Abstract
Strategies to increase antitumor efficacy of oncolytic adenoviruses are actively investigated. We have previously shown that E1B‐55 kDa‐deleted adenovirus, designated Ad5WS1, has therapeutic potential for treating hepatocellular carcinoma (HCC). To achieve HCC‐restricted replication of oncolytic adenovirus, we generated Ad5WS2, an E1B‐55 kDa‐deleted adenovirus with its E1A gene driven by the liver‐specific transthyretin promoter. Our results showed that Ad5WS2 could replicate within tumor cells where the transthyretin gene was expressed. Mouse transthyretin promoter was active in murine and human HCC cells, but relatively quiescent in cells of non‐liver origin. Ad5WS2 caused severe cytolytic effect on HCC cells, but was much attenuated in non‐HCC cells. Peritoneal administration of Ad5WS2 into mice bearing liver tumors grown in ascites resulted in enhanced survival. In an orthotopic HCC model, Ad5WS2, when systemically administered, exerted higher antitumor effects than Ad5WS1. Lack of viral replication in normal organs and minimal hepatic toxicity was noted after Ad5WS2 treatment. Furthermore, the antitumor effect of Ad5WS2 could be enhanced when combined with chemotherapeutic agent cisplatin in the ascites tumor model. These results suggest that E1B‐55 kDa‐deleted adenovirus driven by the transthyretin promoter may be a safer and more efficacious oncolytic agent for the treatment of primary and metastatic HCC. (Cancer Sci 2009; 100: 537–545)
Abbreviations:
- HCC
hepatocellular carcinoma
- MOI
multiplicity of infection
- TCID50
50% tissue culture infective dose
- s.c.
subcutaneously
- i.t.
intratumorally
- i.p.
intraperitoneally
- i.v.
intravenously
- ALT
alanine aminotransferase
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide.( 1 ) Owing to its heterogeneity and ease of tendency to develop multidrug‐resistant phenotypes, HCC is refractory to conventional treatment.( 2 ) The use of conditionally replicating viruses, including adenoviruses, has offered a promising platform for selective cancer treatment.
ONYX‐015, which is one of the most studied oncolytic adenoviruses, can selectively replicate in tumor cells while sparing normal cells. Several clinical trials, such as head and neck cancer, ovarian cancer, and pancreatic cancer, have supported the therapeutic use of ONYX‐015 for the treatment of solid tumors.( 3 , 4 , 5 ) Clinical trials in patients with primary liver cancers or gastrointestinal carcinoma metastatic to liver have also been reported.( 6 , 7 , 8 ) Despite such encouraging results, using oncolytic adenoviruses alone for cancer therapy have so far been disappointing.( 9 ) One of the major barriers to successful clinical translation is the difficulty in successful targeting and quantitative infection of relevant cell types. To increase viral oncolysis, numerous efforts have been made to improve the therapeutic efficacy of oncolytic adenoviruses by expressing transgenes,( 10 , 11 , 12 ) or modifying viral fibers to extend viral tropism.( 13 ) To target oncolytic adenoviruses to liver tumors, E1B‐55 kDa‐deleted adenovirus driven by albumin or α‐fetoprotein promoter has been utilized to control the expression of early viral genes, such as E1A, to achieve tumor‐specific adenoviral replication in HCC cells.( 14 , 15 , 16 , 17 ). Transthyretin is a thyroid hormone transport protein that is secreted into the serum by hepatocytes and into the cerebrospinal fluid by the choroid plexus.( 18 ) A 200‐nucleotide sequence from the 5′ end to the cap site is highly conserved between mouse and human genes. Sequence alignment indicates an 80% homology at the protein level between mouse and human genes.( 19 ) Deletion analysis has identified a crucial element in the 5′‐flanking regulatory region for specific expression in mouse liver and human hepatoma cell lines.( 20 , 21 ) The transthyretin promoter has been used to study the function of transgenes in the liver from transgenic mice.( 22 ) These findings suggest that transthyretin may serve as a specific marker for targeting liver tumors.
In this study, we constructed a liver‐specific E1B‐55 kDa‐deleted adenovirus, designated Ad5WS2, under the control of the transthyretin promoter. Our results showed that Ad5WS2 selectively replicate and hence lyzed HCC cells. Furthermore, Ad5WS2 exhibited high antitumor activity via systemic administration in an orthotopic syngeneic HCC model. The antitumor effect of Ad5WS2 was in synergy with cisplatin in mice bearing liver tumors accompanied with malignant ascites. Therefore, Ad5WS2 represents a potentially applicable anticancer agent for the treatment of primary and metastatic HCC.
Materials and Methods
Cell lines and mice. ML−1 (mouse HCC),( 23 ) CT‐26 (mouse colon cancer), NIH‐3T3 (mouse fibroblast), LL2 (mouse Lewis lung carcinoma), SK‐Hep1, Hep3B (human HCC),( 24 , 25 ) Chang liver (non‐malignant human hepatocytes),( 25 ) HT1376 (human bladder cancer),( 26 ) and 293 (human embryonic kidney) cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 1% glutamine, and 50 µg/mL gentamicin at 37°C. Female BALB/c mice and C57BL/6 mice at the age of 7 weeks were obtained from the Laboratory Animal Center of the National Cheng Kung University. The experimental protocol adhered to the rules of the Animal Protection Act of Taiwan and was approved by the Laboratory Animal Care and Use Committee of the National Cheng Kung University.
Construction of Ad5WS2 oncolytic adenovirus. Ad5WS1, an E1B‐55 kDa‐deleted adenovirus, has been described previously.( 27 ) The 3′ region of the E1A promoter in the backbone of Ad5WS1 was interrupted by the mouse tranthyretin promoter, resulting in generation of Ad5WS2. To insert a multiple cloning site at nucleotide 525 of adenovirus genome, the adenovirus region from bases 343–1003 was generated by polymerase chain reaction (PCR) using pAd5WS1 as the template. The resulting 660‐bp fragment restricted with NotI and SacI was cloned into the pBluescript II SK(+/–) to generate pBSII‐0.7. The adenovirus E1 region from bases 525–2270 was also generated by PCR using pAd5WS1 as the template. The resulting 1500‐bp fragment restricted with XhoI and KpnI was cloned into the XhoI and KpnI sites of pBSII‐0.7, resulting in pBSII‐0.7–1.5. The 2.7 kb‐fragment of pBSII‐0.7‐1.5 containing multiple cloning sites and part of the E1 sequence, was digested with NotI and KpnI and cloned into pAd5WS1, yielding pAd5YS.
The 330‐bp fragment of mouse transthyretin promoter was obtained by PCR using chromosome DNA from mouse embryonic fibroblasts as the template and cloned into the HindIII site of pGL‐Basic (Promega, Madison, WI, US), yielding pGL‐TTRp, a luciferase reporter plasmid driven by the transthyretin promoter. Meanwhile, the transthyretin promoter region was excised from pGL‐TTRp and cloned into the HindIII site of pAd5YS to generate pTTRp/YS. The 3000‐bp fragment of pTTRp/YS was digested with BsrGI and MunI and subcloned into an adenoviral shuttle vector pShuttle,( 28 ) to generate pTTRp/YS/PS, a shuttle vector for generating Ad5WS2. Ad5WS2 virus was then produced using the AdEasy system as previously described.( 28 )
Analysis of transthyretin promoter activity. The transcriptional activity of the transthyretin promoter was assessed as previously described.( 27 ) Briefly, various cells were cotransfected with 2 µg of pGL‐TTRp containing a luciferase expression cassette driven by mouse transthyretin promoter and 1 µg of pTCYLacZ, a β‐galatosidase reporter plasmid driven by the β‐actin promoter,( 29 ) by lipofectin. Cell lysates were harvested 48 h after transfection and their luciferase activities were determined.
