Abstract
The p53 protein exerts its tumor suppressive function mainly by acting as a transcription activator. Two transactivation domains (TADs) located at the amino‐terminus of p53 are required for transcription activation, and the activity of TADs is tightly regulated by post‐translational modifications, such as phosphorylation. We attempted to dissect the functions of the two TADs and phosphorylation within the TADs by analyzing p53 target genes induced by full‐length p53 (FL‐p53), N‐terminally deleted p53 isoform lacking the first TAD (Δ1stTAD) and p53 carrying point mutations at all serine residues within the two TADs (TAD‐S/A). By performing a comprehensive survey by employing microarray expression analysis, the induction of target genes by FL‐p53, Δ1stTAD and TAD‐S/A was analyzed. All p53s showed different target gene induction patterns, suggesting the importance of the two TADs and phosphorylation within the TADs in target gene induction. Although Δ1stTAD showed a marked decrease in the ability to induce genes induced by FL‐p53, Δ1stTAD induced many apoptosis‐related genes that were not induced by FL‐p53, suggesting the roles of these Δ1stTAD‐induced genes in Δ1stTAD‐dependent apoptosis. Approximately 80% of genes induced by FL‐p53 were not induced by TAD‐S/A, including 29 previously reported p53 target genes such as Hdm2 and Bax, emphasizing the importance of phosphorylation within the TADs. These results demonstrate the significance of the regulation and differential roles of the N‐terminal TADs in p53 transcriptional activity. (Cancer Sci 2007; 98: 189–200)
The p53 protein is a transcriptional activator that plays a central role in the response to cellular stress. By inducing its target genes involved in apoptosis induction and cell cycle arrest, p53 exerts its function as a guardian of the cell.( 1 , 2 ) The high incidence of p53 mutation in human cancer illustrates its importance in maintaining the integrity of the cell.
The structure of p53 protein is commonly divided into three functional domains. The amino‐terminal domain, central core DNA‐binding domain and carboxy‐terminal domain.( 3 ) The N‐terminal domain is required for the transcriptional acitivity of p53 protein, and it consists of two transactivation domains (TADs) and a proline‐rich domain. At least one of the two TADs is necessary for transcriptional activity, and the first TAD is dispensable for apoptosis induction.( 4 , 5 ) Recently, an N‐terminally deleted isoform of p53 translated from the internal start codon positioned at amino acid 40, named p53/47 by the authors (termed Δ1stTAD in this manuscript), was found to be expressed endogenously.( 6 , 7 ) This isoform is produced by alternative translation or splicing, and lacks the first TAD. It has been reported that the expression patterns of p53 target genes Bax and p21WAF1 are changed by the presence of Δ1stTAD.( 7 ) This fact points out a possibility that full‐length p53 (FL‐p53) and Δ1stTAD may have different unique DNA‐binding properties. Alternatively, they may have different promoter activating capacities by, for example, recruiting different coactivators to the promoters. Overexpression of Δ1stTAD results in the induction of apoptosis, and this activity is dependent on the transcriptional activity of the protein.( 4 , 5 , 8 ) Furthermore, transgenic mice overexpressing Δ1stTAD show phenotypes of premature aging and growth suppression.( 9 ) Collectively, Δ1stTAD exerts its tumor‐suppressive activity through transcriptional activation of its target genes.
The most common post‐translational modification of p53 is phosphorylation.( 3 ) So far, 17 phosphorylation sites have been reported and nine of them are located within the TADs. Kinases activated upon cellular stress, such as ATM, ATR and p38, are known to phosphorylate the residues within the TADs, and phosphorylation results in an increase of the transcription ability of p53 protein. There are also reports showing that by introducing point mutations to the residues that undergo phosphorylation, apoptosis induction becomes defective.( 10 , 11 , 12 , 13 , 14 , 15 ) Several apoptosis‐related previously known p53 target genes, for example Puma, were not induced efficiently by these mutants.( 12 , 13 , 14 )
In this report, we focused on the functional regulation of p53 through the N‐terminal TADs. We attempted to dissect the functions of the two TADs and phosphorylation within the TADs by analyzing p53 target genes induced by FL‐p53, Δ1stTAD and p53 carrying point mutations at all serine residues within the two TADs (TAD‐S/A). We performed microarray expression analysis using cell lines stably expressing FL‐p53, Δ1stTAD and TAD‐S/A. All p53s showed different target gene induction patterns, suggesting the importance of the first TAD, the second TAD and phosphorylation within the TADs in target gene induction. Although Δ1stTAD showed a decrease in the ability to induce genes induced by FL‐p53, Δ1stTAD induced many apoptosis‐related genes not activated by FL‐p53, which may explain the apoptosis‐inducing activity of Δ1stTAD. Furthermore, approximately 80% of genes induced and 85% of genes repressed by FL‐p53, respectively, were not induced or repressed by the TAD‐S/A, emphasizing the importance of phosphorylation within the TADs. Taken together, these results demonstrate the importance of the N‐terminal first and second TADs in p53 regulation.
Materials and Methods
Transfection and luciferase reporter assay. Transfection and luciferase reporter assays were done basically as described.( 16 ) Transient transfection assays were performed using LIPOFECTAMINE Plus reagent (Invitrogen, Carlsbad, CA). For the luciferase reporter assay, Saos2 cells were seeded in 96‐well dishes and cotransfected with 20 ng of firefly luciferase reporter gene and 60 ng of each p53 gene cloned in pMX‐puro retroviral vector (each construct is expressed from the retroviral LTR promoter) together with 5 ng of renilla luciferase expression vector (pGL4.75[hRluc/CMV] vector, purchased from Promega, Madison WI) as an internal control for transfection efficiency. The firefly luciferase reporter gene containing an artificial p53 binding site, p53‐Luc, was purhcased from STRATAGENE (La Jolla, CA). Cells assayed at the non‐permissive temperature were incubated at 38°C. Cells assayed at the permissive temperature were cultured overnight at 38°C after transfection, and shifted to 32°C for 10 h. Luciferase activity was detected using the Dual‐Luciferase Reporter Assay System (Promega, Madison WI). For the luciferase gene cotransfection growth analysis, 2 × 104 Saos2 cells were seeded in 96‐well dishes and cotransfected with 15 ng of non‐ts version of each p53 gene cloned in pcDNA3 vector together with 2 ng of firefly luciferase expression vector (PicaGene control vector [Wako]). Cells were harvested 48 h post transfection, and analyzed using Luciferase Reporter Assay Reagent (Promega).
Cell culture and establishment of ts stable cell lines expressing different forms of p53. Cell culture was done as described.( 17 ) Stable cell lines expressing ts‐FL‐p53, ts‐Δ1stTAD and ts‐TAD‐S/A were obtained by transducing the retrovirus expressing each p53 to p53‐null Saos2 cells. As control cells, empty retrovirus expressing only the drug resistance gene was also infected. Infection was performed in the presence of polybrene (used at 4 µg/mL, purchased from SIGMA, St. Louis, MO). After infection, cells were selected in puromycin (used at 0.5 µg/mL, purchased from SIGMA, St. Louis, MO). In order to avoid possible disadvantages arising from utilizing clonal cell lines, that is, clonal differences, cells expressing each p53 were maintained as mass cultures. Cells were cultured at 38°C to ensure inactivation of each p53.
Western blotting analysis. Cells were lyzed in lysis buffer containing 50 mM Tris‐HCl (pH 8.0), 1% NP40, 250 mM NaCl, 50 mM NaF, 1 mM Na3VO4, 1 mM protease inhibitor (PMSF, aprotinin, leupeptin) and 1 mM DDT. The whole cell lysates were subjected to protein quantification and analyzed by Western blotting. Anti‐p53 phospho‐Ser15‐specific rabbit polyclonal antibody and anti‐p53 phospho‐Ser46‐specific mouse monoclonal antibody were prepared as described.( 18 ) Anti‐p53 rabbit polyclonal antibody was purchased from Cell Signaling Technology (Danvers, MA), and anti‐actin mouse monoclonal antibody was purchased from SIGMA (St. Louis, MO).
