Abstract
Recent study of murine fibrosarcoma has revealed that platelet‐derived growth factor (PDGF) plays a direct role in promoting lymphangiogenesis and metastatic spread to lymph nodes. Thus, we investigated the relation between PDGF and PDGF receptor (PDGF‐R) expression and lymphatic metastasis in human gastric carcinoma. We examined PDGF‐B and PDGF‐Rβ expression in four human gastric carcinoma cell lines (TMK‐1, MKN‐1, MKN‐45, and KKLS) and in 38 surgical specimens of gastric carcinoma. PDGF‐B and PDGF‐Rβ expression was examined by immunofluorescence in surgical specimens and in human gastric carcinoma cells (TMK‐1) implanted orthotopically in nude mice. Groups of mice (n = 10, each) received saline (control) or PDGF‐R tyrosine kinase inhibitor imatinib. PDGF‐B and PDGF‐Rβ mRNA expression was significantly higher in patients with lymph node metastasis than in those without and was also significantly higher in diffuse‐type carcinoma than in intestinal‐type carcinoma. In surgical specimens, tumor cells expressed PDGF‐B, but PDGF‐Rβ was expressed predominantly by stromal cells. Under culture conditions, expression of PDGF‐B mRNA was found in all of the gastric cell lines, albeit at different levels. In orthotopic TMK‐1 tumors, cancer cells expressed PDGF‐B but not PDGF‐Rβ. PDGF‐Rβ was expressed by stromal cells, including lymphatic endothelial cells. Four weeks of treatment with imatinib significantly decreased the area of lymphatic vessels. Our data indicate that secretion of PDGF‐B by gastric carcinoma cells and expression of PDGF‐Rβ by tumor‐associated stromal cells are associated with lymphatic metastasis. Blockade of PDGF‐R signaling pathways may inhibit lymph node metastasis of gastric carcinoma. (Cancer Sci 2010)
Gastric cancer is one of the most frequent malignancies in the world. The major cause of mortality is metastasis, which relies on de novo formation of blood and lymphatic vessels.( 1 ) Although induction of tumor angiogenesis is known to be a complex process that involves the interplay of a dozen or more tumor‐derived growth factors,( 2 ) how tumors induce lymphangiogenesis is poorly understood.
Among known lymphangiogenic factors, the best‐characterized growth factors are vascular endothelial growth factor C (VEGF‐C) and VEGF‐D.( 3 , 4 , 5 , 6 , 7 , 8 ) Fibroblast growth factor‐2 promotes lymphatic vessel growth in the mouse cornea, but this effect is believed to occur indirectly, via induction of VEGF‐C expression and activation of VEGF receptor 3 (VEGFR‐3) signaling.( 9 ) It is unlikely that VEGF‐C and ‐D and VEGFR‐3 are the sole factors regulating such processes. A range of lymphangiogenic factors produced by tumor cells, endothelial cells, and stromal cells has recently been identified. These include VEGF‐A, and members of the hepatocyte growth factor (HGF) and angiopoietin (Ang) families.( 10 , 11 , 12 ) Additionally, interesting preclinical studies have indicated that platelet‐derived growth factors (PDGFs) and PDGF receptors (PDGF‐Rs) not only promote hemangiogenesis and direct tumor cell growth but are important players in lymphangiogenesis.( 13 )
Members of the PDGF family are often expressed at high levels in many malignant tissues.( 14 ) The PDGF family consists of five isoforms, ‐AA, ‐AB, ‐BB, ‐CC, and ‐DD, usually referred to as PDGF‐A (AA), PDGF‐B (AB and BB), PDGF‐C (CC), and PDGF‐D (DD).( 15 ) Their biological activities are mediated by three forms of the tyrosine kinase receptor encoded by two gene products, PDGF‐Rα and ‐Rβ. PDGF‐Rα binds all possible forms of PDGF except PDGF‐DD, whereas PDGF‐Rβ preferentially binds PDGF‐BB.( 16 ) PDGFs have been found to induce tumor growth by directly stimulating growth of certain types of tumor cells,( 17 ) to stimulate angiogenesis,( 18 ) to recruit pericytes( 19 ) and to control the interstitial fluid pressure in stroma, influencing transvascular transport of chemotherapeutic agents in a paracrine manner.( 20 ) Recently, Cao et al. ( 13 ) showed that expression of PDGF‐B in murine fibrosarcoma cells induced tumor lymphangiogenesis, leading to enhanced lymph node metastasis. However, there is no report concerning the relation between PDGF‐B and PDGF‐Rβ expression and lymphatic metastasis in human gastric carcinoma. Thus, we examined the expression profile of PDGF‐B and PDGF‐Rβ in human gastric carcinoma, and we examined whether blocking PDGF‐R can inhibit lymphangiogenesis of gastric cancer in vivo.
