Abstract
ATP‐binding cassette transporter G2 (ABCG2) is the most recently described transporter of the multidrug‐resistance pump and it promotes resistance to anticancer drugs such as doxorubicin, mitoxantrone, topotecan, and SN‐38. Of the ABCG2 polymorphisms, V12M and Q141K alter the functional activity of the ABCG2 transporter and influence the drug response and various toxicities to chemotherapeutic agents. We therefore evaluated the impact of the ABCG2 V12M and Q141K polymorphisms on the therapeutic outcomes and toxicities of primary rituximab plus cyclophosphamide/doxorubicin/vincristine/prednisone (R‐CHOP) therapy in 145 Korean patients with diffuse large B‐cell lymphoma (DLBCL). ABCG2 V12M and Q141K genotyping was carried out by pyrosequencing of polymerase chain reaction products. The clinical characteristics, treatment outcomes, toxicities of the patients, and the predictive value of the polymorphisms on response, survival, and adverse events to R‐CHOP for 145 patients were analyzed according to the ABCG2 V12M and Q141K polymorphisms. No differences were observed according to ABCG2 Q141K and V12M genotype in patient characteristics, disease characteristics, response, survival, or hematology toxicity profiles in patients with DLBCL who received frontline R‐CHOP chemotherapy. On multivariate analysis, grade I–IV diarrhea was statistically significant according to ABCG2 Q141K polymorphism (the QQ genotype vs the QK or KK genotypes; hazard ratio 2.835; 95% confidence interval 1.432–5.613; P = 0.003). This study demonstrates that the ABCG2 Q141K polymorphism may correlate with chemotherapy‐induced diarrhea in patients with DLBCL who have received frontline R‐CHOP chemotherapy, and this has implications for optimizing treatment with such agents. (Cancer Sci 2008; 99: 2496–2501)
The introduction of rituximab plus cyclophosphamide/ doxorubicin/vincristine/prednisone (R‐CHOP) chemotherapy for treating patients with diffuse large B‐cell lymphoma (DLBCL) has improved treatment outcomes, yet the development of drug resistance to chemotherapeutic agents is still a primary obstacle. One mechanism by which cancer cells can become resistant to chemotherapy is the expression of ATP‐binding cassette (ABC) transporters that use the energy of ATP hydrolysis to transport a wide variety of substrates across the cell membrane. There are three human ABC transporters associated primarily with the multidrug resistance phenomenon: P‐glycoprotein (MDR1),( 1 ) multidrug resistance‐associated protein (MRP),( 2 ) and ATP‐binding cassette transporter G2 (ABCG2).( 3 , 4 ) All three have broad and, to a certain extent, overlapping substrate specificities for transporting the major drugs that are used currently in cancer chemotherapy. ABCG2 is also named breast cancer resistance protein, placenta‐specific ATP binding cassette transporter, and mitoxantrone resistance protein, and it is the most recently described of the three major multidrug‐resistance pumps; its substrates include doxorubicin, mitoxantrone, topotecan, irinotecan, flavopiridol, and methotrexate.( 3 , 4 , 5 , 6 ) Doxorubicin is known as the most active drug for DLBCL patients being treated with R‐CHOP combination chemotherapy, and it is transported by the ABCG2 pump.
Polymorphisms of the ABCG2 gene have been suggested to be a significant factor in a patient's response to medication, toxicities to chemotherapy, and the risk of disease.( 7 , 8 , 9 , 10 , 11 ) In particular, two non‐synonymous polymorphisms, c.34G>A (p.Val12Met, V12M) and c.421C>A (p.Gln141Lys, Q141K), have been detected at relatively high frequencies in most ethnic groups, including Asians, Caucasians, and Africans.( 8 , 12 , 13 , 14 ) These two polymorphisms alter the function of the multidrug resistance transporter ABCG2 through changes in membrane localization and ATPase activity.( 15 ) A recently published study found that the ABCG2 V12M and Q141K polymorphisms are associated with susceptibility to and survival from DLBCL.( 16 )
In the present study, we evaluated the impact of the ABCG2 Q141K and V12M polymorphisms on the therapeutic outcomes and adverse reactions of primary R‐CHOP therapy in 145 patients with DLBCL, including treatment response, overall survival (OS) and event‐free survival (EFS), hematological toxicities, and non‐hematological toxicities.
