Abstract
This study was conducted to determine the impact of a functional tandem repeat minisatellite (MNS16A) polymorphism in the telomerase reverse transcriptase (TERT) gene on the risk of lung cancer, as well as on survival of patients with non‐small‐cell lung cancer (NSCLC). The effect of the MNS16A variable number of tandem repeat (VNTR) polymorphism on the risk of lung cancer was evaluated in a case–control study that consisted of 937 lung cancer patients and 943 healthy controls. The effect of the polymorphism on survival outcome was evaluated in 703 patients with surgically resected NSCLC. Compared with the VNTR‐302 allele, the VNTR‐243 allele was associated with a significantly increased risk of lung cancer (adjusted odds ratio, 1.55; 95% confidence interval [CI], 1.07–2.25; P = 0.02). In addition, the genotypes carrying at least one VNTR‐243 allele were associated with a significantly increased risk of lung cancer compared with the genotypes with no VNTR‐243 allele (adjusted odds ratio, 1.61; 95% CI, 1.09–2.38; P = 0.02). In contrast to the effect of the polymorphism on the risk of lung cancer, the genotypes carrying at least one VNTR‐243 allele were associated with a significantly better overall survival in patients with surgically resected NSCLC (adjusted hazard ratio, 0.51; 95% CI, 0.28–0.93; P = 0.03). These findings suggest that the MNS16A VNTR polymorphism in the TERT gene has dual, conflicting roles in lung carcinogenesis. This polymorphism may increase the risk of lung cancer development, and may improve survival in lung cancer patients. (Cancer Sci 2011; 102: 144–149)
Telomeres are specialized nucleoprotein complexes composed of long arrays of double‐stranded TTAGGG repeats, a G‐rich 3′ single strand overhanging, and associated telomere‐binding proteins.( 1 , 2 ) Telomeres function to cap the ends of chromosomes and protect chromosomes from degradation, end‐to‐end fusion and atypical recombination; thus, telomeres are essential for maintaining the integrity and stability of chromosomes.( 3 , 4 ) In addition to the end protective function, the progressive shortening of telomeres serves as a “mitotic clock”, which limits the lifespan of somatic cells.( 3 , 5 ) Telomeres are maintained by telomerase, a ribonucleoprotein complex that consists of the catalytic component telomerase reverse transcriptase (TERT) and a telomerase RNA template. TERT synthesizes TTAGGG repeats from the RNA template and is the key determinant of telomerase activity.( 6 , 7 , 8 , 9 )
It has been proposed that telomere shortening and telomerase play dual roles in carcinogenesis. During the early phase of cancer development, telomere shortening can induce chromosomal instability, which is perpetuated through fusion–bridge–breakage cycles that increases the risk of cancer development. In this phase, telomerase, the main positive regulator of telomere length, helps to stabilize chromosomal instability and thus reduce cancer development.( 10 , 11 , 12 ) In support of this hypothesis, several studies have reported that individuals with constitutionally short telomeres have a higher risk of developing cancers.( 13 , 14 , 15 ) In addition, it has been reported that humans with dyskeratosis congenita, a hereditary disease with a partial telomerase deficiency, have an increased risk of developing malignant lesions.( 16 , 17 ) In contrast to the early phase of cancer development, after telomerase is re‐activated and the telomere attrition is stabilized, short telomeres inhibit tumor progression. In this later phase of cancer development, activation of telomerase can immortalize transformed cells and thereby promote cancer growth.( 10 , 11 , 12 ) Indeed, several studies have demonstrated that the levels of telomerase expression/activity in cancer tissues correlate with advanced stage and poor prognosis in human cancers.( 18 , 19 )
Several studies have documented considerable inter‐individual variation in telomere length and telomerase activity among healthy individuals of the same age.( 20 , 21 , 22 ) Like many other phenotypic traits, functional polymorphisms in the TERT gene contribute to variation in the telomerase activity in the general population. Recently, the polymorphic tandem repeats minisatellite, MNS16A, in the downstream region of the TERT gene locus was demonstrated to influence the expression of antisense TERT mRNA.( 23 ) Moreover, this MNS16A variable number of tandem repeat (VNTR) polymorphism has been reported to be associated with the risk of breast cancer.( 24 ) Therefore, to investigate the role of the MNS16A VNTR polymorphism on the risk of lung cancer, we conducted a case–control study in a Korean population. In addition, we examined the effect of the MNS16 VNTR polymorphism on the survival outcome of patients with lung cancer.
