molecular characterization of inflammatory genes in sentinel and nonsentinel nodes in melanoma. Clin Cancer Res 2007; 13: 3125–32. [DOI] [PubMed] [Google Scholar]
- 77. Mandriota SJ, Jussila L, Jeltsch M et al . Vascular endothelial growth factor‐C‐mediated lymphangiogenesis promotes tumor metastasis. EMBO J 2001; 20: 672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Skobe M, Hawighhorst T, Jackson DG et al . Induction of tumor lymphangiogenesis by VEGF‐C promotes breast cancer metastasis. Nat Med 2001; 7: 192–8. [DOI] [PubMed] [Google Scholar]
- 79. Stacker SA, Caesar C, Baldwin ME et al . VEGF‐D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 2001; 7: 186–91. [DOI] [PubMed] [Google Scholar]
- 80. Achen MG, McColl BK, Stacker SA. Focus on lymphangiogenesis in tumor metastasis. Cancer Cell 2005; 7: 121–7. [DOI] [PubMed] [Google Scholar]
- 81. Tobler NE, Detmer M. Tumor and lymph node lymphangiogenesis: impact on cancer metastasis. J Leukoc Biol 2006; 80: 691–6. [DOI] [PubMed] [Google Scholar]
- 82. Dadrass SS, Lange‐Asschenfeldt B, Velasco P et al . Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol 2005; 18: 1232–42. [DOI] [PubMed] [Google Scholar]
- 83. Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF‐A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med 2005; 201: 1089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Padera TP, Kadambi A, Di Tomaso E et al . Lymphatic metastasis in the absence of functional intratumoral lymphatics. Science 2002; 296: 1883–6. [DOI] [PubMed] [Google Scholar]
- 85. Wong SY, Haack H, Crowley D et al . Tumor‐secreted vascular endothelial growth factor‐C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis. Cancer Res 2005; 65: 9789–98. [DOI] [PubMed] [Google Scholar]
- 86. Beasley NJ, Prevo R, Banerji S et al . Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res 2002; 62: 1315–20. [PubMed] [Google Scholar]
- 87. Banerji S, Ni J, Wang SX et al . LYVE‐1, new homologue of the CD44 glycoprotein, is a lymph‐specific receptor for hyaluronan. J Cell Biol 1999; 144: 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Breiteneder‐Geleff S, Soleiman A, Kowalski H et al . Angiosarcomas express mized endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 1999; 154: 385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wigle JT, Oliver C. PROX‐1 function is required for the development of the murine lymphatic system. Cell 1999; 98: 769–78. [DOI] [PubMed] [Google Scholar]
- 90. Joukov V, Pajusola K, Kaipainen A et al . A novel vascular endothelial growth factor, VEGF‐C, as a ligand for the Flt4 (VEGFR‐3) and KDR (VEGFR‐2) receptor tyrosine kinases. EMBO J 1996; 15: 290–8. [PMC free article] [PubMed] [Google Scholar]
- 91. Nathanson SD. Insights into the mechanisms of lymph node metastasis. Cancer 2003; 98: 413–23. [DOI] [PubMed] [Google Scholar]
- 92. Karpanen T, Egeblad M, Karkkainen MJ et al . Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res 2001; 61: 1786–90. [PubMed] [Google Scholar]
- 93. Mattila MMT, Ruohola JK, Karpanen T, Jackson DG, Alitaro K, Harkonen PL. VEGF‐C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF‐7 tumors. Int J Caner 2002; 98: 946–51. [DOI] [PubMed] [Google Scholar]
- 94. Wissmann C, Detmer M. Pathways targeting tumor lymphangiogenesis. Clin Cancer Res 2006; 12: 6865–8. [DOI] [PubMed] [Google Scholar]
- 95. Soker S, Takashima S, Miao H, Neufeld G, Klagsbrun M. Neuropilin is expressed by endothelial and tumor cells as isoform‐specific receptor for vascular endothelial growth factor. Cell 1998; 92: 735–45. [DOI] [PubMed] [Google Scholar]
- 96. Salameh A, Galvagni F, Bardelli M, Bussolino F, Oliviero S. Direct recruitment of CRK and GRB2 to VEGR‐3 induce proliferation, migration and survival of endothelial cells through the activation of ERK, AKT and JNK pathways. Blood 2005; 106: 3423–31. [DOI] [PubMed] [Google Scholar]
- 97. Dias S, Choy M, Alitaro K, Rafii S. Vascular endothelial growth factor (VEGF)‐C signaling through FLT‐4 (VEGFR‐3) mediates leukemic cell proliferation, survival, and resistance to chemotherapy. Blood 2002; 99: 2179–84. [DOI] [PubMed] [Google Scholar]
- 98. Roberts N, Kloos B, Cassella M et al . Inhibition of VEGFR‐3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR‐2. Cancer Res 2006; 66: 2650–7. [DOI] [PubMed] [Google Scholar]
- 99. Fidler IJ. Critical determinants of metastasis. Semin Cancer Biol 2002; 12: 89–96. [DOI] [PubMed] [Google Scholar]
- 100. Fidler IJ. Seed and soil revisited: contribution of the organ microenvironment to cancer metastasis. Surg Oncol Clin N Am 2001; 10: 257–69. [PubMed] [Google Scholar]
- 101. Paget S. The distribution of secondary growth in cancer of the breast. Lancet 1989; 1: 571–3. [PubMed] [Google Scholar]
- 102. Dieu MC, Vanbervliet B, Vicari A et al . Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med 1998; 188: 373–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Förster R, Schubel A, Breitfeld D et al . CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999; 99: 23–33. [DOI] [PubMed] [Google Scholar]