Patients, Materials, and Methods
Patient characteristics and treatment protocol. A total of 145 patients who received R‐CHOP chemotherapy between November 2003 and March 2007 as a frontline regimen for DLBCL were included in this retrospective study; these patients were from three hospitals in the Republic of Korea (Gyeongsang National University Hospital, National Cancer Center Research Institute and Hospital, and Pusan National University Hospital). The study protocol was approved by the ethical review boards of Gyeongsang National University Hospital (approval no. 7403‐16), the National Cancer Center Research Institute and Hospital (approval no. NCCCTS‐05‐146), and Pusan National University School of Medicine (approval no. 2006‐16), and the patients gave informed consent. The baseline characteristics of the patients are summarized in Table 1. Overall, among the 145 patients (median age 56 years, 89 men and 56 women), 53 (36.6%) patients had stage 3 or 4 disease, and 38 (26.2%) patients had intermediate to high or high International Prognostic Index (IPI) scores. A median of six cycles of R‐CHOP therapy was given (range one to eight cycles). The patients were treated with 750 mg/m2 cyclophosphamide, 50 mg/m2 doxorubicin, and 1.4 mg/mg2 vincristine up to a maximum dose of 2 mg vinicristin on day 1, with oral administration of 100 mg prednisolone on days 1–5 (CHOP) following infusion of 375 mg/m2 rituximab on day 1. The treatment cycles were repeated every 3 weeks until the maximum of four to six cycles for patients with stage 1 or 2 disease and six to eight cycles for patients with advanced disease. Involved field radiotherapy was allowed for the patients who received four cycles of R‐CHOP with stage 1 and non‐bulky stage 2 disease, or for the patients with advanced bulky disease. The response to R‐CHOP therapy was evaluated after completion of two to three courses of R‐CHOP and 1 month after completion of all the planned cycles of R‐CHOP, and then every 3–6 months.
Table 1.
Patient characteristics and treatment outcomes according to the ABCG2 Q141K and V12M polymorphisms
| ABCG2 Q141K | P‐value | ABCG2 V12M | P‐value | |||||
|---|---|---|---|---|---|---|---|---|
| QK | KK | VV | VM | MM | ||||
| No. patients (%) | 69 (47.6%) | 9 (6.2%) | 71 (49.0%) | 63 (43.4%) | 11 (7.6%) | |||
| Sex (no. men/women) | 39/28 | 45/24 | 5/4 | 0.657 | 44/27 | 37/26 | 8/3 | 0.672 |
| Age (years), median (range) | 55 (17–75) | 59 (39–79) | 0.581 | 57 (17–81) | 55 (17–75) | 59 (39–79) | 0.120 | |
| Disease, n (%) | ||||||||
| Age at least 60 years | 28 (41.8%) | 30 (43.5%) | 4 (44.4%) | 0.975 | 32 (45.1%) | 22 (34.9%) | 8 (72.7%) | 0.428 |
| Stage 3, 4 | 25 (37.3%) | 24 (34.8%) | 4 (44.4%) | 0.839 | 25 (35.2%) | 39 (38.1%) | 7 (36.4%) | 0.942 |
| Elevated LDH | 35 (52.2%) | 37 (53.6%) | 4 (55.6%) | 0.976 | 36 (50.7%) | 34 (54.0%) | 7 (63.6%) | 0.714 |
| Extranodal | 9 (13.4%) | 4 (5.8%) | 2 (22.2%) | 0.166 | 9 (12.7%) | 5 (7.9%) | 1 (9.1%) | 0.661 |
| B symptoms | 11 (16.4%) | 17 (24.6%) | 1 (11.1%) | 0.385 | 17 (23.9%) | 9 (14.3%) | 3 (27.3%) | 0.310 |
| ECOG of at least 2 | 15 (22.4%) | 17 (24.6%) | 1 (11.1%) | 0.658 | 15 (21.1%) | 13 (20.6%) | 5 (45.5%) | 0.174 |
| IPI score, n (%) | 0.878 | 0.123 | ||||||
| 0–2 | 50 (74.6%) | 51 (73.9%) | 6 (66.7%) | 50 (70.4%) | 51 (81.0%) | 6 (54.5%) | ||
| 3–5 | 17 (25.4%) | 18 (26.1%) | 3 (33.3%) | 21 (29.6%) | 12 (19.0%) | 5 (45.5%) | ||
| BCL2 expression, n (%) | 0.065 | 0.719 | ||||||
| Positive | 22 (32.8%) | 17 (24.6%) | 4 (44.4%) | 20 (28.2%) | 20 (31.7%) | 3 (27.3%) | ||