Materials and Methods
Patients and tumor specimens. Endoscopic biopsy specimens (tumor and corresponding normal mucosa) of gastric tissue from 38 patients with gastric carcinoma who later underwent surgical resection at Hiroshima University Hospital were snap‐frozen in liquid nitrogen and stored at −80°C until RNA extraction for quantitative RT‐PCR. Informed consent was obtained from all patients for participation in the study according to the Declaration of Helsinki. Pathology reports and clinical histories were reviewed for accurate staging at the time of surgery. Criteria for staging and histologic classification were those proposed by the Japanese Research Society for Gastric Cancer.( 21 ) Lymph node status was determined by routine pathological examination with the surgical specimens. Two groups of patients, those with lymph node metastasis (node‐positive group, n = 21) and those without (node‐negative group, n = 17), were closely matched for histologic type and depth of invasion. The patient group comprised 34 men and four women with a median age of 66.5 years. All patients had invasive gastric carcinoma in which the tumor invasion was beyond the submucosa.
Cell cultures. Four cell lines established from human gastric carcinomas and human osteosarcoma cell line MG63 were maintained in RPMI‐1640 (Nissui, Tokyo, Japan) with 10% fetal bovine serum (FBS; MA BioProducts, Baltimore, MD, USA). TMK‐1 cell line (a poorly differentiated adenocarcinoma) was`provided by Dr E. Tahara of Hiroshima University. KKLS cell line (an undifferentiated carcinoma) was provided by Dr Y. Takahashi of Chiba University, Chiba, Japan. Two other cell lines (MKN‐1, from an adenosquamous carcinoma, and MKN‐45, from a poorly differentiated adenocarcinoma) as well as MG63 were obtained from the Health Science Research Resources Bank, Osaka, Japan.
Quantitative real‐time RT‐PCR analysis. Total RNA was extracted from gastric carcinoma cell lines and biopsy specimens with an RNeasy Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. cDNA was synthesized from 1 μg total RNA with a first‐strand cDNA synthesis kit (Amersham Biosciences, Piscataway, NJ, USA). After reverse transcription of RNA into cDNA, quantitative RT‐PCR was performed with a LightCycler‐FastStart DNA Master SYBR‐Green I Kit (Roche Diagnostics, Basel, Switzerland) according to the manufacturer’s recommended protocol. Polymerase chain reaction (PCR) reactions were carried out in triplicate. To correct for differences in both RNA quality and quantity between samples, values were normalized to those of β‐actin. The mRNA ratio between gastric carcinoma tissues (T) and corresponding normal mucosa (N) was calculated and expressed as the T/N ratio. Primers for PCR were designed with specific primer analysis software (Primer Designer; Scientific and Educational Software, Durham, NC, USA), and specificity of the sequences was confirmed by FASTA (EMBL Database). Respective primer sequences, annealing temperatures, and PCR cycles were as follows: PDGF‐B forward, CGAGTTGGACCTGAACATGA and PDGF‐B reverse, GTCACCGTGGCCTTCTTAAA (PDGF‐B PCR product, 339 bp; 58°C; 35 cycles); PDGF‐Rβ forward, AGCTACCCCTCAAGGAATCATAG and PDGF‐Rβ reverse, CTCTGGTGGATGGATTAAGACTG (PDGF‐Rβ PCR product, 376 bp; 58°C; 35 cycles); and GAPDH forward, ATCATCCCTGCCTCTACTGG and GAPDH reverse, CCCTCCGACGCCTGCTTCAC (GAPDH PCR product, 188 bp; 55°C; 28 cycles).
Reagents. Imatinib (imatinib mesylate or Gleevec; Novartis Pharma, Basel, Switzerland) is a 2‐phenylaminopyrimidine class protein‐tyrosine kinase inhibitor of PDGF‐R, BCR–ABL, and c‐Kit.( 22 , 23 ) Imatinib was diluted in sterile water for oral administration. Primary antibodies were purchased from as follows: polyclonal rabbit anti‐PDGF‐Rβ and polyclonal rabbit anti‐PDGF‐B subunit from Santa Cruz Biotechnology (Santa Cruz, CA, USA); and rat antimouse Lyve‐1 from R&D Systems (Minneapolis, MN, USA).