- 104. Nakano H, Tamura T, Yoshimoto T et al . Genetic defect in T lymphocyte‐specific homing into peripheral lymph nodes. Eur J Immunol 1997; 27: 215–21. [DOI] [PubMed] [Google Scholar]
- 105. Willimann K, Legler DF, Loetscher M et al . The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur J Immunol 1998; 28: 2025–34. [DOI] [PubMed] [Google Scholar]
- 106. Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity 2000; 12: 121–7. [DOI] [PubMed] [Google Scholar]
- 107. Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol 2002; 2: 957–65. [DOI] [PubMed] [Google Scholar]
- 108. Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002; 2: 563–72. [DOI] [PubMed] [Google Scholar]
- 109. Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA 1998; 95: 258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yoshida R, Nagira M, Imai T et al . EBI1‐ligand chemokine (ELC) attracts a broad spectrum of lymphocytes: activated T cells strongly up‐regulate CCR7 and efficiently migrate toward ELC. Int Immunol 1998; 10: 901–10. [DOI] [PubMed] [Google Scholar]
- 111. Geissmann F, Dieu‐Nosjean MC, Dezutter C et al . Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J Exp Med 2002; 196: 417–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yanagihara S, Komura E, Nagafune J, Watarai H, Yamaguchi Y. EBI1/CCR7 is a new member of dendritic cell chemokine receptor that is up‐regulated upon maturation. J Immunol 1998; 161: 3096–102. [PubMed] [Google Scholar]
- 113. Muller A, Homey B, Soto H et al . Involvement of chemokine receptors in breast cancer metastasis. Nature 2001; 410: 50–6. [DOI] [PubMed] [Google Scholar]
- 114. Takeuchi H, Fujimoto A, Tanaka M, Yamano T, Hsueh E, Hoon DS. CCL21 chemokine regulates chemokine receptor CCR7 bearing malignant melanoma cells. Clin Cancer Res 2004; 10: 2351–8. [DOI] [PubMed] [Google Scholar]
- 115. Mori T, Kim J, Yamano T et al . Epigenetic up‐regulation of C‐C chemokine receptor 7 and C‐X‐C chemokine receptor 4 expression in melanoma cells. Cancer Res 2005; 65: 1800–7. [DOI] [PubMed] [Google Scholar]
- 116. Hjelmstrom P, Fjell J, Nakagawa T, Sacca R, Cuff CA, Ruddle NH. Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am J Pathol 2000; 156: 1133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wiley HE, Gonzalez EB, Maki W, Wu MT, Hwang ST. Expression of CC chemokine receptor‐7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst 2001; 93: 1638–43. [DOI] [PubMed] [Google Scholar]
- 118. Mashino K, Sadanaga N, Yamaguchi H et al . Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res 2002; 62: 2937–41. [PubMed] [Google Scholar]
- 119. Ding Y, Shimada Y, Maeda M et al . Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clin Cancer Res 2003; 9: 3406–12. [PubMed] [Google Scholar]
- 120. Eilber FR, Morton DL, Holmes EC, Sparks FC, Ramming KP. Adjuvant immunotherapy with BCG in treatment of regional‐lymph‐node metastases from malignant melanoma. N Engl J Med 1976; 294: 237–40. [DOI] [PubMed] [Google Scholar]
- 121. Radny P, Caroli UM, Bauer J et al . Phase II trial of intralesional therapy with interleukin‐2 in soft‐tissue melanoma metastases. Br J Cancer 2003; 89: 1620–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Horton HM, Hernandez P, Parker SE, Barnhart KM. Antitumor effects of interferon‐ù: in vivo therapy of human tumor xenografts in nude mice. Cancer Res 1999; 59: 4064–8. [PubMed] [Google Scholar]
- 123. Pan PY, Li Y, Li Q et al . In situ recruitment of antigen‐presenting cells by intratumoral GM‐CSF gene delivery. Cancer Immunol Immunother 2004; 53: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Dranoff G. GM‐CSF‐based cancer vaccines. Immunol Rev 2002; 188: 147–54. [DOI] [PubMed] [Google Scholar]
- 125. Vuylsteke RJ, Molenkamp BG, Gietema HA et al . Local administration of granulocyte/macrophage colony – stimulating factor increases the number and activation state of dendritic cells in the sentinel lymph node of early‐stage melanoma. Cancer Res 2004; 64: 8456–60. [DOI] [PubMed] [Google Scholar]
- 126. Salcedo M, Bercovici N, Taylor R et al . Vaccination of melanoma patients using dendritic cells loaded with an allogeneic tumor cell lysate. Cancer Immunol Immunother 2006; 55: 819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kuwashima N, Nishimura F, Eguchi J et al . Delivery of dendritic cells engineered to secret IFN‐α into central nervous system tumors enhances the efficacy of peripheral tumor cell vaccines: dependence on apoptotic pathways. J Immunol 2005; 175: 2730–40. [DOI] [PubMed] [Google Scholar]
- 128. Chen Z, Warney ML, Backora MW et al . Down‐regulation of vascular endothelial cell growth factor‐C expression using small interfering RNA vectors in mammary tumors inhibits tumor lymphangiogenesis and spontaneous metastasis and enhances survival. Cancer Res 2005; 65: 9004–11. [DOI] [PubMed] [Google Scholar]
- 129. Rubin JB, Kung AL, Klein RS et al . A small‐molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Nat Acad Sci USA 2003; 100: 13513–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tanaka T, Bai Z, Srinoulprasert Y et al . Chemokines in tumor progression and metastasis. Cancer Sci 2005; 96: 317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


