Abstract
Resveratrol, a naturally occurring polyphenolic antioxidant compound present in grapes and red wine, has been reported to hold various biochemical responses. In this preliminary study, we evaluate the chemopreventive potential of resveratrol against bladder cancer and its mechanism of action. Treatment of bladder cancer cells with resveratrol resulted in a significant decrease in cell viability. Resveratrol induced apoptosis through the modulation of Bcl‐2 family proteins and activation of caspase 9 and caspase 3 followed by poly(ADP‐ribose) polymerase degradation. Treatment with resveratrol led to G1 phase cell cycle arrest in T24 cells by activation of p21 and downregulation of cyclin D1, cyclin‐dependent kinase 4, and phosphorylated Rb. Resveratrol also inhibited the phosphorylation of Akt, whereas the phosphorylation of p38 MAPK was enhanced. In addition, resveratrol treatment decreased the expression of vascular endothelial growth factor and fibroblast growth factor‐2, which might contribute to the inhibition of tumor growth on the bladder cancer xenograft model. These findings suggest that reveratrol could be an important chemoprevention agent for bladder cancer. (Cancer Sci 2009)
Bladder cancer is the fifth most common cancer and caused more than 14 100 deaths in the USA in 2008.( 1 ) It is the most expensive cancer to survey and treat because of the need for frequent interval cystourethroscopy, urine cytology, and radiological evaluations. The bladder cancer mortality varies in different countries. The highest rates are noted in European countries, such as Denmark, the UK, Belgium, and Italy, and the lowest rates in Asian countries, such as Japan, China, and Singapore.( 2 ) Populations in South‐East Asia have a 4‐ to 10‐fold lower incidence of, and death from, bladder cancer compared with those in the USA.( 3 ) As studies have proved geographic discrepancies in the incidence of many tumor types, researchers began to examine the impact of diet, nutrient intake, and some chemopreventive agents on tumor development.
Resveratrol, chemically known as 3,5,4’‐trihydroxystilbene, is a naturally occurring polyphenolic antioxidant compound present in grapes, berries, peanuts, and red wine. Studies have suggested that resveratrol can prevent the progression of a wide variety of pathologies, including vascular diseases, ischemic injuries, cancers, and neurodegenerative processes.( 4 , 5 , 6 ) In 1997, Jang et al. showed that resveratrol is a chemopreventive agent through its action on tumor initiation, promotion, and progression.( 7 ) Dozens of reports have shown that resveratrol could inhibit the proliferation of several kinds of tumors such as leukemia, prostate, breast, and colon cancers.( 8 , 9 , 10 , 11 ) However, no study has examined the antitumor effects of resveratrol on bladder cancer. We show for the first time that resveratrol could induce apoptosis and cell cycle arrest of bladder cancer cells and has a potent effect on inhibiting tumor growth in a nude mice xenograft model.
Materials and Methods
Reagents. Resveratrol (>99% pure) and MTT were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The annexin V–FITC apoptosis detection kit was from BD Biosciences (San Jose, CA, USA). The bicinchoninic acid protein assay kit was obtained from Pierce Biotechnology (Rockford, IL, USA). The DNA content analysis kit was from Beckman Coulter (Fullerton, CA, USA). Antibodies against Akt, phosphorylated Akt (Ser473), p38, phosphorylated p38, Rb, phosphorylated Rb, Bad, phosphorylated Bad (Ser112 and Ser136), cleaved caspase 3, caspase 3, and WAF1/p21 were obtained from Cell Signaling Technology, (Beverly, MA, USA). Bcl‐2, Bax, Bcl‐xL, caspase 9, β‐actin, poly(ADP‐ribose) polymerase (PARP), cyclin D1, cyclin‐dependent kinase 4 (CDK4), and antimouse and antirabbit secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture. The human bladder cancer cell line T24 was obtained from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). The cells were cultured in RPMI‐1640 medium (HyClone, Logan, UT, USA) supplemented with 10% heat‐inactivated FBS (JRH Biosciences, Lenexa, KS, USA), 100 U/mL penicillin, and 100 mg/L streptomycin. Cultures were maintained in a humidified atmosphere of 5% CO2 at 37°C.