Materials and Methods
Study population. The case–control study consisted of 937 lung cancer patients and 943 healthy controls. The eligible cases included all patients newly diagnosed with primary lung cancer between January 2004 and June 2006 at Kyungpook National University Hospital (KNUH) in Daegu, Korea, who agreed to participate in the study (participation rate, 96.4%). This case–control study was approved by the Institutional Review Boards of the KNUH and written informed consent was obtained from each participant. After informed consent was obtained, each subject donated 20 mL of blood, which was used for DNA extraction and genotyping. There were no gender, histological or stage restrictions; however, patients ≥75 years of age or those who had a history of cancer were excluded from the present study. The cases included 420 (44.8%) squamous cell carcinomas, 367 (39.2%) adenocarcinomas, 134 (14.3%) small‐cell lung carcinomas (SCLC) and 16 (1.7%) large‐cell carcinomas. The control subjects were randomly selected from a pool of healthy volunteers who visited the general health check‐up center at the KNUH during the same period. The control subjects were frequency matched (1:1) to the cases based on gender and age (±5 years). A total of 2985 (1552 males and 1433 females) of 6972 healthy subjects agreed to participate in the present study (participation rate, 42.8%). Compared with subjects that refused to participate, the enrolled subjects showed similar age and gender distributions. From 2985 healthy volunteers, we randomly selected 943 control subjects by generating a random number using SAS version 8.12 (SAS Institute, Cary, NC, USA). All subjects enrolled in this study were ethnic Koreans who resided in Daegu City or the surrounding regions.
The effect of the MNS16A VNTR polymorphism on survival outcome was evaluated in 703 patients with pathological stages I, II or IIIA (micro‐invasive N2) non‐small‐cell lung cancer (NSCLC) who underwent curative surgical resection at KNUH between September 1998 and August 2006 (n = 391) and Seoul National University Hospital between January 2001 and December 2006 (n = 312). Patients who received chemotherapy or radiotherapy prior to surgery were excluded to avoid the effects on DNA. This study was approved by the Institutional Review Boards of the KNUH and Seoul National University Hospital; written informed consent was obtained from each participant. All tissues were obtained at the time of surgery and then rapidly frozen in liquid nitrogen and stored at −80°C until the genotyping was conducted. The histological type of lung cancers was as follows: 334 cases (47.5%) squamous cell carcinomas; 345 cases (49.1%) adenocarcinomas; and 24 cases (3.4%) large‐cell carcinomas. The pathological staging of the tumors, which was determined according to the International System for Staging Lung Cancer,( 25 ) was as follows: stage I, 465 patients (66.1%); stage II, 135 patients (19.2%); and stage IIIA, 103 patients (14.7%).
Genotyping. A polymerase chain reaction (PCR) was used to genotype the MNS16A VNTR polymorphism with the primer set, as previously reported.( 23 ) The forward primer sequence was 5′‐AGGATTCTGATCTCTGAAGGGTG‐3′ and the reverse primer sequence was 5′‐TCTGCCTGAGGAAGGACGTATG‐3′. The PCR profile consisted of an initial denaturation step of 95°C for 5 min, followed by 35 cycles of 95°C for 30 s, 60°C for 30 s, 72°C for 30 s and a final elongation step of 72°C for 10 min. The PCR products were visualized on 2% agarose gel and stained with ethidium bromide for visualization under UV light. To ensure quality control, the genotyping analysis was performed “blind” with respect to case/control status. Approximately 10% of the samples were randomly selected to be genotyped again by a different researcher and the results were 100% concordant.
Statistical analysis. Differences in the distribution of demographic characteristics and the variant alleles and genotypes of the MNS16A VNTR polymorphism between the cases and controls were compared using the Student’s t‐test for continuous variables and a chi square test for categorical variables. The associations between MNS16A VNTR genotypes and the risk of lung cancer were estimated by computing odds ratios (OR) and the 95% confidence intervals (CI) from logistic regression analysis with and without possible confounding factors (gender as a nominal variable; and age and pack–years of smoking as continuous variables). In addition to the overall association analysis, we performed a stratified analysis by age (median age, ≤62 years, and >62 years), gender and tumor histology to further explore the association between MNS16A VNTR genotypes and the risk of lung cancer in each stratum. For the gene–smoking interaction analyses, we used three approaches to evaluate the consistency of the results: (i) a logistic regression model including the interaction term between the genotype and smoking; (ii) stratified analyses by smoking status; and (iii) genotype–smoking joint effects. Overall survival (OS) was measured from the day of surgery until the date of death or to the date of the last follow up. The survival estimates were calculated using the Kaplan–Meier method. The differences in OS across different genotypes were compared using the log‐rank test. Hazard ratios (HR) and 95% CI were estimated using multivariate Cox proportional hazards models, with adjustment for age (median age, ≤64 vs >64 years), gender (female vs male), smoking status (never‐smokers vs ever‐smokers) and pathological stage (I vs II–IIIA). All analyses were performed using the Statistical Analysis System for Windows (version 9.1).
