Abstract
Although tissue inhibitor of metalloproteinase‐2 (TIMP‐2) is known to be not only an inhibitor of matrix metalloproteinases (MMP) but also a cofactor for membrane‐type 1 MMP (MT1‐MMP)‐mediated MMP‐2 activation, it is still unclear how TIMP‐2 regulates MMP‐2 activation and cleavage of substrates by MT1‐MMP. In the present study we examined the levels of cell‐surface MT1‐MMP, MMP‐2 activation and cleavage of MT1‐MMP substrates in 293T cells transfected with the MT1‐MMP and TIMP‐2 genes. Co‐expression of TIMP‐2 at an appropriate level increased the level of cell‐surface MT1‐MMP, both the TIMP‐2‐bound and free forms, and generated processed MMP‐2 with gelatin‐degrading activity. In contrast, MT1‐MMP substrates testican‐1 and syndecan‐1 were cleaved by the cells expressing MT1‐MMP, which was inhibited by TIMP‐2 even at levels that stimulate MMP‐2 activation. These results suggest that TIMP‐2 environment determines MT1‐MMP substrate choice between direct cleavage of its own substrates and MMP‐2 activation. (Cancer Sci 2007; 98: 563–568)
Abbreviations:
- DMEM
Dulbecco's Modified Eagle Medium
- ECM
extracellular matrix
- MMP
matrix metalloproteinase
- MT
membrane type
- PAGE
polyacrylamide gel electrophoresis
- PBS
phosphate‐buffered saline
- SDS
sodium dodecylsulfate
- TIMP
tissue inhibitor of metalloproteinase
MMP are a family of Zn2+‐dependent enzymes that are known to cleave extracellular matrix proteins under normal and pathological conditions.( 1 , 2 , 3 ) Presently, at least 28 mammalian MMP have been identified by cDNA cloning, and they can be subgrouped into soluble MMP and MT‐MMP.( 3 , 4 ) Although important in the degradation of the extracellular matrix, growing evidence has implicated MMP in specific processing resulting in activation or degradation of cell surface receptors and ligands.( 5 ) Cancer cells make use of MMP for invasion and metastasis. The invading cells are forced to proliferate within embedded dense three‐dimensional matrix composed largely of type I collagen or cross‐linked fibrin. MT1‐MMP has been thought to play a major role in this step.( 5 )
The MMP family is balanced by a family of TIMP. TIMP‐2 preferentially complexes with pro‐MMP‐2,( 6 ) and plays a pivotal role in the MT1‐MMP‐mediated activation process.( 7 , 8 , 9 , 10 ) The active form of MT1‐MMP first binds TIMP‐2 through its catalytic domain, thus docking soluble TIMP‐2 to the cell surface. This TIMP‐2–MT1‐MMP complex subsequently functions as a receptor, allowing pro‐MMP‐2 to form a ternary complex through the interaction of its carboxyl‐terminal domain and the carboxyl‐terminal domain of TIMP‐2. The propeptide of pro‐MMP‐2 is cleaved between Asn37 and Leu38 by an adjacent TIMP‐2‐free MT1‐MMP, generating an activated intermediate form that is further processed to the fully activated form by an intermolecular autocleavage when present at a sufficiently high concentration on the cell surface. When TIMP‐2 exists in excess relative to MT1‐MMP, the first step of this activation process is blocked.( 7 , 8 , 11 , 12 , 13 )
MMP‐2 is involved in various pathological events, including tumor invasion and metastasis, by degrading type IV and V collagens, laminin, fibronectin, elastin and so on.( 1 , 2 ) An active form of MMP‐2 has been frequently detected in tumor tissues, in which MT1‐MMP is always expressed.( 5 ) Thus, MT1‐MMP is thought to contribute to tumor invasion and metastasis by activating MMP‐2. In addition to MMP‐2 activation, MT1‐MMP directly digests various ECM components and cell surface molecules, which also have physiologically and pathologically essential functions.( 5 ) TIMP‐2 is essential for facilitating MT1‐MMP processing of MMP‐2 and we closely compared this here with the roles of TIMP‐2 in cleavage of other substrates by MT1‐MMP.
Materials and methods
Cell culture. Human embryonic kidney 293T cells were obtained from American Type Culture Collection (Rockville, MD, US) and cultured in DMEM (Sigma, St Louis, MO, USA) supplemented with 5% fetal calf serum.