Cell growth/cell viability assay. The effect of resveratrol on the viability of T24 cells was evaluated by MTT assay. Approximately 1.0 × 104 T24 cells were seeded on 96‐well plates. After overnight incubation, the cells were treated with different concentrations of resveratrol (0–200 μm in DMSO) for 24 h. After incubation for the indicated time, MTT (20 μL of 5 mg/mL) was added to each well and incubated at 37°C for 4 h, after which the MTT solution in the medium was aspirated off. To achieve solubilization of the formazan crystal formed in viable cells, 150 μL DMSO was added to each well before the absorbance at 490 nm was measured using an MRX II absorbance reader (Dynex Technologies, Chantilly, VA, USA). Results were expressed as a percentage of growth, with 100% representing control cells treated with DMSO alone.
Apoptosis assessed by flow cytometry. The extent of apoptosis was evaluated by annexin V–FITC and flow cytometry. Cells were grown at a density of 1 × 106 cells in six‐well culture dishes and were treated with different concentrations of resveratrol (0–200 μm in DMSO) for 24 h. Following treatment, the cells were harvested, washed twice with pre‐chilled PBS, and resuspended in 1 × binding buffer at a concentration of 1 × 106 cells/mL. One hundred microliters of such solution was mixed with 5 μL annexin V–FITC and 5 μL propidium iodide for 15 min, then 400 μL 1 × binding buffer was added. Analysis was carried out using a FC500 flow cytometer with CXP software (Beckman Coulter, Fullerton, CA, USA) within 1 h. The percentage of apoptotic cells was assessed by CXP software.
Cell cycle distribution analysis. Cells were plated in six‐well culture dishes at concentrations determined to yield 60–70% confluence within 24 h. Cells were then treated with different concentrations of resveratrol (0–200 μm in DMSO). After 24 h, cells were washed twice with PBS then centrifuged. The pellet was fixed with 70% ethanol for 1 h at 4°C. The cells were washed with PBS and resuspended with propidium iodide solution (0.05 mg/mL) containing RNase, incubated at room temperature in the dark for 30 min. DNA content was then analyzed using the FC500 flow cytometer.
Western blot analysis. Cell were harvested at 24 h following resveratrol treatment, washed, and lysed with lysis buffer (10 mmol/L Tris‐HCl, 0.25 mol/L sucrose, 5 mmol/L EDTA, 50 mmol/L NaCl, 30 mmol/L sodium pyrophosphate, 50 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L PMSF, and 2% cocktail [pH 7.5]). Protein concentration in the resulting lysate was determined using the bicinchoninic acid protein assay. Appropriate amounts of protein (20–30 μg) were separated by electrophoresis in 10–12% Tris‐glycine polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked then incubated overnight with the appropriate primary antibody at dilutions specified by the manufacturer. They were next washed and incubated with the corresponding HRP‐conjugated secondary antibody at 1:1000 dilution in Tris‐buffered saline–Tween 20 (10 mm Tris‐Cl [pH 7.4], 150 mm NaCl, 0.1% Tween‐20). Bound secondary antibody was detected using an enhanced chemiluminescence system (Pierce Biotechnology).
Animal experiments. Male BALB/c‐nude mice (4 weeks old), each weighing 18–20 g, were used for the experiments (supplied by the Shanghai Experimental Animal Center, Chinese Academy of Sciences, Shanghai, China). The mice were housed with free access to food and water on a 12:12 h light:dark cycle with the room temperature maintained at 21°C.
T24 cells (1 × 105 in 10 μL PBS) were injected s.c. into the right flank of the rats. Growth rates of the s.c. tumors were monitored. Tumor size was measured every two days for 28 days. A blinded observer measured tumor length and width. The volume of the tumor was calculated from the formula length × width2 × 0.52,( 12 ) where length and width were tumor diameters measured with calipers in mutually perpendicular directions. At a tumor size of approximately 30 mm3 the mice were divided into three groups. Group A received no treatment. Groups B and C were treated with an i.p. injection of propylene glycol (vehicle, 0.1 mL) or 20 mg/kg resveratrol (in 0.1 mL propylene glycol), once daily for 4 weeks.