Northern blotting analysis. RNA was prepared using an RNeasy Midi kit (QIAGEN, Valencia, CA). Northern blotting was performed as described.( 17 ) Probes were prepared using a BcaBEST labeling kit (TaKaRa, Shiga, Japan), and purified by serial purification using a Probe Quant G‐50 MicroColumn (Amersham, Buckinghamshire HP7 9NA, UK) and NICK Column (Amersham, Buckinghamshire, UK). The full open reading frame of Hdm2, EcoR I‐Sfi I fragment obtained from an EST clone containing p21WAF1 sequence (IMAGE ID 2823668, purchased from Open Biosystems, Huntsville, AL), EcoR I‐Sma I fragment obtained from an EST clone containing GAK sequence (IMAGE ID 6339866, purchased from Open Biosystems), EcoR I‐BamH I fragment obtained from an EST clone containing MUC1 sequence (accession number BM982837, purchased from Open Biosystems), Not I‐Sal I fragment obtained from an EST clone containing TXNIP sequence (IMAGE ID 5784713, purchased from Open Biosystems), Bgl II‐ Bgl II fragment obtained from an EST clone containing IER5 sequence (IMAGE ID 3048356, purchased from Open Biosystems) and Nco I‐ApaL I fragment from reverse transcriptase polymerase chain reaction‐amplified Noxa (containing the full open reading frame) were used for probe preparation.
Microarray expression analysis. The integrity of the total RNA was confirmed using LabChip RNA 6000 Nano chips and a 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany). For gene expression profiling, a Human Genome U133 Plus 2.0 array (Affymetrix, Santa Clara, CA) was used that contained over 54 000 probe sets. Target cRNA preparations from total RNA, hybridization to the microarray, washing and staining with the antibody amplification procedure, and scanning were all carried out according to the manufacturer's instructions. The expression value (Signal) of each gene was calculated and normalized using GeneChip Operating Software version 1.2 (Affymetrix, Santa Clara, CA), so that the mean of Signal values in each experiment was 100, to adjust for minor differences between the experiments. In order to obtain the mean basal expression level of each gene, the average of signal values at 38°C in four cell lines was calculated and used as the standard for the analysis. The change value (Signal Log Ratio) and change call (Increase, Marginal Increase, No Change, Marginal Decrease, or Decrease) for each gene were calculated using Comparison Analysis of the software. To identify genes that were significantly induced by each p53, we selected genes which showed the change call of Increase and the Signal Log Ratio >0.5 (i.e., >1.4‐fold increase) or genes which showed the change call of Decrease and the Signal Log Ratio of <–0.5 (i.e., >1.4‐fold decrease). To eliminate genes that showed similar expression changes in control cells transduced with an empty vector, we further selected genes that were increased or decreased more than 1.2‐fold compared to the changes seen in the control sample. Furthermore, in order to eliminate false positive results, we picked up probes that were selected at more than two time points.
Analysis of p53 binding sites. The p53 binding sites from previously reported p53 target genes were analyzed (shown in supplementary Table S4). First, we aligned the 10 nucleotides derived from each p53 binding site to construct a p53 position weight matrix (PWM), based on a method similar to the method previously described.( 19 ) We grouped the target genes into genes induced by Δ1stTAD and those not induced by Δ1stTAD. We calculated the number of each nucleotide at each position for the group of genes induced by Δ1stTAD, and the total score for each p53 binding site was obtained as the sum of nucleotide frequencies at each position. As shown in Fig. 7A, there were 11 positions where the frequency value was zero. The value zero indicates positions where a particular nucleotide was deleterious for Δ1stTAD binding. Therefore, we assigned a negative value to those positions. We incorporated negative value −92 (corresponding to the difference between the maximum and minimum scores of the sequence with only permitted sequence variations) at 11 zero positions. The PWM obtained was applied to genes not induced by Δ1stTAD to analyze if there was a nucleotide usage preference between each group of genes. The same analysis was done for genes induced by TAD‐S/A and genes not induced by TAD‐S/A.
Figure 7.
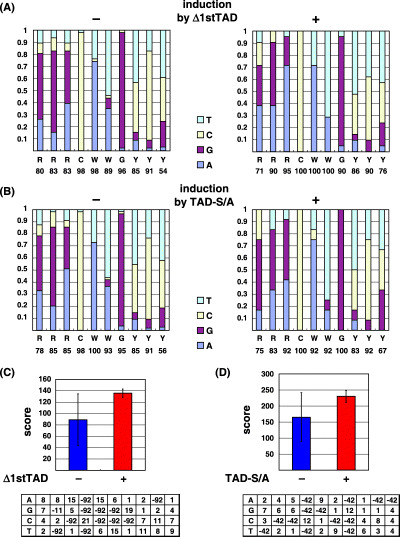
Analysis of p53 binding sites in previously known target genes of p53. (A,B) Nucleotide usage at each position was calculated and the proportion of usage of each nucleotide is shown. Genes induced and not induced by Δ1stTAD (A), and genes induced and not induced by TAD‐S/A (B) were analyzed. (C,D) PWM were obtained for genes induced by Δ1stTAD (C) and TAD‐S/A (D), and are shown at the bottom panels. According to the PWM obtained, scores were calculated for each gene. Average values were obtained for genes induced (+) and not induced (–) by Δ1stTAD (shown in D), and genes induced (+) and not induced (–) by TAD‐S/A (shown in E), respectively, and are shown as a graph.
Results
Construction of temperature‐sensitive FL‐p53, Δ1stTAD and TAD‐S/A. As shown in Fig. 2A, we constructed FL‐p53, Δ1stTAD lacking the first 39 amino acids of FL‐p53 and TAD‐S/A harboring mutations at all serine residues within the first and the second TADs (Ser 6, 9, 15, 20, 33, 37 and 46 were converted to Ala). Each construct carried a temperature sensitive (ts) mutation at position 138 (conversion of Ala to Val) in order to regulate the activity of each p53 by temperature shift.( 20 ) In order to validate the activity of each p53, luciferase reporter assay was performed at 38 and 32°C, non‐permissive and permissive temperatures, respectively. The transcriptional ability of each p53 was tested using reporter plasmid harboring artificial p53 binding sites (containing more than 10 repeats of AGGCAAGTCC) upstream of the luciferase reporter gene. As shown in Fig. 2B, the promoter was efficiently activated at 32°C by ts‐FL‐p53 and ts‐TAD‐S/A (8.7 and 8.3‐fold for ts‐FL‐p53 and ts‐TAD‐S/A, respectively). This result is consistent with the previous reports showing the capability of p53 carrying mutations at six serine residues within the first TAD (serines 6, 9, 15, 20, 33 and 37) to transactivate an artificial p53‐dependent promoter.( 11 ) We speculate that since p53 is not fully phosphorylated under the conditions tested (T. K., unpublished observations), no difference could be detected in the transcription activity between FL‐p53 and TAD‐S/A. On the other hand, Δ1stTAD showed a noticeable reduction of transcriptional activation (2.4‐fold). In addition to its lack of activity with this artificial promoter, Δ1stTAD showed limited activity against all p53‐dependent promoters tested (supplementary Fig. S1 and data not shown). The same results were obtained using a non‐ts version of Δ1stTAD, confirming that the transcription defect on p53‐dependent promoters was not due to the ts mutation (data not shown).( 5 ) Similar results were obtained using the ts constructs against natural p53 target gene promoters Noxa and p21 (Fig. S1).
Figure 2.
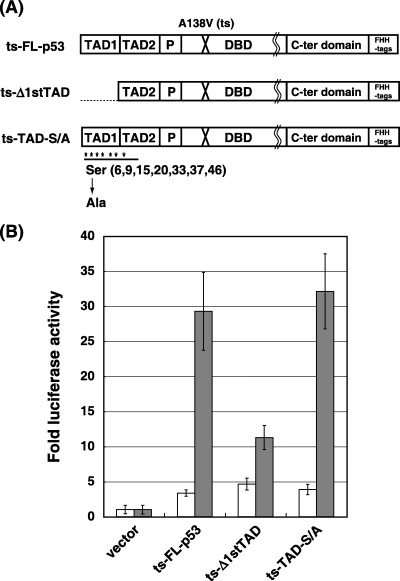
Construction and analysis of ts‐FL‐p53, ts‐Δ1stTAD and ts‐TAD‐S/A. (A) The constructs used in this study are shown. In addition to the ts mutation at codon 138, Δ1stTAD lacks the first 39 amino acids of FL‐p53 and TAD‐S/A carries mutations at all serine residues within the first and the second TAD (Ser 6, 9, 15, 20, 33, 37 and 46 were converted to Ala). Each construct was cloned in pMX‐puro retroviral vector, and is C‐terminally tagged simultaneously with FLAG, HA, and polyhistidine peptide (shown as FHH). TAD, transactivation domain; P, proline‐rich domain; DBD, DNA binding domain. (B) The luciferase reporter assay was performed using the above constructs. The assay was performed using Saos2 cells at non‐permissive (38°C, shown as white bars) and permissive (32°C, shown as gray bars) temperatures.