Western blot analysis. After three washes with cold phosphate‐buffered saline (PBS) containing 1 mmol/L sodium orthovanadate, cells were lysed. Proteins (total protein 20 μg) were separated by SDS‐PAGE and transferred to nitrocellulose transfer membranes (Whatman, Dassel, Germany). The immune complexes were visualized by enhanced chemiluminescence with an ECL Plus Kit (GE Healthcare, Buckinghamshire, UK).
Animals and orthotopic implantation of tumor cells. Male athymic BALB/c nude mice were obtained from Charles River Japan (Tokyo, Japan). The mice were maintained under specific pathogen‐free conditions and used at 5 weeks of age. This study was carried out after permission was granted by the Committee on Animal Experimentation of Hiroshima University.
Subconfluent gastric cancer cells (TMK‐1 or KKLS cells) to be used for implantation were harvested by brief treatment with 0.25% trypsin and 0.02% ethylenediamine tetraacetic acid, and then resuspended to a final concentration of 2.0 × 107 cells/mL Hanks’ solution. With the use of a 30‐gauge needle attached to a 1‐mL syringe, cells (1 × 106 cells in 50 μL) were implanted into the gastric walls in the nude mice under observation with a zoom stereomicroscope. After 4 weeks, the mice were killed, and the tumors were resected for study. The tumors were embedded in OCT compound (Miles, Elkhart, IN, USA), rapidly frozen in liquid nitrogen, and stored at −80°C.
Immunofluorescence staining for PDGF‐B and PDGF‐Rβ, and double staining for PDGF‐Rβ and Lyve‐1. Fresh frozen specimens of human gastric carcinomas as well as human gastric carcinomas growing in nude mice were cut into 8‐μm sections, mounted on positively charged slides, and stored at −80°C. Tissue sections were fixed in cold acetone for 10 min and then washed three times with PBS for 3 min each. Slides were placed in a humidified chamber and incubated with protein blocking solution (5% normal horse serum and 1% normal goat serum in PBS) for 20 min at room temperature. The slides were incubated overnight at 4°C with primary antibody against PDGF‐B or PDGF‐Rβ, then rinsed three times with PBS, incubated for 10 min in protein blocking solution, and incubated for 1 h at room temperature with Cy3‐conjugated goat antirabbit secondary antibody. From this point onwards, the slides were protected from light. The samples were then rinsed three times in PBS. To identify lymph endothelial cells, slides were incubated overnight at 4°C with antibody against Lyve‐1. The sections were rinsed three times with PBS and incubated for 10 min in protein blocking solution. Slides were then incubated for 1 h at room temperature with corresponding Cy5‐conjugated secondary antibody, and the samples were rinsed three times in PBS. 4′,6‐diamidino‐2‐phenylindole (DAPI) nuclear counterstain was applied for 10 min. Samples were then rinsed three times with PBS, and mounting medium was placed on each sample, which was then covered with a glass coverslip. We used Fluoromount/Plus (Diagnostic Bio Systems, Pleasanton, CA, USA) as the mounting medium.
Confocal microscopy. Confocal fluorescence images were obtained at ×203 or ×403 magnification on a Zeiss LSM 510 laser scanning microscopy system (Carl Zeiss, Thornwood, NY, USA) equipped with a motorized Axioplan microscope, argon laser (458/477/488/514 nm, 30 mW), HeNe lasers (543 nm, 1 mW; 633 nm, 5 mW), LSM 510 control and image acquisition software, and appropriate filters (Chroma Technology, Brattleboro, VT, USA). Confocal images were exported to Adobe Photoshop (Adobe Systems, San Jose, CA, USA), and photo montages were prepared for publication. Lymphatic endothelial cells were identified by green fluorescence, whereas PDGF and PDGF‐R were identified by red fluorescence.
Treatment of established human gastric cancer tumors growing in murine gastric walls. Fourteen days after orthotopic implantation of TMK‐1 cells, mice (n = 10 each group) were randomly assigned to receive one of the following two treatments: daily oral gavage of water (control group) or daily oral gavage of imatinib (50 mg/kg, optimal biological dose as determined previously( 17 , 24 )). The treatments continued for 28 days. All therapy experiments were performed twice. The mice bearing orthotopic tumors were euthanized by methophane on day 29. For immunohistochemistry, the tumor tissues were fixed in formalin and embedded in paraffin.