Immunoblot, immunohistochemical and immunofluorescence analysis. Total cell lysates were prepared as previously described.( 27 ) Lysates prepared from liver tissues of HCC patients or mice were homogenized in phosphate‐buffered saline (PBS) containing a protease inhibitor cocktail (Pierce, Rockford, IL, US). These samples were subjected to immunoblot analysis using antibodies against human transthyretin (Dako, Carpinteria, CA, US), adenovirus E1A (M73, Santa Cruz Biotechnology, Santa Cruz, CA, US), adenovirus hexon (abcam, Cambridge, UK), adenovirus fiber (4D2, abcam), and β‐actin (AC‐15, Sigma, St. Louis, MI, US).
Tumor nodules as well as liver and spleen tissues were collected and snap‐frozen. Cryostate sections (5 µm) were prepared and incubated with antibody against human transthyretin (Dako) or adenovirus E1A (clones 20/11 and 2/6, Chemicon, Temecula, CA, US), followed by detection with the Dako LSAB 2 System (Dako) according to the manufacturer's instructions.
Cells were fixed in paraformaldehyde, permeabilized with cold methanol/acetone (90:10 v/v), incubated with antibody against human transthyretin (Dako) and adenovirus fiber (4D2, abcam), and subsequently incubated with fluorescein‐conjugated goat antirabbit IgG (KPL, Gaithersburg, MD, USA) and Texas Red‐conjugated goat antimouse IgG (KPL, Gaithersburg, MD, USA). Nuclei were stained with 50 µg/mL of 4′,6‐diamidino‐2‐phenylindole dihydrochloride (DAPI).
Assay of cell viability and viral replication. The viable cell numbers were determined in cells seeded in 6‐well plates after 3 days of virus infection at a multiplicity of infection (MOI) of 2 by trypan blue exclusion. Viral replication was assessed as previously described.( 27 ) Briefly, cells were infected with either Ad5WS2 or wild‐type adenovirus type 5 (Ad5WT) (MOI = 10). Viruses harvested from both the supernatant and cell lysate at 53 h post‐infection were pooled and titered on 293 cells for 50% tissue culture infective dose (TCID50) calculated by the Reed‐Muench method. The relative virus replication efficiency was calculated by the equation as described previously.( 30 ) The Colorimetric WST‐1 assay (Dojindo Laboratories, Tokyo, Japan) was used to access cell viability according to the manufacturer's instructions. ML‐1 cells were treated with Ad5WS2 (MOI = 50) and cisplatin (10 mM). Four days after treatment, 100 µL of WST‐1 was added to each well and incubated at 37°C for 1 h. The absorbance at 450 nm that stands for cell growth was measured with the reference wavelength at 595 nm.
In vivo antitumor efficacy. ML‐1 and LL2 cells (1 × 106) were inoculated subcutaneously (s.c.) into the right flank of BALB/c and C57BL/6 mice at day 0, respectively. Twelve days after tumor cell inoculation, eight mice were randomly allocated to various treatment groups. Mice were treated intratumorally (i.t.) with Ad5WS2 or Ad5WS1 (2 × 107 plaque‐forming unit (PFU)) at different days. Tumors were measured once a week and the tumor volume was calculated as: (length of tumor) × (width of tumor)2 × 0.45. To establish ascites tumor models, ML‐1 and LL2 cells (2 × 106) were inoculated intraperitoneally (i.p.) into BALB/c and C57BL/6 mice at day 0, respectively. Three days after tumor cell inoculation, seven mice were randomly allocated to various treatment groups. Mice were injected i.p. with Ad5WS2 or Ad5WS1 (5 × 107 PFU) at different days. To establish an orthotopic liver tumor model, ML‐1 cells (2 × 105) were inoculated into the left liver lobe of BALB/c mice at day 0 as previously described.( 31 ) Groups of 6–7 mice bearing orthotopic ML‐1 tumors were injected intravenously (i.v) with Ad5WS2 or Ad5WS1 (2 × 107 PFU) at different days. For combination therapy, mice that had been inoculated with ML‐1 cells (2 × 106) at day 0 were injected i.p. alternatively with four doses (5 × 107 PFU/dose) of Ad5WS2 and four doses of cisplatin (4 mg/kg) at different days. All mice were monitored for survival after tumor inoculation.
Determination of serum ALT (alanine aminotransferase) level. Groups of four mice each were inoculated with ML‐1 cells (2 × 106) at day 0 and then injected i.p. with Ad5WS1 and Ad5WS2 (5 × 107 TCID50) at day 3. Mouse sera collected 19 h, as well as 3 days after virus injection were measured for their ALT concentrations using a vitros DT chemistry system (Johnson & Johnson, Rochester, NY, US).
Statistical analysis. The survival analysis was done using the Kaplan‐Meier survival curve and the log‐rank test. Other statistical differences were assessed with Student's t‐test. P < 0.05 was regarded as statistically significant.
Results
Liver specificity of transthyretin promoter. To ensure transthyretin expression in human liver tissues, we tested the expression of transthyretin protein in six tumor and four non‐tumor tissues, which included three paired samples, from HCC patients (Fig. 1a). The levels of tranthyretin expression in HCC tumor tissues were comparable or even 1.5‐ to 2‐fold higher than those from normal liver tissues determined by densitometric analysis. HCC cells appeared to produce more transthyretin than normal liver cells (P = 0.04) (Supporting Information Fig. S1). We next examined the transcriptional activity of the transthyretin promoter in cells of liver and non‐liver origin using a luciferase reporter assay. The cell lines used included human HCC (Hep3B and SK‐Hep1), human bladder cancer (HT1376), human non‐malignant hepatocytes (Chang liver), murine HCC (ML‐1), and murine lung cancer (LL2) cells. As shown in Fig. 1(b), the transthyretin promoter was active in liver cells, with highest activity in Hep3B cells and moderate activity in Chang liver, ML‐1, and SK‐Hep1 cells. Therefore, the mouse transthyretin promoter may also use human transcription machinery as efficiently as using its mouse counterpart. However, HT1376 and LL2 cells exhibited very low transthyretin promoter activity. To ensure tissue specificity of Ad5WS2 replication, we examined the expression of late viral protein in three cell lines originating from BALB/c mice. As shown in Fig. 2, fiber protein was expressed in ML‐1 cells where the transthyretin gene was active, whereas the virus was scarcely detected in CT‐26 (colon cancer) and NIH‐3T3 cells (normal fibroblast) with low expression of transthyretin (Supporting Information Fig. S2 and Fig. S3). Therefore, among different tissues originating from the same host, adenovirus could only replicate in tumor cells where the transthyretin gene was expressed.
Figure 1.