Plasmid. Expression plasmids for MT1‐MMP, FLAG‐tagged MT1‐MMP (MT1‐MMP‐FLAG), TIMP‐2, FLAG‐tagged testican‐1 (testican‐1‐FLAG) and syndecan‐1 were constructed in pEAK8 vector (EdgeBio Systems, Gaithersburg, MD, USA) as described previously.( 14 , 15 , 16 )
Cell surface biotinylation. Cell surface labeling with biotin and immunoprecipitation were carried out as described previously.( 17 ) Briefly, expression plasmid for MT1‐MMP‐FLAG (700 ng) was cotransfected with increasing amounts of TIMP‐2 plasmid into 293T cells cultured in a 35 mm‐diameter dish coated with poly l‐lysine. At 48 h after transfection, cells were incubated with PBS containing 0.5 µg/mL Biotin Sulfo‐OSu (Wako Pure Chemical Industries, Osaka, Japan) for 30 min at 4°C. The cells were then lysed and were subjected to immunoprecipitation with anti‐FLAG M2 antibody‐conjugated agarose beads. The immunoprecipitated material was separated by 12% SDS‐PAGE, and then blotted with IRDye 800‐conjugated streptavidin (Rockland, Gilbertsville, PA, USA) and anti‐FLAG M2 antibody (Sigma) conjugated with Alexa Fluor 680 (Molecular Probes, Eugene, OR, USA). IRDye 800 and Alexa Fluor 680 were detected using a LI‐COR Odyssey IR imaging system (Lincoln, NE, USA).
Western blotting. Anti‐MT1‐MMP monoclonal antibody (113‐5B7) and anti‐TIMP‐2 monoclonal antibody (11‐1403) were gifts from Daiichi Fine Chemical Co. (Takaoka, Japan). MT1‐MMP and TIMP‐2 in cell lysates were detected by western blotting using anti‐MT1‐MMP antibody and anti‐TIMP‐2 antibody, respectively.
TIMP‐2 binding assay. Recombinant TIMP‐2 (a gift from Daiichi Fine Chemical Co.) was labeled with Alexa Fluor 680. Expression plasmid for MT1‐MMP (100 ng) was cotransfected with increasing amounts of TIMP‐2 plasmid into 293T cells cultured in 48‐well microplates. At 48 h after transfection, cells were incubated with labeled TIMP‐2 (10−8 M) for 1 h, and then washed three times with PBS. Binding of labeled TIMP‐2 was monitored using an Odyssey IR imaging system.
Zymography and gelatin degradation assay. Pro‐MMP‐2 supernatant was prepared from MMP‐2‐transfected 293T cells as described previously.( 13 ) 293T cells cultured in 48‐well microplates were cotrasnfected with MT1‐MMP and TIMP‐2 plasmids in duplicate, cultured for 48 h, and were incubated with 100 µL pro‐MMP‐2 for 1 h. Supernatants and cells dissolved in 100 µL SDS‐PAGE sample buffer were analyzed by gelatin zymography using Alex Fluor 680‐labeled gelatin.( 17 ) Supernatants (10 µL) were examined for gelatin‐degrading activity by incubating with an equal volume of Alex Fluor 680‐labeled gelatin (2 µg/mL) for 1 h. Cells were incubated with 100 µL labeled gelatin (1 µg/mL) in DMEM for 1 h. Labeled gelatin was separated on 10% SDS‐PAGE and monitored using an Odyssey IR imaging system.
Testican‐1 cleavage. Testicans, including testican‐1, interfere with MT1‐MMP‐mediated MMP‐2 activation when coexpressed with MT1‐MMP,( 15 ) and testican‐1 is cleaved by cells expressing MT1‐MMP (Sato, H., unpublished data). Expression plasmid for testican‐1‐FLAG (2 µg) was transfected into 293T cells in 35 mm diameter dishes and culture medium replaced with 2 mL serum‐free DMEM after 48 h. Conditioned medium was harvested after 24 h, diluted 10‐fold with fresh serum‐free DMEM, and used as testican‐1‐FLAG sample. 293T cells cultured in 35 mm‐diameter dishes were cotransfected with MT1‐MMP (700 ng) and increasing amounts of TIMP‐2 plasmid, and cultured for 48 h. Testican‐1‐FLAG (1 mL) was incubated with transfected cells for 3 h, concentrated with trichloroacetic acid, and analyzed by western blotting using anti‐FLAG M2 antibody.
Syndecan‐1 shedding. Expression plasmid for syndecan‐1 (500 ng) was cotransfected with MT1‐MMP (700 ng) and increasing amounts of TIMP‐2 plasmid into 293T cells cultured in 35‐mm dishes. Culture medium was replaced 48 h after transfection with 1 mL serum‐free DMEM, and cells were incubated for a further 24 h. Supernatants were examined by slot blot analysis using antisyndecan‐1 antibody as described previously.( 14 )
Results
Cell‐surface MT1‐MMP. To analyze the level of cell‐surface MT1‐MMP and TIMP‐2 that bound to MT1‐MMP, cell‐surface labeling with biotin and immunoprecipitation were carried out. The cell‐surface level of transfected MT1‐MMP was greatly increased by coexpression of TIMP‐2 (Fig. 1). TIMP‐2 was coprecipitated with MT1‐MMP in a dose‐dependent manner, but the ratio of coprecipitated TIMP‐2 to MT1‐MMP was not constant. Surface MT1‐MMP of cells expressing a low level of TIMP‐2 showed a lower ratio of coprecipitated TIMP‐2 than that of cells expressing higher levels of TIMP‐2. These results suggest that in cells expressing a low level of TIMP‐2, the TIMP‐2 stabilizes both TIMP‐2‐bound and TIMP‐2‐free MT1‐MMP.