Immunohistochemical determination of the area of lymphatic vessels. Paraffin‐embedded tissues were used for immunohistochemical identification of Lyve‐1. Sections were deparaffinized and rehydrated in PBS, microwaved in water for 5 min for antigen retrieval, incubated overnight at 4°C with mouse anti‐Lyve‐1 antibody, and incubated for 1 h at room temperature with a peroxidase‐conjugated rat antimouse antibody. A positive reaction was detected by exposure to stable 3,3′‐diaminobenzidine for 5–10 min. Slides were counterstained with Gill’s hematoxylin. On slides immunolabeled for Lyve‐1, only vessels with typical morphology (including a lumen) were counted as lymphatic vessels because of occasional weak antibody cross‐reactivity with fibroblasts.( 25 ) For quantification of the lymphatic vessel areas, 10 random fields at ×100 magnification were captured for each tumor, and the outline of each lymphatic vessel including a lumen was manually traced. The areas were then calculated with the use of NIH ImageJ software (http://rsbweb.nih.gov/ij/download.html).
Statistical analysis. Results are expressed as mean ± SE. Wilcoxon/Kruskal–Wallis analysis was used to analyze between‐group differences in continuous variables. A P‐value of <0.05 was considered statistically significant.
Results
Expression levels of PDGF‐B and PDGF‐Rβ mRNAs in human gastric carcinoma. We initially examined mRNA expression of PDGF‐B and PDGF‐Rβ by quantitative real‐time PCR. The relative expression levels (T/N ratio) of PDGF‐B and PDGF‐Rβ are shown according to node status in Table 1. Patients with positive lymph nodes showed significantly greater expression of PDGF‐B and PDGF‐Rβ than node‐negative patients (P = 0.03 and P < 0.001, respectively). We also examined the relation between PDGF‐B and PDGF‐Rβ mRNA expression and histologic type of human gastric carcinoma because diffuse‐type gastric carcinoma is known to have abundant stroma and a high probability of lymph node metastasis. Expression of PDGF‐B and PDGF‐Rβ was significantly greater in patients with diffuse‐type gastric carcinoma than in those with intestinal‐type gastric carcinoma (P = 0.03 and P = 0.004, respectively) (Table 1).
Table 1.
Results of quantitative real‐time PCR for mRNA expression of PDGF‐B and PDGF‐Rβ of human gastric carcinoma specimens
| PDGF‐Rβ | PDGF‐B | |||
|---|---|---|---|---|
| Lymph node status | ||||
| Node‐positive (n = 21) | 6.46 ± 1.45 | P = 0.0001 | 5.06 ± 1.88 | P = 0.027 |
| Node‐negative (n = 17) | 0.97 ± 0.29 | 0.95 ± 0.18 | ||
| Histologic type | ||||
| Intestinal (n = 22) | 1.32 ± 0.42 | P = 0.0041 | 1.54 ± 0.71 | P = 0.028 |
| Diffuse (n = 16) | 5.45 ± 1.33 | 5.02 ± 2.16 | ||
PDGF‐B, platelet‐derived growth factor B; PDGF‐Rβ, PDGF receptor β.
Expression of PDGF‐B and PDGF‐Rβ in human gastric carcinoma cell lines growing in culture. We examined expression of PDGF‐B and PDGF‐Rβ in four human gastric carcinoma cell lines derived from different histological types. MG63 cells were used as a positive control for PDGF‐R expression. The results of real‐time PCR and western blot analysis are shown in Figure 1. Under culture conditions, expression of PDGF‐B mRNA was found in all of the gastric cell lines, albeit at different levels. PDGF‐Rβ was not expressed by the cultured gastric carcinoma cell lines.
Figure 1.