| Negative | 9 (13.4%) | 14 (20.3%) | 4 (44.4%) | 12 (16.9%) | 14 (22.2%) | 1 (9.1%) | ||
| Not tested | 36 (53.7%) | 38 (55.1%) | 1 (11.1%) | 39 (54.9%) | 29 (46.0%) | 7 (63.6%) | ||
| Response, n (%) | 0.555 | 0.226 | ||||||
| CR | 47 (70.1%) | 54 (78.3%) | 8 (88.9%) | 54 (76.1%) | 47 (74.6%) | 8 (72.7%) | ||
| PR | 10 (14.9%) | 9 (13.0%) | 0 (0%) | 6 (8.5%) | 10 (15.9%) | 3 (27.3%) | ||
| SD/PD | 10 (14.9%) | 6 (8.7%) | 1 (11.1%) | 11 (15.5%) | 6 (9.5%) | 0 (0%) | ||
| ORR (CR + PR) | 57 (85.1%) | 63 (91.3%) | 8 (88.9%) | 60 (84.5%) | 57 (90.5%) | 11 (100%) | ||
| Survival, n (%) † | 0.150 | 0.826 | ||||||
| Survival | 46 (74.2%) | 56 (81.2%) | 6 (66.7%) | 54 (77.1%) | 47 (77.0%) | 7 (77.8%) | ||
| Relapse | 9 (14.5%) | 4 (5.8%) | 0 (0%) | 8 (11.4%) | 4 (6.6%) | 1 (11.1%) | ||
| Death | 7 (11.3%) | 9 (13.0%) | 3 (33.3%) | 8 (11.4%) | 10 (16.4%) | 1 (11.1%) | ||
Total no. cases evaluated = 140. CR, complete response; ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic Index; LDH, lactate dehydrogenase; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
ABCG2 gene polymorphism study. ABCG2 Q141K and V12M genotyping was carried out by pyrosequencing of the polymerase chain reaction (PCR) products from the ABCG2 gene to determine the presence of the Q141K and V12M polymorphisms. PCR was carried out using a GeneAmp PCR 9700 (Applied Biosystems, Foster City, CA, USA) with an initial denaturation step of 94°C for 5 min, followed by 45 cycles of denaturation at 94°C for 20 s, annealing at 59–60°C for 20 s, and extension at 72°C for 20 s. Final termination of the elongation step was carried out at 72°C for 10 min. The sequences of all of the primers and the details of the annealing temperatures are listed in Table 2. The biotinylated PCR products were prepared for pyrosequencing analysis by the use of a Vaccum Pre Workstation (Biotage AB, Uppsala, Sweden), and the sequencing reactions were carried out on a PSQ 96MA system (Biotage AB) as described by the manufacturer. Sequence analysis software (Biotage AB) was used for measurement of the peak heights, and the results were exported to Microsoft Word for further analysis.
Table 2.
Sequences and information of primers used for pyrosequencing
| Name | Primer sequence (5′ to 3′) | Size (bp) | Polymerase chain reaction (Tm; °C) |
|---|---|---|---|
| ABCG2 V12M | F: CTCTCCAGATGTCTTCCAGTAATG | 110 | 59 |
| R: Biotin‐TCCTTCAGTAAATGCCTTCAGGT | |||
| S: TCGAAGTTTTTATCCCA | |||
| ABCG2 Q141K | F: Biotin‐ACTGCAGGTTCATCATTAGCTAGA | 238 | 60 |
| R: CCGTTCGTTTTTTTCATGATTC | |||
| S: CGAAGAGCTGCTGAGAA | |||
| F, forward; R, reverse; S, sequencing; Tm, melting temperature. | |||
Definitions. The clinical responses to R‐CHOP chemotherapy were scored according to the International Working Group criteria.( 17 ) OS was measured from day 1 of the first cycle of R‐CHOP until the date of death or the last follow up. EFS was calculated from day 1 of the first cycle of R‐CHOP until treatment failure (disease progression or recurrence, or death of any cause).
Side effects. All of the adverse events reported by the patient or observed by the investigator were recorded in predefined categories, and these were evaluated according to the Common Terminology Criteria for Adverse Events of the National Cancer Institute.( 18 ) The hematological toxicity profiles included anemia, leukeocytopenia, neutropenia, and thrombocytopenia, and the non‐hematological toxicities were fever (not neutropenic fever), mucositis, infection, nausea and vomiting, diarrhea, alopecia, and neurotoxicity.