Establishment of FL‐p53, Δ1stTAD and TAD‐S/A cell lines. Since it was demonstrated that each p53 has a ts phenotype, we next introduced ts‐FL‐p53, ts‐Δ1stTAD and ts‐TAD‐S/A into p53‐null Saos2 cells by retrovirus‐mediated gene transfer. Empty retrovirus expressing only the drug resistance gene was also infected and the resultant cells were used as control cells. As shown in Fig. 3C, each p53 was expressed at a comparable level at the non‐permissive temperature (Fig. 3C, top panel, lanes 1, 5 and 9), demonstrating the fact that retrovirus infection and subsequent p53 expression were equally successful in each cell line. We compared the expression of each p53 at the non‐permissive temperature, since each p53 underwent different stabilization levels upon temperature shift to the permissive temperature (see Fig. 3C top panel). In order to exclude the possibility of p53 expressed in the established cell lines as unnaturally high, cells expressing each p53 were maintained as mass culture. Expression level of FL‐p53 was compared with those of endogenously expressed p53, and as expected, it was confirmed that the expression level of FL‐p53 was similar to that of normal human fibroblast after γ‐ray irradiation, and weaker than that of MCF7 cells after adriamycin treatment. (Fig. 3A, compare lane 1 and 5, and lane 7 and 8. For Lane 5, protein extracted from 3‐fold more cells has been loaded compared to lane 1, but lane 5 has approximately 2‐fold more signal than lane 1. The same amount of protein has been loaded for lane 7 and 8). Faint expression of Δ1stTAD was observed in FL‐p53‐ and TAD‐S/A‐expressing cells (Fig. 3C, top panel, lanes 1–4 and 9–12, shown by closed arrow head). This Δ1stTAD may be an alternative translation product arising from FL‐p53 and TAD‐S/A, as judged from its reactivity with antip53 antibody PAb1801 (detects residues 46–55 of FL‐p53) and non‐reactivity with anti‐p53 antibody DO1 (detects residues 20–25 of FL‐p53), and may function to induce genes induced by Δ1stTAD, although at a considerably lower level (T. K. unpublished results). The band detected between FL‐p53 and Δ1stTAD may result from proteolytic cleavage by calpain, owing to the calpain‐sensitive site at residues 13–19 of FL‐p53.
Figure 3.
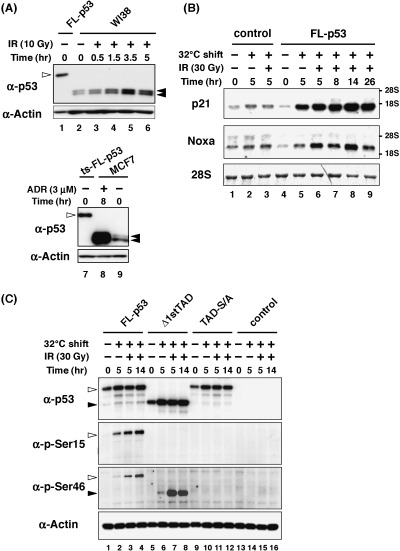
Stabilization and activation of ts‐p53s upon temperature shift to permissive temperature. (A) Expression level of ts‐FL‐p53 was compared with those of endogenously expressed p53. WI38 cells (normal human diploid fibroblast) were subjected to γ‐ray irradiation and harvested at the indicated times. MCF7 cells (p53 wild type) were subjected to adriamycin (3 µM) treatment for 8 h. Whole‐cell lysates were prepared from each sample, and proteins derived from 2 × 104 cells (for ts‐FL‐p53 cells and MCF7 cells) or 6 × 104 cells (for WI38 cells) were loaded per lane. FL‐p53 is shown by open arrow head and endogenous p53 is shown by closed arrow head. Both WI38 and MCF7 cells are cell lines expressing two polymorphic p53 variants, proline or arginine at codon 72, and two bands are detected for p53. Migration of ts‐FL‐p53 is decreased compared to the endogenous p53 because of the C‐terminal tags. (B) The ts‐FL‐p53‐expressing cell line was tested for the ability to induce target genes upon temperature shift to the permissive temperature with or without γ‐ray irradiation. Cells were subjected to γ‐ray irradiation after 2 h of temperature shift to 32°C. Cells were collected at 5, 8, 14, 26 h post‐temperature shift. RNA was purified from each sample and 3 µg of total RNA was used for Northern blotting. Methylene blue staining of 28S ribosomal RNA is shown at the bottom panel to show equal loading of RNA in each lane. (C) Western blotting analysis to show that each p53 is stabilized and phosphorylated upon temperature shift and γ‐ray irradiation. Cells were collected at 5 and 14 h post‐temperature shift with or without γ‐ray irradiation. Whole‐cell lysates were prepared from each sample, and 5 µg (for total p53 and p‐Ser15) or 10 µg (for p‐Ser46) of proteins were loaded. FL‐p53 is shown by open arrow head and Δ1stTAD is shown by closed arrow head.
When these established cell lines were shifted to permissive temperature, control cell line showed no significant change for cells in S phase as deduced from BrdU positive cells (32% and 30% BrdU positive cells at the non‐permissive and permissive temperatures, respectively). On the other hand, the cell lines expressing FL‐p53 (35% and 23% BrdU positive cells at the non‐permissive and permissive temperatures, respectively) and TAD‐S/A (34% and 24% BrdU positive cells at non‐permissive and permissive temperatures, respectively) showed reduction for cells in S phase, indicating that these cells underwent cell cycle arrest. However, the cell line expressing Δ1stTAD showed no significant change for BrdU positive cells at this condition (30% and 29% BrdU positive cells at the non‐permissive and permissive temperatures, respectively). The result is shown in supplementary Fig. S2B. The result was also confirmed by Giemsa staining after culturing each cell line at the non‐permissive and permissive temperatures for 7 days (Fig. S2A). While no difference between each cell line was detected at the non‐permissive temperature (Fig. S2A, left panel), at the permissive temperature, significant decreases in cell numbers were detected for cells expressing FL‐p53 and TAD‐S/A (Fig. S2A, right panel), consistent with the results obtained by analyzing BrdU positive cells. Since parental Saos2 cells undergo p53‐independent apoptosis by γ‐ray irradiation, it was difficult to detect p53‐dependent cell cycle arrest or apoptosis upon γ‐ray irradiation.
Taking the FL‐p53 cell line as a representative cell line, we first determined the conditions suitable for target gene induction. As shown in Fig. 3B, efficient induction of well‐characterized p53 target genes p21WAF1 and Noxa was achieved after 5 h of temperature shift (compare lanes 4 and 5 of each panel). Irradiation with γ‐rays further strengthened the induction of each target gene, as shown in lanes 6–9. As has been reported, target gene induction oscillation was observed following γ‐ray irradiation (lanes 6–9).( 21 ) Strong induction of the target genes was seen in cells after 5 or 14 h of temperature shift with γ‐ray irradiation (Fig. 3B, lanes 6 and 9). We therefore employed these two conditions for the further analysis. We also employed the condition of only a temperature shift for 5 h, in order to discriminate genes induced by p53 without irradiation. We then analyzed the amount of p53 protein and p53 phosphorylation at Ser 15 and 46 as markers for p53 activation. Notably, in each cell line, p53 underwent stabilization after the temperature shift to 32°C without DNA damage (Fig. 3C, top panel lanes 2, 6 and 10). Weak phosphorylation of FL‐p53 was detected with only temperature shift, and the phosphorylation level was increased by γ‐ray irradiation in accordance with FL‐p53 activation (Fig. 3C two panels in the middle, lanes 2–4). Interestingly, strong Ser 46 phosphorylation was observed in γ‐ray irradiated Δ1stTAD‐expressing cells (Fig. 3C, two panels in the middle, lanes 7 and 8). It was previously shown that phosphorylation of Ser 46 is required for the efficient induction of p53‐dependent apoptosis.( 12 , 18 ) The strong phophorylation of this site in Δ1stTAD may contribute to the apoptosis induction by Δ1stTAD. As was expected, neither Ser 15 nor 46 phosphorylation was detected in TAD‐S/A expressing cells (Fig. 3C, two panels in the middle, lanes 9–12).
