Abstract
Approximately 200 types of the cells are qualified as differentiated cells in the human body. If these different types of cells can be separated from each other (or cloned) and obtained in sufficient quantity, it will be beneficial for studying development, morphogenesis, tissue maintenance, cancer and aging, and for reconstructing functional tissues in vitro for regenerative medicine. We produced the transgenic mouse and rat harboring SV40 T‐antigen gene to make the immortalized cell lines in the primary tissue culture and succeeded in establishing many functionally active cell lines from various tissues. Many immortalized cell lines from various tissues are shown to exhibit the unique characteristics of tissue functions and they should be useful as an in vitro model of various tissues for physiological and pharmacological investigations. Future application of these cells to drug screening is discussed. (Cancer Sci 2007; 98: 275–283)
The human body consists of 1012 cells, as a whole, and this enormous number of cells can be classified to approximately 200 types of cells, which are qualified to have individual names and as being distinctly different based on traditional histological classification. These differentiated cells exhibit morphologically and biochemically unique properties and show specific tissue functions. If we hope to know how tissues composed of differentiated cell types are generated during development and are maintained during adult life and what genes (and thus proteins) are required for specialized functions of tissues (or cells), it is required that all these cell types are separated from each other (or cloned) and obtained in sufficient quantity for molecular or biochemical analyses. Recent development in gene technology is making it possible to stock all human genome information separately in each clone as a cDNA or genomic DNA library so that all biological information of the human genome becomes available. Furthermore, by means of bioinformatics, signaling pathways, protein interaction maps and gene regulatory networks can be reconstructed. In a similar sense, if we could clone or stock all the individual cell types of the body of experimental animals, they would be invaluable for studying development, morphogenesis, tissue maintenance, cancer and aging, and for reconstructing functional tissues in vitro for regenerative medicine. This is possible if we can obtain the immortal cell lines with differentiated cell functions similar to the original tissues. However, it is not easy to establish even cell lines of particular interest, because immortalization of the cells seems to occur when rare cells are selected in a population carrying mutations in growth‐restraining genes, and established cell lines are used to lose their original differentiated functions.
Strategy for establishing conditionally immortalized cell lines from SV40 ts‐T‐antigen transgenic mouse and rat
We have studied cell differentiation of erythroleukemia cells; they are maintained in culture as undifferentiated immature cells and can be induced to differentiate into erythroid cells simply by the addition of dimethyl sulfoxide, thus, these cells can be conditionally switched between differentiated and growing states. We found that c‐myc, an oncogene, is down‐regulated upon addition of dimethyl sulfoxide, and if the transferred c‐myc is overexpressed, induction of differentiation is blocked, and we demonstrated that probability of commitment of erythroid differentiation is completely dependent on c‐my levels.( 1 ) This observation leads us to the idea that if some oncogene(s) could be conditionally turned on or off in the cells, the cells could be switched between immature growing and differentiated states. To test this possibility, we made transgenic mouse harboring c‐myc oncogene under the control of metallothionein gene promoter;( 2 ) however, unfortunately, we did not succeed in establishing immortalized cell lines from the tissues of the c‐myc transgenic mice, possibly because heavy metals such as Cd or Zn had to be used to induce metallothionein promoter, and continuous presence of these heavy metals may be toxic for cells in culture.
As an alternative, we produced the transgenic mice harboring SV40 T‐antigen gene because T‐antigen was used to make the immortalized cell lines in the primary tissue culture. We used a temperature‐sensitive (ts) form of T‐antigen, because its activity could be conditionally controlled by temperature shift between the permissive (33°C) and non‐permissive (39°C) temperatures. Using this transgenic mouse, we succeeded in establishing kidney tubule cell lines, the publication of which first appeared in this Journal( 3 ) and then, we reported the establishment of hepatocyte cell lines.( 4 ) During establishment of these two tissue cell lines, we confirmed that a frequency of immortalization of the cells from adult tissues of this transgenic mouse is significantly higher than that from the non‐transgenic mouse and growth of the cell lines are temperature‐dependent. The cells grew at 33°C, but the growth of the cells was significantly prevented at 39°C. The cells expressed large T‐antigen at 33°C but not at 39°C. These cells were not transformed, as judged by the absence of anchorage‐independent growth in soft agar gel and lack of tumor formation in nude mice.
The ts‐T‐antigen gene transgenic mice have the following advantages for establishing conditionally immortalized cell lines. (i) The ts‐T‐antigen is ubiquitously expressed among tissues, thus various tissue cell lines could, in principle, be immortalized. (ii) It is easy to establish cell lines even from cells with slow growth rate. (iii) The cell cloning period is relatively short (2–3 months). (iv) It is not necessary to purify the cells completely at the primary culture, but the functionally active cell clones could be selectable during culture. (v) Thus, it is possible to establish cell lines from tiny tissues containing a small quantity of cells. (vi) Finally, growth and differentiation of the cell lines can be conditionally altered by temperature shift or culture conditions; thus, the established cell lines exhibited functional, morphological and biochemical properties resembling the cells of the original tissues.( 5 , 6 ) We then developed the transgenic rat harboring the ts‐T‐antigen gene and made the rat cell lines available.( 7 ) Many immortalized cell lines exhibited differentiation phenotypes in the non‐permissive temperature. However, in some cases, differentiation phenotypes are detected even in the permissive temperature when T‐antigen is active, but they are manifested after the growth is arrested. In both cases, the differentiation seemed to be coupled with entry to Go phase of cell cycles. In some cell lines, with the shift to 39°C, the cell lines not only were arrested in growth, but also exhibited cell death by apoptosis.( 8 , 9 )
We succeeded in establishing many functionally active cell lines from various tissues of both transgenic mice and rats by collaboration with many expert scientists in cell differentiation and confirmed that the ts‐T‐antigen gene transgenic mice and rats have many advantages for efficient immortalization of the cells from various tissues and the established cells may be used for reconstruction of tissue functions (1, 2 and Table 2) and the properties and possible application of these functional active cell lines are described in this review (Table 2).
Figure 1.
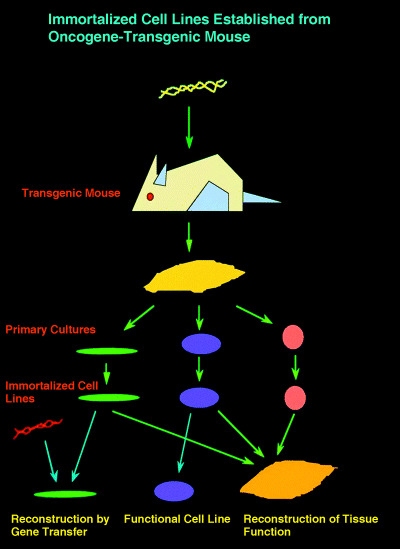
The flow of derivation of the immortal cell lines from various tissues of the temperature‐sensitive T‐antigen transgenic mouse and practical use.
Figure 2.