Figure 1.
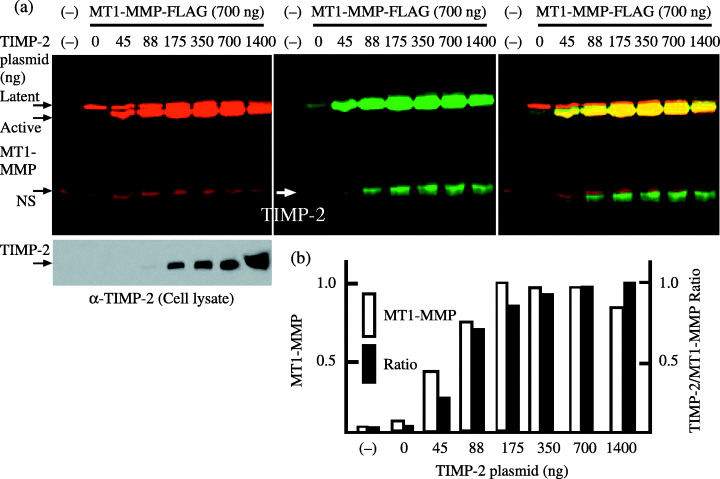
Cell‐surface membrane‐type (MT) 1‐matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase‐2. (a) The expression plasmid for MT1‐MMP‐FLAG (700 ng) was cotransfected with the indicated amounts of tissue inhibitor of metalloproteinase‐2 plasmid into 293T cells. At 48 h after transfection, cells were labeled with biotin, and were analyzed as described in ‘Materials and Methods’. Cell‐surface MT1‐MMP and tissue inhibitor of metalloproteinase‐2 (TIMP‐2) coprecipitated with MT1‐MMP were detected with IRDye 800‐conjugated streptavidin (middle panel), whereas MT1‐MMP latent and active forms were both detected with anti‐FLAG M2 antibody labeled with Alexa Fluor 680 (left upper panel). Aliquots of cell lysates were examined for the expression of TIMP‐2 by western blotting using anti‐TIMP‐2 antibody (left lower panel). Merged 800 and 680 signals are shown in the right panel. NS, non‐specific band. (b) Cell‐surface MT1‐MMP levels and the ratio of TIMP‐2 to MT1‐MMP signal measured from the middle panel are shown. The highest MT1‐MMP level (175 ng) and TIMP‐2/MT1‐MMP ratio (1400 ng) were arbitrarily set to 1, and the levels of other transfections were adjusted accordingly. The data represent one of three different experiments showing similar results.
To confirm this, a TIMP‐2‐binding experiment was carried out using fluorescence‐labeled TIMP‐2 (Fig. 2a). Because MT1‐MMP is protected from autodegradation by the MMP inhibitor BB94 in its TIMP‐2‐free form (Fig. 2b), binding of labeled TIMP‐2 was highest in cells transfected with MT1‐MMP plasmid alone in the presence of BB94. Binding of labeled TIMP‐2 was decreased by cotransfection of TIMP‐2 plasmid in proportion to the transfected TIMP‐2 levels in the presence of BB94. This suggests that TIMP‐2 coexpressed with MT1‐MMP in the presence of BB94 may bind to MT1‐MMP. Cell‐surface biotinylation and immunoprecipitation experiments confirmed the binding of TIMP‐2 with MT1‐MMP in cotransfected cells in the presence of BB94 (Fig. 2c). In the absence of BB94, binding of labeled TIMP‐2 to the cells transfected with MT1‐MMP plasmid alone was low. Binding of labeled TIMP‐2 was enhanced by cotransfection of a small amount of TIMP‐2 plasmid relative to MT1‐MMP plasmid, but this was attenuated by the cotransfection of higher amounts of TIMP‐2 plasmid. This confirms that MT1‐MMP on the surface of cells expressing a low level of TIMP‐2 is partly free from TIMP‐2.
Figure 2.