Results
Association of MNS16A VNTR genotypes with the risk of lung cancer. The demographics of the cases and controls enrolled in the case–control study are shown in Table 1. There were no significant differences in the mean age or gender distribution between the cases and controls. However, there were more current smokers among the cases than the controls (P < 0.001), and the number of pack–years in smokers was significantly higher in the cases than in the controls (40.5 ± 20.2 vs 30.9 ± 17.0 pack–years; P < 0.001). These differences were subsequently controlled for in the multivariate analyses.
Table 1.
Characteristics of the case–control study population
| Variable | Cases (n = 937)† | Controls (n = 943) | P |
|---|---|---|---|
| Age (years) | 61.7 ± 9.2 | 61.5 ± 9.1 | 0.67‡ |
| Gender | |||
| Male | 738 (78.8) | 739 (78.4) | 0.83§ |
| Female | 199 (21.2) | 204 (21.6) | |
| Smoking status | |||
| Current | 541 (57.7) | 340 (36.1) | <0.001§ |
| Former | 224 (23.9) | 301 (31.9) | |
| Never | 172 (18.4) | 302 (32.0) | |
| Pack–years¶ | 40.5 ± 20.2 | 30.9 ± 17.0 | <0.001‡ |
| Histological type | |||
| Squamous cell carcinoma | 420 (44.8) | ||
| Adeno carcinoma | 367 (39.2) | ||
| Small‐cell carcinoma | 134 (14.3) | ||
| Large‐cell carcinoma | 16 (1.7) | ||
†Numbers in parentheses indicate percentage. ‡t‐test. §χ2 test. ¶In current and former smokers.
In the present study, seven VNTR genotypes by four different sized VNTR alleles (VNTR‐302, VNTR‐272, VNTR‐243, and VNTR‐333) were identified. The distributions of the VNTR alleles and genotypes among the cases were significantly different from the controls (P = 0.02 and P = 0.05, respectively; Table 2). Compared with the VNTR‐302 allele, the VNTR‐243 allele was associated with a significantly increased risk of lung cancer (adjusted OR [aOR], 1.55; 95% CI, 1.07–2.25; P = 0.02). When the VNTR‐302/VNTR‐302 genotype, the most common genotype in both cases and controls (87.4% and 89.1%, respectively), was used as a reference group, the VNTR‐302/VNTR‐243 genotype was associated with a significantly increased risk of lung cancer (aOR, 1.54; 95% CI, 1.04–2.29; P = 0.03). We next classified the four VNTR alleles as long (L), middle (M) or short (S) alleles as follows: L, VNTR‐302 or ‐333; M, VNTR‐272; and S, VNTR‐243. The seven genotypes were then categorized into the following three groups: group I, L/L genotype; group II, L/M or M/M genotype; and group III, L/S, M/S or S/S genotype. As shown in Table 2, group III carrying at least one S allele was associated with a significantly increased risk of lung cancer compared with group I and the combined group I + II (aOR, 1.59; 95% CI, 1.08–2.34; P = 0.02; and aOR, 1.61, 95% CI, 1.09–2.38; P = 0.02, respectively).
Table 2.
Logistic regression analyses for associations between telomerase reverse transcriptase (TERT) MNS16A variable number of tandem repeat genotypes and the risk of lung cancer
| Allele/genotype | Cases, n (%) | Controls, n (%) | Adjusted† OR (95% CI) | P |
|---|---|---|---|---|
| Allele | ||||
| 302 alleles | 1749 (93.3) | 1781 (94.4) | 1.00 | |
| 272 alleles | 44 (2.3) | 57 (3.0) | 0.82 (0.54–1.26) | 0.37 |
| 243 alleles | 80 (4.3) | 48 (2.5) | 1.55 (1.07–2.25) | 0.02 |
| 333 alleles | 1 (0.1) | 0 (0.0) | – | – |
| P = 0.02 | ||||
| Genotype | ||||
| 302/302 | 819 (87.4) | 840 (89.1) | 1.00 | |
| 302/272 | 37 (4.0) | 55 (5.8) | 0.75 (0.48–1.18) | 0.21 |
| 272/272 | 3 (0.3) | 1 (0.1) | 2.15 (0.21–21.66) | 0.52 |
| 302/243 | 73 (7.8) | 46 (4.9) | 1.54 (1.04–2.29) | 0.03 |
| 272/243 | 1 (0.1) | 0 (0.0) | – | – |
| 243/243 | 3 (0.3) | 1 (0.1) | 1.70 (0.28–10.39) | 0.57 |
| 302/333 | 1 (0.1) | 0 (0.0) | – | – |
| P = 0.047 | ||||
| Group of genotype‡ | ||||
| L/L | 820 (87.5) | 840 (89.1) | 1.00 | |
| L/M + M/M | 40 (4.3) | 56 (5.9) | 0.75 (0.48–1.17) | 0.20 |
| L/S + M/S + S/S | 77 (8.2) | 47 (5.0) | 1.59 (1.08–2.34) | 0.02 |
| P = 0.005 | ||||
| L/L + L/M + M/M | 860 (91.8) | 896 (95.0) | 1.00 | |
| L/S + M/S + S/S | 77 (8.2) | 47 (5.0) | 1.61 (1.09–2.38) | 0.02 |
| P = 0.005 | ||||
CI, confidence interval; OR, odds ratio. †Adjusted for age, gender and pack–years of smoking. ‡L, VNTR‐302 or ‐333 allele; M, VNTR‐272 allele; and S, VNTR‐243 allele.