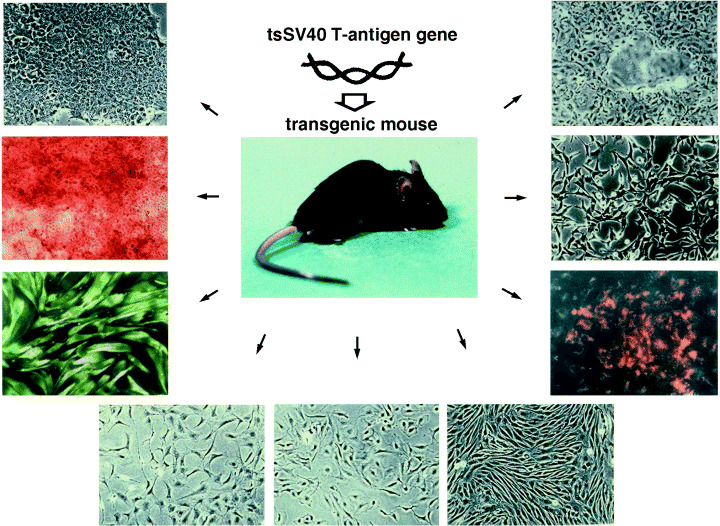
The established cell lines from the transgenic mouse kidney tubule cell line (top in the right), kidney endothelial cell line (middle in the right), vascular endothelial cell (bottom in the right), three bone marrow stromal cell lines (bottom), hepatocyte cell line (top in the left), gastric mucous cell line (middle in the left) and smooth muscle cell line (bottom in the left).
Table 2.
The cell lines established from the transgenic mouse and rat described in this review
| Adrenocortical precursor cell |
| Neuronal cell |
| Oligodendrocyte cell |
| Brain pericyte cell |
| Type 2 astrocyte cell |
| Brain capillary endothelial cell |
| Choroid plexus cell |
| Retinal Müller cell |
| Retinal capillary endothelial cell |
| Retinal pericyte cell |
| Periodontal ligament cell |
| Gingival epithelium cell |
| Tracheal epithelial cell |
| Vascular smooth muscle cell |
| Vascular endothelial cell |
| Vascular lymphatic cell |
| Gastric mucosal cell |
| Hepatocyte |
| Small intestine epithelial cell |
| Colonic epithelial cell |
| Kidney tubule cell |
| Macula densa cell |
| BM stromal cells |
| Hematopoietic cells |
| Mesenchymal stem cells |
| Dendritic cell |
| Mast Cell |
| Syncytiotrophoblast cell |
| Natural killer cell |
| Chondrocyte cell |
| Tendon cell |
Properties of the immortalized cell lines with differentiation potentials
Bone marrow (BM) stromal cells that create hematopoietic micorenvironment. Multipotent stem cells such as hematopoietic stem cells and neuronal stem cells are regulated by tissue microenvironment (or niche). Since the stromal cells in BM are known to create the hematopoietic microenvironment, we established a variety of BM stromal cell lines from the transgenic mouse, including fibroblasts, endothelial cells and preadipocytes. We examined whether they supported hematopoiesis in the in vitro culture and showed that BM stromal cells stimulated expansion of the hematopoietic stem cells and their progenitors (erythroid, monocyte/macrophage, granulocyte, and monocyte‐granulocyte).( 10 , 11 ) When the sorted hematopoietic stem cells (lineage marker negative (Lin−)/c‐Kit+/Sca1+) were cocultured with stromal cells, they could form cobblestones committed to both myeloid and lymphoid lineages in vitro.( 11 ) Both cobblestone formations require c‐Kit function, but VLA‐4‐VCAM‐1 interaction plays different parts in the two lineages.( 12 ) To characterize the BM hematopoietic micorenvironment, we searched genes required for erythropoietic stimulatory activity of stromal cells by gene expression profiling using DNA microarray. By comparison between the cell lines that support erythropoiesis and the cell lines that do not, we found that 138 genes were up‐regulated and 282 genes were down‐regulated in the supported cells.( 13 ) Among them, Tenascin‐C (TN‐C) was one of the up‐regulated genes and we demonstrated that addition of exogenous TN‐C enhanced the number of erythroid colonies in the non‐supported cells, while the number of erythroid colonies in the presence of TN‐C siRNA was significantly lower in the supported cells. Thus, we concluded that TN‐C is responsible for the stromal cell‐dependent erythropoiesis.
Then, we searched the genes whose expression levels were correlated with the stem cell supportive ability of stromal cells by a differential display method.( 14 ) The isolated gene is termed MysPDZ because it is an unconventional myosin containing a KE (lysine and glutamine)‐rich domain and a PDZ domain. MysPDZ is classified to a new type XVIII myosin.( 15 ) The XVIII myosins consisted of MYO18A (MysPDZ) and MYO18B, and MYO18B was shown to be a candidate tumor suppressor gene in human lung cancer.( 16 ) MysPDZ has tissue‐specific spliced variants in blood cells, muscle, and brain and we showed that MysPDZ can self‐associate through its C‐terminus coiled‐coil domain( 17 ) and the KE‐rich and the PDZ domains are required for the codistribution with actin fibers and the localization in inner surface of cell membrane of MysPDZ, respectively.( 18 ) Recently, the t(8;17) (p11;q23) in the 8p11 myeloproliferative syndrome was shown to fuse MYO18A to FGFR1.( 19 ) MysPDZ may be a multifunctional protein and is involved in the hematopoietic supporting activity of stromal cells through its multidomain structures.
Stroma‐dependent immature hematopoietic cell lines. We established several stroma‐dependent hematopoietic cell lines to examine the regulatory mechanisms of hematopoiesis by stromal cells.( 20 , 21 , 22 , 23 , 24 ) Among them, a primitive hematopoietic cell line, THS119, was established from BM Lin−/Sca‐1+ cells of the transgenic mice after lengthy passaging by coculture with TBR59 stromal cells.( 21 ) THS119 cells exhibited immature primitive hematopoietic cells such as forming cobblestones and surface phenotypes (Lin−/c‐Kit+/Sca1+), and multiple expressions of both earlier developmental markers of myeloid, lymphoid and the hematopoietic cell‐specific transcription factors. Growth of THS119 cells is ts T‐antigen‐dependent and stromal cell‐dependent. We found that cobblestone formation of these cells requires their invasion to the stromal cell layers and the invasion could be triggered by addition of sphingosine‐1‐phosphate or lysophosphatidic acid and was dependent on both Rho‐ and Ras‐signaling pathways through endothelial differentiation gene receptor −2 (Edg‐2).( 25 ) These results suggest the importance of specific lipids and their specific receptors on the invasive activity of primitive hematopoietic cells in the hematopoietic microenvironment.
We established another stroma‐dependent cell line, DFC‐28, from long‐term BM culture.( 26 ) When they are cultured on MSS62 (spleen stromal) cells, they exhibit immature phenotypes, but when they are cultured on TBR31‐1(BM stromal) cells, they are differentiated into a very early B‐lymphoid stage. Interestingly, the differentiation phenotypes were reverted to the immature state by replacing stromal cells back to MSS62 from TBR31‐1, indicating that the differentiation phenotypes of DFC‐28 could be reversibly controlled via direct contact with stromal cells in the microenvironment. We found that the expression of ephrinB2 in DFC‐28 cells and BM hematopoietic cells was induced by coculture with MSS62 cells and ephrinB2–EphB4 interaction modulates the migration and colonization of the hematopoietic cells in the local stromal microenvironment.( 27 )
BM stromal cell lines exhibiting properties of mesenchymal stem cells. BM is believed to contain multipotential stromal stem cells and we showed that most of BM stromal cell lines exhibited properties of cells of mesenchymal lineages( 28 ) and one of stromal cell line, TBR31‐2, is induced to express tissue‐specific differentiation markers for adipocyte, osteoblast, chondrocyte, and muscle cell, thus, exhibited properties of mesenchymal stem cells.( 29 ) One of the stromal cells exhibited early vascular progenitor properties and can be differentiated into cardiomyocytes( 30 ) while other cells (TBR‐B) could express smooth muscle‐specific genes including h1‐calponin, h‐caldesmon, SM22α and α‐actin at the defined culture condition.( 31 )
Hematopoietic cell lines for immunological studies
Dendritic cell line. The uptake of an antigen and its presentation to specific T cells by dendritic cells is a primary event in initiation of humoral and cellular immune responses as well as the induction of cytotoxic T cells. Immortalized dendritic cell line (SVDC) was established from the transgenic mice and was shown to have antigen‐presenting capacity in vitro as well as an augmenting effect on humoral and cellular immune responses in vivo.( 32 ) SVDC cells could be continuously cultured for more than 12 months in the presence of GM‐CSF. SVDC augmented OVA‐specific T cell proliferation efficiently in vitro, and elicited OVA‐specific IgG production in vivo on the adoptive transfer of pulsed SVDC into naive mice. Interestingly, SVDC exhibited significantly high cross‐priming ability compared to dendritic cells in a short‐term culture, thus leading to their extremely high effectiveness in inducing antitumor immunity in vivo, suggesting that SVDC is useful for the studies of antigen presentation and dendritic cell vaccination to elicit specific immune responses.