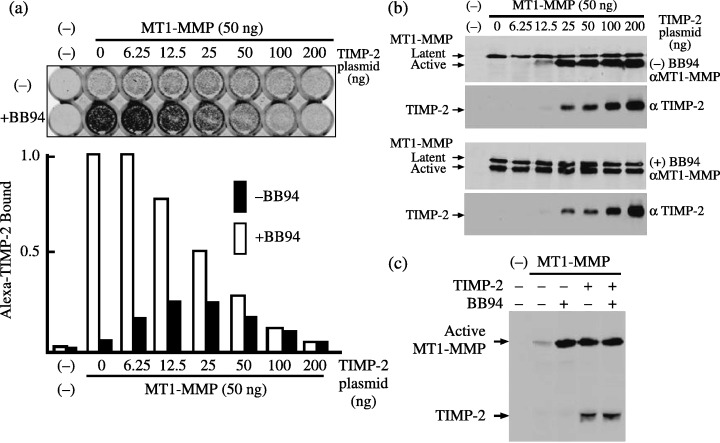
Binding of tissue inhibitor of metalloproteinase‐2 (TIMP‐2) to the cells expressing membrane‐type (MT) 1‐matrix metalloproteinase (MMP). (a) Control plasmid or expression plasmid for MT1‐MMP (50 ng) was cotransfected with the indicated amounts of TIMP‐2 plasmid into 293T cells cultured in 48‐well plates. Cells were cultured in the presence or absence of 1 µM BB94 for 48 h, and then the TIMP‐2 binding experiment was carried out as described in ‘Materials and Methods’. Binding of Alexa Fluor 680‐labeled TIMP‐2 was monitored using an Odyssey IR imaging system (upper panel) and relative fluorescence intensity is shown (lower panel). The fluorescence intensity obtained from the cells trasnfected with MT1‐MMP plasmid alone (lane 0) in the presence of BB94 was arbitrarily set to 1, and the levels of other transfections were adjusted accordingly. The data represent one of four different experiments showing similar results. (b) Cell lysates from the above transfected cells were examined for the expression of MT1‐MMP and TIMP‐2 by western blotting using anti‐MT1‐MMP and ‐TIMP‐2 antibodies, respectively. (c) The expression plasmid for MT1‐MMP‐FLAG (700 ng) was cotranfected with TIMP‐2 plasmid (1400 ng) in the presence or absence of BB94, and cell‐surface biotinylated TIMP‐2 coprecipitated with MT1‐MMP was detected as described in Fig. 1. Note that TIMP‐2 was coprecipitated with MT1‐MMP from both cells treated and untreated with BB94.
Effect of TIMP‐2 on pro‐MMP‐2 activation. The effect of TIMP‐2 expression on MT1‐MMP‐mediated pro‐MMP‐2 activation and the gelatin‐degrading activity of activated MMP‐2 was examined. Incubation of pro‐MMP‐2 with the cells transfected with MT1‐MMP plasmid alone resulted in a faint conversion from latent to active MMP‐2, which was enhanced by the cotransfection of TIMP‐2 plasmid dependent on the plasmid concentration (Fig. 3a). Gelatin was digested most intensively by MMP‐2 activated by the cells cotransfected with MT1‐MMP and 25 ng TIMP‐2 plasmids, where the level of active MMP‐2 was lower than in cells cotransfected with larger amounts of TIMP‐2 plasmid. Conversion to active MMP‐2 was most effective in cells cotransfected with MT1‐MMP and 50 ng or more TIMP‐2 plasmids; however, MMP‐2 activated by those cells showed lower gelatin‐degrading activity. Degradation of gelatin by the cells or the supernatant was suppressed by either TIMP‐1 or TIMP‐2, indicating that gelatin degradation is mediated by MMP‐2 and not MT1‐MMP (Fig. 3b). These results suggest that only MT1‐MMP, a part of which is bound by TIMP‐2, processed pro‐MMP‐2 to generate TIMP‐2‐free active MMP‐2. At higher TIMP‐2 concentrations, pro‐MMP‐2 was still efficiently converted to active form by MT1‐MMP; however, this MMP‐2 was inactive, probably due to binding of TIMP‐2. In order to confirm that gelatin degradation is not mediated by MT1‐MMP but by MMP‐2, cells transfected with MT1‐MMP alone or cotransfected with MT1‐MMP and TIMP‐2 were incubated directly with gelatin (Fig. 3c). These cells did significantly degrade gelatin.
Figure 3.
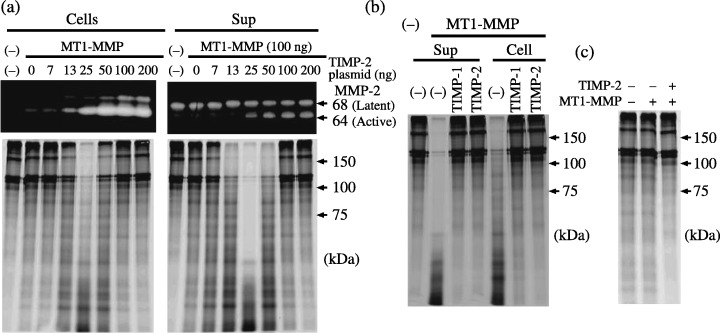
Effect of tissue inhibitor of metalloproteinase‐2 (TIMP‐2) on matrix metalloproteinase (MMP)‐2 activation by membrane‐type (MT) 1‐MMP. (a) Control plasmid or expression plasmid for MT1‐MMP (100 ng) was cotransfected with the indicated amounts of TIMP‐2 plasmid into 293T cells cultured in 48‐well plates in duplicate. At 48 h after transfection, cells were incubated with MMP‐2 for 1 h. Then, supernatants and cells were analyzed by gelatin zymography (upper panel) and for gelatin‐degradation activity (lower panel) as described in ‘Materials and Methods’. (b) 293T cells transfected with control plasmid or expression plasmids for MT1‐MMP (100 ng) and TIMP‐2 (25 ng) were incubated with MMP‐2, and gelatin degradation was examined as above in the presence or absence of 1 µg/mL TIMP‐1 or TIMP‐2. (c) 293T cells were transfected with control plasmid or expression plasmids for MT1‐MMP (100 ng) and TIMP‐2 (25 ng) as indicated, and were incubated with labeled gelatin for 1 h at 48 h after transfection.