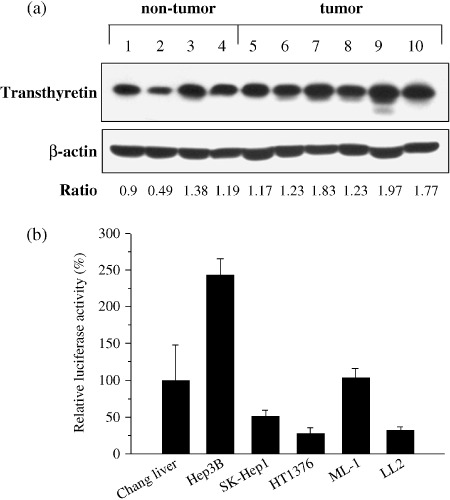
Detections of transthyretin protein and transcriptional activity of transthyretin promoter in various human and murine cells. (a) Transthyretin protein was produced in the non‐tumor (Lanes 1–4) and tumor (Lanes 5–10) parts of the liver from hepatocellular carcinoma (HCC) patients, as determined by immunoblot analysis. Lanes 1 and 6, 3 and 9, and 4 and 7 were paired samples. Expression of β‐actin served as the quantitative control. P = 0.04 for tumor group vs. non‐tumor group by unpaired Student's t‐test. (b) The activity of the transthyretin promoter was higher in liver cell lines compared with non‐liver cell lines. Cells were transfected with pGL‐TTRp and the transfection efficiency was standardized to the cotransfected plasmid pTCYLacZ expressing β‐galatosidase driven by the β‐actin promoter. Relative luciferase activity was indicated by comparing each cell line with Chang liver cells. Each value represents the mean ± SD (n = 3).
Figure 2.
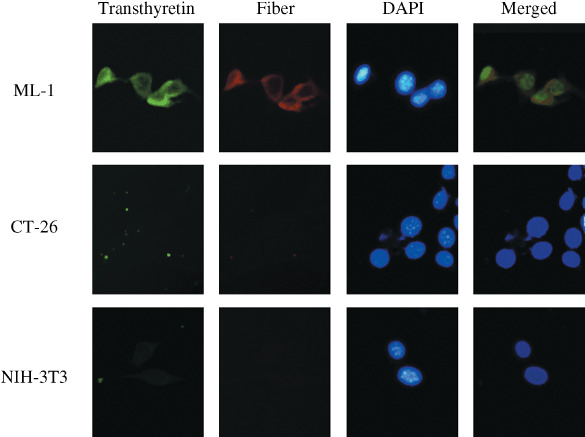
Detections of transthyretin and viral protein in Ad5WS2 infected cells. Cells were infected with Ad5WS2 (MOI = 3) for 48 h and immunofluorescent double stainings were performed with rabbit antitransthyretin antibody by fluorescein and mouse antifiber antibody by Texas‐Red. Nuclei were counterstained with 4’,6‐diamidino‐2‐phenylindole dihydrochloride (DAPI). The merged column represents the superposition of the cells stained with antitransthyretin and antifiber, and DAPI to visualize colocalization. Representative sections are shown (original magnification ×400).
Replication specificity of Ad5WS2 in HCC cells. We have previously shown the antitumor efficacy of Ad5WS1 in HCC and bladder cancer in animal models.( 27 , 32 ) We next investigated whether Ad5WS2 selectively induced cytolytic effect in HCC cells (Supporting Information Fig. S4). The viability of Hep3B or SK‐Hep1 cells infected with Ad5WS2 was lower than that infected with Ad5WS1, whereas cell survival was decreased to a similar extent in Ad5WS2‐infected ML‐1 cells as in their Ad5WS1‐infected counterparts (Fig. 3a). Notably, the viability of Ad5WS2‐ or Ad5WS1‐infected Chang liver cells did not differ significantly from that of their mock‐infected counterparts. By contrast, whereas Ad5WS1 induced significant cell death in HT1376 and LL2 cells, Ad5WS2 did not cause significant cell death in these cells. To test the replication of Ad5WS2 in cell lines from different origins, the level of Ad5WS2 produced at 53 h post‐infection was determined and normalized against that of Ad5WT produced in the same cell line. The absolute virus titers of Ad5WS2 produced in ML‐1, Hep3B and SK‐Hep1 cells were 5 × 107 TCID50/mL, 1.7 × 108 TCID50/mL and 1 × 107 TCID50/mL, respectively. Figure 3(b) shows that the relative viral production was highest in Hep3B cells, followed by SK‐Hep1 and ML‐1 cells. By contrast, Ad5WS2 production reached only 0.41% and 16% of Ad5WT in HT1376 and LL2 cells, respectively, which exhibited a relatively resistant phenotype to Ad5WS2 infection. Notably, Chang liver cells, which were immortalized human hepatocytes with moderate transthyretin promoter activity, were also resistant to Ad5WS2 infection (2.35% relative to Ad5WT). Immunoblot analysis revealed that E1A protein was identified in both Hep3B and ML‐1 cells albeit to a low level of protein in Chang liver cells 36 h after Ad5WS2 infection (Fig. 3c). Hexon and fiber proteins, were also detected not only in human Hep3B HCC cells but also in mouse ML‐1 HCC cells following Ad5WS2 infection at 48 h post‐infection (Fig. 3d). However, hexon protein was undetectable, and fiber proteins were present at a negligible level in Ad5WS2‐infected Chang liver cells. Taken together, these results suggest that replication of Ad5WS2 was restricted to HCC cells.
Figure 3.
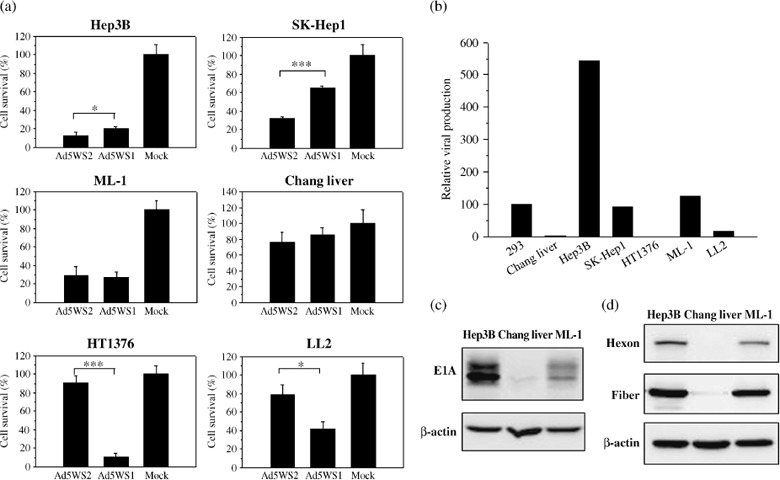
Selective replication of Ad5WS2 in human and murine hepatocellular carcinoma (HCC) cell lines. (a) Ad5WS2 induced more cell death in human and murine HCC cells, but was highly attenuated in non‐HCC cells. Cells were infected with Ad5WS2 or Ad5WS1 at an multiplicity of infection (MOI) of 2. The viable cell numbers were determined after 3 days by trypan blue exclusion. Each value represents the mean ± SD (n = 3). *P < 0.05; ***P < 0.001.(b) Human and murine HCC cells produced higher viral titers than cancer cells from non‐liver origin following infection with Ad5WS2. Various cells were infected with Ad5WS2 or Ad5WT at an MOI of 10. Fifty‐three hours post‐infection, viral titers were determined in 293 cells by the TCID50 method. The amount of Ad5WS2 produced was normalized against the amount of Ad5WT produced in the same cell line. Relative viral production was indicated by comparing each cell line with 293 cells. (c) Expression of adenoviral E1A protein was detected in Hep3B and ML‐1 cells at 36 h post‐infection with Ad5WS2 at an MOI of 10. (d) Expressions of adenoviral hexon and fiber proteins were detected in Hep3B and ML‐1 cells at 48 h post‐infection with Ad5WS2 at an MOI of 1.