Mast cell lines. Mast cells play crucial roles in innate immunity to parasitic and bacterial infections as well as in hypersensitivity. Immortalized MC lines (SVMCs) from BM cells were established from the transgenic mice.( 33 ) On culture with nerve growth factor, SCF and IL‐3, SVMC showed morphologic and biochemical changes from mucosal mast cell‐like to connective‐tissue mast cell‐like phenotypes. These SVMC lines exhibited a significantly enhanced proliferation rate, and a higher responsiveness to the high affinity Fc receptor for IgE‐mediated intracellular calcium mobilization and degranulation than those of BM‐derived cultured mast cells.
Natural killer (NK) cell lines. NK cells belong to an important lymphocyte population that eliminates transformed cells and invades pathogens without any prior sensitization. NK cells possess not only natural killing activity against non‐self and altered‐self cells but also exhibit cytokine production and antibody‐dependent cell‐mediated cytotoxicity (ADCC). Immortalized murine NK cell clones were established and these NK cell lines continuously proliferated for more than 30 months in the presence of IL‐2. Some cell lines contained azurophilic granules in the cytoplasm and retained the NK cell functions such as natural killing activity, cytokine production and ADCC.( 34 )
Vascular smooth muscle cell lines. The dedifferentiation of vascular smooth muscle cells plays a critical role in the progression of atherosclerosis and restenosis after angioplasty. Thus, factors that stimulate smooth muscle cell differentiation should be useful for therapy for these diseases. A novel smooth muscle cell line (SVS) established from the transgenic mice retains the expression of specific markers for smooth muscle cells, such as smooth muscle myosin‐1, calponin and SM22α.( 35 ) We found that one BM stromal cell line, TBR‐B, can be induced to smooth muscle cells in the defined culture medium.( 31 ) Since the differentiation stage of SVS and TBR‐B cells can be controlled by culture temperature, they should be quite valuable tools to study the regulation of phenotypic modulation of smooth muscle cells, and ascorbic acid was shown to be a potent factor inducing the expression of h1‐calponin and α‐actin.( 36 ) Thus, both SVS and TBR‐B cells will prove useful for identifying compounds that regulate the differentiation state of vascular smooth muscle cells and for discovery of new antiatherosclerotic drugs.
Blood vascular and lymphatic endothelial cell lines. While the basic biology of blood vascular endothelial cells has been well documented, little is known about that of lymphatic endothelial cells, despite their importance under normal and pathological conditions. The culture of lymphatic endothelial cells is important to obtain a better understanding of their respective roles in vascular physiology and pathology including wound healing, lymphedema, lymphangiogenesis, and lymphatic tumor metastasis. The lymphatic endothelial (TR‐LE) cell lines established from the transgenic rat( 37 ) expressed lymphatic endothelial markers VEGFR‐3, LYVE‐1, Prox‐1 and podoplanin as well as endothelial markers CD31, Tie‐2 and VEGFR‐2. On the contrary, the simultaneously established vascular endothelial cell lines (TR‐BE) expressed CD31, Tie‐2 and VEGFR‐2, but not lymphatic endothelial markers. Thus, both cell lines would be important tools for the studies of lymphatic and vascular endothelial cell functions in vitro and tumor metastasis.
Chondrocyte cell lines from the articular and costal cartilages. To understand the physiology and pathology of the cartilage, chondrocyte‐like cell lines derived from articular cartilage (TC6)( 38 , 39 , 40 , 41 , 42 ) and those (MCC‐2, ‐5, and ‐35) from the costal cartilage( 43 ) of transgenic mice were established. Both of them exhibited spindle‐like or polygonal morphology and these cells formed alcian blue‐positive nodules after confluence and expressed mRNA of the genes encoding cartilage‐specific proteins. Using TC6 cells, the effect of several factors such as FGF and BMP‐2, and regulation by the osteogenesis‐related transcription factors have been extensively studied.( 38 , 39 , 40 , 41 , 42 , 43 )
Tendon cell lines. Development of the musculoskeletal system requires coordinated formation of distinct types of tissues, including bone, cartilage, muscle, and tendon. The tendon cell lines (TT‐E4, TT‐G11, and TT‐D6) established from the Achilles tendon of the transgenic mice( 44 ) expressed tendon‐related genes such as scleraxis, Six1, EphA4, and they also expressed some osteoblast‐related genes such as osteopontin and Cbfal. These cell lines formed hard fibrous connective tissue when implanted onto chorioallantoic membrane in ovo and also formed tendon‐like tissues when they were implanted into defects made in patella tendon in mice. Interestingly, these cell lines were shown to possess mesenchymal stem cell‐like properties, suggesting the existence of mesenchymal stem cell in tendon tissue.
The cell lines from the teeth
Periodontal ligament cell lines. The periodontal ligament is a connective tissue located between the cementum of teeth and the alveolar bone of the mandibula. It plays an integral role in the maintenance and regeneration of periodontal tissue. The periodontal ligament cells (PDL‐L2) established from the transgenic mouse( 45 ) express genes such as alkaline phosphatase, type I collagen, periostin, runt‐related transcription factor‐2 (Runx2) and EGF receptor, but does not express genes such as bone sialoprotein and osteocalcin. Using this cell line, the homeobox protein Msx2 was shown to be a key factor in preventing ligaments and tendons from mineralizing.( 46 )
Gingival epithelium cell lines. Gingival epithelium has three distinct anatomical types: the stratified squamous keratinized gingival epithelium, the non‐keratinized sulcular epithelium, and the junctional epithelium. Two gingival epithelial cell lines (GE1 and GE6) established from the transgenic mouse( 47 ) grew in a pavement arrangement and solely formed multilayers similar to the stratified oral epithelium. These cells exhibited the phenotype of non‐keratinized sulcular epithelium, which possessed the potency undergoing keratinization in highly stratified cultures as oral gingival epithelium.
Gastro‐intestinal cell lines
Gastric mucosal cell lines. The gastric gland of the stomach is composed of several differentiated cell types such as mucus‐producing pit (surface mucous) cells, acid‐secreting parietal cells, and pepsinogen‐secreting zymogenic cells. The gastric surface mucous cell line GSM06 established from the transgenic mice( 48 ) produced periodic acid‐Schiff positive glycoconjugates that formed a mucous sheet like the gastric surface mucosa in the stomach at 39°C.( 49 ) Insulin markedly increased the production of glycoconjugates( 50 ) and suppression of furin, a proprotein‐processing endoprotease, retarded cell growth, but accelerated cell differentiation.( 51 ) An air–liquid interface promoted the differentiation of GSM06 cells in a reconstruction culture with nitrocellulose membrane and collagen gel.( 52 ) GSM06 cells were used for evaluation of factors controlling the level of cell restitution in vitro to study repair mechanisms in cell defect type mucosal injuries, such as peptic ulcers.( 53 , 54 ) GSM06 cells have been used for an in vitro model that closely resembles in vivo conditions leading to infection by H. pylori, whose chronic infection is one of the major causes of gastritis resulting in various disease states.( 55 , 56 ) In addition to GSM06 cells, three distinct types of cells were established from the transgenic mice; (i) MGE12‐1, MGE3‐2, and MGE509 cells expressing pit cell markers, (ii) MGE02, MGE503, and MGE511 cells expressing pit and zymogenic cell markers, and (iii) MGE507 and MGE727 cells expressing pit zymogenic and parietal cell markers.( 57 ) These gastric epithelial cell lines seem to reflect different stages of development of gastric mucosal cells. Rat gastric epithelial cell lines (RGE1, RGE2) were also established.( 58 ) Thus, these conditionally immortalized gastric cell lines will provide a useful in vitro model of gastric epithelium.