RT‐PCR analysis. The expression of vascular endothelial growth factor (VEGF) and fibroblast growth factor‐2 (FGF‐2) from the tissue samples was studied by RT‐PCR. Total RNA was extracted from tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. cDNA was generated using the M‐MuLV reverse transcription system (Fermentas, Hanover, MD, USA). The cDNA samples were then subjected to PCR analysis using the following primers: VEGF sense, 5′‐ACTTTCTGCTGTCTTGGGTG‐3′; VEGF antisense, 5′‐ TGCTGTAGGAAGCTCATCTC‐3′; FGF‐2 sense, 5′‐GAGAAGAGCGACCCTCACA‐3′; FGF‐2 antisense, 5′‐TAGCTTTCTGCCCAGGTCC‐3′; β‐actin sense, 5′‐ATGGATGACGATATCGCTGCG‐3′, β‐actin antisense, 5′‐GAAGGTCTCAAACATGATCTGG‐3′. PCR reaction conditions were as follows: initial denaturation at 94°C for 4 min and 32 cycles of amplification (94°C for 45 s, 57°C for 45 s, and 72°C for 60 s), followed by a final extension step for 10 min at 72°C. The PCR products were analyzed on 1.5% agarose gel.
Statistical analysis. Statistical significance was compared between various treatment groups and controls using anova. Data were considered statistically significant when P‐values were <0.05.
Results
Cytotoxicity effects of resveratrol on T24 cells. To determine the inhibitory effects of resveratrol on T24 cells, cytotoxicity assays were carried out as described above. Figure 1 shows the survival curves of T24 cells treated with various concentrations of resveratrol for different exposure times. The survival curves shifted to the left with longer drug exposures. The inhibitory concentration 50% values for resveratrol treatment were estimated to be 250, 180, and 160 μm for 12, 24, and 48 h, respectively. The control cells were treated with DMSO (0.1%), which did not affect cell proliferation, viability, or morphology (data not shown). These data indicate that resveratrol exerts a significant cytotoxic effect upon T24 cells in a dose‐ and time‐dependent manner.
Figure 1.
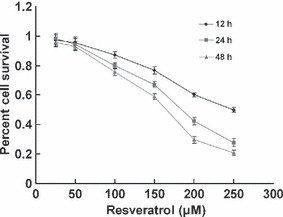
Cytotoxicity effects of resveratrol on T24 bladder cancer cells. Cell proliferation and viability were determined by an MTT assay. Reduced cell viability was observed with resveratrol treatment (25–250 μm) at 12, 24, and 48 h. The data are presented as mean ± SD.
Induction of apoptosis of T24 cells by resveratrol. In view of the above‐mentioned growth‐inhibitory effects, we were interested in determining whether resveratrol also induced apoptosis in this cell line. After treatment for 24 h, it was observed that treatment of T24 cells with 0–100 μm resveratrol increased the number of early apoptotic cells from 4.0% to 14.1%, which decreased from 14.1% to 10.0% after treatment with 150 μm resveratol. The number of late apoptotic cells increased from 2.6% to 36.7% (200 μm resveratol). The total percent of apoptotic cells was directly related to resveratrol concentration, increasing from 6.6% (control) to 47.5% (200 μm resveratrol) (Fig. 2). Consistent with the cytotoxicity assay, the results revealed that resveratrol induced cellular apoptosis of T24 cells in a dose‐dependent manner.
Figure 2.