Statistical analysis. The clinical data were analyzed according to information available as of December 2007. The primary objective of the current study was to correlate the ABCG2 Q141K and V12M polymorphisms with the response and survival outcomes, including OS and EFS to R‐CHOP therapy. The secondary objectives were to correlate the ABCG2 Q141K and V12M polymorphisms with the hematological and non‐hemtological toxicities of R‐CHOP therapy. The clinical characteristics, treatment outcomes, and toxicities of the patients were compared using χ2‐tests, Fisher's exact tests, or Mann–Whitney U‐tests according to the ABCG2 Q141K and V12M polymorphisms. Logistic regression analysis was conducted to determine the predictive value of the polymorphisms on response and adverse reaction to R‐CHOP for the 145 patients who had available both the ABCG2 Q141K and V12M polymorphism data. The variables included stage (stages 1 and 2 vs 3 and 4), IPI score (0–2 vs 3–5), age (<60 vs ≥60 years), performance status (Eastern Cooperative Oncology Group [ECOG] 0 and 1 vs ≥2), lactate dehydrogenase level (normal vs beyond normal range), extranodal involvement (≤1 vs ≥2 sites), B symptoms (absence vs presence), hematological toxicity (grade 0–II vs III–IV or grade 0 vs grade I–IV), non‐hematological toxicity (grade 0–II vs III–IV or grade 0 vs grade I–IV), and ABCG2 Q141K (QQ, QK, and KK) and ABCG2 V12M (VV, VM, and MM) genotypes. Multivariate analyses were carried out on 145 patients. Odds ratios (OR) and 95% confidence intervals (CI) for relative risks were also calculated by logistic regression analysis. Survival estimates were calculated using the Kaplan–Meier method. A cut‐off P‐value of 0.05 was adopted for all statistical analyses. The hazard ratio (HR) and 95% CI were also estimated. The statistical data were obtained using SPSS software package, version 11.5 (SPSS, Chicago, IL, USA).
Results
Frequency of ABCG2 Q141K and V12M polymorphisms. In the frontline R‐CHOP therapy group, the distribution of the QQ, QK, and KK genotypes of the ABCG2 Q141K polymorphism was 46.2, 47.6, and 6.2%, respectively (Table 1). The distribution of the VV, VM, and MM genotypes of the ABCG2 V12M polymorphism was 49.0, 43.4, and 7.6%, respectively (Table 1). The observed allele frequencies were consistent with the Hardy–Weinberg equilibrium.
Patient characteristics according to the ABCG2 Q141K and V12M polymorphisms. The patients’ characteristics are summarized in Table 1. In brief, no differences in the patient and disease characteristics were observed according to the ABCG2 Q141K and V12M polymorphisms.
Response to frontline R‐CHOP therapy according to the ABCG2 Q141K and V12M polymorphisms. Of the 145 patients who were evaluable for their response to chemotherapy, the overall response rate (ORR) was 88.3% (128 of 145 patients) with a complete response (CR) rate of 75.2% (109 of 145 patients), and a partial response (PR) rate of 13.1% (19 of 145 patients). As shown in Table 1, no significant difference in the response rate or the ORR was observed according to the ABCG2 Q141K and V12M polymorphisms. No statistically significant difference in the response rate or ORR was observed according to the ABCG2 Q141K and V12M polymorphisms.
Survival analysis according to the ABCG2 Q141K and V12M polymorphisms. At a median follow‐up duration of 16.4 months (range 0.13–41.4 months), 13 (9.0%) patients had relapsed or progressed, with 19 (13.1%) deaths and five (3.4%) follow‐up losses. The 1‐ and 2‐year OS rates were 90% ± 3 and 79% ± 6%, respectively, whereas the 1‐ and 2‐year EFS rates were 87% ± 3 and 69% ± 6%, respectively. When comparing OS and EFS according to the ABCG2 Q141K and V12M polymorphisms, neither the Q141K nor V12M polymorphisms had any impact on OS or EFS (Fig. 1).
Figure 1.
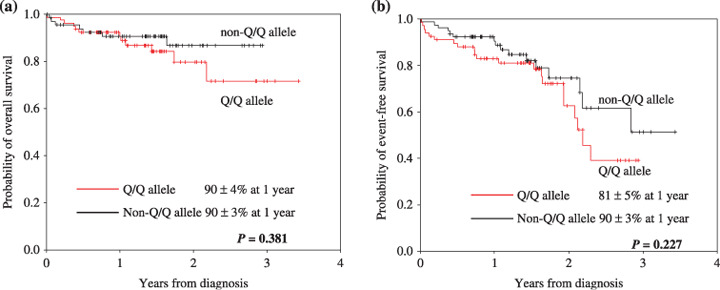
Survival curve after frontline rituximab plus cyclophosphamide–doxorubicin–vincristine–prednisone (R‐CHOP) therapy according to the ABCG2 Q141K genotype.