Colonic epithelial cell lines. Colonic epithelial cell line, MCE301( 59 ) showed epithelial‐like morphology and maintained tight connections with neighboring cells. Electron microscopic studies showed that the cells formed microvilli‐like structures on the cell surface and junctional complexes such as tight junctions and desmosomes between the cells. The cells expressed typical markers for cytosketal, basement membrane and junctional complex and colonic mucin, indicating that the cells originate from mucus‐secreting cells. Butyrate is known to provide major respiratory fuel for colonic epithelial cells, playing an essential role in the maintenance of homeostasis of the colonic epithelium, and sodium butyrate was shown to induce cell growth arrest and the differentiation of MCE301 cells.( 60 , 61 )
Small intestine epithelial cell lines. Small intestine epithelial cell lines (TR‐SIE) established from the transgenic rats( 62 ) had a polygonal morphology and expressed cytokeratin and villin. TR‐SIE cells expressed several intestinal transporter mRNAs, thus, could be used for study physiological transport functions in the intestine.
Male genital cell lines
Sertoli and Leydig cells. The process of spermatogenesis occurs within the seminiferous tubules of the testis and requires the cooperative functions of several cell types, including peritubular, Leydig and Sertoli cells. A Leydig cell line, TTE1( 63 ) showed alkaline phosphatase and 3 γ‐hydroxysteroid dehydrogenase (HSD), cytokeratin and vimentin. The mouse Sertoli cell line, TTE3( 64 ) expressed mRNAs encoding SCF, inhibin‐α, transferrin, follicle‐stimulating hormone receptor and sulfated glycoprotein‐2, similar to the original cells. The rat Sertoli cell line, RT3‐3, showed biochemical features similar to the mouse Sertoli cell line TTE3. Moreover, treatment with sodium butyrate and retinoic acid significantly elevated levels of SGP‐2 and/or transferrin.( 65 ) Androgen receptor‐regulated gene transcription plays indispensable roles in maintaining spermatogenesis in Sertoli cells, and TTE3 cells expressed coregulators (PSPC1, NONO and SFPQ) associating with androgen receptor.( 66 )
The epididymal epithelial cell lines. The epididymal epithelium secretes proteins in a highly regulated and regionalized manner, so that maturing spermatozoa encounter discrete microenvironments as they transit through the epididymis. The established epididymal epithelial cell lines( 67 ) express the androgen receptor as well as markers of the murine epididymal epithelium, such as PEB (phosphatidye ethanolamine binding)‐like protein, E‐RABP (epididymal retinoic acid‐binding protein), and EP17 (epididymal protein of 17 kDa), and the promoter of the epididymal lipocalin gene required for tissue‐ and region‐specific expression was defined using these cell lines;( 68 ) thus, these cell lines should be valuable tools for studying the regulation of epididymal specific gene expression.
Transport systems of the tissue–blood barriers. The transport functions at the tissue–blood barriers such as the blood–brain barrier (BBB), the blood–cerebrospinal fluid (BCSFB) barrier, the blood–retinal barrier (BRB) and the blood–placental barrier (BPB) are important for organ‐selective drug targeting and for toxicological and pharmacological drug screening studies. The use of the cultured cells from these tissues have become increasingly important for studying their transport functions in vitro; however, it had been difficult to make a primary culture of these cells from rats or mice, because, for examples, brain capillary cells account for only 0.1% to 0.2% of the total cell volume of the brain and the choroid plexus accounts for only about 0.25% of the weight of the entire brain of these animals, thus, it is hoped to establish the immortalized cell lines exhibiting the transport activities similar to the original tissues. The cell lines were established to study physiological transport functions at the blood–tissue barriers.
The blood–brain barrier (BBB) cell lines. The brain capillary endothelial cell lines established from mice (TM‐BBB)( 69 ) and from rats (TR‐BBB)( 70 ) expressed von Willebrand factor, a typical endothelial marker and exhibited acetylated low‐density lipoprotein uptake activity. Several transporter activities specific for BBB were detected in these cell lines. The established brain pericyte cell line (TR‐PCT) expressed pericyte markers such as ICAM‐1, PDGFRβ, angiopoietin‐1, and osteopontin and α‐smooth muscle actin.( 71 ) At the defined condition, they developed von Kossa stain‐positive nodules, a marker of calcification.
The blood‐cerebrospinal fluid (BCSFB) barrier cell lines. The choroid plexus cell lines (TR‐CSFB) expressed the typical choroid plexus epithelial cell marker, transthyretin, and possessed Na+, K+‐ATPase on their apical side and exhibited amino acid transport activity similar to in vivo studies.( 72 )
The blood–retinal barrier (BRB) cell lines. The retina is a highly differentiated tissue involved in the function of sight and has a blood–retinal barrier (BRB) to prevent any non‐specific transport of substances from the circulating blood. The retinal capillary endothelial cell lines (TR‐iBRBs) had a spindle‐fiber shape morphology, expressed von Willebrand factor, and internalized acetylated low‐density lipoprotein.( 73 ) Moreover, they expressed VEGFR‐2, GLUT1, 180 Kda‐P‐glycoprotein, multidrug resistant proteins (mdr 1a, mdr 1b and mdr 2), and exhibited 3‐O‐methyl‐D‐glucose uptake activity. Retinal pericytes surround retinal capillary endothelial cells have been proposed to play a role in regulation of endothelial cell proliferation. The established rat retinal pericyte cell lines (TR‐rPCTs)( 74 ) expressed typical pericyte markers and morphologies as similar to the brain pericyte cell line (TR‐PCT). Co‐culture studies of TR‐rPCT1 and TR‐iBRB2 cells suggested that physical contact between pericytes and retinal endothelial cells is important for the growth of retinal endothelial cells. The studies with the established rat retinal Müller cell lines (TR‐MUL)( 75 ) demonstrated an importance of the maintenance of the glutathione concentration in the retina and of the neuroprotective role in Müller cells.
The blood‐placental barrier (BPB) cell lines. The placenta can undergo rapid changes after implantation, and take over not only the complete nutrition of the growing fetus but also its waste excretion. Syncytiotrophoblasts, which form a continuous barrier between the maternal and fetal circulation, play an essential role in restriction of drug delivery through the blood–placental barrier (BPB). The established rat syncytiotrophoblast cell lines (TR‐TBTs)( 76 ) had a syncytium‐like morphology, expressed cytokeratins and syncytiotrophoblast markers, and carrier‐mediated transport functions specific to the BPB. In addition, the expression patterns of nucleoside and neurotransmitter transporters are shown to be quite similar in the BPB and BBB.