The effect of the S allele on the risk of lung cancer was further examined after stratifying the subjects according to age, gender, smoking status and tumor histology. The effect of the S allele on the risk of lung cancer was similar in younger and older individuals (Table 3). When stratified according to smoking status, the effect of the S allele was significant in ever‐smokers (aOR, 1.75; 95% CI, 1.13–2.72; P = 0.01), but not in never‐smokers (aOR, 1.16; 95% CI, 0.53–2.56; P = 0.71). When stratified according to tumor histology, the effect of the S allele on lung cancer risk was observed in three major histological types of lung cancer.
Table 3.
Association between telomerase reverse transcriptase (TERT) MNS16A variable number of tandem repeat genotypes and the risk of lung cancer according to age, gender, smoking status and histological types of lung cancer
| Variables | Case | Control | Adjusted OR (95% CI) | P | ||
|---|---|---|---|---|---|---|
| Group I + II† | Group III† | Group I + II† | Group III† | |||
| Age (years) | ||||||
| ≤62 | 431 (91.9) | 38 (8.1) | 487 (95.1) | 25 (4.9) | 1.63 (0.95–2.78)‡ | 0.08 |
| >62 | 429 (91.7) | 39 (8.3) | 409 (94.9) | 22 (5.1) | 1.57 (0.89–2.76)‡ | 0.12 |
| Gender | ||||||
| Male | 677 (91.7) | 61 (8.3) | 705 (95.4) | 34 (4.6) | 1.67 (1.06–2.62)§ | 0.03 |
| Female | 183 (92.0) | 16 (8.0) | 191 (93.6) | 13 (6.4) | 1.40 (0.65–3.02)§ | 0.39 |
| Smoking status | ||||||
| Never | 159 (92.4) | 13 (7.6) | 287 (95.0) | 15 (5.0) | 1.16 (0.53–2.56)¶ | 0.71 |
| Ever | 701 (91.6) | 64 (8.4) | 609 (95.0) | 32 (5.0) | 1.75 (1.13–2.72)¶ | 0.01 |
| Histology†† | ||||||
| Squamous cell carcinoma | 388 (92.4) | 32 (7.6) | 896 (95.0) | 47 (5.0) | 1.58 (0.93–2.68)‡‡ | 0.09 |
| Adeno carcinoma | 337 (91.8) | 30 (8.2) | 896 (95.0) | 47 (5.0) | 1.61 (0.99–2.62)‡‡ | 0.05 |
| Small‐cell carcinoma | 122 (91.0) | 12 (9.0) | 896 (95.0) | 47 (5.0) | 2.12 (1.05–4.31)‡‡ | 0.04 |
CI, confidence interval; OR, odds ratio. †Group I, L/L genotype; group II, L/M + M/M genotype; and group III, L/S + M/S + S/S genotype. ‡Adjusted for gender and pack–years of smoking. §Adjusted for age and pack–years of smoking. ¶Adjusted for age and gender. ††Sixteen large‐cell carcinomas were excluded from this analysis. ‡‡Adjusted for age, gender and pack–years of smoking.
In addition to the stratification analysis, the joint effect of the MNS16A VNTR genotypes and smoking status on the risk of lung cancer was also determined (Table 4). When the never‐smokers with the group I + II genotype was used as the reference group, the heavy smokers with the group III genotype were found to have the highest risk of lung cancer (aOR, 9.56; 95% CI, 5.42–16.87; P = 6 × 10−14).
Table 4.
