Abstract
There is no standard second‐line chemotherapy treatment for recurrent or metastatic urothelial cancer (MUC). The purpose of this phase II study was to evaluate the efficacy and toxicity of the three‐drug combination of paclitaxel, ifosfamide, and nedaplatin (TIN). Patients with MUC were eligible after treatment failure with methotrexate, vinblastine, doxorubicin, and cisplatin, or gemcitabine and cisplatin. Doses for TIN therapy were paclitaxel 175 mg/m2 on day 1, ifosfamide 1500 mg/m2 on days 1–3, and nedaplatin 70 mg/m2 on day 1, every 4 weeks. Tumor response, the primary efficacy parameter, was assessed according to unidimensional measurements (Response Evaluation Criteria in Solid Tumors criteria, version 1.0). Secondary efficacy parameters were overall survival (OS) and progression‐free survival (PFS). Toxicity was assessed according to the National Cancer Institute Common Toxicity Criteria, version 3.0. A total of 45 patients (13 females and 32 males) with MUC were evaluable for response and toxicity. The overall response rate was 40.0%. Median PFS time was 4.0 months (95% confidence interval [CI], 4.6–11.6). Median OS time was 8.9 months (95% CI, 10.5–18.9). Grade 3 or 4 hematologic adverse events were neutropenia (95.6%), anemia (15.6%), and thrombocytopenia (17.8%). The most common grade 3 or 4 non‐hematologic adverse events were anorexia (4.4%) and elevated aspartate transaminase/alanine transaminase (2.2%). No toxic death was observed. The main limitation of this study is that only 10 patients (22.2%) who were previously treated with gemcitabine and cisplatin were included. In conclusion, TIN as second‐line treatment for MUC is an active regimen with a manageable toxicity profile. (Cancer Sci 2011; 102: 1171–1175)
Urothelial carcinoma of the bladder is the fourth most common cancer in men.( 1 ) Systemic chemotherapy has been the mainstay of management for metastatic urothelial cancer (MUC).( 2 ) Cisplatin‐based combinations have evolved as the standard for first‐line systemic therapy for MUC. The methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) regimen was reported to show an impressive complete remission rate of approximately 40–50% with substantial toxicity and a toxic death rate of 3–4% in MUC.( 3 , 4 ) The gemcitabine and cisplatin (GC) regimen provides almost the same response rate as MVAC with less toxicity.( 5 ) Neither of the two combinations proved superior over the other with prolongation of survival up to 14.8 and 13.8 months for MVAC and GC, respectively.( 6 ) With cisplatin‐containing combination chemotherapy, patients with lymph node metastases only, good performance status, and adequate renal function may achieve excellent response rates, including a high degree of complete responses, with up to 20% of patients achieving long‐term disease‐free survival.
However, no standard therapy has been established for patients pretreated for MUC.( 7 ) Recently, new combination regimens, including paclitaxel and gemcitabine,( 8 , 9 , 10 ) and paclitaxel and carboplatin,( 11 ) indicated the efficacy and tolerability of paclitaxel‐based therapy for the treatment of such patients. It has also been shown that ifosfamide is one of the most promising agents in salvage chemotherapy for MUC when it is given with paclitaxel( 12 ) or gemcitabine.( 13 , 14 ) Combination regimens using three agents, for example, paclitaxel, cisplatin, and methotrexate,( 15 ) gemcitabine, ifosfamide, and cisplatin,( 16 ) or paclitaxel, ifosfamide, and nedaplatin,( 17 ) may provide higher response rates and longer survival than those with two agents. A preliminary study( 18 ) indicated that nedaplatin had a higher inhibition index than cisplatin in all tissues from 12 urothelial cancer patients by the histoculture drug response assay.( 19 ) The results suggest that nedaplatin can be effective for patients with progressive disease after cisplatin‐based chemotherapies, such as MVAC or GC, although nedaplatin is cross‐resistant to cisplatin.( 20 ) Based on these data, this phase II study was carried out to evaluate the efficacy and toxicity of a regimen combining paclitaxel, ifosfamide, and nedaplatin (TIN) for MUC.