The cell lines from brain
The neuronal cell lines from the suprachiasmatic nucleus. The suprachiasmatic nucleus (SCN, a small nucleus 0.5 mm in diameter) of the anterior hypothalamus is the center of an internal biological clock in mammals. Glutamate is the neurotransmitter of the retino‐hypothalamic tract responsible for mediating the circadian actions of light in rodents and N‐methyl‐d‐aspartate (NMDA) receptors are shown to be principally involved in photic resetting of the biological clock in vivo and in slice culture. To study the precise cellular mechanisms of the resetting, a neuronal cell line, N14.5, derived from the SCN of the transgenic rat was established.( 77 ) At defined culture conditions, these cells showed neuronal round cell body with neurite extensions, and expressed neuronal markers and vasoactive intestinal peptide which is expressed in the ventrolateral region of the SCN. The cells expressed NMDA receptors, particularly NR1 and NR2B. NMDA activated phosphorylation of p44/42 mitogen‐activated protein kinase and increased expression level of Per1 and Per2 mRNA. These results suggest that the N14.5 is a novel neuronal cell line that expressed a functional NMDA receptor, derived from the ventrolateral region of the SCN; thus, the N14.5 cells may be a useful tool to elucidate numerous chronobiological processes, especially the resetting mechanism induced by an external light signal.
Oligodendrocyte cell line. During establishment of the N14.5 cells, an oligodendrocyte cell line, OLP6, was established( 78 ) and the OLP6 cells expressed the oligodendrocytic markers; 2′‐3′‐cyclic‐nucleotide 3′‐phosphodiesterase, galactocerebroside and Edg‐2. The OLP6 cells underwent apoptosis upon serum withdrawal at 39°C, but lysophosphatidic acid inhibited this apoptosis and promoted the expression of myelin basic protein through the activation of Edg‐2, suggesting that lysophosphatidic acid promoted the maturation of OLP6 cells in the immature oligodendrocyte stage. OLP6 cells should provide a potent model system for studying the precise mechanism involved in stepwise differentiation of oligodendrocytes.
Kidney cell lines
Kidney tubule cell lines. A kidney tubule cell line (TKC2)( 3 ) was first established from distal tubules and the cells exhibited characteristics such as dome formation and stimulation of cAMP synthesis by vasopressin.( 79 ) Then, renal cell lines from definite nephron segments were established by microdissection of kidneys of the transgenic mice.( 80 ) Cells derived from distal tubule, cortical and outer medullary collecting duct possessed their cyclic AMP response to vasopressin, like their original nephron segment.( 81 ) The vasopressin‐responsive early proximal tubule (S1) cells retained a unique morphology specific to the proximal tubule. On the other hand, cells derived from terminal proximal tubules (S3 segment) formed a cobblestone‐like confluent monolayer, and did not respond to vasopressin like their fresh segments. Thus, these cell lines are the first in the kidney to define the segmental origin and to maintain some differentiated unique functions. They are valuable for studies on intrarenal site‐specific actions and possible mechanisms of action of pharmacological and toxic substances.( 82 )
Macula densa cell line. The macula densa is involved in the tubuloglomerular feedback (TGF) system in the kidney and specifically expresses neuronal nitric oxide synthase (nNOS). The established cell line (NE‐MD) showed a time‐dependent increase in signals of the nNOS with furosemide treatment; thus, this newly developed macula densa cell line will be useful in studies of the TGF sytem.( 83 )
Ongoing studies
The derivation of mutation‐specific cell lines. While many mutant mice with targeted disruption in particular genes have recently been generated, it is difficult to determine the precise function of the gene, because they died before birth in many cases, due to their participation in embryogenesis. If the tissue cell line with the disrupted gene could be established, the gene function could be more easily examined. Similarly, the tissue‐specific cell lines from spontaneously appearing mutant mice or transgenic mice may be useful in the study of gene functions in vitro. A genetic cross of T‐transgenic mice with these mutant mice could be used to generate cell lines with particular gene disruption or alteration. These studies are ongoing in collaboration. Once the tissue cell line with the mutant gene can be established, the gene function may be more easily examined and the established cell lines may be applicable for the screening of drugs targeted for the particular gene or protein.
Analysis of global gene expression of the immortalized cell lines. Many immortalized cell lines from various tissues that exhibit the unique characteristics of tissue functions should be useful as in vitro models of various tissues for physiological and pharmacological investigations, but, to use these cell lines, it is necessary to get more precise information about the gene expression profiles. Owing to recent advancements in gene analysis technology, the gene expression profiles of these cell lines may be easily examined by DNA microarray analysis. Tabuchi et al. analyzed the expression profiles of several immortalized cell lines. When colonic epithelial cells (MCE301) were treated with sodium butyrate, the cell growth was arrested and induction of differentiation accompanying an increase in alkaline phosphatase activity was observed. Butyrate is known to modulate gene transcription through the inhibition of histone deacetylase, and the resulting histone hyperacetylation consequently facilitates the accessibility of transcription factors to DNA binding sites. DNA microarray analysis showed that butyrate down‐regulated 25 genes and up‐regulated 88 genes. Among them, levels of cyclin D1 and proliferating cell nuclear antigen (PCNA) decreased, whereas the levels of integrin β1 and osteopontin increased.( 57 ) Further analysis suggested the genetic networks for cellular development and cell cycles or canonical pathways for fatty acid biosynthesis and pyrimidine metabolism, respectively.( 58 ) Tabuchi et al.( 61 ) also showed that changes in gene expression were unique depending on the culture condition and drug treatment using Sertoli TTE3 cells.
Conclusions
Our collaborative studies indicated that these conditionally immortalized cell lines exhibit the unique characteristics of tissues, and therefore should be useful as an in vitro model of various tissues for physiological and pharmacological investigations and for toxicological and pharmacological drug screening studies. Our method could be used to construct a whole tissue cell library of the body of transgenic animals, and to identify all distinctive cell types such as unidentified cell types that may possess new functions, in addition to the identified cell types. The whole tissue cell library could be used to make whole panels of differentiated cell‐specific gene (or protein) expression profiles combined with the mouse (and rat) genome information. This information will be of great benefit in many biological analyses in the development of drug screening systems.
Furthermore, these immortalized cells having specific functions, may offer an alternative to experiments using living animals and thereby offering a solution to ethical issues.
Acknowledgments
I would like to thank many colleagues for collaboration in establishing various cell lines. The work was sported by grants form the Ministry of Education, Culture, Sports, Science and Technology in Japan.