Antitumor efficacy of Ad5WS2 in mice bearing ML‐1 tumors. Given that immunocompetent mouse tumor models are more relevant to clinical applications and that murine ML‐1 cells are permissive for Ad5WS2 replication, we employed the ML‐1 tumor model for animal studies. Tumor growth was first evaluated in ML‐1 tumor‐bearing BALB/c mice and LL2 lung tumor‐bearing C57BL/6 mice injected i.t. with Ad5WS2 or Ad5WS1. The growth of ML‐1 tumors was significantly retarded in Ad5WS2‐treated (P = 0.007) or Ad5WS1‐treated (P = 0.002) mice compared with that in PBS‐treated mice (Fig. 4a). However, in LL2 tumor‐bearing mice, whereas Ad5WS1 significantly retarded tumor growth (P = 0.00007), Ad5WS2 only exhibited marginal antitumor effect compared with phosphate buffered saline (PBS) treatment (P = 0.09) (Fig. 4b). We also used replication‐defective AdLacZ to infect ML‐1 and LL2 cells and confirmed similar susceptibility of the two cell lines to adenoviral infection (data not shown). Consistent with in vitro data (Fig. 3), these results indicate that Ad5WS2 was cytolytic to tumor cells in which transthyretin promoter was active, which resulted in suppressing tumor growth. We next used an ML‐1 ascites tumor model to test whether Ad5WS2 could systemically target HCC cells in vivo. We treated mice bearing ML‐1 ascites tumors with Ad5WS2 or Ad5WS1. In tumor sections from animals treated with Ad5WS2 or Ad5WS1, hexon protein‐positive tumor cells were detected in peritoneal tumor nodules, suggesting that active replication of either Ad5WS2 or Ad5WS1 may have occurred (Fig. 4c). In a separate experiment, we treated mice bearing ML‐1 or LL2 ascites tumors with Ad5WS2 or Ad5WS1. Figure 4(d) shows that in mice bearing ML‐1 ascites tumors, Ad5WS2 treatment (P = 0.0034) significantly prolonged the survival time, whereas Ad5WS1 treatment (P = 0.0572) only marginally enhanced mouse survival compared with PBS treatment. The median survival in the Ad5WS2‐treated group is 36 days, longer than Ad5WS1 (31 days) and PBS‐treated ones (30 days). Nevertheless, as shown in Fig. 4(e), Ad5WS2 treatment failed to enhance the survival time of the mice bearing LL2 ascites tumors compared with PBS treatment (P = 0.934). Collectively, these results suggest more potent inhibition of liver tumors by Ad5WS2 than by Ad5WS1 in mice. As orthotopic liver tumors more closely resemble the biological characteristics of the human tumor, we next used ML‐1 cells grown in the liver of their syngeneic hosts to evaluate the bio‐distribution of Ad5WS2 after i.v. injection. Immunoblot analysis revealed that virus fiber protein was only detected in liver tumors but not normal liver and spleen tissues (Fig. 5a). Figure 5(b) shows that Ad5WS2‐treated mice exhibited enhanced survival compared to PBS‐treated (P = 0.008) or Ad5WS1‐treated (P = 0.0257) animals. Notably, Ad5WS2 was more efficacious than Ad5WS1 in prolonging the survival time and increasing the survival rate in mice bearing orthotopic liver tumors (P = 0.0257). Importantly, three of seven Ad5WS2‐treated mice remained alive at the time of sacrifice. In a separate experiment, we i.v. treated mice bearing ML‐1 orthotopic tumors with Ad5WS2 or Ad5WS1. In contrast to Ad5WS1‐treated animals, virus protein expression was restricted only in Ad5WS2‐treated tumor sections, indicating more specific spreading of Ad5WS2 (Fig. 5c). A mild transient elevation of ALT levels was noted at 19 h post‐infection compared to the control counterpart (P = 0.016) and back to the normal range at 3 days post‐infection (Fig. 5d). However, Ad5WS1‐treated mice exhibited higher ALT levels at 19 h and 3 days after administration compared to the Ad5WS2‐treated ones (P = 0.032, P = 0.0006, respectively). There was a positive correlation between the viral load in livers and serum ALT levels, suggesting that the specificity of Ad5WS2 may contribute to decreased hepatic toxicity through systemic administration.
Figure 4.
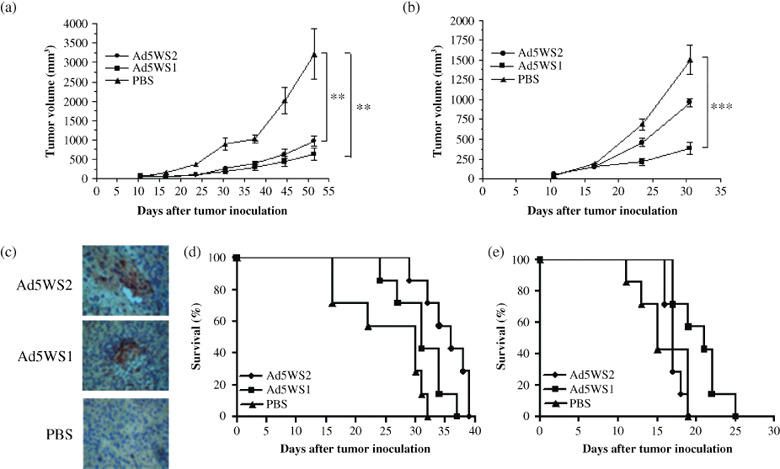
Antitumor effects of Ad5WS2 on subcutaneous and ascites ML‐1 tumors. (a) Ad5WS2 and Ad5WS1 exhibited similar antitumor activity against ML‐1 tumors. (b) Ad5WS1, but not Ad5WS2, exerted antitumor activity against LL2 tumor. ML‐1 (a) and LL2 (b) cells (1 × 106), which were inoculated subcutaneously into the flanks of BALB/c and C57BL/6 mice at day 0, respectively. The mice were treated intratumorally (i.t.) with Ad5WS2 or Ad5WS1 (2 × 107 PFU) every other day from day 12 to day 20. The tumor volume is presented as mean ± SE (n = 8). **P < 0.01; ***P < 0.001. (c) Adenoviral hexon protein was detected in the tumor nodules from mice bearing ML‐1 ascites tumors after systemic administration of Ad5WS2 or Ad5WS1. Groups of four mice were injected i.p. with Ad5WS2 or Ad5WS1 (5 × 107 PFU/dose), or with PBS at days 3, 5, 7, and 9, and sacrificed at day 15. Representative sections are shown (original magnification × 200). (d) Ad5WS2 was more efficacious than Ad5WS1 in enhancing the survival of mice bearing ML‐1 ascites tumors. (e) Ad5WS1 but not Ad5WS2 prolonged the survival time of mice bearing LL2 ascites tumors. ML‐1 (d) and LL2 (e) cells (2 × 106) were inoculated i.p. into groups of seven BALB/c and C57BL/6 mice at day 0, respectively. Mice were injected i.p. with Ad5WS2 or Ad5WS1 at a dose of 5 × 107 PFU at days 3, 5, 7, and 9. Kaplan‐Meier survival curves are shown and analyzed by the log rank test. P = 0.0034 for Ad5WS2 vs. PBS in (d); P = 0.0224 for Ad5WS1 vs. PBS and P = 0.0105 for Ad5WS2 vs. Ad5WS1 in (e).
Figure 5.