Side effects according to the ABCG2 Q141K and V12M polymorphisms. The treatment‐associated grade III–IV side effects encompassed mainly neutropenia (77/145, 46.9%) and leukocytopenia (61/145, 42.1%) (Table 3). No significant differences of such hematological toxicities as anemia, leukocytopenia, neutropenia, and thrombocytopenia were observed according to the ABCG2 Q141K and V12M alleles. Among the severe non‐hematological side effects (grade III–IV), fever, mucositis, infection, and diarrhea were observed more frequently in the patients with the non‐QQ alleles of the ABCG Q141K polymorphisms. Of them, fever and infection were statistically significant according to the ABCG2 Q141K polymorphism (QQ vs QK or KK genotype; P = 0.037 and 0.046, respectively). When considering the presence of toxicity (grade I–IV), regardless of severity, differences for fever (P = 0.007) and diarrhea (P = 0.002) in the non‐hematological toxicity profiles were noted and these were statistically significant according to the ABCG2 Q141K polymorphism (Table 4). On multivariate analysis, grade I–IV diarrhea was the statistically significant factor according to the ABCG2 Q141K polymorphism (HR 2.835; 95% CI 1.432–5.613; P = 0.003).
Table 3.
Hematological and non‐hematological toxicity outcomes according to the ABCG2 Q141K and V12M polymorphisms (grade 0–II vs grade III–IV)
| ABCG2 Q141K | P‐value | ABCG2 V12M | P‐value | |||
|---|---|---|---|---|---|---|
| QK or KK | VV | VM or MM | ||||
| No. patients (%) | 78 (53.8%) | 71 (49.0%) | 74 (51.0%) | |||
| Grade III–IV, n (%) | ||||||
| Anemia | 9 (13.4%) | 9 (11.5%) | 0.730 | 10 (14.1%) | 8 (10.8%) | 0.550 |
| Leukocytopenia | 29 (43.3%) | 32 (41.0%) | 0.784 | 33 (46.5%) | 28 (37.8%) | 0.292 |
| Neutropenia | 38 (56.7%) | 39 (50.0%) | 0.419 | 38 (53.5%) | 39 (52.7%) | 0.921 |
| Thrombocytopenia | 4 (6.0%) | 11 (14.1%) | 0.109 | 9 (12.7%) | 6 (8.1%) | 0.367 |
| Grade III–IV, n (%) | ||||||
| Fever | 8 (11.9%) | 20 (25.6%) | 0.037 | 16 (22.5%) | 12 (16.2%) | 0.335 |
| Mucostis | 11 (16.4%) | 22 (28.2%) | 0.091 | 19 (26.8%) | 14 (18.9%) | 0.260 |
| Infection | 9 (13.4%) | 21 (27.5%) | 0.046 | 13 (18.3%) | 17 (23.0%) | 0.488 |
| Nausea and vomiting | 2 (3.0%) | 5 (6.4%) | 0.337 | 3 (4.2%) | 4 (5.4%) | 0.740 |
| Diarrhea | 2 (3.0%) | 9 (11.5%) | 0.052 | 4 (5.6%) | 7 (9.5%) | 0.384 |
| Alopecia | 27 (40.3%) | 38 (48.7%) | 0.309 | 32 (45.1%) | 33 (44.6%) | 0.954 |
| Neurotoxicity | 2 (3.0%) | 4 (5.1%) | 0.518 | 3 (4.2%) | 3 (4.1%) | 0.959 |
| Bold and italic values significant at P < 0.05. | ||||||
Table 4.
Non‐hematological toxicity outcomes according to ABCG2 Q141K genotype (grade 0 vs grade I–IV)
| ABCG2 Q141K | P‐value | ||
|---|---|---|---|
| QK or KK | |||
| No. patients, n (%) | 67 (46.2%) | 78 (53.8%) | |
| Grade I–IV, n (%) | |||
| Fever | 18 (26.9%) | 38 (48.7%) | 0.007 |
| Mucositis | 43 (64.2%) | 50 (64.1%) | 0.992 |
| Infection | 24 (35.8%) | 40 (51.3%) | 0.062 |
| Diarrhea | 21 (31.3%) | 44 (56.4%) | 0.002 |
Bold and italic values significant at P < 0.05.
Discussion
Among several naturally occurring ABCG2 polymorphisms, V12M in exon 2 and Q141K in exon 5 occur in most racial groups, but they occur with a higher allellic frequency in Asians. In the present study, the frequencies of two polymorphisms, V12M and Q141K, were 0.293 and 0.300, respectively, and these values were comparable to those reported in the literature for Asians (0.230–0.241 and 0.150–0.360, respectively).( 8 , 10 , 16 , 19 , 20 , 21 ) When the frequencies of the present study were compared with those reported in the literature for Asian populations, the frequencies of Q141K in these Korean DLBCL patients and the Asian healthy controls, including Korean, Chinese, and Malaysian, were statistically insignificant using the χ2‐test (Table 5).( 8 , 10 , 16 , 19 ) Unlike a previous study that reported that ABCG2 Q141K is a candidate susceptibility gene in Chinese DLBCL patients,( 16 ) our findings suggest the ABCG2 Q141K polymorphism may not be associated with an increased risk of DLBCL in Koreans. However, the allele frequency of ABCG2 V12M in the present study was higher than that of the Korean healthy controls, and the association between ABCG2 V12M and disease susceptibility should be further investigated (Table 5).