References
- 1. Kume T‐U, Takada S, Obinata M. Probability that the commitment of murine erythroleukemia cell differentiation is determined by the c‐myc level. J Mol Biol 1988; 202: 779–86. [DOI] [PubMed] [Google Scholar]
- 2. Suzuki M, Yosinaga K, Obinata M, Furusawa M, Abe K. Specific defect in spermatogenesis induced in the c‐myc transgenic mice. Genes Cells 1996; 1: 1077–86. [DOI] [PubMed] [Google Scholar]
- 3. Yanai Y, Satoh T, Kyo S, Abe K, Suzuki M, Obinata M. A tubule cell line established from transgenic mice harboring temperature‐sensitive Simian Virus 40 large T‐antigen gene. Jpn J Cancer Res 1991; 82: 1344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yanai Y, Suzuki M, Obinata M. Hepatocyte cell lines established from transgenic mice harboring temperature‐sensitive Simian Virus 40 large T‐antigen gene. Exp Cell Res 1991; 197: 50–6. [DOI] [PubMed] [Google Scholar]
- 5. Obinata M. The conditionally immortalized cell lines with differentiated functions established from the temperature‐sensitive T‐antigen transgenic mice. Genes Cells 1997; 2: 224–35. [DOI] [PubMed] [Google Scholar]
- 6. Obinata M. Possible applications of conditionally mmortalized tissue cell lines with differentiation functions (Mini review for the breakthroughs and views). Biochem Biophys Res Commun 2001; 286: 667–72. [DOI] [PubMed] [Google Scholar]
- 7. Takahashi R, Hirabayashi M, Yanai N, Obinata M, Ueda M. Establsihment of the tsA58 transgenic rats as a source of conditionally immortalized cell lines. Exp Animals 1999; 48: 255–61. [DOI] [PubMed] [Google Scholar]
- 8. Yanai Y, Obinata M. Apoptosis is induced at nonpermissive temperature by a transient increase in p53 in cell lines immortalized with ts SV40 T‐antigen gene. Exp Cell Res 1994; 211: 296–300. [DOI] [PubMed] [Google Scholar]
- 9. Taher A, Yanai N, Obinata M. Properties of incompletely immortalized cell lines generated from the line established from temperature‐sensitive SV40 T‐antigen transgenic mice. Exp Cell Res 1995; 219: 332–8. [DOI] [PubMed] [Google Scholar]
- 10. Kameoka J‐I, Yanai N, Obinata M. Bone marrow stromal cells selectively stimulate the rapid expansion of lineage‐restricted myeloid progenitors. J Cell Physiol 1995; 164: 55–64. [DOI] [PubMed] [Google Scholar]
- 11. Okuyama R, Koguma M, Yanai N, Obinata M. Bone marrow stromal cells induce myeloid and lymphoid development of the sorted hematopoietic stem cells in vitro. Blood 1995; 86: 2590–7. [PubMed] [Google Scholar]
- 12. Koguma M, Matsuda K‐I, Okuyama R, Yanai N, Obinata M. Selective proliferation of lymphoid cells from lineage‐ c‐Kit+ Sca1+ cells by a clonal bone marrow stromal cell line. Exp Hematol 1998; 26: 280–7. [PubMed] [Google Scholar]
- 13. Seki M, Kameoka J, Takahashi S et al. Identification of Tenascin‐C as a key molecule determing the stromal cell dependent erythropoiesis. Exp Hematol, 2006; 34: 519–327. [DOI] [PubMed] [Google Scholar]
- 14. Furusawa T, Ikawa S, Yanai N, Obinata M. Isolation of a novel PDZ‐containing myosin from hematopoietic supportive bone marrow stromal cell lines. Biophys Biochem Res Commn 2000; 270: 67–75. [DOI] [PubMed] [Google Scholar]
- 15. Sellers JR. Myosins: a diverse superfamily. Biochimbiophys Acta 2000; 1496: 3–22. [DOI] [PubMed] [Google Scholar]
- 16. Nishioka M, Kohno T, Tani M et al. MYO18B, a candidate tumor suppressor gene at chromosome 22q12.1, deleted, mutated, and methylated in human lung cancer. Proc Natl Acad Sci USA 2002; 99: 12 269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mori K, Furusawa T, Okubo T et al. Genome structure and differential expression of two isoforms of a novel PDZ‐containing myosin (MysPDZ) (Myo18A). J Biochem (Tokyo) 2003; 133: 405–13. [DOI] [PubMed] [Google Scholar]
- 18. Mori K, Matsuda K, Furusawa T, Kawata M, Inoue T, Obinata M. Subcellular localization and dynamics of MysPDZ (Myo18A) in live mammalian cells. Biochem Biophys Res Commun 2005; 326: 491–8. [DOI] [PubMed] [Google Scholar]
- 19. Walz C, Chase A, Schoch C et al. The t(8; 17)(p11;q23) in the 8p11 myeloproliferative syndrome fuses MYO18A to FGFR1. Leukemia 2005; 19: 1005–9. [DOI] [PubMed] [Google Scholar]
- 20. Matsuda K, Koguma M, Okuyama R et al. A novel stromal cell‐dependent B lymphoid stem‐like cell line that induces immunoglobulin gene rearrangement. J Biochem 1999; 125: 602–12. [DOI] [PubMed] [Google Scholar]
- 21. Yanai N, Matsui N, Matsuda K‐I et al. A novel stromal cell‐dependent hematopoietic cell line established from temperature‐sensitive SV40 T‐antigen transgenic mice. Exp Hematol, 1999; 27: 1087–96. [DOI] [PubMed] [Google Scholar]
- 22. Okubo T, Yanai N, Watanabe S, Arai K‐I, Obinata M. Effect of human granulocyte‐macrophage colony stimulating factor (hGM‐CSF) on lymphoid and myeloid differentiation of sorted hematopoietic stem cells from hgm‐csf receptor gene transgenic mice. J Biochem 2000; 127: 591–6. [DOI] [PubMed] [Google Scholar]
- 23. Okubo T, Yanai N, Obinata M. Self‐renewal and differentiation of a novel bipotent myeloid progenitor cells in the stroma‐dependent culture. Exp Hematol 2000; 28: 651–9. [DOI] [PubMed] [Google Scholar]
- 24. Okubo T, Matsui N, Yanai N, Obinata M. A stromal cell dependent immature hematopoietic cell line generated from long‐term bone marrow culture. Cell Struct Funct 2000; 25: 133–9. [DOI] [PubMed] [Google Scholar]
- 25. Yanai N, Matsui N, Furusawa T, Okubo T, Obinata M. Sphingosine‐1‐phosphate and lysophosphatidic acid trigger invasion of primitive hematopoietic cells into stromal cell layers. Blood 2000; 96: 139–44. [PubMed] [Google Scholar]
- 26. Okubo T, Yanai N, Ikawa S, Obinata M. Reversible switching of expression of c‐kit and Pax‐5 in immature hematopoietic progenitor cells by stromal cells. Exp Hematol 2002; 30: 1193–201. [DOI] [PubMed] [Google Scholar]
- 27. Okubo T, Yanai N, Obinata M. Stromal cells modulate ephrinB2 expression and transmigration of hematopoietic cells. Exp Hematol 2006; 34: 330–8. [DOI] [PubMed] [Google Scholar]
- 28. Okuyama R, Yanai N, Obinata M. Differentiation capacity towards mesenchymal cell lineages of bone marrow stromal cells established from temperature‐sensitive SV40 T‐antigen transgenic mouse. Exp Cell Res 1995; 218: 424–9. [DOI] [PubMed] [Google Scholar]
- 29. Negishi Y, Kudo A, Obinata A et al. Multipotency of a bone marrow stromal cell line, TBR31‐2, established from ts SV40 T antigen gene transgenic mice. Biophys Biochem Res Commn 2000; 268: 450–5. [DOI] [PubMed] [Google Scholar]
- 30. Karibe A, Okubo T, Yagi T et al. Adult bone marrow cells with early vascular progenitor properties can differentiate into cardiomyocytes. Circulation 2002; 106 (Suppl): Meeting abstract. [Google Scholar]
- 31. Arakawa E, Hasegawa K, Yanai N, Obinata M, Matsuda Y. A mouse bone marrow stromal cell line, TBR‐B, shows inducible expression of smooth muscle‐specific genes. FEBS Lett 2000; 481: 193–6. [DOI] [PubMed] [Google Scholar]