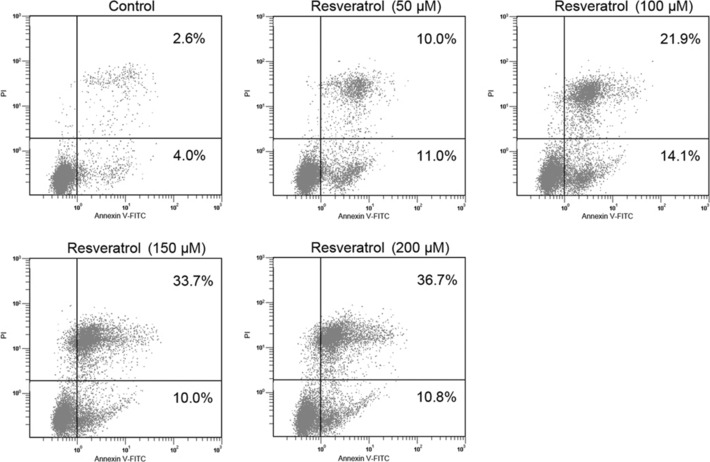
Dose‐dependent apoptosis induced by treatment with resveratrol in T24 bladder cancer cells. Cells treated with various concentrations of resveratrol were double‐stained with annexin V and PI and analyzed by flow cytometry. The gate setting distinguished between living (bottom left), necrotic (top left), early apoptotic (bottom right), and late apoptotic (top right) cells.
Cell cycle accumulation at G1 phase after treatment with resveratrol. As the induction of apoptosis might be mediated through the regulation of the cell cycle, we also examined the effect of resveratrol on cell cycle perturbations. Compared with the vehicle‐treated controls, resveratrol treatment resulted in an appreciable arrest of T24 cells in G1 phase of the cell cycle. It was observed that the G1 phase population of the untreated cells was 48.9% and the percentage of cells in G1 phase was significantly increased (73.1%, 77.0%, 78.1%, and 72.1% cells at 50, 100, 150, and 200 μm concentrations of resveratrol, respectively; Fig. 3) after 24 h of treatment. This increase in the G1 cell population was accompanied with a concomitant decrease of cell numbers in S phase and G2–M phases in T24 cell lines. After 24 h, the percentage of the sub‐G1 cell population increased, suggesting that the blockage in G1 phase results in the triggering of the apoptotic program.
Figure 3.
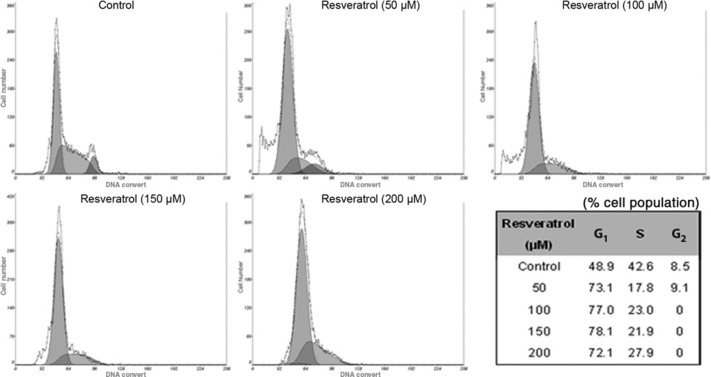
Cell cycle analysis of T24 bladder cancer cells treated with resveratrol. Cells were cultured with different concentrations of resveratrol for 24 h and then stained with propidium iodide. The DNA content was analyzed by flow cytometry. G1, S, and G2 indicate cell cycle phases.
Induction in WAF‐1/p21 and inhibition in Rb, cyclin D1, and CDK4. Because our results indicated that resveratrol treatment caused G1 phase cell cycle arrest, we further examined the effect of resveratrol on cell cycle‐regulatory molecules operative in the G1 phase. We assessed WAF‐1/p21 protein, the key regulator of G1–S phase transition. Using immunoblot analysis, we found that resveratrol treatment resulted in a significant dose‐dependent induction of WAF‐1/p21. The results also revealed that resveratrol treatment of T24 cells leads to a dose‐dependent decrease in protein expression of phosphorylated Rb, cyclin D1, and CDK4 (Fig. 4A).
Figure 4.