Materials and Methods
Eligibility. Patients eligible for study were those with a histological diagnosis of urothelial carcinoma of the bladder, ureter, or renal pelvis who had progressive or recurring disease, measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria, were previously treated with MVAC or GC, aged ≥20 years, with an Eastern Cooperative Oncology Group performance status of 0–2 and with adequate bone marrow (neutrophil count ≥1000 cells/μL, platelets ≥50 000 cells/μL), hepatic (aspartate transaminase [AST] and alanine transaminase [ALT] ≤1.5 × upper limit of normal, unless there were liver metastases, in which case AST and ALT ≤5.0 × upper limit of normal), and renal function (creatinine clearance [CCr] ≥30 mL/min). Creatinine clearance was calculated according to the following formula: creatinine clearance (mL/min) = (urine creatinine/serum creatinine) × urine volume (mL)/(time [h] × 60]. All previous therapies for urothelial cancer had to be discontinued for ≥4 weeks before study entry and all acute toxic effects, excluding alopecia or peripheral neuropathy, of any prior therapy had to be recovered from. Life expectancy had to be ≥12 weeks and all patients needed to provide signed informed consent.
Exclusion criteria were: a serious uncontrolled medical disorder or active infection that would impair the ability to receive study treatment; known allergy or hypersensitivity to paclitaxel, ifosfamide, or nedaplatin; concomitant malignancy other than urothelial cancer; and female patients who were pregnant or lactating.
This study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Approval to carry out the study was obtained from the institutional review board before the start of the trial.
Treatment plan. Paclitaxel was given at 175 mg/m2 for 3 h by i.v. infusion on day 1 after premedication (given 1 h previous) consisting of dexamethasone 20 mg, ranitidine 50 mg, and diphenhydramine 50 mg. Ifosfamide was given at 1500 mg/m2 per day i.v. on days 1, 2, and 3 for 4 h/day (total dose 4500 mg/m2). Mesna uroprotection at 300 mg/m2 i.v. was given with the ifosfamide infusion, then at 3 and 6 h afterwards. Nedaplatin was given at 70 mg/m2 i.v., for 3 h on day 1 with vigorous pre‐ and post‐hydration and mannitol. The chemotherapy schedule was recycled every 28 days. If the CCr was <60 mL/min, the doses of ifosfamide and nedaplatin were reduced to 3600 mg/m2 and 70 mg/m2, respectively, in the case of 45 ≤ CCr < 60 mL/min, and 3375 mg/m2 and 35 mg/m2, respectively, in the case of 30 ≤ CCr < 45 mL/min. If a complete response (CR), partial response (PR), or stable disease (SD) response was obtained and the disease site was considered to be resectable after TIN treatment, salvage surgery or radiation therapy was carried out.
Evaluation. Tumor response, the primary efficacy parameter, was assessed every two cycles according to RECIST criteria, version 1.0.( 21 ) Complete response required the total disappearance of all evidence of cancer for at least 4 weeks. Partial response (PR) required a more than 30% reduction in the sum of the longest diameters of the target lesions without any new lesions for at least 4 weeks. The response status was reviewed by a panel of independent experts. Secondary efficacy parameters were overall survival (OS) and progression‐free survival (PFS). The qualitative and quantitative toxic effects were graded in agreement with National Cancer Institute Common Toxicity Criteria, version 3.0.
Statistical analysis. For the primary end‐point, the percentage of change in the sum of the longest diameter of the target lesion from baseline to the best response was calculated and recorded in a waterfall plot. For the secondary end‐point, PFS was calculated from the first day of treatment to the date of the first documented disease progression or death from any cause. The Standard Southwest Oncology Group Phase II design( 22 ) was used to plan this study on the assumption that the regimen would not be of interest if the true response rate was <20%, but that it would be of interest if the response rate was 40% or more. Alpha (the significance level) and 1‐beta (the power) were set as 0.05 and 0.90, respectively. If more than four of the first 25 patients obtained a response, planned accrual of 20 more eligible patients was to be carried out. Overall survival was defined as the time between the first day of treatment and date of death from any cause. Patients remaining on‐study or alive at time of analyses were censored at the date of the last follow‐up. The probability of PFS and OS was estimated using the Kaplan–Meier method.