- 32. Ebihara S, Endo S, Ito K et al. Immortalized dendritic cell line with efficient cross‐priming ability established from transgenic mice harboring the temperature‐sensitive SV40 large T‐antigen gene. J Biochem (Tokyo) 2004; 136: 321–8. [DOI] [PubMed] [Google Scholar]
- 33. Kanehira M, Kaifu T, Maya K et al. Novel Mast Cell Lines with Enhanced Proliferative and Degranulative Abilities Established from Temperature‐Sensitive SV40 Large T Antigen Transgenic Mice. J Biochem (Tokyo) 2006; 140: 211–20. [DOI] [PubMed] [Google Scholar]
- 34. Iizuka S, Kaifu T, Nakamura A, Obinata M, Takai T. Establishment and functional characterization of novel natural killer cell lines derived from a temperature‐sensitive SV40 large T antigen transgenic mouse. J Biochem (Tokyo) 2006; 140: 255–65. [DOI] [PubMed] [Google Scholar]
- 35. Hasegawa K, Arakawa E, Oda S, Yanai N, Obinata M, Matsuda Y. Novel smooth muscle cell lines from transgenic mice harboring temperature‐sensitive SV40 large T‐antigen gene. temperature‐dependent expression of smooth muscle myosin heavy chain‐1 and calponin genes. J Mol Cell Cardiol 1997: 29: 2177–86. [DOI] [PubMed] [Google Scholar]
- 36. Arakawa E, Hasegawa K, Irie J et al. L‐ascorbic acid stimulates expression of smooth muscle‐specific markers in smooth muscle cells both in vitro and in vivo. J Cardiovasc Pharmacol 2003; 42: 745–51. [DOI] [PubMed] [Google Scholar]
- 37. Matsuo M, Koizumi K, Yamada S et al. Establishment and characterization of conditionally immortalized endothelial cell lines from the thoracic duct and inferior vena cava of tsA58/EGFP double‐transgenic rats. Cell Tissue Res 2006; 326: 749–758. [DOI] [PubMed] [Google Scholar]
- 38. Mataga N, Tamura M, Yanai N et al. Establishment of a novel chondrocyte‐like cell line derived from transgenic mice harboring the temperature‐sensitive simian virus 40 large T‐antigen gene. J Bone Miner Res 1996; 11: 1646–54. [DOI] [PubMed] [Google Scholar]
- 39. Kawa‐uchi T, Nifuji A, Mataga N et al. Fibroblast growth factor downregulates expression of a basic helix‐loop‐helix‐type transcription factor, scleraxis, in a chondrocyte‐like cell line, TC6. J Cell Biochem 1998; 70: 468–77. [DOI] [PubMed] [Google Scholar]
- 40. Takazawa Y, Nifuji A, Mataga N, Yamauchi Y, Kurosawa H, Noda M. Articular cartilage cells immortalized by a temperature sensitive mutant of SV40 large T antigen survive and form cartilage tissue in articular cartilage environment. J Cell Biochem 1999; 75: 338–45. [PubMed] [Google Scholar]
- 41. Sekiya I, Tsuji K, Koopman P et al. SOX9 enhances aggrecan gene promoter/enhancer activity and is up‐regulated by retinoic acid in a cartilage‐derived cell line, TC6. J Biol Chem 2000; 275: 10 738–44. [DOI] [PubMed] [Google Scholar]
- 42. Takazawa Y, Tsuji K, Nifuji A, Kurosawa H, Ito Y, Noda M. An osteogenesis‐related transcription factor, core‐binding factor A1, is constitutively expressed in the chondrocytic cell line TC6, and its expression is upregulated by bone morphogenetic protein‐2. J Endocrinol 2000; 165: 579–86. [DOI] [PubMed] [Google Scholar]
- 43. Kitaoka E, Satomura K, Hayashi E et al. Establishment and characterization of chondrocyte cell lines from the costal cartilage of SV40 large T antigen transgenic mice. J Cell Biochem 2001; 81: 571–82. [DOI] [PubMed] [Google Scholar]
- 44. Salingcarnboriboon R, Yoshitake H, Tsuji K et al. Establishment of tendon‐derived cell lines exhibiting pluripotent mesenchymal stem cell‐like property. Exp Cell Res 2003; 287: 289–300. [DOI] [PubMed] [Google Scholar]
- 45. Saito Y, Yoshizawa T, Takizawa F et al. A cell line with characteristics of the periodontal ligament fibroblasts is negatively regulated for mineralization and Runx2/Cbfa1/Osf2 activity, part of which can be overcome by bone morphogenetic protein‐2. J Cell Sci 2002; 115: 4191–200. [DOI] [PubMed] [Google Scholar]
- 46. Yoshizawa T, Takizawa F, Iizawa F et al. Homeobox protein MSX2 acts as a molecular defense mechanism for preventing ossification in ligament fibroblasts. Mol Cell Biol 2004; 24: 3460–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hatakeyama S, Ohara‐Nemoto Y, Yanai N, Obinata M, Hayashi S, Satoh M. Establishment of gingival epithelial cell lines from transgenic mice harboring temperature sensitive simian virus 40 large T‐antigen gene. J Oral Pathol Med 2001; 30: 296–304. [DOI] [PubMed] [Google Scholar]
- 48. Sugiyama N, Tabuchi Y, Horiuchi T, Obinata M, Furusawa M. Establishment of gastric surface mucous cell lines from transgenic mice harboring temperature‐sensitive Simian Virus 40 large T‐antigen gene. Exp Cell Res 1993; 209: 382–7. [DOI] [PubMed] [Google Scholar]
- 49. Tabuchi Y, Sugiyama N, Horiuchi T, Furuhama K, Furusawa M. Insulin stimulates production of glycoconjugate layers on the cell surface of gastric surface mucous cell line GSM06. Digestion 1997; 58: 28–33. [DOI] [PubMed] [Google Scholar]
- 50. Konda Y, Yokota H, Kayo T et al. Proprotein‐processing endoprotease furin controls the growth and differentiation of gastric surface mucous cells. J Clin Invest 1997; 99: 1842–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ootani A, Toda S, Fujimoto K, Sugihara H. An air‐liquid interface promotes the differentiation of gastric surface mucous cells (GSM06) in culture. Biochem Biophys Res Commun 2000; 271: 741–6. [DOI] [PubMed] [Google Scholar]
- 52. Suzuki H, Masaoka T, Minegishi Y, Motosugi Y, Miura S, Ishii H. Lansoprazole promotes gastric mucosal cell proliferation and migration by activating p44/p42 mitogen‐activated protein kinase. Wound Repair Regen 2004; 12: 93–9. [DOI] [PubMed] [Google Scholar]
- 53. Nakajima T, Konda Y, Izumi Y et al. Gastrin stimulates the growth of gastric pit cell precursors by inducing its own receptors. Am J Physiol Gastrointest Liver Physiol 2002; 282: 359–66. [DOI] [PubMed] [Google Scholar]
- 54. Kanai M, Konda Y, Nakajima T, Izumi Y, Takeuchi T, Chiba T. TGF‐alpha inhibits apoptosis of murine gastric pit cells through an NF‐kappaB‐dependent pathway. Gastroenterology 2001 (Jul); 121: 56–67. [DOI] [PubMed] [Google Scholar]
- 55. Zhang S, Yanaka A, Tauchi M et al. Hyperosmotic stress enhances interleukin‐1beta expression in Helicobacter pylori‐infected murine gastric epithelial cells in vitro. J Gastroenterol Hepatol 2006; 21: 759–66. [DOI] [PubMed] [Google Scholar]
- 56. Takahashi T, Matsumoto T, Nakamura M et al. A novel in vitro infection model of Helicobacter pylori using mucin‐producing murine gastric surface mucous cells. Helicobacter 2004; 9: 302–12. [DOI] [PubMed] [Google Scholar]
- 57. Tabuchi Y, Arai Y, Shioya H et al. New gastric epithelial cell lines from mice transgenic for temperature‐sensitive simian virus 40 large T antigen show distinct types of cell differentiation. Digestion 2003; 67: 71–81. [DOI] [PubMed] [Google Scholar]