Interaction of telomerase reverse transcriptase (TERT) MNS16A variable number of tandem repeat genotypes and smoking on lung cancer risk
| Smoking status | Genotype, group I + II† | OR (95% CI)‡ | P‡ | Genotype, group III† | OR (95% CI)‡ | P‡ |
|---|---|---|---|---|---|---|
| Never | 159/287 | 1.00 | 13/15 | 1.23 (0.56–2.68) | 0.61 | |
| Ever | 701/609 | 5.40 (3.67–7.94) | 1 × 10−17 | 64/32 | 9.56 (5.42–16.87) | 6 × 10−15 |
CI, confidence interval; OR, odds ratio. †Group I, L/L genotype; group II, L/M + M/M genotype; and group III, L/S + M/S + S/S genotype. ‡Data were calculated by logistic regression, with never‐smokers with group I + II genotypes as a reference group and adjusted for age and gender.
Association of MNS16A genotypes with survival outcome of patients with lung cancer. We evaluated the associations between MNS16A VNTR genotypes and the survival outcome of patients with surgically resected NSCLC. The clinical and pathological characteristics of the patients and the association with OS are shown in Table 5. There were 249 deaths (35.4%). The estimated 5‐year OS for all patients was 60.5%. Upon univariate analysis, the age, gender, smoking status and pathological stage were significantly associated with OS. Patients with at least one S allele (group III) had a significantly better OS compared with the group I + II patients (adjusted HR [aHR], 0.51, 95% CI, 0.58–0.93; P = 0.03; Table 6 and Fig. 1). Based on multivariate survival analysis using Cox’s proportional hazard model, the MNS16 VNTR polymorphism was an independent prognostic factor along with the age (HR for >64 years old vs≤64 years old, 1.41; 95% CI, 1.09–1.83; P = 0.01), gender (HR for male vs female, 2.50; 95% CI, 1.53–4.09; P = 0.0003) and pathological stage (HR for stage II or III vs stage I, 1.83; 95% CI, 1.40–2.39; P < 0.0001).
Table 5.
Univariate analysis for overall survival by demographics, smoking status, histological type, pathological stage and adjuvant chemotherapy
| Variables | No. cases | No. deaths (%)† | 5Y‐OSR (%)‡ | Log‐rank P |
|---|---|---|---|---|
| Overall | 703 | 249 (35.4) | 60.5 | |
| Age (years) | ||||
| ≤64 | 400 | 128 (32.0) | 64.8 | 0.01 |
| >64 | 303 | 121 (39.9) | 54.6 | |
| Gender | ||||
| Male | 517 | 208 (40.2) | 55.7 | <0.0001 |
| Female | 186 | 41 (22.0) | 74.5 | |
| Smoking status | ||||
| Never | 216 | 61 (28.2) | 69.0 | 0.003 |
| Ever | 487 | 188 (38.6) | 56.7 | |
| Histological type | ||||
| Squamous cell carcinoma | 334 | 127 (38.0) | 57.8 | 0.14 |
| Adenocarcinoma | 345 | 111 (32.2) | 63.6 | |
| Large cell carcinoma | 24 | 11 (45.8) | 54.4 | |
| Pathological stage | ||||
| I | 465 | 137 (29.5) | 64.9 | <0.0001 |
| II | 135 | 62 (45.9) | 56.7 | |
| IIIA | 103 | 50 (48.5) | 45.8 | |
| Adjuvant treatment§ | ||||
| Yes | 65 | 36 (55.4) | 40.7 | 0.37 |
| No | 110 | 37 (33.6) | 47.0 | |
†Row percentage. ‡Five‐year overall survival rate (5Y‐OSR) indicates proportion of survival derived from Kaplan–Meier analysis. §Of the patients with pathological stage II + IIIA, 55 cases received chemotherapy, eight cases received radiotherapy and two cases received both chemotherapy and radiotherapy.
Table 6.
Overall survival according to telomerase reverse transcriptase (TERT) MNS16A variable number of tandem repeat genotypes
| Genotype group† | No. cases (%)‡ | No. deaths (%)§ | 5Y‐OSR (%)¶ | Log‐rank P | Adjusted HR†† (95% CI) | P†† |
|---|---|---|---|---|---|---|
| Group I | 618 (87.9) | 225 (36.4) | 59.3 | 0.11 | 1.00 | |
| Group II | 36 (5.1) | 13 (36.1) | 58.9 | 0.96 (0.55–1.68) | 0.88 | |
| Group III | 49 (7.0) | 11 (22.5) | 75.9 | 0.51 (0.27–0.93) | 0.03 | |
| Group I + II | 654 (93.0) | 238 (36.4) | 59.3 | 0.04 | 1.00 | |
| Group III | 49 (7.0) | 11 (22.5) | 75.9 | 0.51 (0.28–0.93) | 0.03 |
5Y‐OSR, 5‐year overall survival rate; CI, confidence interval; HR, hazard ratio. †Group I, L/L genotype; group II, L/M + M/M genotype; and group III, L/S + M/S + S/S genotype. ‡Column percentage. §Row percentage. ¶Five‐year survival rate, proportion of survival derived from Kaplan–Meier analysis. ††HR, 95% CI and their corresponding P‐values were calculated using multivariate Cox proportional hazard models, adjusted for age, gender, smoking status, histological type, pathological stage and adjuvant therapy.