Effect of TIMP‐2 on cleavage of MT1‐MMP substrates. The effect of TIMP‐2 expression on cleavage of the MT1‐MMP substrates testican‐1 and syndecan‐1 was examined. Testican‐1‐FLAG incubated with cells transfected with MT1‐MMP was cleaved efficiently by the cells transfected with MT1‐MMP without any TIMP‐2 (Fig. 4). Transfected TIMP‐2 did not show any effect at low concentrations but suppressed testican‐1 cleavage at higher concentrations. Syndecan‐1 was previously shown to be cleaved at the G82–L83 and G245–L246 peptide bonds by MT1‐MMP.( 14 ) Shedding of syndecan‐1 was stimulated by transfection of MT1‐MMP plasmid alone, and this was suppressed by cotransfection of TIMP‐2 in proportion to TIMP‐2 plasmid concentration (Fig. 5). Shedding of syndecan‐1 and cleavage of testican‐1 were inhibited by TIMP‐2 but not by TIMP‐1, confirming the involvement of MT1‐MMP.
Figure 4.
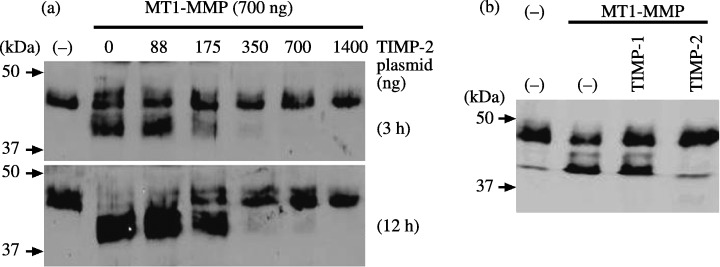
Effect of tissue inhibitor of metalloproteinase‐2 (TIMP‐2) on testican‐1 cleavage by membrane‐type (MT) 1‐matrix metalloproteinase (MMP). Testican‐1‐FLAG sample was incubated with 293T cells mock‐transfected or cotransfected with MT1‐MMP (700 ng) and indicated amounts of TIMP‐2 plasmid for 3 h. Cleavage of testican‐1‐FLAG was analyzed by western blotting using anti‐FLAG M2 antibody (left panel). Testican‐1‐FLAG sample was incubated with cells mock‐transfected or transfected with MT1‐MMP plasmid in the presence or absence of TIMP‐1 or TIMP‐2 (1 µg/mL) for 3 h, and was analyzed as above (right panel).
Figure 5.
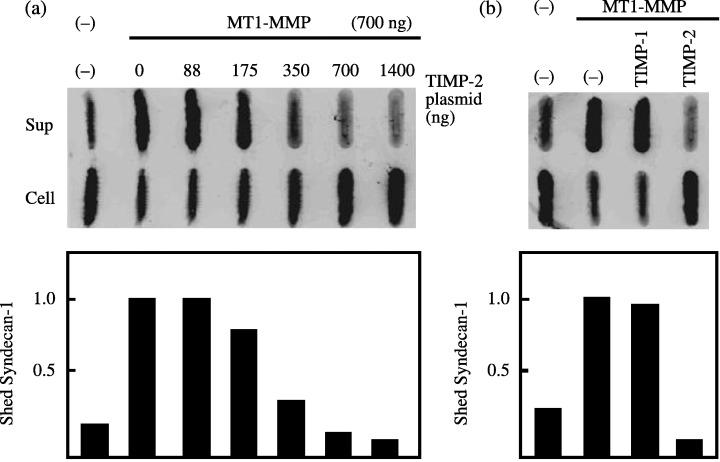
Effect of tissue inhibitor of metalloproteinase‐2 (TIMP‐2) on syndecan‐1 shedding by membrane‐type (MT) 1‐matrix metalloproteinase (MMP). (a) Expression plasmid for syndecan‐1 was cotransfected with control plasmid (lane –) or MT1‐MMP plasmid and indicated amount of TIMP‐2 plasmid into 293T cells, and the culture medium was replaced with serum‐free Dulbecco's Modified Eagle Medium (DMEM) at 48 h after transfection. Medium was harvested after 12 h, and was analyzed for shed syndecan‐1 by dot blot analysis using anti‐syndecan‐1 antibody. (b) Cells cotransfected with syndecan‐1 and MT1‐MMP plasmids were cultured in serum‐free DMEM in the presence or absence of TIMP‐1 or TIMP‐2 (1 µg/mL), and shed syndecan‐1 was analyzed as above. The data represent one of three different experiments showing similar results. The highest shed syndecan‐1 level for each experiment was arbitrarily set to 1, and the levels of other transfections were adjusted accordingly.