- 58. Tabuchi Y, Arai Y, Ohta S et al. Development and characterization of conditionally immortalized gastric epithelial cell lines from transgenic rats harboring temperature‐sensitive simian virus 40 large T‐antigen gene. Cell Struct Funct 2002; 27: 71–9. [DOI] [PubMed] [Google Scholar]
- 59. Tabuchi Y, Ohta S, Arai Y et al. Establishment and characterization of a colonic epithelial cell line MCE301 from transgenic mice harboring temperature‐sensitive simian virus 40 large T‐antigen gene. Cell Struct Funct 2000; 25: 297–307. [DOI] [PubMed] [Google Scholar]
- 60. Tabuchi Y, Arai Y, Kondo T, Takeguchi N, Asano S. Identification of genes responsive to sodium butyrate in colonic epithelial cells. Biochem Biophys Res Commun 2002; 293: 1287–94. [DOI] [PubMed] [Google Scholar]
- 61. Tabuchi Y, Takasaki I, Doi T, Ishii Y, Sakai H, Kondo T. Genetic networks responsive to sodium butyrate in colonic epithelial cells. FEBS Lett 2006; 580: 3035–41. [DOI] [PubMed] [Google Scholar]
- 62. Hosoya K, Tomi M, Takayama M et al. Transporter mRNA expression in a conditionally immortalized rat small intestine epithelial cell line (TR‐SIE). Drug Metab Pharmacokinet 2004; 19: 264–9. [DOI] [PubMed] [Google Scholar]
- 63. Ohta S, Tabuchi Y, Yanai N, Asano S, Fuse H, Obinata M. Establishment of a leydig cell line TTE1 from transgenic mice harboring temperature‐sensitive simian virus 40 large T‐antigen gene. Arch Androl 2002; 348: 43–51. [DOI] [PubMed] [Google Scholar]
- 64. Tabuchi Y, Ohta S, Kondo T et al. Development of a conditionally immortalized testicular Sertoli cell line TTE3 expressing Sertoli cell‐specific genes from mice transgenic for temperature‐sensitive simian virus 40 large t‐antigen gene. J Urol 2002; 167: 1538–45. [PubMed] [Google Scholar]
- 65. Tabuchi Y, Takahashi R, Ueda M, Obinata M. Development of a Conditionally Immortalized Testicular Sertoli Cell Line RTS3‐3 from Adult Transgenic Rats Harboring Temperature‐Sensitive Simian Virus 40 Large T‐antigen Gene. Cell Struct Funct 2003; 28: 87–95. [DOI] [PubMed] [Google Scholar]
- 66. Kuwahara S, Ikei A, Taguchi Y. PSPC1, NONO, and SFPQ are expressed in mouse sertoli cells and may function as coregulators of androgen receptor‐mediated transcription. Biol Reprod 2006; 75: 352–359. [DOI] [PubMed] [Google Scholar]
- 67. Araki Y, Suzuki K, Matusik RJ, Obinata M, Orgebin‐Crist MC. Immortalized epididymal cell lines from transgenic mice overexpressing temperature‐sensitive simian virus 40 large T‐antigen gene. J Androl 2002; 23: 854–69. [PubMed] [Google Scholar]
- 68. Suzuki K, Yu X, Chaurand P et al. Epididymis‐specific promoter‐driven gene targeting: a transcription factor which regulates epididymis‐specific gene expression. Mol Cell Endocrinol 2006; 250: 184–9. [DOI] [PubMed] [Google Scholar]
- 69. Hosoya K, Tetsuka K, Nagase K et al. Conditionally immortalized brain capillary endothelial cell lines established from a transgenic mouse harboring temperature‐sensitive simian virus 40 large T‐antigen gene. AAPS Pharmsci 2000; 2: E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hosoya K‐I, Takashima T, Tetsuka K et al. mRna expression and transport characterization of conditionally immortalized rat brain capillary endothelial cell lines; a new in vitro BBB model for drug targeting. J Drug Target 2000; 8: 357–70. [DOI] [PubMed] [Google Scholar]
- 71. Asashima T, Iizasa H, Terasaki T et al. Newly developed rat brain pericyte cell line, TR‐PCT1, responds to transforming growth factor‐beta1 and beta‐glycerophosphate. Eur J Cell Biol 2002; 81: 145–52. [DOI] [PubMed] [Google Scholar]
- 72. Kitazawa T, Hosoya K, Watanabe M et al. Characterization of the amino acid transport of new immortalized choroid plexus epithelial cell lines: a novel in vitro system for investigating transport functions at the blood–cerebrospinal fluid barrier. Pharm Res 2001; 18: 16–22. [DOI] [PubMed] [Google Scholar]
- 73. Hosoya K, Tomi M, Ohtsuki S et al. Conditionally immortalized retinal capillary endothelial cell lines (TR‐iBRB) expressing differentiated endothelial cell functions derived from a transgenic rat. Exp Eye Res 2001; 72: 163–72. [DOI] [PubMed] [Google Scholar]
- 74. Kondo T, Hosoya K, Hori S et al. Establishment of conditionally immortalized rat retinal pericyte cell lines (TR‐rPCT) and their application in a co‐culture system using retinal capillary endothelial cell line (TR‐iBRB2). Cell Struct Funct 2003; 28: 145–53. [DOI] [PubMed] [Google Scholar]
- 75. Tomi M, Funaki T, Abukawa H et al. Expression and regulation of 1‐cystine transporter, system xc‐, in the newly developed rat retinal Muller cell line (TR‐MUL). Glia 2003; 43: 208–17. [DOI] [PubMed] [Google Scholar]
- 76. Kitano T, Iizasa H, Terasaki T et al. Polarized glucose transporters and mRNA expression properties in newly developed rat syncytiotrophoblast cell lines, TR‐TBTs. J Cell Physiol 2002; 193: 208–18. [DOI] [PubMed] [Google Scholar]
- 77. Matsushita T, Amagai Y, Terai K, Kojima T, Obinata M, Hashimoto S. A novel neuronal cell line derived from the ventrolateral region of the suprachiasmatic nucleus. Neuroscience 2006; 140: 849–56. [DOI] [PubMed] [Google Scholar]
- 78. Matsushita T, Amagai Y, Soga T, Terai K, Obinata M, Hashimoto S. A novel oligodendrocyte cell line OLP6 shows the successive stages of oligodendrocyte development: Late progenitor, immature and mature stages. Neuroscience 2005; 136: 115–21. [DOI] [PubMed] [Google Scholar]
- 79. Takeuchi K, Yanai N, Takahashi N et al. Different cellular actions of vasopression‐induced adenosine 3′, 5′‐monophosphate formation in an immortalized renal tubule cell line, TKC2. Biochem Biophys Res Commn 1994; 202: 680–7. [DOI] [PubMed] [Google Scholar]
- 80. Sekine T, Hosoyamada M, Haga‐Mizuno A et al. Ammonia production in cell lines established from transgenic mice harboring temperature‐sensitive simian virus 40 large T‐antigen gene. Contrib Nephrol 1994; 110: 98–102. [DOI] [PubMed] [Google Scholar]
- 81. Takeda M, Hosoyamada M, Shirato I, Obinata M, Suzuki M, Endou H. Establishment of vasopressin‐responsive early proximal tubular cell lines derived from transgenic mice harboring temperature‐sensitive simian virus 40 large T‐antigen gene. Biochem Mol Biol Int 1995; 37: 507–15. [PubMed] [Google Scholar]
- 82. Hosoyamada M, Obinata M, Endou H. Cisplatin‐induced toxicity in immortalized renal cell lines established from transgenic mice harboring temperature sensitive SV40 large T‐antigen gene. Arch Toxicol 1996; 70: 284–92. [DOI] [PubMed] [Google Scholar]
- 83. Yasuoka Y, Kawada H, Suzuki Y et al. Establishment of a mouse macula densa cell line with an nNOS promoter driving EGFP expression. Jpn J Physiol 2005; 55: 365–72. [DOI] [PubMed] [Google Scholar]