Figure 1.
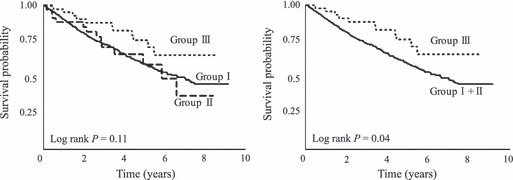
Kaplan–Meier overall survival curves according to telomerase reverse transcriptase (TERT) MNS16A variable number of tandem repeat (VNTR) genotypes. Group I, L/L genotype; group II, L/M + M/M genotype; and group III, L/S + M/S + S/S genotype.
Discussion
Telomerase plays an important role in the development and progression of lung cancer. Therefore, it is assumed that functional polymorphisms that affect TERT gene expression or activity might contribute not only to the susceptibility to lung cancer but also to the prognosis of patients with lung cancer. In the present study, we investigated the effect of the MNS16A VNTR on the risk of lung cancer and the prognosis of patients with surgically resected NSCLC. Interestingly, the VNTR‐243 allele was associated with an increased risk of lung cancer, whereas it was associated with a better survival outcome in patients with lung cancer. These findings suggest that alteration of the antisense TERT mRNA expression level by the MNS16A VNTR has a dual, conflicting role in lung carcinogenesis. Specifically, the MNS16A VNTR‐243 allele may increase the risk of lung cancer development and may improve survival in lung cancer patients.
Wang et al. ( 23 ) reported that the MNS16A VNTR is located in the putative promoter region of the antisense TERT mRNA transcript and identified four different sized alleles (VNTR‐333, ‐302, ‐272 and ‐243). The core sequence of MNS16A VNTR is a 23 bp tandem repeat or a 26 bp sequence with a CAT insertion. Wang et al. ( 23 ) found that the VNTR‐302 allele, which contains two 23 bp repeats and three 26 bp repeats, has a significantly lower promoter activity and antisense TERT mRNA expression when compared with the VNTR‐243 allele, which contains one 23 bp repeat and two 26 bp repeats. Based on this finding, they suggested that promoter activity depends on the length of the MNS16A VNTR and the 26 bp sequence with a CAT insertion functions as a repressor for the promoter of antisense TERT mRNA. There is growing evidence that naturally occurring antisense RNA transcripts negatively regulate gene expression by modulating sense RNA transcription, pre‐mRNA splicing and mRNA stability, transport and translation.( 26 , 27 ) Therefore, although the biological function of the antisense TERT mRNA remains unknown, it is likely that the antisense TERT mRNA acts as a repressor of TERT expression. Given that the VNTR‐243 allele has a significantly higher antisense TERT mRNA expression compared with the VNTR‐302 allele,( 23 ) it can be expected that the VNTR‐243 allele has reduced TERT expression and thus contributes to carcinogenesis. In accordance with this hypothesis, it has been reported that the MNS16A VNTR is associated with the risk of breast cancer and malignant glioma, as well as the prognosis of patients with brain cancer.( 24 , 28 , 29 )
A major strength of the present study was that the MNS16A VNTR was evaluated in relation to the susceptibility to lung cancer and prognosis of lung cancer patients, which may have a higher value in unraveling the role of the MNS16A VNTR during the multi‐step process of carcinogenesis. It has been proposed that telomerase may play both anti‐ and pro‐tumorigenic roles depending on the phase of carcinogenesis. In the earlier phases of cancer development, telomerase can help to control chromosomal instability by maintaining telomere length and suppress cancer initiation. In contrast, in the later phases of cancer development, telomerase is abnormally upregulated and facilitates tumor progression.( 10 , 11 , 12 , 30 , 31 ) In agreement with this proposal, we demonstrated dual roles of the MNS16A VNTR in lung carcinogenesis: the higher production allele VNTR‐243, for antisense TERT mRNA, was associated with an increased risk of lung cancer, whereas it was associated with a better survival outcome of patients with lung cancer, an opposing effect on the risk of lung cancer.