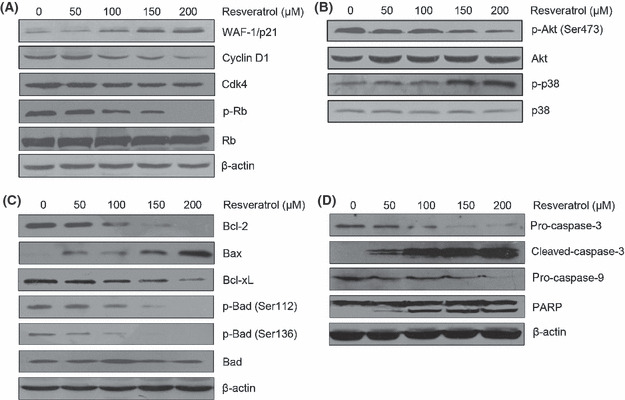
Expression of proteins changed in cells treated with different concentrations of resveratrol for 24 h. (A) Resveratrol upregulated the expression of WAF‐1/p21 but downregulated the expression of cyclin D1, cyclin‐dependent kinase 4 (CDK4), and phosphorylated (p‐) Rb. (B) Resveratrol inhibited Akt phosphorylation and enhanced p38 MAPK activation. (C) Resveratrol inhibited the expression of anti‐apoptotic gene products Bcl‐2 and Bcl‐xL but upregulated the expression of apoptotic gene product Bax. The levels of p‐Bad at Ser112 and Ser136 were decreased by treatment with resveratrol. (D) Resveratrol treatment activated caspase 3 and caspase 9 and resulted in a cleavage of poly(ADP‐ribose) polymerase (PARP). The data shown here are from a representative experiment repeated three times with similar results.
Resveratrol inhibits Akt and enhances p38 MAPK activation. To determine whether regulation of the Akt and p38 MAPK signal pathways is necessary for resveratrol‐induced apoptosis, we investigated the expression and phosphorylation levels of Akt and p38 MAPK after treatment with various concentrations of resveratrol for 24 h. As shown in Figure 4(B), the phosphorylation of Akt was decreased, but the phosphorylation of p38 was increased. The results suggested that the levels of both phosphorylated Akt and phosphorylated p38 are dose‐dependent in response to resveratrol.
Resveratrol‐induced apoptosis mediated by Bcl‐2 family modulation and caspase activation. To further characterize this cell‐specific apoptotic effect of resveratrol in bladder cancer cells, we analyzed the levels of Bcl‐2 family proteins and the activation of caspases. We observed that the anti‐apoptotic Bcl‐2 and Bcl‐xL expressions were downregulated, whereas the level of pro‐apoptotic Bax expression was markedly upregulated. Resveratrol treatment also caused a decrease in the levels of phosphorylated Bad at Ser112 and Ser136 (Fig. 4C). As shown in Figure 4(D), resveratrol treatment was found to result in a significant increase in cleaved caspase 3 and resulted in a dose‐dependent cleavage of PARP, which is indicative of induction of apoptosis.
Antitumor effects of resveratrol on bladder cancer xenograft model. As resveratrol had cytotoxic effects on T24 cells, we studied its antitumor effects in vivo. Nude mice were s.c. inoculated with 1 × 105 T24 cells in the right flank followed by no treatment (control; group A), i.p. injection of propylene glycol (vehicle; group B), or a resveratrol concentration of 20 mg/kg for 4 weeks (group C). Figure 5 shows the tumor growth in the resveratrol group was significantly slower than in the vehicle and control groups. In the first 10 days, there were no differences in tumor volumes among the three groups (P > 0.05). In contrast, the tumor volumes in groups A and B were significantly larger than in group C from days 12 to 28 (P < 0.05). The results indicated that treatment with a resveratrol concentration of 20 mg/kg daily exerted antitumor effects on bladder cancer xenografts in vivo.
Figure 5.