Discussion
The evaluation of TIMP‐2 level in human tumor tissues is still controversial, and additional research is needed to elucidate further the roles of TIMP‐2 in tumor tissues. MT1‐MMP turnover occurs through autodegradation and endocytosis depending on cell type.( 18 , 19 , 20 , 21 , 22 ) Because 293T cells synthesize low levels of TIMP‐2,( 23 ) turnover of cell‐surface MT1‐MMP is high due to autodegradation and cell‐surface levels are low (Fig. 1). Activation of pro‐MMP‐2 is initiated through a tri‐molecular complex composed of MT1‐MMP, TIMP‐2 and pro‐MMP‐2, and TIMP‐2‐free MT1‐MMP adjacent to this complex cleaves the amino‐terminus of pro‐MMP‐2 to generate the activation intermediate form.( 3 ) However, it is not clear how the TIMP‐2‐bound and TIMP‐2‐free forms of MT1‐MMP localize adjacently on the cell surface. We demonstrate here for the first time that low levels of TIMP‐2 increased not only the TIMP‐2‐bound form but also the free form of MT1‐MMP on the cell‐surface. MT1‐MMP forms dimers or oligomers through its hemopexin domain,( 24 ) and TIMP‐2‐free MT1‐MMP in the oligomer is thought to be subjected to autodegradation by adjacent TIMP‐2‐free MT1‐MMP. We conclude that TIMP‐2‐bound MT1‐MMP in the oligomer may interfere with mutual autodegradative interactions between TIMP‐2‐free MT1‐MMP molecules.
The model of MT1‐MMP substrate determination by TIMP‐2 is shown in Fig. 6. The optimal TIMP‐2 levels for pro‐MMP‐2 activation allowed partial TIMP‐2 binding of MT1‐MMP (Fig. 1), maximum binding of alexa–TIMP‐2 to MT1‐MMP in the absence of BB‐94 (Fig. 2), and maximal MMP‐2 gelatin‐degrading activity (Fig. 3). Lower levels did not protect MT1‐MMP sufficiently from degradation (Fig. 1), and higher levels allowed pro‐MMP‐2 processing, but presumably blocked the MMP‐2 activity (Fig. 3). It should be emphasized that TIMP‐2‐free MT1‐MMP shows the most active proteolytic activity, which in turn induces rapid turnover of itself due to autodegradation. Thus, the cell‐surface active MT1‐MMP level is very low in the absence of MT1‐MMP inhibitor, such as TIMP‐2 and BB94 (1, 2), but it still cleaves testican‐1 and syndecan‐1 most effectively (4, 5). MT1‐MMP that has escaped autodegradation through TIMP‐2 binding appears inactive against direct substrates for MT1‐MMP. Pro‐MMP‐2 activation could be achieved by MT1‐MMP at a wide range of TIMP‐2 levels, but gelatin‐degrading activity was only seen in a limited range (Fig. 3). This is consistent with the previous report using BS‐C‐1 cells infected with recombinant vaccinia viruses expressing MT1‐MMP and TIMP‐2.( 25 ) Tri‐molecular complex formation may be a dynamic process in which TIMP‐2 may transiently interact with MT1‐MMP, pro‐MMP‐2 and activated MMP‐2 dependent on its concentration. Interaction of pro‐MMP‐2 with MT1‐MMP bound to TIMP‐2 may cause partial replacement of TIMP‐2 to generate TIMP‐2‐free MT1‐MMP, which in turn processes pro‐MMP‐2. Activated MMP‐2 is then inactivated by binding to TIMP‐2.
Figure 6.
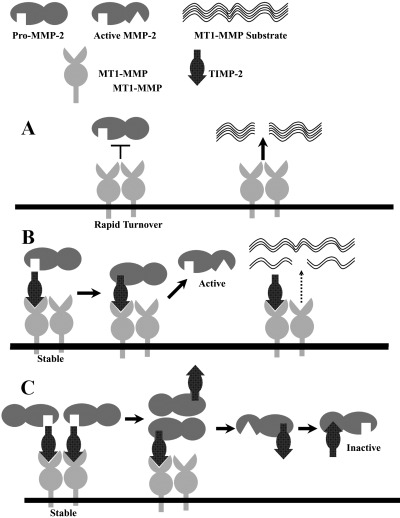
Model of membrane‐type (MT) 1‐matrix metalloproteinase (MMP) substrate determination by tissue inhibitor of metalloproteinase‐2 (TIMP‐2). (a) At low or no TIMP‐2, TIMP‐2‐free MT1‐MMP digests its own substrates such as testican‐1, syndecan‐1 and presumably collagens, but it does not activate pro‐MMP‐2. TIMP‐2‐free MT1‐MMP shows the most active proteolytic activity, which in turn causes rapid turnover due to intensive autodegradation. (b) At appropriate levels of TIMP‐2, binding of TIMP‐2 protects MT1‐MMP (TIMP‐2‐bound and ‐free) from autodegradation, and the complex serves as a receptor for pro‐MMP‐2, which is then cleaved by adjacent TIMP‐2‐free MT1‐MMP to generate active MMP‐2. Because a part of MT1‐MMP binds to TIMP‐2, it digests its own substrates less effectively. (c) When MT1‐MMP is saturated with TIMP‐2, pro‐MMP‐2 may transiently replace TIMP‐2 to generate TIMP‐2‐free MT1‐MMP, which then cleaves pro‐MMP‐2, but activated MMP‐2 is finally blocked by TIMP‐2.