Few studies have investigated the MNS16A VNTR in relation to human cancers. Wang et al. ( 24 ) have reported that the VNTR‐243 and VNTR‐272 alleles are associated with a significantly increased risk of breast cancer compared with the VNTR‐302 allele in a Chinese population. In addition, Wang et al. ( 29 ) have reported that the genotypes with at least one VNTR‐302 or VNTR‐333 allele exhibited a worse survival outcome compared with the homozygous genotypes of the VNTR‐243 or VNTR‐272 allele in non‐Hispanic white patients with glioblastomas multiforme. These two studies are in agreement with our findings that the VNTR‐243 allele was associated with an increased risk of lung cancer, whereas it was associated with a better survival outcome in patients with lung cancer. However, although the VNTR‐272 allele was combined with the VNTR‐243 allele as one group in the previous studies,( 24 , 29 ) we combined the VNTR‐272 and VNTR‐302 alleles as one group. Like the VNTR‐302 allele, the VNTR‐272 allele contains three 26 bp repeats, a repressor for promoter of antisense TERT mRNA. In light of the putative function of the VNTR‐272 allele, therefore, it is more reasonable that the VNTR‐272 allele is combined with the VNTR‐302 allele as one group.
In the present study, we detected a joint effect of MNS16A VNTR genotypes and smoking on the risk of lung cancer. Such a finding is biologically plausible because smoking has been shown to induce telomere shortening and to increase telomerase activity;( 32 , 33 ) therefore, polymorphisms, including the MNS16A VNTR that can influence telomere shortening, may have a synergistic effect with smoking on lung cancer development.
There is growing evidence that inherited genotypes might affect the disease outcome by influencing the biological characteristics of the disease or an individual’s response to a specific therapy. In view of this point, the present study was aimed at evaluating the role of the MNS16A VNTR polymorphism as a prognostic factor in patients with lung cancer. SCLC is a rapidly progressive malignancy, which is usually a systemic disease at the time of initial presentation. Consequently, in the case of SCLC, surgery is reserved for selected patients and chemotherapy with or without radiotherapy is the standard therapy for most patients. Therefore, the survival analysis of the present study was limited to patients with NSCLC who underwent curative surgical resection. Future studies are needed to evaluate the role of the MNS16A VNTR as a predictive factor for survival outcome in SCLC and NSCLC patients receiving a specific therapy.
Genetic polymorphisms often show a lot of ethnic variation. In the present study, the frequencies of the VNTR‐302, ‐272 and ‐243 alleles among the healthy controls were 0.944, 0.030 and 0.025, respectively, which were significantly different from those of healthy Chinese (0.947, 0.012 and 0.041, respectively).( 24 ) Therefore, further studies are needed to clarify the association between the TERT MNS16A VNTR and lung cancer in diverse ethnic populations.
In conclusion, we found that the TERT MNS16A VNTR polymorphism exerts a dual, conflicting effect on the risk and prognosis of lung cancer. This finding suggests that a VNTR polymorphism in the TERT gene can be used not only as a marker for the genetic susceptibility to lung cancer, but also as a prognostic marker for patients with lung cancer. Our findings need to be validated by further functional studies as well as well‐designed larger studies with diverse ethnic populations.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This study was supported by the National R&D Program for the Cancer Control Ministry of Health and Welfare (0720550‐2), and the Regional Technology Innovation Program of the Ministry of Commerce, Industry and Energy (RTI04‐01‐01) of Republic of Korea.