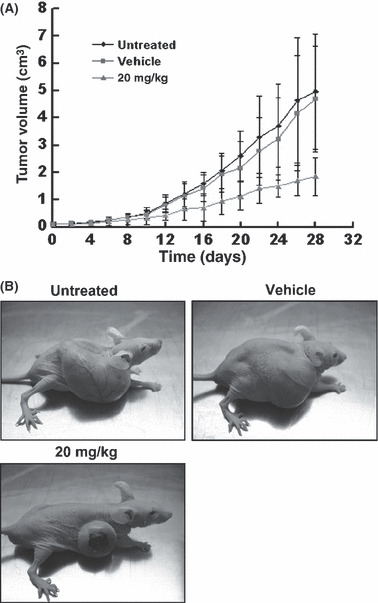
Effects of resveratrol on bladder cancer xenografts in vivo. (A) Tumor volume growth in nude mice treated with i.p. injection of resveratrol (20 mg/kg per day) for 4 weeks. The data are presented as mean ± SD. Daily average tumor volumes for each group were compared throughout the course of the experiment using ANOVA. (B) Photographs show the implanted tumors in representative mice at the day 28.
Resveratrol suppressed expression of VEGF and FGF‐2 in T24 cells. VEGF and FGF‐2 gene expression was measured in resveratrol‐treated T24 cells and in tumors of mice in different groups for four consecutive weeks (Fig. 6A, B). These data suggested that the expression level of VEGF and FGF‐2 was decreased after treatment with resveratrol both in vitro and in vivo.
Figure 6.
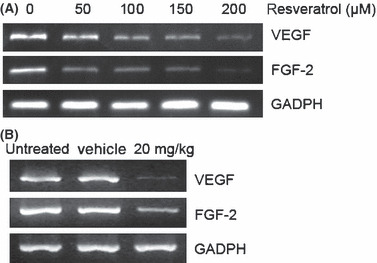
Resveratrol inhibited the expression of vascular endothelial growth factor (VEGF) and fibroblast growth factor‐2 (FGF‐2) in both T24 bladder cancer cells and tumor tissue. Gene expression was analyzed by RT‐PCR. Representative data are shown from three independent experiments with identical results.
Discussion
Resveratrol is currently being evaluated as a potential cancer chemoprevention agent. It has gained much attention for its anticancer activities in both cell culture and animal carcinogenesis models. Although studies have shown that resveratrol imparts antiproliferative effects against several cancer types, there is no report on its effects on bladder cancers. The present work provides evidence for the first time that resveratrol has a chemopreventive/therapeutic potential against bladder cancer.
One of the most effective anti‐apoptotic survival pathways in mammals is constituted by phosphoinositide 3‐kinase (PI3K)/Akt. Constitutive Akt signaling is believed to promote proliferation and increased cell survival, thereby contributing to cancer progression; recent studies have shown that Akt is constitutively active in several types of human cancers, including bladder cancer.( 13 , 14 , 15 , 16 , 17 ) Consistent with previous studies, our data showed that resveratrol treatment resulted in significant dose‐dependent inhibition in phosphorylated Akt (at Ser473) in tumor cells. As Akt is a downstream target of PI3K, the inhibition of Akt phosphorylation indicated that resveratrol treatment could lead to downregulation of PI3K and serine/threonine protein kinases. Akt inhibits apoptosis through multiple pathways, including mitochondrial pathways,( 18 ) therefore we assessed the effect of resveratrol on Bcl‐2 family proteins and caspases. The present study indicates that resveratrol treatment downregulates Bcl‐2 and Bcl‐xL protein but upregulates Bax protein. Also, the resveratrol‐mediated inactivation of Akt in our model is associated with reduced Ser112 and Ser136 phosphorylation of Bad. We found that resveratrol treatment of T24 cells activated caspase 3 and caspase 9, and induced the cleavage of caspase 3 and PARP. It is suggested that the apoptosis of T24 cells induced by resveratrol might act through the mitochondrial pathways.