Two populations of TIMP‐2 binding sites were reported on the membrane of concanavalin A‐treated fibroblasts, one sensitive and the other insensitive to synthetic hydroxamate inhibitors.( 26 ) In our transfected 293T cell experimental system, TIMP‐2 binding was totally dependent on the level of TIMP‐2‐free MT1‐MMP on the cell surface (Fig. 2). Pretreatment of MT1‐MMP‐expressing cells with BB94 protected TIMP‐2‐free MT1‐MMP from autodegradation and labeled TIMP‐2 could bind to MT1‐MMP by replacing BB94 (Fig. 2b). BB94 did not inhibit complex formation between MT1‐MMP and TIMP‐2 in cotransfected cells (Fig. 2c), which accounts for the reduced binding of labeled TIMP‐2 by cotransfection of TIMP‐2 plasmid in proportion to the expressed TIMP‐2 levels in the presence of BB94. The idea that TIMP‐2 replaces BB94 which binds to MT1‐MMP is consistent with the previous report of synthetic MMP inhibitors stimulating TIMP‐2‐dependent MMP‐2 activation by MT1‐MMP.( 19 ) It should be noticed that excess BB94 in the binding reaction inhibited binding of labeled TIMP‐2 (data not shown); however, it can not release TIMP‐2 that is already bound to MT1‐MMP. In contrast, TIMP‐2 can replace BB94 bound to cell‐surface MT1‐MMP unless BB94 exists in excess in culture.
Thus, TIMP‐2 concentration dictates MT1‐MMP substrate choice. In the absence of TIMP‐2 and at low TIMP‐2 concentrations, MT1‐MMP cleaves testican‐1 and syndecan‐1 directly. A variety of substrates for MT1‐MMP were reported other than ECM components, such as adherence molecules, cytokines and growth factors, and cell surface receptors.( 5 ) MT1‐MMP plays essential roles in not only tumor invasion and metastasis but also tumor cell growth in a three‐dimensional matrix composed largely of type I collagen or cross‐linked fibrin.( 27 ) These multifunctions of MT1‐MMP appear to be involved in the regulation of various events taking place at the cell–ECM interface. In higher concentrations of TIMP‐2, MT1‐MMP is involved in ECM degradation through the activation of MMP‐2, possibly as a resolving wave to remove degradation byproducts (denatured collagens) after direct ECM degradation. It remains to be explored which function of MT1‐MMP is most associated with malignancy of tumors.
Acknowledgment
This work was supported in part by a Grant in Aid for Scientific Research on Priority Areas (18013023) from the Ministry of Education, Culture, Sports, Sciences and Technology.
References
- 1. Woessner JFJ. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 2004; 5: 2145–54. [PubMed] [Google Scholar]
- 2. Birkedal HH, Moore WG, Bodden MK et al. Matrix metalloproteinases. a review. Crit Rev Oral Biol Med 1993; 4: 197–250. [DOI] [PubMed] [Google Scholar]
- 3. Seiki M. Membrane‐type matrix metalloproteinases. APMIS 1999; 107: 137–43. [DOI] [PubMed] [Google Scholar]
- 4. Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem 1999; 274: 21 491–4. [DOI] [PubMed] [Google Scholar]
- 5. Sato H, Takino T, Miyamori H. Roles of membrane‐type matrix metalloproteinase‐1 in tumor invasion and metastasis. Cancer Sci 2005; 96: 212–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howard EW, Banda MJ. Binding of tissue inhibitor of metalloproteinases 2 to two distinct sites on human 72‐kDa gelatinase: Identification of a stabilization site. J Biol Chem 1991; 266: 17 972–7. [PubMed] [Google Scholar]
- 7. Strongin AY, Collier I, Bannikov G et al. Mechanism of cell surface activation of 72‐kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem 1995; 270: 5331–8. [DOI] [PubMed] [Google Scholar]
- 8. Kinoshita T, Sato H, Okada A et al. TIMP‐2 promotes activation of progelatinase A by membrane‐type 1 matrix metalloproteinase immobilized on agarose beads. J Biol Chem 1998; 273: 16 098–103. [DOI] [PubMed] [Google Scholar]
- 9. Butler GS, Butler MJ, Atkinson SJ et al. The TIMP2 membrane type 1 metalloproteinase ‘receptor’ regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem 1998; 273: 871–80. [DOI] [PubMed] [Google Scholar]
- 10. Zucker S, Drews M, Conner C et al. Tissue inhibitor of metalloproteinase‐2 (TIMP‐2) binds to the catalytic domain of the cell surface receptor, membrane type 1‐matrix metalloproteinase 1 (MT1‐MMP). J Biol Chem 1998; 273: 1216–22. [DOI] [PubMed] [Google Scholar]