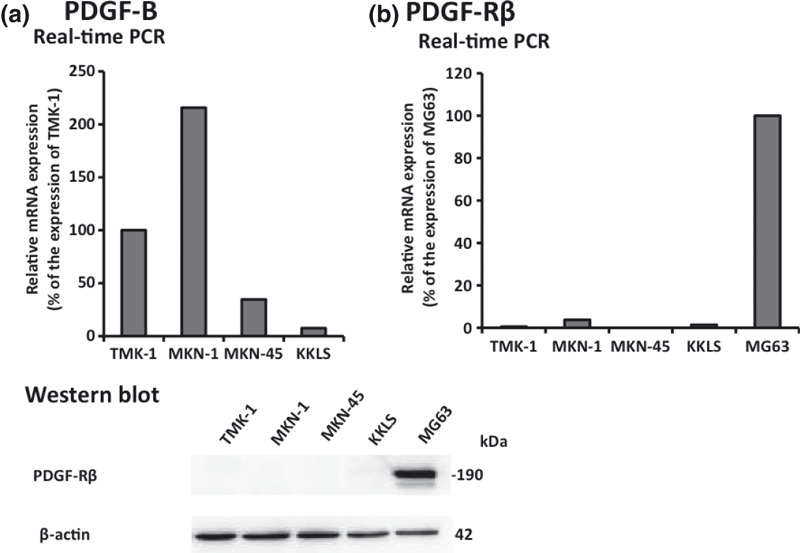
Expression of platelet‐derived growth factor B (PDGF‐B) and PDGF receptor β (PDGF‐Rβ) in gastric carcinoma cell lines. (a) Gastric cancer cell lines constitutively expressed mRNA for PDGF‐B subunit at various levels. (b) PDGF‐Rβ was not expressed by the cultured gastric carcinoma cell lines.
Immunolocalization of PDGF‐B and PDGF‐Rβ in human gastric carcinoma tissues and in human gastric carcinoma cells growing in the mouse stomachs. We next confirmed PDGF‐B and PDGF‐Rβ expression in vivo. We used TMK‐1 and KKLS cells for animal models because the other cell lines (MKN‐1 and MKN‐45) were difficult to grow in the mouse gastric walls. Representative photomicrographs are shown in Figure 2. Because TMK‐1 tumors had more abundant stroma than KKLS tumors, it was convenient to use TMK‐1 tumors to evaluate PDGF‐B and PDGF‐Rβ expression in stroma. In the surgical specimens and the orthotopic xenograft models, tumor cells expressed PDGF‐B, but PDGF‐Rβ was expressed predominantly by stromal cells (Fig. 2a–d).
Figure 2.
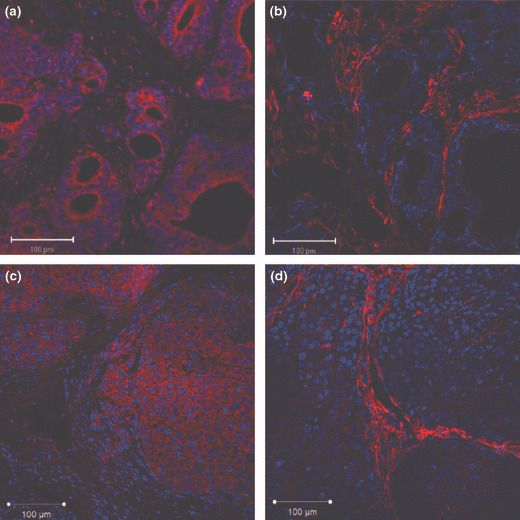
Immunohistochemical detection of platelet‐derived growth factor B (PDGF‐B) and PDGF receptor β (PDGF‐Rβ) in gastric carcinoma. (a,b) Immunolocalization of PDGF‐B and PDGF‐Rβ in human gastric carcinoma tissues. (c,d) Immunolocalization of PDGF‐B and PDGF‐Rβ in an orthotopic xenograft model (TMK‐1 cell line). Expression of PDGF‐B (a,c) and PDGF‐Rβ (b,d) in tumor tissue appears red.
Immunohistochemical analysis of PDGF‐Rβ and Lyve‐1 expression in TMK‐1 tumors. To identify whether tumor‐associated lymphatic vessels express PDGF‐Rβ, we analysed co‐localization of PDGF‐Rβ and Lyve‐1 (lymphatic endothelial cells). Co‐localization of green (Lyve‐1) and red (PDGF‐Rβ) fluorescence appeared as yellow fluorescence, indicating that tumor‐associated lymphatic vessels expressed PDGF‐Rβ. PDGF‐Rβ was expressed occasionally on lymphatic endothelial cells, especially on enlarged and tortuous lymphatic vessels located immediately adjacent to tumor nests (Fig. 3a–g). Lymphatic vessels in normal tissue or intratumoral lymphatic vessels did not express PDGF‐Rβ (Fig. 3h).
Figure 3.