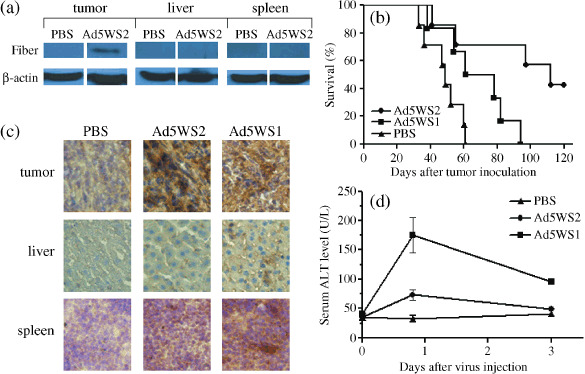
Antitumor effects of Ad5WS2 on orthotopic ML‐1 tumors. (a) Adenoviral fiber protein was detected in the tumor nodules from mice bearing ML‐1 orthotopic tumors but not normal liver and spleen tissue after systemic administration of virus. ML‐1 cells (2 × 105) were inoculated into the left liver lobe of BALB/c mice at day 0. Groups of four mice bearing orthotopic ML‐1 tumors were then injected i.v. with Ad5WS2 (2 × 107 PFU) or PBS at days 30, and sacrificed at day 33. Expression of β‐actin served as the quantitative control. (b) Ad5WS2 exerted higher antitumor efficacy than Ad5WS1 in mice bearing orthotopic ML‐1 tumors. ML‐1 cells (2 × 105) were inoculated into the left liver lobe of BALB/c mice at day 0. Groups of 6–7 mice bearing orthotopic ML‐1 tumors were then injected i.v. with Ad5WS2 or Ad5WS1 (2 × 107 PFU) at days 15, 17, and 19. Kaplan‐Meier survival curves are shown and analyzed by the log rank test. P = 0.008 for Ad5WS2 vs. PBS, P = 0.0305 for Ad5WS1 vs. PBS, and P = 0.0257 for Ad5WS2 vs. Ad5WS1 in (b). (c) Adenoviral E1A protein was detected in the tumor nodules from mice bearing ML‐1 orthotopic tumors but not normal liver and spleen tissue after systemic administration of viruses albeit to substantial levels of E1A protein in the Ad5WS1‐treated group. Groups of four mice bearing orthotopic ML‐1 tumors were injected i.v. with Ad5WS2 or Ad5WS1 (2 × 107 PFU) at day 30, and sacrificed at day 33. Representative sections are shown (original magnification × 400). (d) Mild and transient elevation of serum alanine aminotransferase (ALT) levels are shown in ML‐1 tumor‐bearing mice receiving Ad5WS2. Sera were collected 19 h, as well as 3 days after virus injection and measured for ALT levels. Each value represents the mean ± SE (n = 4).
Additive antitumor efficacy of Ad5WS2 in the combination of cisplatin on mice bearing ascites ML‐1 tumors. To further enhance the antitumor effect of Ad5WS2, we used the combination therapy of Ad5WS2 with cisplatin. Cells treated with cisplatin were given only moderate cytolytic effect (Fig. 6a; P = 0.037). However, the combination treatment of Ad5WS2 plus cisplatin significantly reduced tumor cell growth (P = 0.00015). In mice bearing ML‐1 ascites tumors, either Ad5WS2 (P = 0.0066) or cisplatin (P = 0.0004) alone prolonged survival compared with PBS treatment (Fig. 6b). Of note, combination therapy significantly increased the survival time of the animals compared with either Ad5WS2 (P = 0.0002) or cisplatin treatment alone (P = 0.0011). Collectively, these results demonstrate that systemic administration of Ad5WS2, in particular combined with cisplatin, prolonged the survival of the animals bearing liver tumors grown orthotopically or in ascites.
Figure 6.
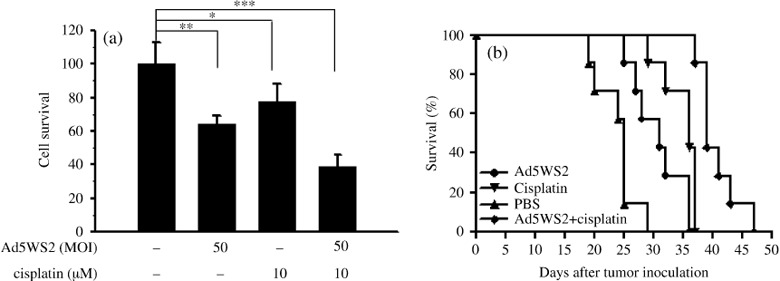
Antitumor effects of Ad5WS2 were enhanced in combination with cisplatin on ML‐1 ascite tumors. (a) Ad5WS2 induced more cell death in combination with cisplatin. ML‐1 cells (5000 cells/well) which were treated with Ad5WS2 (MOI = 50) and cisplatin (10 µm) or with either treatment alone. The viable cell numbers were determined after 4 days by WST‐1 assay. Each value represents the mean ± SD (n = 4). *P < 0.05; **P < 0.01; ***P < 0.001. (b) Ad5WS2 in combination with cisplatin exerted higher antitumor efficacy compared with either Ad5WS2 or cisplatin treatment alone. Groups of seven mice were inoculated i.p. with ML‐1 cells (2 × 106) at day 0. For single agent treatment, mice were treated i.p. with either Ad5WS2 (5 × 107 PFU) at days 3, 5, 7, and 9 or cisplatin at days 11, 13, 15, and 17. For combination treatment, four doses Ad5WS2 (5 × 107 PFU/dose) were injected i.p. at days 3, 5, and 7, and then treated i.p. with cisplatin at days 11, 13, 15, and 17. Kaplan‐Meier survival curves are shown and analyzed by the log rank test. P = 0.0002 for Ad5WS2 plus cisplatin vs. Ad5WS2, P = 0.0011 for Ad5WS2 plus cisplatin vs. cisplatin, P = 0.0004 for cisplatin vs. PBS, and P = 0.0066 for Ad5WS2 vs. PBS in (b).
Discussion
In this study, we exploited Ad5WS2, an E1B‐55 kDa‐deleted adenovirus driven by the transthyretin promoter for HCC treatment. Ad5WS2 was derived from Ad5WS1,( 27 ) which is similar to ONYX‐015, frequently used in gene therapy,( 33 ) by replacing its internal E1A promoter with the transthyretin promoter. This is the first time that transthyretin promoter has been used to construct a liver tumor‐specific oncolytic virus. Although transthyretin was also consistently expressed in the brain, the endogenous brain expression was ~10–20% of that observed in the liver. Yan et al. reported that in transgenic mice carrying 1–2 copies of the regulatory regions of gene, which was sufficient for high‐level hepatic expression, showed very low, nearly undetectable transgene expression in the brain.( 18 ) No transgene expression was detected in the spleen, salivary gland, heart, lung, intestine and striated muscle. This regulatory region contains several binding sites for hepatocyte nuclear factors (HNFs). It is believed that HNF‐1, HNF‐3, and HNF‐4 were highly enriched in liver but was lacking in the choroids plexus tissues, conferring to the different gene expressions in tissues.( 34 ) The same regulatory region (330 bp) was used as the promoter of Ad5WS2. Therefore, the damage to the choroids plexus could be reduced to a minimum. Moreover, Ad5WS2 also harbored deletion in the E1B‐55 kDa‐protein. Giving that the functions of E1B‐55 kDa include RNA export, host protein shutoff, as well as p53 degradation. O'shea et al. demonstrated that the oncolytic selectivity of E1B‐55 kDa‐deleted adenovirus was determined by a property of tumor cells to efficiently export late viral RNA in the absence of E1B‐55 kDa, a propensity not shared by normal cells.( 35 ) Thus, permissive liver tumor cells could support Ad5WS2 replication by providing the late function of E1B‐55 kDa. One of the viral late RNAs, fiber, could be detected in Hep3B cells, but not in the Chang liver cells after Ad5WS2 infection (Supporting Information Fig. S5). Moreover, we had also observed significantly reduced fiber expression, and a distinct lack of hexon expression at 48 h post‐infection with Ad5WS2 in Chang liver cells compared to the Hep3B and ML‐1 cells. As with non‐malignant human liver cells, although Chang liver cells exhibited significant transthyretin promoter activity, no efficient viral production was detected after Ad5WS2 infection. Based on these observations, we suggest that the tumor‐selective replication of Ad5WS2 may be determined using a similar mechanism proposed by O'shea. However, we could not rule out other unprecedented differences existing between tumor and normal cells that might confer to the discrepant replication ability of Ad5WS2.