p53‐dependent gene expression changes identified by microarray analysis. RNA samples were prepared from the established cell lines cultured at 32°C for 5 h with or without γ‐ray irradiation and cells cultured at 32°C for 14 h with γ‐ray irradiation. Using these RNA samples, microarray analysis was performed according to the procedure described in Materials and Methods. In order to set up the optimum cut‐off value for p53 target gene analysis, we first selected genes induced or repressed by FL‐p53 more than 1.2‐fold, which we consider to be the minimum difference in expression that is experimentally significant. To eliminate probes that were also induced or repressed in control cells resulting from temperature shift and γ‐ray irradiation, probes showing more than 1.2‐fold difference in expression compared to the control cells were selected. We then selected previously reported p53‐inducible genes among these selected genes. The p53 target genes listed in the TranSignal p53 Target Gene Array (B‐Bridge, Sunnyvale, CA) and the p53‐inducible genes described in the manuscript by Kho et al. were combined and analyzed as to whether or not they are induced in our experimental system.( 22 ) Out of 178 p53‐inducible genes, 36 genes were induced, and the minimum induction value was 1.4‐fold for LITAF and IER3, and the maximum was 24‐fold for Hdm2 (supplementary Table S1). Therefore, to obtain all of the genes induced by p53, we further selected probes with the cut‐off value of 1.4‐fold. Besides, to eliminate false positives that may arise by the analysis due to the low cut‐off value, we selected probes that were induced by each p53 at more than two time points.
By selecting probes as described above, in total, 545 probe sets were up‐regulated and 768 probe sets were down‐regulated by FL‐p53 (Fig. 4). To be specific, there were 2045 probe sets up‐regulated more than 1.4‐fold at more than two time points in cells expressing FL‐p53, but within these probe sets, 1500 probe sets were also up‐regulated in the control cells in a p53‐independent manner. On the other hand, 21 143 probe sets were down‐regulated more than 1.4‐fold at more than two time points in cells expressing FL‐p53, and within these probe sets, 20 375 probes were also down‐regulated in the control cells. When cells expressing FL‐p53 were shifted to the permissive temperature, only 1371 and 2023 probes were up‐ or down‐regulated, and within these probe sets, 634 and 1006 probe sets were also up‐ or down‐regulated in the control cells (i.e. 737 and 1017 probe sets were up‐ or down‐regulated in a p53‐dependent manner upon temperature shift to the permissive temperature). Therefore, huge numbers of genes were down‐regulated especially upon γ‐ray irradiation in a p53‐independent manner. Among the probes up‐ or down‐regulated by FL‐p53, 154 and 115 or 162 and 119 probes were up‐ or down‐regulated by Δ1stTAD and TAD‐S/A, respectively. Overall, both genes transactivated and transrepressed by FL‐p53 were not activated or repressed efficiently in the cell lines expressing Δ1stTAD and TAD‐S/A. The groups of genes induced by each p53 overlapped each other, but each showed specific patterns of gene induction or repression (Fig. 4A,B). It is noteworthy that approximately 80% or 85% of the probe set induced or repressed by FL‐p53 was not efficiently induced or repressed in cells expressing TAD‐S/A, emphasizing the impact of N‐terminal phosphorylation on the transcriptional activity of p53.
Figure 4.
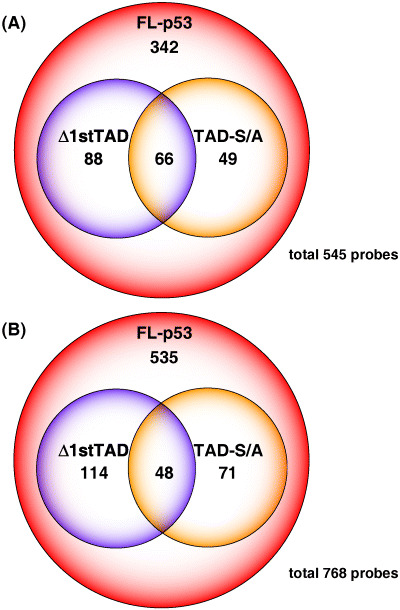
Probes up‐regulated and down‐regulated by FL‐p53, Δ1stTAD and TAD‐S/A. GeneChip analysis was done as described in Materials and Methods, and GeneChip probes that showed more than 1.4‐fold of increase (A) or decrease (B) were identified.
Previously reported p53 target genes are differentially induced by Δ1stTAD and TAD‐S/A. We next analyzed 36 previously reported p53 target genes from the up‐regulated genes described above. Among these target genes, most of the genes were induced after 5 h of temperature shift without γ‐ray irradiation by FL‐p53 (29 genes, shown by capitalized YES in Table S1). We therefore consider genes up‐regulated only by temperature shift to be good candidates for direct p53 target genes in our experimental system. In addition to FL‐p53, Δ1stTAD and TAD‐S/A also induced genes merely by temperature shift, and those genes are potential direct targets of each p53 (shown by capitalized YES in supplementary Tables S1 and S2, and shown in shaded columns in supplementary Table S3).
According to the expression patterns, we grouped the previously reported target genes into four types, and typical patterns of gene induction are shown in Fig. 5 (the genes are listed in Table S1). There were 22 genes induced only by FL‐p53 (type I), seven genes induced by FL‐p53 and Δ1stTAD (type II), five genes induced by FL‐p53 and TAD‐S/A (type III) and two genes induced by all p53s (type IV). The transcription‐inducing ability of Δ1stTAD toward previously reported p53 target genes seemed to be weak compared to FL‐p53, and many genes were not induced by Δ1stTAD (see Fig. 5 and Table S1). The first TAD therefore seems to be essential for efficient induction of these genes. Besides, it has been reported that the transactivation properties of Δ1stTAD are different from those of FL‐p53, and Bax is preferentially induced by Δ1stTAD.( 7 ) This was also confirmed in our experimental system (see Table S1, type II genes). Many p53 target genes were not induced by TAD‐S/A, again accentuating the importance of post‐translational modification in the N‐terminal TADs.
Figure 5.
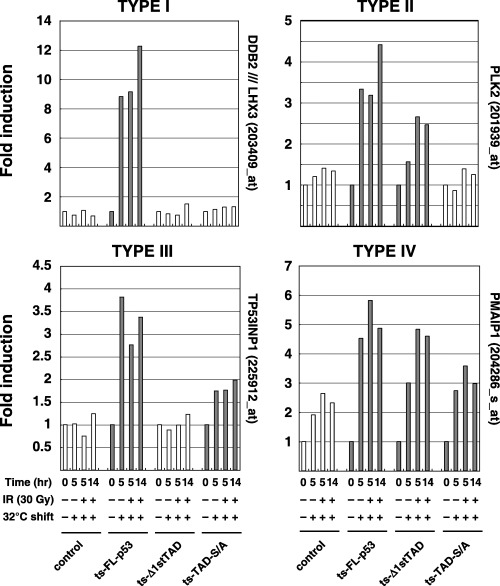
Typical patterns for each type of gene expression pattern are shown. Target genes were divided into four types, which were induced only by FL‐p53 (I), induced by FL‐p53 and Δ1stTAD (II), induced by FL‐p53 and TAD‐S/A (III) or induced by all p53s (IV). Data obtained from cell lines that were determined to have a p53‐dependent increase are shown by shadowed bars.
In order to confirm the reliability of the microarray analysis, expression patterns of representative p53 target gene Hdm2 and a potential p53 target gene IER5 were also analyzed by Northern blotting. The results obtained by Northern blotting and microarray analysis are compared (Fig. 6A top and middle panels). Both genes were confirmed to be induced specifically by FL‐p53 (type I gene, dependent on the 1st TAD and phosphorylation within the TAD), and the results obtained by two different methods were consistent, confirming the reliability of the microarray analysis performed.
Figure 6.