Results
Patient characteristics. Between April 2005 and January 2010, 45 patients with advanced transitional cell cancer were entered into this phase II study. Because a response was obtained in 36.0% of the first 25 patients, 20 additional patients were enrolled in this study. All patients were assessable for efficacy and safety. The baseline characteristics of the patients are presented in Table 1.
Table 1.
Baseline characteristics of patients with metastatic urothelial carcinoma who participated in this study
| Characteristic | n | % |
|---|---|---|
| No. of patients | 45 | — |
| Median age, years (range) | 68 (35–78) | — |
| Male:female | 32:13 | — |
| ECOG performance status | ||
| 0 | 35 | 77.8 |
| 1 | 6 | 13.3 |
| 2 | 4 | 8.9 |
| Primary site | ||
| Bladder | 26 | 57.7 |
| Renal pelvis | 13 | 28.9 |
| Ureter | 6 | 13.3 |
| Metastatic sites | ||
| Lymph nodes | 31 | 68.9 |
| Pelvic mass | 5 | 11.1 |
| Lung | 15 | 33.3 |
| Liver | 9 | 20.0 |
| Bone | 6 | 13.3 |
| Adrenal | 1 | 2.2 |
| Ovary | 1 | 2.2 |
| Duodenum | 1 | 2.2 |
| Peritoneum | 1 | 2.2 |
| Contralateral renal pelvis | 1 | 2.2 |
| No. of sites with tumor involvement | ||
| 1 | 25 | 55.6 |
| 2 | 14 | 31.1 |
| ≥3 | 6 | 13.3 |
| Prior chemotherapy | ||
| MVAC | 35 | 77.8 |
| GC | 10 | 22.2 |
—, Not applicable; ECOG, Eastern Cooperative Oncology Group; GC, gemcitabine and cisplatin; MVAC, methotrexate, vinblastine, doxorubicin, and cisplatin.
Extent of exposure. Altogether 108 cycles were given with a median of two cycles per patient (range, 1–6). In eight (17.8%) patients the drug doses were reduced due to renal dysfunction. Seven patients stopped therapy after only one cycle, either because of progressive disease (two patients), patient choice to withdraw from treatment (four patients), or the adverse effect of AST/ALT elevation (one patient). Fifteen (33.3%) patients were treated with three or more cycles of therapy.
Responses to treatment. Of the 45 patients, two CR and 16 PR were obtained (Table 2). Plots of serial tumor measurements over time and a waterfall plot of the best response, to better characterize antitumor activity, showed that 28 (62.2%) of the 45 patients had some degree of tumor reduction (Fig. 1). The overall response rate was 40.0%. Overall, at follow‐up, five (11.1%) patients were progression free. The median PFS and OS were 4.0 months (95% confidence interval [CI], 4.6–11.6) and 8.9 months (95% CI, 10.5–18.9), respectively (Fig. 2). Responses stratified by the first‐line chemotherapy and disease site are shown in 3, 4, respectively. There was no significant difference in the objective response (OR) rate, PFS, or OS between patients who received MVAC and those who received GC as the first‐line therapy (37.1%vs 50.0%, median 4.0 vs 5.6 months, and median 8.8 vs 9.9 months, respectively). Six (13.3%) patients (one CR, four PR and one SD) underwent salvage surgery or radiation therapy after TIN therapy. Of these six patients, three (50.0%) were followed‐up for 50, 50, and 49 months after the salvage surgery without recurrence.
Table 2.
Best overall response to paclitaxel, ifosfamide, and nedaplatin as second‐line chemotherapy regimen in 45 patients with metastatic urothelial carcinoma
| Response | No. of patients (%) |
|---|---|
| CR | 2 (4.4) |
| PR | 16 (35.6) |
| SD | 15 (33.3) |
| PD | 12 (26.7) |
| Total | 45 (100) |
CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 1.
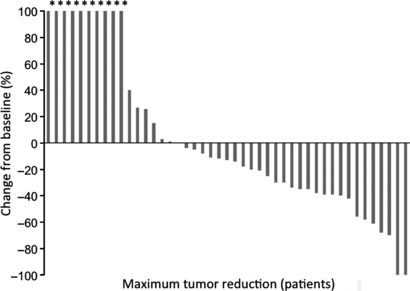
Waterfall plot of percentage changes showing the best response in each patient with metastatic urothelial carcinoma who underwent second‐line chemotherapy treatment with paclitaxel, ifosfamide, and nedaplatin. *Appearance of a new lesion.