Table 5.
ABCG2 Q141K and V12M genotype frequencies in Asian healthy subjects and cancer patients from the literature and the present study †
| Polymorphism | No. cases | Race | Disease status | Genotype frequency | Allele frequency | P‐value | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|
| W/W | W/M | M/M | W | M | ||||||
| c. 421C>A (Q141K) | 145 | Korea | Diffuse large B‐cell lymphoma | 67 (46.2%) | 69 (47.6%) | 9 (6.2%) | 0.700 | 0.300 | This study | |
| 275 | Korea | Healthy control | Not described | 0.720 | 0.280 | 0.306 | ( 10 ) | |||
| 376 | Chinese | Healthy control | 181 (48.1%) | 162 (43.1%) | 33 (8.8%) | 0.697 | 0.303 | 0.849 | ( 16 ) | |
| 95 | Chinese | Healthy control | 41 (43.2%) | 43 (45.3%) | 11 (11.6%) | 0.66 | 0.34 | 0.205 | ( 8 ) | |
| 94 | Chinese | Healthy control | 49 (52.1%) | 38 (40.4%) | 7 (7.4%) | 0.72 | 0.28 | 0.588 | ( 19 ) | |
| 97 | Malays | Healthy control | 54 (55.7%) | 33 (34.0%) | 10 (10.3%) | 0.73 | 0.27 | 0.278 | ( 19 ) | |
| 45 | Chinese | Cancer such as lung, colon, and genitourinary tract | 22 (48.9%) | 23 (51.1%) | 0 (0%) | 0.74 | 0.26 | 0.358 | ( 19 ) | |
| Malays | ||||||||||
| Indians | ||||||||||
| 156 | Chinese | Diffuse large B‐cell lymphoma | 60 (38.5%) | 80 (51.3%) | 16 (10.3%) | 0.64 | 0.36 | 0.023 | ( 16 ) | |
| 105 | Korea | Non‐small cell lung cancer | 59 (56.2%) | 42 (40.0%) | 4 (3.8%) | 0.762 | 0.238 | 0.035 | ( 20 ) | |
| c. 34G>A (V12M) | 145 | Korea | Diffuse large B‐cell lymphoma | 71 (49.0%) | 63 (43.4%) | 11 (7.6%) | 0.707 | 0.293 | This study | |
| 275 | Korea | Healthy control | Not described | 0.770 | 0.230 | 0.001 | ( 10 ) | |||
† Based on the reported ABCG2 Q141K and V12M allele frequencies in Asian population, we compared the frequencies of ABCG2 Q141K polymorphisms using χ2‐tests. M, mutant type; W, wild type.
The present study demonstrated that the ABCG2 Q141K and V12M polymorphisms were not predictive of the response, OS, or EFS to R‐CHOP chemotherapy in patients with DLBCL. No association of the ABCG2 Q141K and V12M polymorphisms with response and survival was noted in the present study for the following reasons: (1) the relatively small number of patients; (2) the relatively short period of follow up may not have been enough to see a significant difference in survival; and (3) other unknown chemoresistance mechanisms are probably important in the mechanism of R‐CHOP action, and this would be expected to affect the response and survival of DLBCL patients. Accordingly, further study is necessary to reach a clear conclusion on this issue.