- 11. Cao J, Sato H, Takino T, Seiki M. The C‐terminal region of membrane type matrix metalloproteinase is a functional transmembrane domain required for pro‐gelatinase A activation. J Biol Chem 1995; 270: 801–5. [DOI] [PubMed] [Google Scholar]
- 12. Atkinson SJ, Crabbe T, Cowell S et al. Intermolecular autolytic cleavage can contribute to the activation of progelatinase A by cell membranes. J Biol Chem 1995; 270: 30 479–85. [DOI] [PubMed] [Google Scholar]
- 13. Sato H, Kinoshita T, Takino T et al. Activation of a recombinant membrane type 1‐matrix metalloproteinase (MT1‐MMP) by furin and its interaction with tissue inhibitor of metalloproteinases (TIMP)‐2. FEBS Lett 1996; 393: 101–4. [DOI] [PubMed] [Google Scholar]
- 14. Endo K, Takino T, Miyamori H et al. Cleavage of syndecan‐1 by membrane type matrix metalloproteinase‐1 stimulates cell migration. J Biol Chem 2003; 278: 40 764–70. [DOI] [PubMed] [Google Scholar]
- 15. Nakada M, Yamada A, Takino T et al. Suppression of membrane‐type 1 matrix metalloproteinase (MMP)‐mediated MMP‐2 activation and tumor invasion by testican 3 and its splicing variant gene product, N‐Tes. Cancer Res 2001; 61: 8896–902. [PubMed] [Google Scholar]
- 16. Li Y, Aoki T, Mori Y et al. Cleavage of lumican by membrane‐type matrix metalloproteinase‐1 abrogates this proteoglycan‐mediated suppression of tumor cell colony formation in soft agar. Cancer Res 2004; 64: 7058–64. [DOI] [PubMed] [Google Scholar]
- 17. Ahmad M, Takino T, Miyamori H et al. Cleavage of amyloid‐β precursor protein (APP) by membrane‐type matrix metalloproteinases. J Biochem 2006; 139: 517–26. [DOI] [PubMed] [Google Scholar]
- 18. Hernandez‐Barrantes S, Toth M, Bernardo MM et al. Binding of active (57 kDa) membrane type 1‐matrix metalloproteinase (MT1‐MMP) to tissue inhibitor of metalloproteinase (TIMP)‐2 regulates MT1‐MMP processing and pro‐MMP‐2 activation. J Biol Chem 2000; 275: 12 080–9. [DOI] [PubMed] [Google Scholar]
- 19. Toth M, Bernardo MM, Gervasi DC et al. Tissue inhibitor of metalloproteinase (TIMP)‐2 acts synergistically with synthetic matrix metalloproteinase (MMP) inhibitors but not with TIMP‐4 to enhance the (membrane type 1)‐MMP‐dependent activation of pro‐MMP‐2. J Biol Chem 2000; 275: 41 415–23. [DOI] [PubMed] [Google Scholar]
- 20. Galvez BG, Matias‐Roman S, Yanez‐Mo M et al. Caveolae are a novel pathway for membrane‐type 1 matrix metalloproteinase traffic in human endothelial cells. Mol Biol Cell 2004; 15: 678–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uekita T, Itoh Y, Yana I et al. Cytoplasmic tail‐dependent internalization of membrane‐type 1 matrix metalloproteinase is important for its invasion‐promoting activity. J Cell Biol 2001; 155: 1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Remacle A, Murphy G, Roghi C. Membrane type I‐matrix metalloproteinase (MT1‐MMP) is internalised by two different pathways and is recycled to the cell surface. J Cell Sci 2003; 116: 3905–16. [DOI] [PubMed] [Google Scholar]
- 23. Miyamori H, Takino T, Kobayashi Y et al. Claudin promotes activation of pro‐matrix metalloproteinase‐2 mediated by membrane‐type matrix metalloproteinases. J Biol Chem 2001; 276: 28 204–11. [DOI] [PubMed] [Google Scholar]
- 24. Itoh Y, Takamura A, Ito N et al. Homophilic complex formation of MT1‐MMP facilitates proMMP‐2 activation on the cell surface and promotes tumor cell invasion. EMBO J 2001; 20: 4782–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bernardo MM, Fridman R. TIMP‐2 (tissue inhibitor of metalloproteinase‐2) regulates MMP‐2 (matrix metalloproteinase‐2) activity in the extracellular environment after pro‐MMP‐2 activation by MT1 (membrane type 1)‐MMP. Biochem J 2003; 374: 739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Itoh Y, Ito A, Iwata K et al. Plasma membrane‐bound tissue inhibitor of metalloproteinases (TIMP)‐2 specifically inhibits matrix metalloproteinase 2 (gelatinase A) activated on the cell surface. J Biol Chem 1998; 273: 24 360–7. [DOI] [PubMed] [Google Scholar]
- 27. Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three‐dimensional extracellular matrix. Cell 2003; 114: 33–45. [DOI] [PubMed] [Google Scholar]