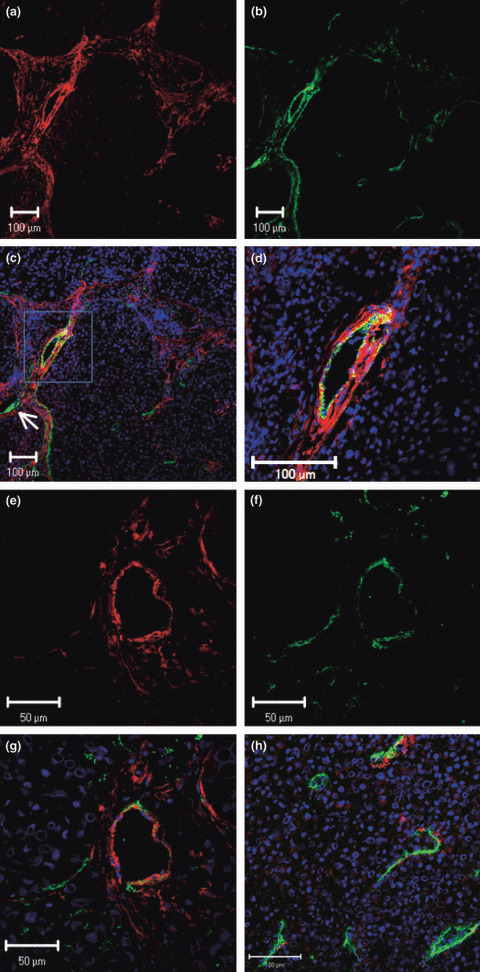
Fluorescence double‐labeled immunohisto‐chemistry (IHC) of TMK‐1 human gastric cancer cells growing in nude mice. Representative images show IHC for PDGF‐Rβ in red and Lyve‐1 (lymphatic endothelial marker) in green. (a–g) PDGF‐Rβ was detected in lymphatic endothelial cells on enlarged and tortuous lymphatic vessels located immediately adjacent to tumor nests. Small lymphatic vessels (arrow) did not express PDGF‐Rβ. (h) Intratumoral lymphatic vessels did not express PDGF‐Rβ.
Treatment of human gastric carcinoma growing in mouse stomachs. We determined the effects of imatinib, PDGF‐Rβ thyrosine kinase inhibitor, on lymphatic vessels in tumors growing up from implantation of TMK‐1 human gastric carcinoma cells into the stomachs of nude mice. The tumors treated with imatinib had reduced areas of lymphatic vessels in comparison to areas of lymphatic vessels in control tumors (P < 0.05) (Fig. 4).
Figure 4.
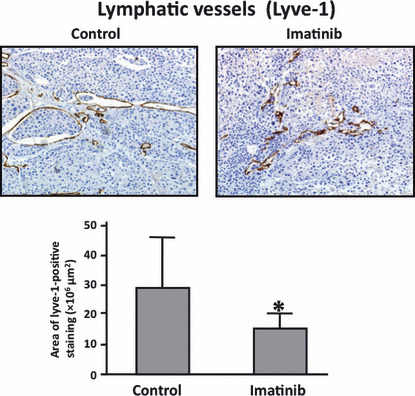
Immunohistochemistry for Lyve‐1 in TMK‐1 orthotopic gastric tumors with and without imatinib treatment. Treatment with imatinib significantly reduced the area of lymphatic vessels. *P < 0.05; bars, SE.
Discussion
Lymph node metastasis is a common clinical finding in many human cancers and is associated with both aggressive disease and poor prognosis. Genetic and epigenetic alterations of tumor cells often lead to amplified expression of multiple growth factors that contribute to tumor growth, angiogenesis and lymphangiogenesis.( 26 )
VEGF‐C and VEGF‐D through interaction with VEGFR‐3 expressed on lymphatic endothelial cells were once thought to be the only direct lymphangiogenic factors.( 27 ) However, tumors with high lymphatic metastatic ability express additional growth factors at high levels, suggesting that these factors may contribute to tumor lymphangiogenesis.( 26 ) In an extensive study in which Cao et al. ( 13 ) implanted PDGF‐A and PDGF‐B in mouse corneas, both factors were shown to induce growth of lymphatic vessels, although PDGF‐B was more potent than PDGF‐A. Additionally, its main receptor, PDGF‐Rβ, was detected on the induced lymphatic endothelial cells. VEGF‐C/‐D and VEGFR‐3 antagonists did not inhibit PDGF‐B‐induced lymphangiogenesis. PDGF‐B was also shown to activate intracellular signaling components by phosphorylation of Akt, Src, and Erk. In the present study, patients with positive lymph nodes showed significantly greater expression of PDGF‐B and PDGF‐Rβ than that of node‐negative patients.