Several groups have reported that the Q141K genotype is associated with altered pharmacokinetic parameters of some ABCG2 substrates.( 8 , 15 , 22 ) Notably, the Q141K genotype has the signature of recent positive selection and it is the strongest in the Asian population,( 23 ) suggesting that there is some advantageous property for this genotype. The Q141K polymorphism has been associated with lower expression and activity of ABCG2 and with higher accumulation of ABCG2 substrates.( 24 ) On univariate analysis, the present study showed statistically significant results for non‐hematological toxicity such as grade I–IV diarrhea, grade I–IV fever, grade III–IV fever, and grade III–IV infection, according to ABCG2 Q141K polymorphism (QQ genotype vs non‐QQ genotype, P = 0.002, 0.007, 0.037, and 0.046, respectively). On multivariate analysis, the present study demonstrated that the ABCG2 Q141K polymorphism was associated with grade I–IV diarrhea (P = 0.003), just like a previous report for which the ABCG2 Q141K polymorphism was associated with diarrhea in gefitinib‐treated patients with non‐small cell cancer.( 11 ) Diarrhea is one of the most common side effects of chemotherapy.( 25 ) Chemotherapy‐induced diarrhea significantly affects quality of life and it may result in early death either directly, from life‐threatening sequelae, or indirectly, from adjustments in the cancer treatment, which result in suboptimal therapy.( 26 ) Of the R‐CHOP agents, cyclophosphamide and doxorubicin are associated with high rates of diarrhea.( 27 ) The risk factors that appear to be associated with an increased incidence of chemotherapy‐induced diarrhea are elderly patients (>65 years), female, a low performance status (ECOG 2 or more), and genetic polymorphisms that affect drug metabolism and distribution. Until now, the genetic polymorphisms associated with chemotherapy‐induced diarrhea were studied mainly by examining genetic defects in uridine diphosphate glucoronosyltransferase isoform 1A1. Therefore, the present study demonstrates the necessity of study for determining the association between ABCG2 Q141K polymorphism and adverse reactions to chemotherapy. Nonetheless, due to a fairly small number of patients, results from these analyses may not be representative of the larger patient population. Further larger, more well‐defined studies including the multifactorial factors determining the interindividual variability are warranted for using this polymorphism to optimize R‐CHOP‐based chemotherapy before they are developed into routine clinical tests.
To the best of our knowledge, the present study provides the first evidence that adverse events related to R‐CHOP treatment with ABCG2 substrate drugs are linked to a common functional polymorphism in the ABCG2 gene for DLBCL patients. The mechanism underlying the functional impact of the ABCG2 Q141K amino acid change is not entirely known, but it is most likely associated with reduced protein levels and altered ATPase activity.( 15 ) The association we observed between ABCG2 Q141K genotype status and observed clinical adverse reactions such as diarrhea, infection, and fever may reflect a role for this transporter in the metabolism and elimination pathways of R‐CHOP, especially doxorubicin.
In summary, the present study demonstrates that the ABCG2 Q141K polymorphism is associated with chemotherapy‐induced diarrhea in patients with DLBCL who have received frontline R‐CHOP chemotherapy. ABCG2 common polymorphisms do not appear to play a role in overall disease susceptibility, phenotype, response to or survival after R‐CHOP chemotherapy in the Korean population. However, the association between the presence of ABCG2 polymorphisms and adverse reactions to R‐CHOP chemotherapy for patients with DLBCL clearly need to be replicated in additional patient populations, and the present study introduces the potential for practical pharmaceutical implications for optimizing treatment with such agents.
Acknowledgments
This study was supported by grants from the special clinical fund of Gyeongsang National University Hospital.
References
- 1. Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem 1993; 62: 385–427. [DOI] [PubMed] [Google Scholar]
- 2. Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance‐associated proteins. J Natl Cancer Inst 2000; 92: 1295–302. [DOI] [PubMed] [Google Scholar]
- 3. Doyle LA, Yang W, Abruzzo LV et al . A multidrug resistance transporter from human MCF‐7 breast cancer cells. Proc Natl Acad Sci USA 1998; 95: 15 665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miyake K, Mickley L, Litman T et al . Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone‐resistant cells: demonstration of homology to ABC transport genes. Cancer Res 1999; 59: 8–13. [PubMed] [Google Scholar]