Cell cycle regulation is one important mechanism of anti‐proliferation in cancers. In this study, we investigated the cell cycle distribution after treatment with resveratrol and found that resveratrol induced cell cycle accumulation at G1 phase. We observed that after the treatment with 50 μm resveratrol, the cell cycle was significant accumulated at G1 phase. Similar results were observed in A431 cells and HepG2 cells.( 18 , 19 ) Cyclin D1–CDK4 complexes play a crucial role in the transition of cells from G1 to S phase through inactivation of Rb tumor suppressor by phosphorylation at serine residues, and their functions are negatively regulated by CDK inhibitors, such as p21. On this line, our data of resveratrol‐induced cell cycle arrest at G1 phase were well correlated with the upregulation of p21 and the downregulation of cyclin D1, CDK4, and phosphorylated Rb.( 20 , 21 , 22 , 23 )
The MAPK pathway has a central role in many cellular signaling processes, and p38 is one of the major components of this pathway.( 24 , 25 ) In recent years, studies have proved the functions of p38 MAPK in the negative regulation of cell cycle progression, which suggested its role as a potential tumor suppressor. Studies showed that activation of p38 MAPK might contribute to the negative regulation of cyclin D1 and CDK4, which leads to the arrest of cell cycle progression at G1–S transition.( 26 , 27 ) In the present study, we found that resveratrol induces the phosphorylation of p38 MAPK and the changes in cyclin D1 and CDK4, consistent with other studies.
Because resveratrol caused cytotoxic effects and apoptosis in T24 cells, we further investigated the effects of resveratrol in vivo. We observed that resveratrol significantly slowed the growth of tumors. The mechanisms of the antitumor activity of resveratrol are not yet fully understood. The induction of apoptosis might be one of the mechanisms because resveratrol causes apoptosis of leukemia, prostate, breast, colon, and bladder cancers, as indicated in this study.( 8 , 9 , 10 , 11 ) In recent years, resveratrol has been found to inhibit tumor‐induced neovascularization.( 28 , 29 ) Angiogenesis is important for tumor growth and progression. The development of angiogenesis is stimulated by cytokines and growth factors, and the expression of these cytokines and growth factors are correlated with the pathological neovascularization circumstances.( 30 ) Among these angiogenic factors, VEGF and FGF‐2 are important angiogenic factors and essential for cancers. ( 31 , 32 , 33 ) In this study, we found that resveratrol inhibited VEGF and FGF‐2 expression in both in vitro and in vivo studies. Thus, the inhibition of tumorigenesis might contribute to the antitumor effect of resveratrol on bladder cancer.
In conclusion, our study indicated that resveratrol caused dose‐ and time‐dependent cytotoxicity and induction of apoptosis in bladder cancer T24 cells. Resveratrol induced accumulation of T24 cells at G1 phase of the cell cycle. Akt and p38 MAPK may both play an important role in the chemopreventive effects of this polyphenolic agent for bladder cancer. In the nude mice xenograft model, resveratrol was capable of inhibiting tumor growth. To the best of our knowledge, this is the first report showing the antitumor effects of resveratrol on bladder cancer. However, further investigations of the detailed molecular mechanism involved in the resveratrol‐induced apoptosis are necessary.
Acknowledgments
We thank Dr. Long‐Cheng Li for his critical review of, and editorial assistance with, the manuscript. This work was supported by the Administration of Traditional Chinese Medicine of Zhejiang Province, China (Grant no. 2009CA057).