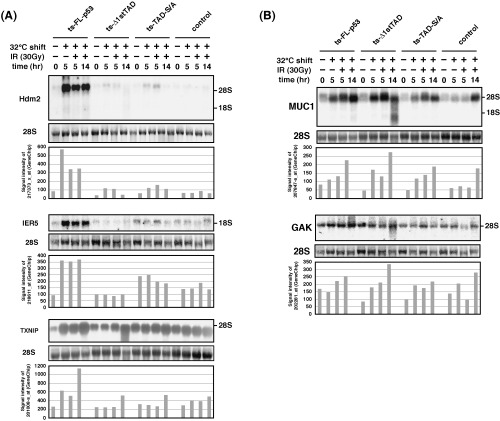
Genes induced in a manner dependent on p53 N‐terminal phosphorylation and expression of Δ1stTAD. (A) Expressions of genes that are induced by FL‐p53 but not by TAD‐S/A (Hdm2, IER5 and TXNIP) were analyzed by Northern blotting. The results obtained by microarray analyses are also shown for comparison. (B) Expressions of genes that are induced by Δ1stTAD were analyzed by Northern blotting. The results obtained by microarray analyses are also shown.
Induction of genes involved in apoptosis. It has been reported that mutants carrying mutations at serine residues that undergo phosphorylation show defects in apoptosis‐inducing ability.( 11 , 14 ) As shown in supplementary Table S1, most of the proapoptotic genes among the previously reported p53 target genes were not induced by TAD‐S/A (see Table S1, shown in shaded columns). We therefore speculated that the inability of TAD‐S/A to induce apoptosis was due to the defect in transcription activation of proapoptotic genes. Attempting to analyze genes involved in apoptosis comprehensively, we sorted out 6630 probes by searching the probes that have gene ontology terms for ‘apoptosis’, and analyzed if they are induced by each p53. FL‐p53 induced 97 apoptosis‐related genes, and within these genes, 31 genes were previously reported p53 target genes (shown by shaded columns in supplementary Table S2). As expected, only 23 genes were induced by TAD‐S/A, supporting our hypothesis described above (Table 2). Genes involved in apoptosis induction, such as RYBP (transcription regulator and apoptosis inducer), TXNIP (proapoptotic gene), ING3 (growth inhibitor and apoptosis inducer, which also increases the transactivation ability of p53) and caspase 2 were not induced by TAD‐S/A.( 23 , 24 , 25 ) On the other hand, in spite of its apoptosis‐inducing ability, out of 97 genes induced by FL‐p53, only 29 genes were induced by Δ1stTAD. Since there was a report suggesting the possibility that Δ1stTAD may have a different target gene induction ability from that of FL‐p53, we analyzed genes induced by Δ1stTAD but not by FL‐p53.( 7 ) Interestingly, there were 69 apoptosis‐related genes induced by Δ1stTAD but not by FL‐p53 (supplementary Table S3). These included P53BP2 (ASPP2), apoptosis‐stimulating factor and p53 activator, or TIAL1, which is an apoptosis inducer.( 26 , 27 ) These genes may contribute to apoptosis induction by Δ1stTAD. On the other hand, TAD‐S/A also induced 43 genes that are not induced by FL‐p53. Among these genes, 11 genes were also induced by Δ1stTAD, and there were 32 genes induced only by TAD‐S/A. We do not know the physiological relevance of these genes induced only by TAD‐S/A, and further examination is required for this issue.
Selected apoptosis‐related probe sets induced by FL‐p53, Δ1stTAD and TAD‐S/A were combined, and hierarchical clustering analysis was performed. The dendrogram represents the correlation of each gene expression pattern, and closer distance reflects closer relationship between gene expression patterns. As shown in supplementary Fig. S3, genes induced by FL‐p53 and genes induced by Δ1stTAD independent of FL‐p53 were clustered in distinct branches, and the most prominent clusters of FL‐p53‐inducible genes and Δ1stTAD‐inducible genes are shown by black bars at the right of the dendrogram in Fig. S3. This result clearly depicted a distinct difference in gene expression profile between genes induced by FL‐p53 and Δ1stTAD, and confirmed that the microarray analysis performed was successful in discriminating genes induced by each p53.
Furthermore, to confirm the reliability of the microarray analysis performed, expression pattern of TXNIP, from the list of apoptosis‐related gene, was also analyzed by Northern blotting (Fig. 6A bottom panel). It was confirmed that TXNIP was induced specifically by FL‐p53, and the result obtained by Northern blotting matched well with the result obtained by microarray analysis.
Analysis of genes induced by Δ1stTAD. From the genes identified as Δ1stTAD‐inducible genes, MUC1 (also induced by FL‐p53 and TAD‐S/A) and GAK (induced only by Δ1stTAD) were analyzed by Northern blotting. Both proteins are suggested to have important roles in tumorigenesis. MUC1 protein is reported to regulate p53 transcription activity and down‐regulation of GAK results in cellular transformation.( 28 , 29 ) As shown in Fig. 6B, the results obtained by Northern blotting and microarray analysis were consistent with each other.
It has been reported that Δ1stTAD has an ability to induce apoptosis in a manner dependent on the activity of the second TAD. The activity of the second TAD is lost when a double point mutation at residues 53 and 54 was introduced, and this mutant is inert in inducing apoptosis. We therefore analyzed whether MUC1 and GAK are induced in the course of Δ1stTAD‐dependent apoptosis. In order to detect Δ1stTAD‐dependent apoptosis, luciferase gene cotransfection growth analysis was performed. Control empty pcDNA3 vector, FL‐p53, Δ1stTAD,Δ1stTAD‐W53QF54S or an inactive mutant of p53 (V143A) were transfected together with luciferase gene which is expressed from a constitutively active CMV promoter. As shown in Fig. 1A, and 48 h post transfection, luciferase activity in Δ1stTAD‐W53QF54S and p53‐V143A expressing cells were similar to that of the control cells, showing that the conditions of the cells expressing these proteins were similar to the cells without their expression. On the other hand, the activity of luciferase was reduced to 32% and 53% of that of the control cells in FL‐p53 or Δ1stTAD expressing cells, indicating that cells expressing FL‐p53 or Δ1stTAD underwent apoptosis. The apoptotic phenotypes of these cells were also confirmed by microscopic analysis (data not shown). We next analyzed whether MUC1 and GAK were induced in the course of this Δ1stTAD‐dependent apoptosis. As shown in Fig. 1B, cells were transfected with Δ1stTAD or Δ1stTAD‐W53QF54S at the same condition as in Fig. 1A, and expressions of the genes were analyzed. Both MUC1 and GAK were induced by Δ1stTAD, and inductions of both genes were diminished when the cells were transfected with Δ1stTAD‐W53QF54S. It was also confirmed that the non‐ts version of Δ1stTAD induces MUC1 and GAK. Taken together, both MUC1 and GAK were induced by Δ1stTAD in a manner dependent on the activity of the second TAD, and were induced in the course of Δ1stTAD‐dependent apoptosis, in agreement with the involvement of Δ1stTAD‐dependent genes in apoptosis.
Analysis of p53 binding sites used by each p53. The consensus binding motif of p53 is composed of two copies of 5′‐RRRCWWGYYY‐3′ separated by 0–13 base pairs (R: purine; Y: pyrimidine; W: A or T).( 30 ) It is also known that the binding sites show significant degeneracy, and the degenerative nature of the binding sites may contribute to regulation of the target genes in response to diverse cellular signals (see binding sites listed in supplementary Table S4). Since each p53 showed specific preferences for gene induction, we speculated that there may be specific binding motif preferences for each p53. Therefore, we examined differences in nucleotide usage of the binding sites preferred by each p53. First, we grouped well‐characterized p53‐target genes into two groups, namely, genes induced (type II and IV) or not induced (type I and III) by Δ1stTAD (Table S4). Then, we counted the nucleotides used at each position for genes induced and not induced by Δ1stTAD as shown in Fig. 1A. Unexpectedly, there were no distinct differences in the nucleotide usage frequency at each position. However, in contrast to the genes induced only by FL‐p53, CWW trinucleotides in the core‐binding element (CWWG) were conserved among the genes induced by Δ1stTAD. There was also a preference for A compared to G in the first three nucleotides and T to C in the last three nucleotides. In order to evaluate the difference of nucleotide usage in more detail, we constructed a Δ1stTAD PWM similar to those described by Miled et al. (see Materials and Methods and Fig. 7C). Then, we applied the PWM to the genes that are induced and not induced by Δ1stTAD, and scores were obtained for each gene. We then plotted the average value of the scores obtained. As shown in Fig. 7C, there were no significant differences in the average of the scores obtained for the genes induced and not induced by Δ1stTAD (shown by + and – columns in the graph). The same analysis was done for the genes induced and not induced by TAD‐S/A. For genes induced by TAD‐S/A, no stuffer sequence was found between the consensus 10 mer sequence, and the C and G of the core binding site (CWWG) were conserved (supplementary Table S4 and Fig. 7B). The TAD‐S/A PWM was also obtained and applied to genes induced and not induced by TAD‐S/A, and scores were calculated for each gene. As shown in Fig. 7D, no marked difference in the average of the scores for genes induced and not induced by TAD‐S/A was observed. Collectively, these results suggest that the nucleotide usage of the p53 binding site derived from promoters activated by FL‐p53, Δ1stTAD and TAD‐S/A may not differ. Since there was a limitation in the number of p53 binding sites analyzed, there is a possibility that subtle differences in the preference of the binding sites may exist. However, we speculate that the global structures, and not the nucleotide sequences of the promoters, may be the main determinant for the discrimination by each p53 of its preferred binding sites.