Figure 2.
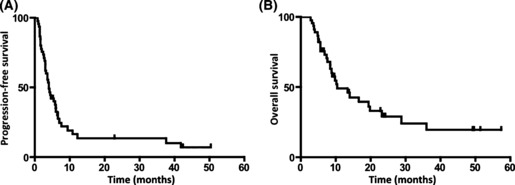
Progression‐free survival (A) and overall survival (B) of urothelial cancer patients treated with paclitaxel, ifosfamide, and nedaplatin as second‐line chemotherapy.
Table 3.
Objective response (OR) to paclitaxel, ifosfamide, and nedaplatin (TIN) as second‐line chemotherapy in 45 patients with metastatic urothelial carcinoma, stratified by response to first‐line chemotherapy
| Response to first‐line chemotherapy | Response to TIN | |||
|---|---|---|---|---|
| n | CR (%) | PR (%) | OR (%) | |
| CR | 2 | 1 (50.0) | 1 (50.0) | 2 (100.0) |
| PR | 6 | 0 (0.0) | 4 (66.7) | 4 (66.7) |
| SD | 12 | 0 (0.0) | 2 (16.7) | 2 (16.7) |
| PD | 12 | 0 (0.0) | 4 (33.3) | 4 (33.3) |
| NE | 13 | 1 (7.7) | 5 (38.5) | 6 (46.2) |
| Total | 45 | 2 (4.4) | 16 (35.6) | 18 (40.0) |
CR, complete response; NE, not evaluable (neoadjuvant or adjuvant chemotherapy); PD, progressive disease; PR, partial response; SD, stable disease.
Table 4.
Objective response (OR) to paclitaxel, ifosfamide, and nedaplatin (TIN) as second‐line chemotherapy in 45 patients with metastatic urothelial carcinoma, stratified by disease sites
| Disease site | Response to TIN | |||
|---|---|---|---|---|
| n | CR (%) | PR (%) | OR (%) | |
| Multiple organs | 19 | 1 (5.3) | 3 (15.8) | 4 (21.1) |
| Lymph nodes | 19 | 1 (5.3) | 9 (47.4) | 10 (52.6) |
| Lung | 4 | 0 (0.0) | 3 (75.0) | 3 (75.0) |
| Duodenum | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ovary | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Local | 1 | 0 (0.0) | 1 (100.0) | 1 (100.0) |
| Total | 45 | 2 (4.4) | 16 (35.6) | 18 (40.0) |
CR, complete response; PR, partial response.
Toxicity. There were no toxicity‐related deaths with TIN. Grade 3/4 leukopenia, neutropenia, thrombocytopenia, anemia, anorexia, and elevated AST/ALT were noted in 42 (93.3%), 43 (95.6%), 8 (17.8%), 8 (17.8%), 2 (4.4%), and 1 (2.2%) patients, respectively (Table 5). The median onsets of neutropenia and thrombocytopenia for all grades were both 11 days. Ten (22.2%) patients displayed febrile neutropenia and were treated with antibiotics until the neutrophil count recovered and the fever abated. Granulocyte‐colony stimulating factor was given as a treatment for neutropenia a median of five times per course (range, 0–11). Peripheral neuropathy was observed in seven (15.6%) patients, but no grade 3/4 neuropathy occurred. Another common side‐effect was alopecia in 100%.
Table 5.