- 5. Maliepaard M, Van Gastelen MA, De Jong LA et al . Overexpression of the BCRP/MXR/ABCP gene in a topotecan‐selected ovarian tumor cell line. Cancer Res 1999; 59: 4559–63. [PubMed] [Google Scholar]
- 6. Kawabata S, Oka M, Shiozawa K et al . Breast cancer resistance protein directly confers SN‐38 resistance of lung cancer cells. Biochem Biophys Res Commun 2001; 280: 1216–23. [DOI] [PubMed] [Google Scholar]
- 7. Yanase K, Tsukahara S, Mitsuhashi J, Sugimoto Y. Functional SNPs of the breast cancer resistance protein – therapeutic effects and inhibitor development. Cancer Lett 2006; 234: 73–80. [DOI] [PubMed] [Google Scholar]
- 8. De Jong FA, Marsh S, Mathijssen RH et al . ABCG2 pharmacogenetics: ethnic differences in allele frequency and assessment of influence on irinotecan disposition. Clin Cancer Res 2004; 10: 5889–94. [DOI] [PubMed] [Google Scholar]
- 9. Tamura A, Wakabayashi K, Onishi Y et al . Re‐evaluation and functional classification of non‐synonymous single nucleotide polymorphisms of the human ATP‐binding cassette transporter ABCG2. Cancer Sci 2007; 98: 231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee SS, Jeong HE, Yi JM et al . Identification and functional assessment of BCRP polymorphisms in a Korean population. Drug Metab Dispos 2007; 35: 623–32. [DOI] [PubMed] [Google Scholar]
- 11. Cusatis G, Gregorc V, Li J et al . Pharmacogenetics of ABCG2 and adverse reactions to gefitinib. J Natl Cancer Inst 2006; 98: 1739–42. [DOI] [PubMed] [Google Scholar]
- 12. Backstrom G, Taipalensuu J, Melhus H et al . Genetic variation in the ATP‐binding cassette transporter gene ABCG2 (BCRP) in a Swedish population. Eur J Pharm Sci 2003; 18: 359–64. [DOI] [PubMed] [Google Scholar]
- 13. Iida A, Saito S, Sekine A et al . Catalog of 605 single‐nucleotide polymorphisms (SNPs) among 13 genes encoding human ATP‐binding cassette transporters: ABCA4, ABCA7, ABCA8, ABCD1, ABCD3, ABCD4, ABCE1, ABCF1, ABCG1, ABCG2, ABCG4, ABCG5, and ABCG8 . J Hum Genet 2002; 47: 285–310. [DOI] [PubMed] [Google Scholar]
- 14. Honjo Y, Morisaki K, Huff LM et al . Single‐nucleotide polymorphism (SNP) analysis in the ABC half‐transporter ABCG2 (MXR/BCRP/ABCP1). Cancer Biol Ther 2002; 1: 696–702. [DOI] [PubMed] [Google Scholar]
- 15. Mizuarai S, Aozasa N, Kotani H. Single nucleotide polymorphisms result in impaired membrane localization and reduced ATPase activity in multidrug transporter ABCG2. Int J Cancer 2004; 109: 238–46. [DOI] [PubMed] [Google Scholar]
- 16. Hu LL, Wang XX, Chen X et al . BCRP gene polymorphisms are associated with susceptibility and survival of diffuse large B‐cell lymphoma. Carcinogenesis 2007; 28: 1740–4. [DOI] [PubMed] [Google Scholar]
- 17. Cheson BD, Horning SJ, Coiffier B et al . Report of an international workshop to standardize response criteria for non‐Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999; 17: 1244–1253. [DOI] [PubMed] [Google Scholar]
- 18. National Institutes of Health, US Department of Health and Human Services, Cancer Therapy Evaluation Program . Terminology Criteria for Adverse Effects, Version 3.0. Bethesda: National Cancer Institute, 2003. [Google Scholar]
- 19. Jada SR, Lim R, Wong CI et al . Role of UGT1A1*6, UGT1A1*28 and ABCG2 c.421C>A polymorphisms in irinotecan‐induced neutropenia in Asian cancer patients. Cancer Sci 2007; 98: 1461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han JY, Lim HS, Yoo YK et al . Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with irinotecan – pharmacokinetics and clinical outcome in patients with advanced non‐small cell lung cancer. Cancer 2007; 110: 138–47. [DOI] [PubMed] [Google Scholar]
- 21. Korenaga Y, Naito K, Okayama N et al . Association of the BCRP C421A polymorphism with nonpapillary renal cell carcinoma. Int J Cancer 2005; 117: 431–4. [DOI] [PubMed] [Google Scholar]
- 22. Morisaki K, Robey RW, Ozvegy‐Laczka C et al . Single nucleotide polymorphisms modify the transporter activity of ABCG2. Cancer Chemother Pharmacol 2005; 56: 161–72. [DOI] [PubMed] [Google Scholar]
- 23. Wang Z, Wang J, Tantoso E et al . Signatures of recent positive selection at the ATP‐binding cassette drug transporter superfamily gene loci. Hum Mol Genet 2007; 16: 1367–80. [DOI] [PubMed] [Google Scholar]
- 24. Imai Y, Nakane M, Kage K et al . C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low‐level drug resistance. Mol Cancer Ther 2002; 1: 611–16. [PubMed] [Google Scholar]
- 25. Arnold RJ, Gabrail N, Raut M, Kim R, Sung JC, Zhou Y. Clinical implications of chemotherapy‐induced diarrhea in patients with cancer. J Support Oncol 2005; 3: 227–32. [PubMed] [Google Scholar]
- 26. Richardson G, Dobish R. Chemotherapy induced diarrhea. J Oncol Pharm Pract 2007; 13: 181–98. [DOI] [PubMed] [Google Scholar]
- 27. National Cancer Institute, US National Institutes of Health . Gastrointestinal Complications – Diarrhea. 2003. Available from URL: http://www.cancer.gov/cancertopics/pdq/supportivecare/gastrointestinalcomplications/healthprofessional/page6