Figure 1.
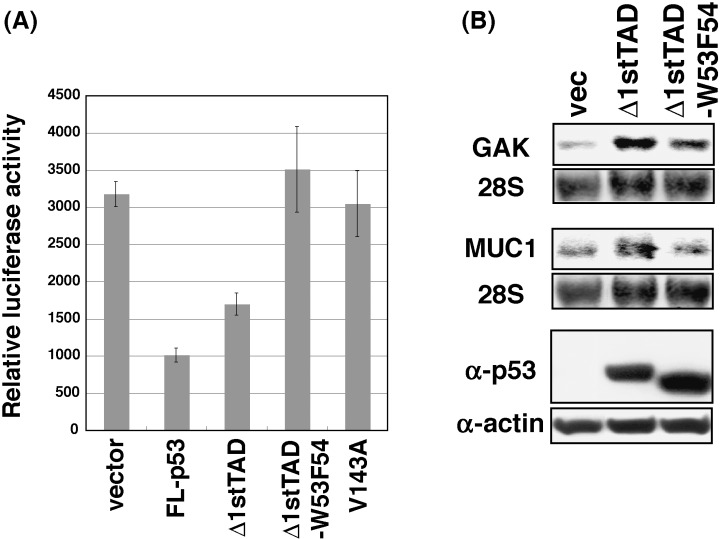
Δ1stTAD‐inducible genes in Δ1stTAD‐dependent apoptosis. (A) Luciferase gene cotransfection growth analysis was performed. Saos2 cells (2 × 104 cells) were transfected with 15 ng of pcDNA3, pcDNA3‐FL‐p53, pcDNA3‐Δ1stTAD, pcDNA3‐Δ1stTAD‐W53F54 or pcDNA3‐V143A together with 2 ng of PicaGene control vector. Cells were harvested 48 h post‐transfection, and luciferase activities were measured. (B) Expressions of GAK and MUC1 were analyzed by Northern blotting. Saos2 cells (5 × 106 cells) were transfected with 3.75 µg of pcDNA3, pcDNA3‐Δ1stTAD or pcDNA3‐Δ1stTAD‐W53F54. Cells were harvested 24 h post‐transfection and analyzed by Northern blotting as in Fig. 3B. Expression of each p53 was analyzed by Western blotting (bottom panel).
Discussion
Currently, many reports identifying novel p53 target genes utilizing GeneChip or ChIP‐on‐chip techniques have been published.( 19 , 22 , 31 , 32 , 33 , 34 , 35 ) However, no detailed analysis has been done to show the functional relevance of the p53 N‐terminal domain. In this study, we constructed cell lines stably expressing a ts form of FL‐p53, Δ1stTAD and TAD‐S/A, and analyzed the genes induced by each p53 upon temperature shift to the permissive temperature with or without γ‐ray irradiation. As shown in Fig. 8, we expected that by comparing the genes induced by each p53, we would be able to show the differential roles of the first and the second TAD and the significance of phosphorylation within the TADs. The results met our expectation, and each p53 induced different sets of genes. Since Δ1stTAD and TAD‐S/A were not able to induce all of the target genes induced by FL‐p53, both the first and the second TAD, and phosphorylation within the TADs are required for the full activation of p53 target genes.
Figure 8.
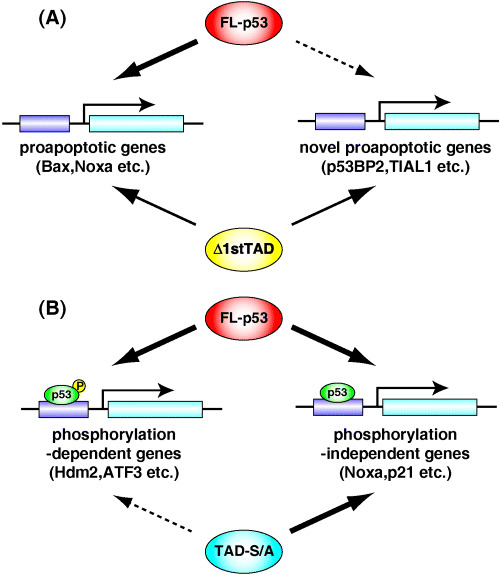
Model of regulation of p53 by its N‐terminal TAD. (A) p53 devoid of the 1st TAD has decreased transcriptional ability against p53 target genes induced by FL‐p53. However, it can induce target genes not induced by FL‐p53, and therefore has apoptosis‐inducing activity. (B) The p53 target genes can be divided into two categories: genes induced independently of phosphorylation within the TADs and genes that are dependent on such phosphorylation. TAD, transactivation domain.
Through the analysis described in this study, we were able to cast light on two interesting issues. First, we were able to show that the phosphorylation of serine residues (Ser 6, 9, 15, 20, 33, 37 and 46) within the TADs has a significant impact on target gene induction by p53. The importance of the modification of serine residues within the TADs has been suggested in several reports. Knock‐in mice harboring a missense mutation converting Ser 18 (corresponding to Ser 15 of human p53) to Ala showed defects in apoptosis and target gene induction,( 13 ) while mice with a missense mutation converting Ser 23 (corresponding to Ser 20 of human p53) to Ala showed defects in apoptosis induction and developed tumors at a high rate, but target gene induction (p21WAF1 and Mdm2) was normal.( 15 ) In addition, in several reports utilizing a non‐phosphorylatable p53 with a point mutation at Ser 46, double mutations at Ser 15 and 20, double mutations at Ser 33 and 37, and combined mutations at Ser 6, 9, 15, 20, 33 and 37, it was shown that the apoptosis and target gene induction abilities of these mutants were defective.( 10 , 11 , 12 , 13 , 14 ) Furthermore, a p53 target gene involved in apoptosis induction, p53AIP1, was fully regulated by Ser 46‐phosphorylated p53.( 18 ) However, no analysis using mutants carrying mutations at all serine residues within the TADs, nor any comprehensive survey, was performed in the previous studies. In this study, we performed an exhaustive investigation by utilizing cells stably expressing phosphorylation‐proficient (FL‐p53) and phosphorylation‐deficient p53 (TAD‐S/A), and by utilizing the powerful GeneChip technology to analyze the genes induced in a p53‐ and phosphorylation‐dependent manner. By this approach, it was shown that approximately 80% of the genes induced by FL‐p53 were not induced and 85% of the genes repressed by FL‐p53 were not repressed by TAD‐S/A (Fig. 4). The genes that were expressed in a phosphorylation‐dependent manner included previously reported p53 target genes such as Hdm2 and ATF3, and those that were expressed in a phosphorylation‐independent manner included p21WAF1 and Noxa (see supplementary Table S1). Interestingly, the expression of many pro‐apoptotic genes previously known as p53 target genes, such as BNIP3L, FAS and TNFRSF10B, was dependent on phosphorylation within the TADs. We also identified potential novel p53 target genes involved in apoptosis that are dependent and independent of the phosphorylation of p53 TADs (see supplementary Table S2). The genes that showed phosphorylation dependency may be involved in apoptosis induction that is dependent on the phosphorylation status of p53. Although we do not know whether all of the genes identified are induced directly by p53, we speculate that genes induced only by 5 h of temperature shift to the permissive temperature are potential direct target genes (shown by capitalized YES in supplementary Tables S1,S2).