Because it has been generally thought that adenovirus is unable to complete the replication cycle in normal murine tissues, such as lung and fibroblasts, most preclinical studies for replication‐selective adenoviruses assess in vivo antitumor efficacy using human tumor xenograft models in immunodeficient mice. Giving that antiviral immune responses have impacts on antitumor efficacy of oncolytic adenoviruses, syngeneic murine tumor models in immunocompetent mice provide more clinically relevant systems for these studies. In mice, intravenous administration of adenovirus type 5 resulted in viral replication within the liver, as evidenced by detections of viral late proteins, as well as virus particles, and a dramatic increase of viral levels 48 h after infection.( 36 ) In mouse cell lines, it has also been shown that mouse epidermal cells are permissive for replication of human adenovirus type 5.( 37 ) Some mouse tumor cell lines can support viral uptake, gene expression, and replication of oncolytic adenoviruses.( 38 ) To this end, we show that not only viral early protein E1A, but also late proteins hexon and fiber were detected in Ad5WS2‐infected ML‐1 cells, suggesting that productive replication of Ad5WS2 occurred.
Invasion of HCC to the peritoneum causes malignant ascites, which is frequently found in patients with advanced HCC.( 39 ) To be more clinically plausible, in our study, we systemically injected virus in mice bearing tumors. The profile of neutralization antibody was monitored before we set up the regimen. After one i.p. injection, only low to moderate levels of neutralization antibody were detected against the virus within 2 weeks (Supporting Information Table S1). Therefore, to reduce the possible effects of neutralization antibody on the virus, we injected mice i.p. and i.v. with Ad5WS2 three and four times, respectively, at intervals of 2 days. In mice bearing ascites ML‐1 tumors and orthotopic ML‐1 tumors, treatment with Ad5WS2 significantly prolonged survival as compared with Ad5WS1. However, Ad5WS1 and Ad5WS2 exhibited similar effects against ML‐1 cells in the in vitro cytotoxicity analysis and mice subcutaneous tumor models. The replication rate of adenoviruses may partially account for the divergent therapeutic effect of these two viruses. In a plaque development assay in 293 cells, we found that Ad5WS1 replicated faster than Ad5WS2 (Supporting Information Fig. S6). When delivered systemically, a slower replication rate of Ad5WS2 may have accounted for a lower viral yield found in the mouse liver, which may have resulted in lower hepatic toxicity. Nevertheless, the E1A protein was also detected in the liver of Ad5WS2‐treated mice, albeit to a much lower level, than that of Ad5WS1‐treated ones. Another factor contributing to the lower toxicity of Ad5WS2 is the liver‐specific transthyretin promoter that drives E1A expression. Since expression of E1A in Ad5WS1 is controlled by its native constitutive E1A promoter, Ad5WS1 would express E1A in all tissues even though efficient replication does not occur. As a result, toxicity of the transduced normal cells may occur due to E1A‐induced apoptosis and other pleiotropic effects.( 40 ) Immunological responses raised by rapid viral replication and non‐specific expression of viral protein resulted in more serious acute inflammation after systemic treatment of Ad5WS1. The increased serum ALT levels in Ad5WS1‐treated mice demonstrated more serious liver toxicity than those in Ad5WS2‐treated ones. These effects caused by immune responses are the reasons we did not observe cytotoxicity of Ad5WS1 against Chang liver in the in vitro study. Rather, immune responses also play essential roles against tumors. Viruses have evolved to kill cells by direct cell death machinery and fairly brisk immune responses. Endo et al. reported that tumor cell death caused by oncolytic adenoviruses could prime dendritic cells with tumor‐associated antigens.( 41 ) The cytotoxic T‐lymphocytes then activated by these antigen‐presenting cells would further eliminate the virus‐infected cells. Ad5WS2 tends to replicate in liver tumor cells, which may initiate more effective antitumor immune responses in liver tumors. Therefore, through systemic administration, immune responses occurred in the Ad5WS1‐ and Ad5WS2‐treated animals might be different and thus resulted in diverse therapeutic effect of these two viruses.
In conclusion, our in vitro and in vivo results demonstrate that transthyretin promoter‐driven Ad5WS2 exhibits more potent antitumor efficacy than Ad5WS1 for the treatment of HCC in the tumor models closely resembling the clinical settings. Therefore, this study may have clinical implications in exploring E1B‐55 kDa‐deleted adenovirus under the transcriptional control of the transthyretin promoter for the treatment of primary and metastatic HCC.
Supporting information
Fig. S1. Detections of transthyretin protein in human hepatocellular carcinoma (HCC). Cytoplasmic expression of transthyretin in non‐tumor and tumor parts of the liver from HCC patients was detected by immunohistochemistry (original magnification ×400).
Fig. S2. Detections of viral protein in Ad5WS2‐infected tissues. Expression of adenoviral E1A and fiber proteins were detected in ML‐1 cells at 36 h, 48 h, respectively, postinfection with Ad5WS2 at an multiplicity of infection (MOI) of 3, but not CT‐26 and NIH‐3T3 cells, indicating a restricted viral replication in liver tumor cells. (Expression of β‐actin served as the quantitative control).
Fig. S3. Detections of transthyretin and viral protein in Ad5WS2 infected tissues. The doubling staining was performed on tissues removed from animals after systemic administration of Ad5WS2. The result was shown above ML‐1 cells (2 × 105) were inoculated into the left liver lobe of BALB/c mice at day 0. Groups of 4 mice bearing orthotopic ML‐1 tumors were then injected i.v. with Ad5WS2 (2 × 107 plaque‐forming unit (PFU)) at days 30, and sacrificed at day 33. Consistent with the in vitro data, the late viral protein was detected in liver tumor tissues. These data indicated Ad5WS2 could only replicate in tumor cells where transthyretin gene is active.
Fig. S4. Cytolytic effects of Ad5WS2, Ad5WS1, or Ad5WT on various cells. Cytopathic effect (CPE) was observed in all the cell lines tested following Ad5WT infection, whereas it was detected in all the tumor cell lines after Ad5WS1 infection. Ad5WS2, however, only induced CPE in hepatocellular carcinoma (HCC) cell lines. Cells were infected with varying doses of Ad5WS2, Ad5WS1, or Ad5WT, and monitored for CPE by crystal violet staining at 4–7 days postinfection.
Fig. S5. Detection of fiber mRNA expression in Ad5WS2‐infected Hep3B cells by RT‐PCR analysis. RNA was isolated using the quickGene RNA cultured cell HC kits (FujiPhoto Film Co., Ltd. Life Science Product Division, Tokyo, Japan). RNA (1 µg) was then reverse‐transcribed into cDNA according to the manufacture’s instruction (ABgene, Surrey, UK). PCR was performed on the samples in triplicate with specific primers for L5‐fiber [5′‐CGG CCT CCG AAC GGT ACT‐3′(forward) and 5′‐TCT TGC GCG CTT CAT CTT G‐3′ (reverse)]; and for β‐actin [5′‐GAG AAG CTG TGC TAT GTT GCT CT‐3′(forward) and 5′‐ATG ATC TTG ATC TTC ATG GTG CT‐3′ (reverse)]. The reaction conditions were denaturing at 95°C (30 s), annealing at 56°C (30 s) and extension at 72°C (1 min). The reactions were completed by a final extension at 72°C (7 min).