References
- 1. Rhodes D, Fairall L, Simonsson T, Court R, Chapman L. Telomere architecture. EMBO Rep 2002; 3: 1139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Gene Dev 2005; 19: 2100–10. [DOI] [PubMed] [Google Scholar]
- 3. Blasco MA. Telomeres and human disease: aging, cancer and beyond. Nat Rev Genet 2005; 6: 611–22. [DOI] [PubMed] [Google Scholar]
- 4. Murnane JP. Telomeres and chromosome instability. DNA Repair 2006; 5: 1082–92. [DOI] [PubMed] [Google Scholar]
- 5. Allsopp RC, Vaziri H, Patterson C et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA 1992; 89: 10114–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blackburn EH. Switching and signaling at the telomere. Cell 2001; 106: 661–73. [DOI] [PubMed] [Google Scholar]
- 7. Liu D, O’Connor MS, Qin J et al. Telosome, a mammalian telomere‐associated complex formed by multiple telomeric proteins. J Biol Chem 2004; 279: 51338–42. [DOI] [PubMed] [Google Scholar]
- 8. Chan SW, Blackburn EH. New ways not to make ends meet: telomerase, DNA damage proteins and heterochromatin. Oncogene 2002; 21: 553–63. [DOI] [PubMed] [Google Scholar]
- 9. Collins K, Mitchell JR. Telomerase in the human organism. Oncogene 2002; 21: 564–79. [DOI] [PubMed] [Google Scholar]
- 10. Meyerson M. Role of telomerase in normal and cancer cells. J Clin Oncol 2000; 18: 2626–34. [DOI] [PubMed] [Google Scholar]
- 11. Hackett JA, Greider CW. Balancing instability: dual roles for telomerase and telomere dysfunction in tumorigenesis. Oncogene 2002; 21: 619–26. [DOI] [PubMed] [Google Scholar]
- 12. Londono‐Vallejo JA. Telomere instability and cancer. Biochimie 2008; 90: 73–82. [DOI] [PubMed] [Google Scholar]
- 13. Wu X, Amos CI, Zhu Y et al. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst 2003; 95: 1211–18. [DOI] [PubMed] [Google Scholar]
- 14. McGrath M, Wong JYY, Michaud D et al. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev 2007; 16: 815–19. [DOI] [PubMed] [Google Scholar]
- 15. Jang JS, Choi YY, Lee WK et al. Telomere length and the risk of lung cancer. Cancer Sci 2008; 99: 1385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature 1999; 402: 551–5. [DOI] [PubMed] [Google Scholar]
- 17. Vulliamy TJ, Marrone A, Knight SW et al. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood 2006; 107: 2680–5. [DOI] [PubMed] [Google Scholar]
- 18. Hiyama E, Hiyama K. Clinical utility of telomerase in cancer. Oncogene 2002; 21: 643–9. [DOI] [PubMed] [Google Scholar]
- 19. Gertler R, Rosenberg R, Stricker D et al. Telomere length and human telomerase reverse transcriptase expression as markers for progression and prognosis of colorectal carcinoma. J Clin Oncol 2004; 22: 1807–14. [DOI] [PubMed] [Google Scholar]
- 20. Hastie ND, Dempster M, Dunlop MG et al. Telomere reduction in human colorectal carcinoma and with ageing. Nature 1990; 346: 866–8. [DOI] [PubMed] [Google Scholar]
- 21. Iwama H, Ohyashiki K, Ohyashiki JH et al. Telomere length and telomerase activity with age in peripheral blood cells obtained from normal individuals. Hum Genet 1998; 102: 397–402. [DOI] [PubMed] [Google Scholar]
- 22. Flenck RW, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci USA 1998; 95: 5607–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang L, Soria JC, Chang YS et al. Association of a functional tandem repeats in the downstream of human telomerase gene and lung cancer. Oncogene 2003; 22: 7123–9. [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Hu Z, Liang J et al. A tandem repeat of human telomerase reverse transcriptase (hTERT) and risk of breast cancer development and metastasis in Chinese women. Carcinogenesis 2008; 29: 1197–201. [DOI] [PubMed] [Google Scholar]
- 25. Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009; 136: 260–71. [DOI] [PubMed] [Google Scholar]
- 26. Knee R, Murphy PR. Regulation of gene expression by natural antisense RNA transcripts. Neurochem Int 1997; 31: 379–92. [DOI] [PubMed] [Google Scholar]
- 27. Timmons JA, Good L. Does everything now make (anti)sense? Biochem Soc Trans 2006; 34: 1148–50. [DOI] [PubMed] [Google Scholar]
- 28. Carpentier C, Lejeune J, Gros F et al. Association of telomerase gene hTERT polymorphism and malignant gliomas. J Neurooncol 2007; 84: 249–53. [DOI] [PubMed] [Google Scholar]
- 29. Wang L, Wei Q, Wang LE et al. Survival prediction in patients with glioblastoma multiforme by human telomerase genetic variation. J Clin Oncol 2006; 24: 1627–32. [DOI] [PubMed] [Google Scholar]
- 30. Chadeneau C, Hay K, Hirte HW et al. Telomerase activity associated with acquisition of malignancy in human colorectal cancer. Cancer Res 1995; 55: 2533–6. [PubMed] [Google Scholar]
- 31. Tang R, Cheng AJ, Wang JY, Wang TCV. Close correlation between telomerase expression and adenomatous polyp progression in multistep colorectal carcinogenesis. Cancer Res 1998; 58: 4052–4. [PubMed] [Google Scholar]
- 32. Valdes AM, Andrew T, Gardner JP et al. Obesity, cigarette smoking, and telomere length in women. Lancet 2005; 366: 662–4. [DOI] [PubMed] [Google Scholar]
- 33. Yim HW, Slebos RJC, Randell SH et al. Smoking is associated with increased telomerase activity in short‐term cultures of human bronchial epithelial cells. Cancer Lett 2007; 246: 24–33. [DOI] [PubMed] [Google Scholar]