Another interesting issue was raised by analyzing genes induced by Δ1stTAD. It has been reported that Δ1stTAD has apoptosis‐inducing ability, and the ability relies on the transactivation ability of the protein.( 4 , 5 ) However, no target gene has been identified that explains this phenomenon. By the analysis performed in this study, it was shown that Δ1stTAD induced many apoptosis‐related genes that were not induced by FL‐p53. These genes included pro‐apototic genes such as P53BP2 (ASPP2) and TIAL1, and the apoptosis induction by Δ1stTAD may be explained by induction of these genes.( 26 , 27 ) Genes suggested to play important roles in tumorigenesis, such as MUC1 and GAK, were also shown to be induced by Δ1stTAD.( 28 , 29 ) It has been reported that this N‐terminally deleted form of p53, Δ1stTAD, is expressed endogenously. The highest ratio between the expression levels of Δ1stTAD and FL‐p53 was reported to be observed in non‐stressed cells, and the level of Δ1stTAD equaled or exceeded that of FL‐p53 (R. O. unpublished observations).( 6 )Δ1stTAD lacks the Hdm2 binding site, located at amino acids 18–26 of FL‐p53, and therefore escapes the degradation caused by the ubiquitin E3 ligase activity of Hdm2. The binding of Hdm2 is regulated by phopshorylation of FL‐p53 at residues Ser 15 and 20, and FL‐p53 is dissociated from Hdm2 upon DNA damage which induces p53 phosphorylation.( 2 , 3 ) Disruption of the FL‐p53‐Hdm2 complex by FL‐p53 phosphorylation results in the accumulation of FL‐p53 and a decreased ratio between Δ1stTAD and FL‐p53. Hdm2 is also known to be frequently amplified in human cancers, and FL‐p53 is degraded easily in these cells.( 3 ) However, since Δ1stTAD escapes from degradation by Hdm2, it may exert tumor‐suppressive functions through transcription activation of genes involved in tumor suppression, such as GAK.( 29 ) In addition, Δ1stTAD was demonstrated to be strongly phosphorylated at Ser 46 upon DNA damage, which is a residue reported to be involved in p53‐dependent apoptosis.( 12 , 18 ) Analyzing the ability of Δ1stTAD with a mutation at Ser 46 may reveal the importance of this modification in target gene induction by Δ1stTAD. Taken together, we can conceive that the genes induced by Δ1stTAD may contribute to the tumor‐suppressive function of p53, especially under non‐stressed conditions or in cancer cells with Hdm2 overexpression.
Analysis of the p53 binding sites located at the promoters induced by each p53 failed to discover distinct differences in the binding sites preferred by each p53. We therefore speculate that the global structure of the promoter and/or the ability of each p53 to activate the promoters (for example, by recruiting cofactors) is the main determinant of gene induction by each p53. Transcriptional coactivator p300 binds to p53 in the N‐terminal domain of p53, and the association is increased by phosphorylation at Ser 15.( 36 , 37 ) One explanation for the difference of the transcriptional abilities of the p53s may be the difference of p300 binding. We are now investigating the functions of genes specifically induced by p53 phosphorylated within the TADs and by Δ1stTAD. By analyzing these genes, the functional regulation of p53 by the N‐terminal domain of p53 should be clarified in more detail in the future studies.
Supporting information
Fig. S1. Activation of Noxa and p21 promoter by ts‐FL‐p53, ts‐Δ1stTAD and ts‐TAD‐S/A. The luciferase reporter assay was performed using the constructs described in Fig. 2A. The assay was performed using H1299 cells at non‐permissive (38°C, shown as white bars) and permissive (32°C, shown as gray bars) temperatures. The results obtained by Noxa promoter (A) and p21 promoter (B) is shown.(1,2)
Fig. S2. Analysis of each cell lines cultured at the permissive temperature. (A) Each cell line (2 × 105 cells were plated in a 6‐well dish) was cultured for 7 days at the non‐permissive (left panel) or permissive temperature (right panel) and stained by Giemsa. (B) Each cell line (7 × 105, cells were plated in a 6 cm dish) was plated and cultured at the non‐permissive and permissive temperature for 24 h. Cells were treated with 10 M BrdU for 1 h and analyzed for BrdU‐positive cells by FACS. Changes in ratio of S phase cells by temperature shift to the permissive temperature were calculated and are shown as a graph.
Fig. S3. Hierarchical clustering of apoptosis‐related probes induced by FL‐p53, Δ1stTAD and TAD‐S/A. Selected apoptosis‐related genes induced by FL‐p53, Δ1stTAD and TAD‐S/A at more than two time points were combined, and hierarchical clustering analysis was performed by the method of Eisen using software from http://rana.lbl.gov/EisenSoftware.htm. (3) Probes induced by each combination of p53 were shown by boxes in colors indicated at the right of the dendrogram. Consecutive clusters of probes containing more than 20 probes induced by any of the p53 were shown by black bars at the right of the dendrogram. Probes induced by FL‐p53 were divided into two branches. One branch contained probes also induced by TAD‐S/A, and the other contained probes also induced by Δ1stTAD and all p53s.
Table S1. Analysis of previously known p53 target genes
Table S2. Analysis of apoptosis‐related genes induced by FL‐p53
Table S3. List of apoptosis‐related genes induced by Δ1stTAD
Table S4. p53 binding sites from previously known target genes of p53
References for Supplementary Figures and Tables
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Acknowledgments
We are grateful to K. Fujinaka for technical assistance and W. Satoh for critical reading of the manuscript. This study was supported by the program for promotion of Fundamental Studies in Health Sciences of the Pharmaceuticals and Medical Devices Agency (PMDA) (to T. O. and H. I.), a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to Y. T.), a Grant‐in‐Aid for Third Term Comprehensive Control Research for Cancer from the Ministry of Health, Labor and Welfare, Japan (to Y. T.), and Research Grants from the Princess Takamatsu Cancer Research Fund and Takeda Science Foundation (to Y. T.).
References
- 1. Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer 2002; 2: 594 – 604. [DOI] [PubMed] [Google Scholar]
- 2. Prives C. Signaling to p53: breaking the MDM2‐p53 circuit. Cell 1998; 95: 5 – 8. [DOI] [PubMed] [Google Scholar]
- 3. Bode AM, Dong Z. Post‐translational modification of p53 in tumorigenesis. Nat Rev Cancer 2004; 4: 793 – 805. [DOI] [PubMed] [Google Scholar]
- 4. Zhu J, Zhang S, Jiang J, Chen X. Definition of the p53 functional domains necessary for inducing apoptosis. J Biol Chem 2000; 275: 39927 – 34. [DOI] [PubMed] [Google Scholar]
- 5. Zhu J, Zhou W, Jiang J, Chen X. Identification of a novel p53 functional domain that is necessary for mediating apoptosis. J Biol Chem 1998; 273: 13030 – 6. [DOI] [PubMed] [Google Scholar]
- 6. Courtois S, Verhaegh G, North S et al. DeltaN‐p53, a natural isoform of p53 lacking the first transactivation domain, counteracts growth suppression by wild‐type p53. Oncogene 2002; 21: 6722 – 8. [DOI] [PubMed] [Google Scholar]
- 7. Yin Y, Stephen CW, Luciani MG, Fahraeus R. p53 Stability and activity is regulated by Mdm2‐mediated induction of alternative p53 translation products. Nat Cell Biol 2002; 4: 462 – 7. [DOI] [PubMed] [Google Scholar]
- 8. Venot C, Maratrat M, Sierra V, Conseiller E, Debussche L. Definition of a p53 transactivation function‐deficient mutant and characterization of two independent p53 transactivation subdomains. Oncogene 1999; 18: 2405 – 10. [DOI] [PubMed] [Google Scholar]
- 9. Maier B, Gluba W, Bernier B et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev 2004; 18: 306 – 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Turenne GA, Paul P, Laflair L, Price BD. Activation of p53 transcriptional activity requires ATM's kinase domain and multiple N‐terminal serine residues of p53. Oncogene 2001; 20: 5100 – 10. [DOI] [PubMed] [Google Scholar]