Fig. S6. Plaque development assay. Viral titer was determined by plaque assay. Visible plaques were counted every day 96 h after infection. Each point represents the average of duplicate determination.
Table S1. Titer of neutralization antibody against adenoviruses. Mice (n = 4) were intraperitoneally injected with Ad5WS2 (2 × 108 PFU) or PBS. Mice serum samples were collected before (pre‐immune) and after virus injection. Neutralization antibody titers were analyzed by determining the ability of serum to inhibit the infection of Ad5WT to 293 cells. Various dilutions (1/2, 1/4, 1/8, 1/16, 1/32) of antibodies pre‐incubated with the Ad5WT (100 TCID50) for 1 h were added to 90% confluent 293 cells. Cells were then incubated for 4 days, and sera were scored positive for neutralization when the protection of cytopathic effect was 50%.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Acknowledgments
This work was supported by grants (NSC 92‐3112‐B‐006‐007, NSC 93‐3112‐B‐006‐008, and NSC 95‐3112‐B‐006‐013 to A.‐L.S and NSC 94‐2320‐B‐273‐004 to J.‐L.H) from the National Science Council, Taiwan.
References
- 1. Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet 1999; 353: 1253–7. [DOI] [PubMed] [Google Scholar]
- 2. Huang CC, Wu MC, Xu GW et al . Overexpression of the MDR1 gene and P‐glycoprotein in human hepatocellular carcinoma. J Natl Cancer Inst 1992; 84: 262–4. [DOI] [PubMed] [Google Scholar]
- 3. Nemunaitis J, Khuri F, Ganly I et al . Phase II trial of intratumoral administration of ONYX‐015, a replication‐selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol 2001; 19: 289–98. [DOI] [PubMed] [Google Scholar]
- 4. Heise C, Ganly I, Kim YT, Sampson‐Johannes A, Brown R, Kirn D. Efficacy of a replication‐selective adenovirus against ovarian carcinomatosis is dependent on tumor burden, viral replication and p53 status. Gene Ther 2000; 7: 1925–9. [DOI] [PubMed] [Google Scholar]
- 5. Hecht JR, Bedford R, Abbruzzese JL et al . A Phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX‐015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res 2003; 9: 555–61. [PubMed] [Google Scholar]
- 6. Habib N, Salama H, Abd El Latif Abu Median A et al . Clinical trial of E1B‐deleted adenovirus (dl1520) gene therapy for hepatocellular carcinoma. Cancer Gene Ther 2002; 9: 254–9. [DOI] [PubMed] [Google Scholar]
- 7. Reid T, Galanis E, Abbruzzese J et al . Intra‐arterial administration of a replication‐selective adenovirus (dl1520) in patients with colorectal carcinoma metastatic to the liver: a phase I trial. Gene Ther 2001; 8: 1618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reid T, Galanis E, Abbruzzese J et al . Hepatic arterial infusion of a replication‐selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res 2002; 62: 6070–9. [PubMed] [Google Scholar]
- 9. Everts B, Van Der Poel HG. Replication‐selective oncolytic viruses in the treatment of cancer. Cancer Gene Ther 2005; 12: 141–61. [DOI] [PubMed] [Google Scholar]
- 10. Chen MJ, Green NK, Reynolds GM et al . Enhanced efficacy of Escherichia coli nitroreductase/CB1954 prodrug activation gene therapy using an E1B‐55K‐deleted oncolytic adenovirus vector. Gene Ther 2004; 11: 1126–36. [DOI] [PubMed] [Google Scholar]
- 11. Pei Z, Chu L, Zou W et al . An oncolytic adenoviral vector of Smac increases antitumor activity of TRAIL against HCC in human cells and in mice. Hepatology 2004; 39: 1371–81. [DOI] [PubMed] [Google Scholar]
- 12. Gao Q, Zhou J, Huang X et al . Selective targeting of checkpoint kinase 1 in tumor cells with a novel potent oncolytic adenovirus. Mol Ther 2006; 13: 928–37. [DOI] [PubMed] [Google Scholar]
- 13. Schmitz M, Graf C, Gut T et al . Melanoma cultures show different susceptibility towards E1A‐, E1B‐19 kDa‐ and fiber‐modified replication‐competent adenoviruses. Gene Ther 2006; 13: 893–905. [DOI] [PubMed] [Google Scholar]
- 14. Hallenbeck PL, Chang YN, Hay C et al . A novel tumor‐specific replication‐restricted adenoviral vector for gene therapy of hepatocellular carcinoma. Hum Gene Ther 1999; 10: 1721–33. [DOI] [PubMed] [Google Scholar]
- 15. Li Y, Yu DC, Chen Y et al . A hepatocellular carcinoma‐specific adenovirus variant, CV890, eliminates distant human liver tumors in combination with doxorubicin. Cancer Res 2001; 61: 6428–36. [PubMed] [Google Scholar]
- 16. Ohashi M, Kanai F, Tateishi K et al . Target gene therapy for alpha‐fetoprotein‐producing hepatocellular carcinoma by E1B55k‐attenuated adenovirus. Biochem Biophys Res Commun 2001; 282: 529–35. [DOI] [PubMed] [Google Scholar]
- 17. Takahashi M, Sato T, Sagawa T et al . E1B‐55K‐deleted adenovirus expressing E1A‐13S by AFP‐enhancer/promoter is capable of highly specific replication in AFP‐producing hepatocellular carcinoma and eradication of established tumor. Mol Ther 2002; 5: 627–34. [DOI] [PubMed] [Google Scholar]
- 18. Yan C, Costa RH, Darnell JE Jr, Chen JD, Van Dyke TA. Distinct positive and negative elements control the limited hepatocyte and choroid plexus expression of transthyretin in transgenic mice. EMBO J 1990; 9: 869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wakasugi S, Maeda S, Shimada K, Nakashima H, Migita S. Structural comparisons between mouse and human prealbumin. J Biochem (Tokyo) 1985; 98: 1707–14. [DOI] [PubMed] [Google Scholar]
- 20. Wakasugi S, Maeda S, Shimada K. Structure and expression of the mouse prealbumin gene. J Biochem (Tokyo) 1986; 100: 49–58. [DOI] [PubMed] [Google Scholar]
- 21. Costa RH, Lai E, Darnell JE Jr. Transcriptional control of the mouse prealbumin (transthyretin) gene: both promoter sequences and a distinct enhancer are cell specific. Mol Cell Biol 1986; 6: 4697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tannour‐Louet M, Porteu A, Vaulont S, Kahn A, Vasseur‐Cognet M. A tamoxifen‐inducible chimeric Cre recombinase specifically effective in the fetal and adult mouse liver. Hepatology 2002; 35: 1072–81. [DOI] [PubMed] [Google Scholar]
- 23. Chen SH, Hu CP, Chang CM. Hepatitis B virus replication in well differentiated mouse hepatocyte cell lines immortalized by plasmid DNA. Cancer Res 1992; 52: 1329–35. [PubMed] [Google Scholar]
- 24. Chang WH, Chen CH, Gau RJ et al . Effect of baicalein on apoptosis of the human HepG2 cell line was induced by mitochondrial dysfunction. Planta Med 2002; 68: 302–6. [DOI] [PubMed] [Google Scholar]
- 25. Lin YL, Liu CC, Lei HY et al . Infection of five human liver cell lines by dengue‐2 virus. J Med Virol 2000; 60: 425–31. [DOI] [PubMed] [Google Scholar]
- 26. Chang FL, Lai MD. The relationship between p53 status and anticancer drugs‐induced apoptosis in nine human bladder cancer cell lines. Anticancer Res 2000; 20: 351–5. [PubMed] [Google Scholar]