Toxicity profile of 45 patients who underwent treatment with paclitaxel, ifosfamide, and nedaplatin as second‐line chemotherapy for metastatic urothelial carcinoma
| Toxicity | Grade (all cycles), no. of patients (%) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Hematologic | ||||
| Leukopenia | 0 (0) | 2 (4.4) | 21 (46.7) | 21 (46.7) |
| Neutropenia | 0 (0) | 1 (2.2) | 2 (4.4) | 41 (91.1) |
| Thrombocytopenia | 10 (22.2) | 10 (22.2) | 5 (11.1) | 3 (6.7) |
| Anemia | 10 (22.2) | 14 (31.1) | 7 (15.6) | 0 (0.0) |
| Febrile neutropenia | 10 (22.2) | |||
| Non‐hematologic | ||||
| Anorexia | 12 (26.7) | 13 (28.9) | 2 (4.4) | 0 (0.0) |
| Vomiting | 3 (6.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Peripheral neuropathy | 0 (0.0) | 7 (15.6) | 0 (0.0) | 0 (0.0) |
| AST/ALT, elevated | 0 (0.0) | 0 (0.0) | 1 (2.2) | 0 (0.0) |
| Creatinine, elevated | 0 (0.0) | 1 (2.2) | 0 (0.0) | 0 (0.0) |
| Alopecia | 0 (0.0) | 45 (100.0) | 0 (0.0) | 0 (0.0) |
Toxic effects were graded in agreement with National Cancer Institute Common Toxicity Criteria, version 3.0. ALT, alanine transaminase; AST, aspartate transaminase.
Discussion
Although urothelial cancer is chemosensitive, there are insufficient data to provide a recommendation on standard second‐line chemotherapy.( 6 ) Various regimens have been investigated in an attempt to prolong survival for first‐line chemotherapy‐resistant MUC. In second‐line chemotherapy for MUC, a paclitaxel‐based regimen, such as paclitaxel and cisplatin, or paclitaxel and carboplatin, provided 30–70% OR rates and a median OS of 7.9–11.5 months.( 8 , 9 , 10 , 11 ) Phase II trials of a gemcitabine/ifosfamide regimen had a 21–22% OR with a median OS of 4.8–9.0 months.( 13 , 14 ) A paclitaxel/ifosfamide regimen gave a 15% OR with a median OS of 8 months.( 12 ) Thus, combinations of two anticancer drugs had similar results, that is, a median OS of 6–12 months.
A phase II study of gemcitabine, ifosfamide, and cisplatin (gemcitabine 800 mg/m2, ifosfamide 1000 mg/m2, and cisplatin 30 mg/m2, on days 1, 8, and 15 on a 28‐day cycle) for MUC( 16 ) provided an OR of 40.8% with a median OS of 9.5 months. However, the intended schedule of weekly doses given three times per cycle was not deliverable due to hematologic toxicity. Thus significant toxicity can be expected and is of concern, especially in patients who have undergone several courses of first‐line chemotherapy. Shinohara et al. ( 17 ) invented the TIN regimen by modification of paclitaxel/ifosfamide/cisplatin( 23 ) with the aim of higher relative dose intensity with lower toxicities for MUC patients who had received prior chemotherapy. They used nedaplatin, a second‐generation platinum complex with lower renal and gastrointestinal toxicities than cisplatin,( 24 ) instead of cisplatin, and reported a surprising result, a 75% OR (16% CR + 59% PR) with a median OS of 22 months.
The OR of 40% and the median survival of 8.9 months in this study are similar to those for paclitaxel/gemcitabine( 8 , 9 , 10 ) rather than the first report on TIN by Shinohara et al. ( 17 ) One of the reasons explaining this is a difference in patient characteristics between the two studies of TIN. First, this study included only eight (17.8%) patients who had a CR or PR after the first‐line chemotherapy, which indicated a good response to TIN (Table 3). In contrast, 14 (43.8%) such patients were treated by TIN in Shinohara’s study. Another possibility is that Shinohara’s study included fewer patients with visceral metastases (lung and liver metastasis in 18.8% and 6.3% of the patients, respectively) than others,( 8 , 9 , 10 , 11 ) including this study. Nineteen (42.2%) of the patients in this study had multiple organ diseases, which showed lower responses to TIN than lymph node or lung metastasis (Table 4).
Grade 3/4 neutropenia, thrombocytopenia, and anemia were noted in 43 (95.6%), eight (17.8%), and seven (15.6%) patients, respectively. Although most of the patients displayed hematological toxicity, these adverse effects were manageable and no delay in the start of chemotherapy was required. One patient stopped the therapy because of AST/ALT elevation due to allergic hepatitis induced by the polyoxyethylated castor oil solvent used for paclitaxel. No other patient discontinued this treatment due to toxic effects. Thus, the TIN regimen was well tolerated with hematological toxicity being the most significant side‐effect.