Tumor blood vessels have been shown to differ morphologically from their normal counterparts. The endothelial cells are structurally and functionally abnormal and can acquire cytogenetic abnormalities while in the tumor microenvironment.( 28 ) Tumor lymphatic vessels appear to be structurally disorganized, tortuous, and leaky.( 13 ) These leaky tumoral lymphatics could provide a vulnerable structural basis for tumor cell invasion into the lymphatic system. Like tumor blood cells, tumor‐associated lymphatic vessels have been recently shown to have differentially expressed genes. Clasper et al. ( 29 ) compared gene expression of purified lymphatic endothelial cells from highly metastatic fibrosarcoma and from dermal tissue. They found differential expression of some 792 genes that code for a variety of proteins including components of endothelial junctions, subendothelial matrix, and vessel growth/patterning. In our orthotopic gastric cancer model, Lyve‐1‐positive lymphatic vessels were shown to express PDGF‐Rβ; not all lymphatic vessels expressed PDGF‐Rβ. PDGF‐Rβ was expressed occasionally on lymphatic endothelial cells, especially on enlarged and tortuous lymphatic vessels located immediately adjacent to tumor nests, whereas lymphatic vessels in normal tissue or intratumoral small lymphatic vessels did not express PDGF‐Rβ. Additionally, we did not find PDGF‐Rβ expression on lymphatic vessels of orthotopic tumors grown up from KKLS cells, which express low levels of PDGF‐B (data not shown). In general, tumor cells in a neoplasm are biologically heterogeneous, and their phenotype can be modified by the organ microenvironment.( 30 ) Our data indicate that tumor‐associated lymphatic vessels are also biologically heterogeneous and that interplay between lymphatic vessels and tumor cells may have a more significant effect on the endothelial phenotype than previously anticipated. Additionally, blockade of PDGF‐Rβ signaling by oral administration of the PDGF‐R tyrosine kinase inhibitor imatinib significantly reduced the area of lymphatic vessels in our orthotopic mouse model of gastric cancer. In our experiment, lymph node metastasis was not inhibited by treatment with imatinib alone (control, 8/10 vs imatinib treatment, 7/10). To inhibit lymph node metastasis, reduction of the lymphatic vessel area seems to be insufficient. Combination therapy of imatinib with cytotoxic chemotherapeutic drugs may be needed to inhibit lymph node metastasis. Together, these findings indicate that PDGF‐Rβ is preferentially expressed by activated, proliferating lymphatic endothelium but not by quiescent lymphatic vessels in normal tissue, a finding with important implications for the potential therapeutic use of targeted PDGF‐Rβ‐blocking strategies.
In conclusion, we found PDGF‐B secreted by tumor cells and PDGF‐Rβ expressed by stromal cells including lymphatic endothelial cells to be associated with lymphatic metastasis in gastric carcinoma. Thus, blockage of PDGF‐induced lymphangiogenesis may be a reasonable approach to prevention and treatment of lymphatic metastasis.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This work was carried out with the kind cooperation of the Analysis Center of Life Science, Hiroshima University, Hiroshima, Japan, and we thank Novartis Pharma K.K. (Basel, Switzerland) for providing the imatinib used in the study. This work was supported, in part, by Grants‐in‐Aid for Cancer Research from the Ministry of Education, Culture, Science, Sports and Technology of Japan and from the Ministry of Health, Labor and Welfare of Japan.