- 27. Hsieh JL, Wu CL, Lee CH, Shiau AL. Hepatitis B virus X protein sensitizes hepatocellular carcinoma cells to cytolysis induced by E1B‐deleted adenovirus through the disruption of p53 function. Clin Cancer Res 2003; 9: 338–45. [PubMed] [Google Scholar]
- 28. He TC, Zhou S, Da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 1998; 95: 2509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shiau AL, Chen YL, Liao CY, Huang YS, Wu CL. Prothymosin alpha enhances protective immune responses induced by oral DNA vaccination against pseudorabies delivered by Salmonella choleraesuis. Vaccine 2001; 19: 3947–56. [DOI] [PubMed] [Google Scholar]
- 30. Heise C, Sampson‐Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. ONYX‐015, an E1B gene‐attenuated adenovirus, causes tumor‐specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med 1997; 3: 639–45. [DOI] [PubMed] [Google Scholar]
- 31. Lee CH, Wu CL, Tai YS, Shiau AL. Systemic administration of attenuated Salmonella choleraesuis in combination with cisplatin for cancer therapy. Mol Ther 2005; 11: 707–16. [DOI] [PubMed] [Google Scholar]
- 32. Hsieh JL, Wu CL, Lai MD, Lee CH, Tsai CS, Shiau AL. Gene therapy for bladder cancer using E1B‐55 kD‐deleted adenovirus in combination with adenoviral vector encoding plasminogen kringles 1–5. Br J Cancer 2003; 88: 1492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bischoff JR, Kirn DH, Williams A et al . An adenovirus mutant that replicates selectively in p53‐deficient human tumor cells. Science 1996; 274: 373–6. [DOI] [PubMed] [Google Scholar]
- 34. Costa RH, Van Dyke TA, Yan C, Kuo F, Darnell JE Jr. Similarities in transthyretin gene expression and differences in transcription factors: liver and yolk sac compared to choroid plexus. Proc Natl Acad Sci USA 1990; 87: 6589–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O'Shea CC, Johnson L, Bagus B et al . Late viral RNA export, rather than p53 inactivation, determines ONYX‐015 tumor selectivity. Cancer Cell 2004; 6: 611–23. [DOI] [PubMed] [Google Scholar]
- 36. Duncan S. Infection of mouse liver by human adenovirus type 5. J General Virol 1978; 40: 45–61. [DOI] [PubMed] [Google Scholar]
- 37. Ganly I, Mauntner V, Balmain A. Productive replication of human adenoviruses in mouse epidermal cells. J Virol 2000; 74: 2895–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hallden G, Hill R, Wang Y et al . Novel immunocompetent murne tumor models for the assessment of replicaiotn‐competent oncolytic adenovirus efficacy. Mol Ther 2003; 8: 412–24. [DOI] [PubMed] [Google Scholar]
- 39. Kew MC, Dos Santos HA, Sherlock S. Diagnosis of primary cancer of the liver. Br Med J 1971; 4: 408–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shenk T, Flint J. Transcriptional and transforming activities of the adenovirus E1A proteins. Adv Cancer Res 1991; 57: 47–85. [DOI] [PubMed] [Google Scholar]
- 41. Endo Y, Sakai R, Ouchi M et al . Virus‐mediated oncolysis induces danger signal and stimulates cytotoxic T‐lymphocyte activity via proteasome activator upregulation. Oncogene 2008; 27: 2375–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Detections of transthyretin protein in human hepatocellular carcinoma (HCC). Cytoplasmic expression of transthyretin in non‐tumor and tumor parts of the liver from HCC patients was detected by immunohistochemistry (original magnification ×400).
Fig. S2. Detections of viral protein in Ad5WS2‐infected tissues. Expression of adenoviral E1A and fiber proteins were detected in ML‐1 cells at 36 h, 48 h, respectively, postinfection with Ad5WS2 at an multiplicity of infection (MOI) of 3, but not CT‐26 and NIH‐3T3 cells, indicating a restricted viral replication in liver tumor cells. (Expression of β‐actin served as the quantitative control).
Fig. S3. Detections of transthyretin and viral protein in Ad5WS2 infected tissues. The doubling staining was performed on tissues removed from animals after systemic administration of Ad5WS2. The result was shown above ML‐1 cells (2 × 105) were inoculated into the left liver lobe of BALB/c mice at day 0. Groups of 4 mice bearing orthotopic ML‐1 tumors were then injected i.v. with Ad5WS2 (2 × 107 plaque‐forming unit (PFU)) at days 30, and sacrificed at day 33. Consistent with the in vitro data, the late viral protein was detected in liver tumor tissues. These data indicated Ad5WS2 could only replicate in tumor cells where transthyretin gene is active.
Fig. S4. Cytolytic effects of Ad5WS2, Ad5WS1, or Ad5WT on various cells. Cytopathic effect (CPE) was observed in all the cell lines tested following Ad5WT infection, whereas it was detected in all the tumor cell lines after Ad5WS1 infection. Ad5WS2, however, only induced CPE in hepatocellular carcinoma (HCC) cell lines. Cells were infected with varying doses of Ad5WS2, Ad5WS1, or Ad5WT, and monitored for CPE by crystal violet staining at 4–7 days postinfection.
Fig. S5. Detection of fiber mRNA expression in Ad5WS2‐infected Hep3B cells by RT‐PCR analysis. RNA was isolated using the quickGene RNA cultured cell HC kits (FujiPhoto Film Co., Ltd. Life Science Product Division, Tokyo, Japan). RNA (1 µg) was then reverse‐transcribed into cDNA according to the manufacture’s instruction (ABgene, Surrey, UK). PCR was performed on the samples in triplicate with specific primers for L5‐fiber [5′‐CGG CCT CCG AAC GGT ACT‐3′(forward) and 5′‐TCT TGC GCG CTT CAT CTT G‐3′ (reverse)]; and for β‐actin [5′‐GAG AAG CTG TGC TAT GTT GCT CT‐3′(forward) and 5′‐ATG ATC TTG ATC TTC ATG GTG CT‐3′ (reverse)]. The reaction conditions were denaturing at 95°C (30 s), annealing at 56°C (30 s) and extension at 72°C (1 min). The reactions were completed by a final extension at 72°C (7 min).
Fig. S6. Plaque development assay. Viral titer was determined by plaque assay. Visible plaques were counted every day 96 h after infection. Each point represents the average of duplicate determination.
Table S1. Titer of neutralization antibody against adenoviruses. Mice (n = 4) were intraperitoneally injected with Ad5WS2 (2 × 108 PFU) or PBS. Mice serum samples were collected before (pre‐immune) and after virus injection. Neutralization antibody titers were analyzed by determining the ability of serum to inhibit the infection of Ad5WT to 293 cells. Various dilutions (1/2, 1/4, 1/8, 1/16, 1/32) of antibodies pre‐incubated with the Ad5WT (100 TCID50) for 1 h were added to 90% confluent 293 cells. Cells were then incubated for 4 days, and sera were scored positive for neutralization when the protection of cytopathic effect was 50%.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item