Salvage surgery after the chemotherapy might have favorably impacted the observed survival in this series. However, postchemotherapy metastasectomy is still controversial. Dodd et al. ( 25 ) reported that salvage surgery after MVAC contributed to long‐term survival with a 5‐year survival of 33%. Abe et al. ( 26 ) showed the impact of metastasectomy on survival in patients with MUC. The median OS was 42 months for patients who underwent postchemotherapy surgery, which was significantly longer than that for patients without surgery (10 months).( 26 ) In contrast, Otto et al. ( 27 ) reported that surgical resection had no impact on survival but only on the quality of life of patients with symptomatic disease. This study showed that patients with a small number of metastatic sites who underwent salvage resection had more favorable survival. Although there is a limitation because of the small number of patients in this study, properly selected patients may be considered for consolidation surgery.
The main limitation of this study is that only 10 patients (22.2%) who were previously treated with GC were included. Recently, more patients with MUC undergo GC rather than MVAC as their first‐line chemotherapy because of the anticancer activity and lower toxicity of GC, which means that there are fewer gemcitabine‐naïve patients at the time of second‐line chemotherapy. Although paclitaxel/gemcitabine is hematologically less toxic than TIN, a regimen other than gemcitabine should be considered. Further studies are needed including more GC‐failure patients.
In conclusion, TIN therapy is a tolerable and active regimen for treating MUC after MVAC or GC failure. We suggest that TIN can be one of the options for second‐line chemotherapy for MUC patients in the GC era. Salvage surgery can be offered to patients with a good response to TIN, which may provide longer survival.
Disclosure Statement
The authors have no conflict of interest.
References
- 1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59: 225–49. [DOI] [PubMed] [Google Scholar]
- 2. Vaishampayan U. Systemic therapy of advanced urothelial cancer. Curr Treat Options Oncol 2009; 10: 256–66. [DOI] [PubMed] [Google Scholar]
- 3. Sternberg CN, Yagoda A, Scher HI et al. Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium. Efficacy and patterns of response and relapse. Cancer 1989; 64: 2448–58. [DOI] [PubMed] [Google Scholar]
- 4. Loehrer PJ Sr, Einhorn LH, Elson PJ et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 1992; 10: 1066–73. [DOI] [PubMed] [Google Scholar]
- 5. von der Maase H, Sengelov L, Roberts JT et al. Long‐term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005; 23: 4602–8. [DOI] [PubMed] [Google Scholar]
- 6. Stenzl A, Cowan NC, De Santis M et al. The updated EAU guidelines on muscle‐invasive and metastatic bladder cancer. Eur Urol 2009; 55: 815–25. [DOI] [PubMed] [Google Scholar]
- 7. Bachner M, De Santis M. Second‐line therapy in bladder cancer. Curr Opin Urol 2009; 19: 533–9. [DOI] [PubMed] [Google Scholar]
- 8. Matsumoto K, Irie A, Satoh T, Okazaki M, Iwamura M, Baba S. Gemcitabine and paclitaxel chemotherapy as a second‐line treatment for advanced or metastatic urothelial carcinoma. Int J Urol 2007; 14: 1000–4; discussion 4. [DOI] [PubMed] [Google Scholar]
- 9. Kanai K, Kikuchi E, Ohigashi T et al. Gemcitabine and paclitaxel chemotherapy for advanced urothelial carcinoma in patients who have received prior cisplatin‐based chemotherapy. Int J Clin Oncol 2008; 13: 510–4. [DOI] [PubMed] [Google Scholar]
- 10. Suyama T, Ueda T, Fukasawa S et al. Combination of gemcitabine and paclitaxel as second‐line chemotherapy for advanced urothelial carcinoma. Jpn J Clin Oncol 2009; 39: 244–50. [DOI] [PubMed] [Google Scholar]