References
- 1. Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 2003; 3: 453–8. [DOI] [PubMed] [Google Scholar]
- 2. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000; 407: 249–57. [DOI] [PubMed] [Google Scholar]
- 3. Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev 2002; 82: 673–700. [DOI] [PubMed] [Google Scholar]
- 4. Karkkainen MJ, Haiko P, Sainio K et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 2004; 5: 74–80. [DOI] [PubMed] [Google Scholar]
- 5. Makinen T, Jussila L, Veikkola T et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor‐3. Nat Med 2001; 7: 199–205. [DOI] [PubMed] [Google Scholar]
- 6. Mandriota SJ, Jussila L, Jeltsch M et al. Vascular endothelial growth factor‐C‐mediated lymphangiogenesis promotes tumor metastasis. EMBO J 2001; 20: 672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Skobe M, Hawighorst T, Jackson DG et al. Induction of tumor lymphangiogenesis by VEGF‐C promotes breast cancer metastasis. Nat Med 2001; 7: 192–8. [DOI] [PubMed] [Google Scholar]
- 8. Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer 2002; 2: 573–83. [DOI] [PubMed] [Google Scholar]
- 9. Kubo H, Cao R, Brakenhielm E, Makinen T, Cao Y, Alitalo K. Blockade of vascular endothelial growth factor receptor‐3 signaling inhibits fibroblast growth factor‐2‐induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci U S A 2002; 99: 8868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gale NW, Thurston G, Hackett SF et al. Angiopoietin‐2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin‐1. Dev Cell 2002; 3: 411–23. [DOI] [PubMed] [Google Scholar]
- 11. Kajiya K, Hirakawa S, Ma B, Drinnenberg I, Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J 2005; 24: 2885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF‐A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med 2005; 201: 1089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cao R, Bjorndahl MA, Religa P et al. PDGF‐BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell 2004; 6: 333–45. [DOI] [PubMed] [Google Scholar]
- 14. Pietras K, Sjoblom T, Rubin K, Heldin CH, Ostman A. PDGF receptors as cancer drug targets. Cancer Cell 2003; 3: 439–43. [DOI] [PubMed] [Google Scholar]
- 15. Heldin CH, Eriksson U, Ostman A. New members of the platelet‐derived growth factor family of mitogens. Arch Biochem Biophys 2002; 398: 284–90. [DOI] [PubMed] [Google Scholar]
- 16. Li X, Eriksson U. Novel PDGF family members: PDGF‐C and PDGF‐D. Cytokine Growth Factor Rev 2003; 14: 91–8. [DOI] [PubMed] [Google Scholar]
- 17. Uehara H, Kim SJ, Karashima T et al. Effects of blocking platelet‐derived growth factor‐receptor signaling in a mouse model of experimental prostate cancer bone metastases. J Natl Cancer Inst 2003; 95: 458–70. [DOI] [PubMed] [Google Scholar]
- 18. Risau W, Drexler H, Mironov V et al. Platelet‐derived growth factor is angiogenic in vivo. Growth Factors 1992; 7: 261–6. [DOI] [PubMed] [Google Scholar]
- 19. Ostman A. PDGF receptors‐mediators of autocrine tumor growth and regulators of tumor vasculature and stroma. Cytokine Growth Factor Rev 2004; 15: 275–86. [DOI] [PubMed] [Google Scholar]
- 20. Pietras K. Increasing tumor uptake of anticancer drugs with imatinib. Semin Oncol 2004; 31: 18–23. [DOI] [PubMed] [Google Scholar]
- 21. Japanese Research Society for Gastric Cancer . Japanese classification of gastric carcinoma. Tokyo: Kanehara, 1999. [Google Scholar]
- 22. Druker BJ, Tamura S, Buchdunger E et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr‐Abl positive cells. Nat Med 1996; 2: 561–6. [DOI] [PubMed] [Google Scholar]
- 23. Buchdunger E, Cioffi CL, Law N et al. Abl protein‐tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c‐kit and platelet‐derived growth factor receptors. J Pharmacol Exp Ther 2000; 295: 139–45. [PubMed] [Google Scholar]
- 24. Hwang RF, Yokoi K, Bucana CD et al. Inhibition of platelet‐derived growth factor receptor phosphorylation by STI571 (Gleevec) reduces growth and metastasis of human pancreatic carcinoma in an orthotopic nude mouse model. Clin Cancer Res 2003; 9: 6534–44. [PubMed] [Google Scholar]
- 25. Valencak J, Heere‐Ress E, Kopp T, Schoppmann SF, Kittler H, Pehamberger H. Selective immunohistochemical staining shows significant prognostic influence of lymphatic and blood vessels in patients with malignant melanoma. Eur J Cancer 2004; 40: 358–64. [DOI] [PubMed] [Google Scholar]
- 26. Folkman J. Looking for a good endothelial address. Cancer Cell 2002; 1: 113–5. [DOI] [PubMed] [Google Scholar]
- 27. Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 2002; 1: 219–27. [DOI] [PubMed] [Google Scholar]
- 28. Hida K, Hida Y, Amin DN et al. Tumor‐associated endothelial cells with cytogenetic abnormalities. Cancer Res 2004; 64: 8249–55. [DOI] [PubMed] [Google Scholar]
- 29. Clasper S, Royston D, Baban D et al. A novel gene expression profile in lymphatics associated with tumor growth and nodal metastasis. Cancer Res 2008; 68: 7293–303. [DOI] [PubMed] [Google Scholar]
- 30. Fidler IJ, Poste G. The cellular heterogeneity of malignant neoplasms: implications for adjuvant chemotherapy. Semin Oncol 1985; 12: 207–21. [PubMed] [Google Scholar]