- 11. Unger T, Sionov RV, Moallem E et al. Mutations in serines 15 and 20 of human p53 impair its apoptotic activity. Oncogene 1999; 18: 3205 – 12. [DOI] [PubMed] [Google Scholar]
- 12. Mayo LD, Seo YR, Jackson MW et al. Phosphorylation of human p53 at serine 46 determines promoter selection and whether apoptosis is attenuated or amplified. J Biol Chem 2005; 280: 25953 – 9. [DOI] [PubMed] [Google Scholar]
- 13. Chao C, Hergenhahn M, Kaeser MD et al. Cell type‐ and promoter‐specific roles of Ser18 phosphorylation in regulating p53 responses. J Biol Chem 2003; 278: 41028 – 33. [DOI] [PubMed] [Google Scholar]
- 14. Kaeser MD, Pebernard S, Iggo RD. Regulation of p53 stability and function in HCT116 colon cancer cells. J Biol Chem 2004; 279: 7598 – 605. [DOI] [PubMed] [Google Scholar]
- 15. MacPherson D, Kim J, Kim T et al. Defective apoptosis and B‐cell lymphomas in mice with p53 point mutation at Ser 23. Embo J 2004; 23: 3689 – 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oda E, Ohki R, Murasawa H et al. Noxa, a BH3‐only member of the Bcl‐2 family and candidate mediator of p53‐induced apoptosis. Science 2000; 288: 1053 – 8. [DOI] [PubMed] [Google Scholar]
- 17. Ohki R, Nemoto J, Murasawa H et al. Reprimo, a new candidate mediator of the p53‐mediated cell cycle arrest at the G2 phase. J Biol Chem 2000; 275: 22627 – 30. [DOI] [PubMed] [Google Scholar]
- 18. Oda K, Arakawa H, Tanaka T et al. p53AIP1, a potential mediator of p53‐dependent apoptosis, and its regulation by Ser‐46‐phosphorylated p53. Cell 2000; 102: 849 – 62. [DOI] [PubMed] [Google Scholar]
- 19. Miled C, Pontoglio M, Garbay S, Yaniv M, Weitzman JB. A genomic map of p53 binding sites identifies novel p53 targets involved in an apoptotic network. Cancer Res 2005; 65: 5096 – 104. [DOI] [PubMed] [Google Scholar]
- 20. Yamato K, Yamamoto M, Hirano Y, Tsuchida N. A human temperature‐sensitive p53 mutant p53Val‐138: modulation of the cell cycle, viability and expression of p53‐responsive genes. Oncogene 1995; 11: 1 – 6. [PubMed] [Google Scholar]
- 21. Lahav G, Rosenfeld N, Sigal A et al. Dynamics of the p53‐Mdm2 feedback loop in individual cells. Nat Genet 2004; 36: 147 – 50. [DOI] [PubMed] [Google Scholar]
- 22. Kho PS, Wang Z, Zhuang L et al. p53‐regulated transcriptional program associated with genotoxic stress‐induced apoptosis. J Biol Chem 2004; 279: 21183 – 92. [DOI] [PubMed] [Google Scholar]
- 23. Danen‐van Oorschot AA, Voskamp P, Seelen MC et al. Human death effector domain‐associated factor interacts with the viral apoptosis agonist Apoptin and exerts tumor‐preferential cell killing. Cell Death Differ 2004; 11: 564 – 73. [DOI] [PubMed] [Google Scholar]
- 24. Minn AH, Hafele C, Shalev A. Thioredoxin‐interacting protein is stimulated by glucose through a carbohydrate response element and induces beta‐cell apoptosis. Endocrinology 2005; 146: 2397 – 405. [DOI] [PubMed] [Google Scholar]
- 25. Nagashima M, Shiseki M, Pedeux RM et al. A novel PHD‐finger motif protein, p47ING3, modulates p53‐mediated transcription, cell cycle control, and apoptosis. Oncogene 2003; 22: 343 – 50. [DOI] [PubMed] [Google Scholar]
- 26. Samuels‐Lev Y, O’Connor DJ, Bergamaschi D et al. ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell 2001; 8: 781 – 94. [DOI] [PubMed] [Google Scholar]
- 27. Taupin JL, Tian Q, Kedersha N, Robertson M, Anderson P. The RNA‐binding protein TIAR is translocated from the nucleus to the cytoplasm during Fas‐mediated apoptotic cell death. Proc Natl Acad Sci USA 1995; 92: 1629 – 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei X, Xu H, Kufe D. Human MUC1 oncoprotein regulates p53‐responsive gene transcription in the genotoxic stress response. Cancer Cell 2005; 7: 167 – 78. [DOI] [PubMed] [Google Scholar]
- 29. Zhang L, Gjoerup O, Roberts TM. The serine/threonine kinase cyclin G‐associated kinase regulates epidermal growth factor receptor signaling. Proc Natl Acad Sci USA 2004; 101: 10296 – 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. El‐Deiry WS, Kern SE, Pietenpol JA, Kinzler KW, Vogelstein B. Definition of a consensus binding site for p53. Nat Genet 1992; 1: 45 – 9. [DOI] [PubMed] [Google Scholar]
- 31. Burns TF, El‐Deiry WS. Microarray analysis of p53 target gene expression patterns in the spleen and thymus in response to ionizing radiation. Cancer Biol Ther 2003; 2: 431 – 43. [DOI] [PubMed] [Google Scholar]
- 32. Jen KY, Cheung VG. Identification of novel p53 target genes in ionizing radiation response. Cancer Res 2005; 65: 7666 – 73. [DOI] [PubMed] [Google Scholar]
- 33. Spurgers KB, Coombes KR, Meyn RE et al. A comprehensive assessment of p53‐responsive genes following adenoviral‐p53 gene transfer in Bcl‐2‐expressing prostate cancer cells. Oncogene 2004; 23: 1712 – 23. [DOI] [PubMed] [Google Scholar]
- 34. Wei CL, Wu Q, Vega VB et al. A global map of p53 transcription‐factor binding sites in the human genome. Cell 2006; 124: 207 – 19. [DOI] [PubMed] [Google Scholar]
- 35. Yoon H, Liyanarachchi S, Wright FA et al. Gene expression profiling of isogenic cells with different TP53 gene dosage reveals numerous genes that are affected by TP53 dosage and identifies CSPG2 as a direct target of p53. Proc Natl Acad Sci USA 2002; 99: 15632 – 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coutts AS, La Thangue NB. The p53 response: emerging levels of co‐factor complexity. Biochem Biophys Res Commun 2005; 331: 778 – 85. [DOI] [PubMed] [Google Scholar]
- 37. Lambert PF, Kashanchi F, Radonovich MF, Shiekhattar R, Brady JN. Phosphorylation of p53 serine 15 increases interaction with CBP. J Biol Chem 1998; 273: 33048 – 53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Activation of Noxa and p21 promoter by ts‐FL‐p53, ts‐Δ1stTAD and ts‐TAD‐S/A. The luciferase reporter assay was performed using the constructs described in Fig. 2A. The assay was performed using H1299 cells at non‐permissive (38°C, shown as white bars) and permissive (32°C, shown as gray bars) temperatures. The results obtained by Noxa promoter (A) and p21 promoter (B) is shown.(1,2)
Fig. S2. Analysis of each cell lines cultured at the permissive temperature. (A) Each cell line (2 × 105 cells were plated in a 6‐well dish) was cultured for 7 days at the non‐permissive (left panel) or permissive temperature (right panel) and stained by Giemsa. (B) Each cell line (7 × 105, cells were plated in a 6 cm dish) was plated and cultured at the non‐permissive and permissive temperature for 24 h. Cells were treated with 10 M BrdU for 1 h and analyzed for BrdU‐positive cells by FACS. Changes in ratio of S phase cells by temperature shift to the permissive temperature were calculated and are shown as a graph.
Fig. S3. Hierarchical clustering of apoptosis‐related probes induced by FL‐p53, Δ1stTAD and TAD‐S/A. Selected apoptosis‐related genes induced by FL‐p53, Δ1stTAD and TAD‐S/A at more than two time points were combined, and hierarchical clustering analysis was performed by the method of Eisen using software from http://rana.lbl.gov/EisenSoftware.htm. (3) Probes induced by each combination of p53 were shown by boxes in colors indicated at the right of the dendrogram. Consecutive clusters of probes containing more than 20 probes induced by any of the p53 were shown by black bars at the right of the dendrogram. Probes induced by FL‐p53 were divided into two branches. One branch contained probes also induced by TAD‐S/A, and the other contained probes also induced by Δ1stTAD and all p53s.
Table S1. Analysis of previously known p53 target genes
Table S2. Analysis of apoptosis‐related genes induced by FL‐p53
Table S3. List of apoptosis‐related genes induced by Δ1stTAD
Table S4. p53 binding sites from previously known target genes of p53
References for Supplementary Figures and Tables
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item