- 11. Kouno T, Ando M, Yonemori K et al. Weekly paclitaxel and carboplatin against advanced transitional cell cancer after failure of a platinum‐based regimen. Eur Urol 2007; 52: 1115–22. [DOI] [PubMed] [Google Scholar]
- 12. Sweeney CJ, Williams SD, Finch DE et al. A Phase II study of paclitaxel and ifosfamide for patients with advanced refractory carcinoma of the urothelium. Cancer 1999; 86: 514–8. [DOI] [PubMed] [Google Scholar]
- 13. Pectasides D, Aravantinos G, Kalofonos H et al. Combination chemotherapy with gemcitabine and ifosfamide as second‐line treatment in metastatic urothelial cancer. A phase II trial conducted by the Hellenic Cooperative Oncology Group. Ann Oncol 2001; 12: 1417–22. [DOI] [PubMed] [Google Scholar]
- 14. Lin CC, Hsu CH, Huang CY et al. Gemcitabine and ifosfamide as a second‐line treatment for cisplatin‐refractory metastatic urothelial carcinoma: a phase II study. Anticancer Drugs 2007; 18: 487–91. [DOI] [PubMed] [Google Scholar]
- 15. Tu SM, Hossan E, Amato R, Kilbourn R, Logothetis CJ. Paclitaxel, cisplatin and methotrexate combination chemotherapy is active in the treatment of refractory urothelial malignancies. J Urol 1995; 154: 1719–22. [PubMed] [Google Scholar]
- 16. Pagliaro LC, Millikan RE, Tu SM et al. Cisplatin, gemcitabine, and ifosfamide as weekly therapy: a feasibility and phase II study of salvage treatment for advanced transitional‐cell carcinoma. J Clin Oncol 2002; 20: 2965–70. [DOI] [PubMed] [Google Scholar]
- 17. Shinohara N, Harabayashi T, Suzuki S et al. Salvage chemotherapy with paclitaxel, ifosfamide, and nedaplatin in patients with urothelial cancer who had received prior cisplatin‐based therapy. Cancer Chemother Pharmacol 2006; 58: 402–7. [DOI] [PubMed] [Google Scholar]
- 18. Nakaigawa N, Miyoshi Y, Hattori Y et al. Chemotherapy based on a chemosensitivity test in urothelial cancer. Urol View (in Japanese) 2008; 6: 109–15. [Google Scholar]
- 19. Furukawa T, Kubota T, Hoffman RM. Clinical applications of the histoculture drug response assay. Clin Cancer Res 1995; 1: 305–11. [PubMed] [Google Scholar]
- 20. Desoize B, Madoulet C. Particular aspects of platinum compounds used at present in cancer treatment. Crit Rev Oncol Hematol 2002; 42: 317–25. [DOI] [PubMed] [Google Scholar]
- 21. Therasse P, Arbuck SG, Eisenhauer EA et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 22. Green SJ, Dahlberg S. Planned versus attained design in phase II clinical trials. Stat Med 1992; 11: 853–62. [DOI] [PubMed] [Google Scholar]
- 23. Bajorin DF, McCaffrey JA, Dodd PM et al. Ifosfamide, paclitaxel, and cisplatin for patients with advanced transitional cell carcinoma of the urothelial tract: final report of a phase II trial evaluating two dosing schedules. Cancer 2000; 88: 1671–8. [PubMed] [Google Scholar]
- 24. Cao W, Xu C, Lou G et al. A phase II study of paclitaxel and nedaplatin as first‐line chemotherapy in patients with advanced esophageal cancer. Jpn J Clin Oncol 2009; 39: 582–7. [DOI] [PubMed] [Google Scholar]
- 25. Dodd PM, McCaffrey JA, Herr H et al. Outcome of postchemotherapy surgery after treatment with methotrexate, vinblastine, doxorubicin, and cisplatin in patients with unresectable or metastatic transitional cell carcinoma. J Clin Oncol 1999; 17: 2546–52. [DOI] [PubMed] [Google Scholar]
- 26. Abe T, Shinohara N, Harabayashi T et al. Impact of multimodal treatment on survival in patients with metastatic urothelial cancer. Eur Urol 2007; 52: 1106–13. [DOI] [PubMed] [Google Scholar]
- 27. Otto T, Krege S, Suhr J, Rubben H. Impact of surgical resection of bladder cancer metastases refractory to systemic therapy on performance score: a phase II trial. Urology 2001; 57: 55–9. [DOI] [PubMed] [Google Scholar]