References
- 1. Jemal A, Siegel R, Ward E et al. Cancer statistics, 2008. CA Cancer J Clin 2008; 58: 71–96. [DOI] [PubMed] [Google Scholar]
- 2. Paneau C, Schaffer P, Bollack C. Epidemiology of bladder cancer. Ann Urol 1992; 26: 281–93. [PubMed] [Google Scholar]
- 3. Silverman DT, Hartge P, Morrison AS, Devesa SS. Epidemiology of bladder cancer. Hematol Oncol Clin North Am 1992; 6: 1–30. [PubMed] [Google Scholar]
- 4. Bradamante S, Barenghi L, Villa A. Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev 2004; 22: 169–88. [DOI] [PubMed] [Google Scholar]
- 5. Wang Q, Xu J, Rottinghaus GE et al. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res 2002; 958: 439–47. [DOI] [PubMed] [Google Scholar]
- 6. Russo A, Palumbo M, Aliano C, Lempereur L, Scoto G, Renis M. Red wine micronutrients as protective agents in Alzheimer‐like induced insult. Life Sci 2003; 72: 2369–79. [DOI] [PubMed] [Google Scholar]
- 7. Jang M, Cai L, Udeani GO et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997; 275: 218–20. [DOI] [PubMed] [Google Scholar]
- 8. Clément MV, Hirpara JL, Chawdhury SH, Pervaiz S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling‐dependent apoptosis in human tumor cells. Blood 1998; 92: 996–1002. [PubMed] [Google Scholar]
- 9. Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N. Resveratrol‐caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3’‐kinase/Akt pathway and Bcl‐2 family proteins. Mol Cancer Ther 2006; 5: 1335–41. [DOI] [PubMed] [Google Scholar]
- 10. Tang HY, Shih A, Cao HJ, Davis FB, Davis PJ, Lin HY. Resveratrol‐induced cyclooxygenase‐2 facilitates p53‐dependent apoptosis in human breast cancer cells. Mol Cancer Ther 2006; 5: 2034–42. [DOI] [PubMed] [Google Scholar]
- 11. Tessitore L, Davit A, Sarotto I, Caderni G. Resveratrol depresses the growth of colorectal aberrant crypt foci by affecting bax and p21(CIP) expression. Carcinogenesis 2000; 21: 1619–22. [PubMed] [Google Scholar]
- 12. Boehm T, Folkman J, Browder T, O’Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 1997; 390: 404–7. [DOI] [PubMed] [Google Scholar]
- 13. Osaki M, Oshimura M, Ito H. PI3K‐Akt pathway: its functions and alterations in human cancer. Apoptosis 2004; 9: 667–76. [DOI] [PubMed] [Google Scholar]
- 14. Wetzker R, Rommel C. Phosphoinositide 3‐kinases as targets for therapeutic intervention. Curr Pharm Des 2004; 10: 1915–22. [DOI] [PubMed] [Google Scholar]
- 15. Fresno Vara JA, Casado E, De Castro J, Cejas P, Belda‐Iniesta C, González‐Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev 2004; 30: 193–204. [DOI] [PubMed] [Google Scholar]
- 16. Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene 2003; 22: 8983–98. [DOI] [PubMed] [Google Scholar]
- 17. Luo J, Manning BD, Cantley LC. Targeting the PI3K‐Akt pathwayin human cancer: rationale and promise. Cancer Cell 2003; 4: 257–62. [DOI] [PubMed] [Google Scholar]
- 18. Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide‐regulated kinases: kinase activation by phosphoinositide‐dependent phosphorylation. Annu Rev Biochem 1999; 68: 965–1014. [DOI] [PubMed] [Google Scholar]
- 19. Ahmad N, Adhami VM, Afaq F, Feyes DK, Mukhtar H. Resveratrol causes WAF‐1/p21‐mediated G(1)‐phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin Cancer Res 2001; 7: 1466–73. [PubMed] [Google Scholar]
- 20. Notas G, Nifli AP, Kampa M, Vercauteren J, Kouroumalis E, Castanas E. Resveratrol exerts its antiproliferative effect on HepG2 hepatocellular carcinoma cells, by inducing cell cycle arrest, and NOS activation. Biochim Biophys Acta 2006; 1760: 1657–66. [DOI] [PubMed] [Google Scholar]
- 21. Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1‐phase progression. Genes Dev 1999; 13: 1501–12. [DOI] [PubMed] [Google Scholar]
- 22. Jacks T, Weinberg RA. Cell‐cycle control and its watchman. Nature 1996; 381: 643–4. [DOI] [PubMed] [Google Scholar]
- 23. Macleod KF, Sherry N, Hannon G et al. p53‐dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev 1995; 9: 935–44. [DOI] [PubMed] [Google Scholar]
- 24. Shapiro GI. Cyclin‐dependent kinase pathways as targets for cancer treatment. J Clin Oncol 2006; 24: 1770–83. [DOI] [PubMed] [Google Scholar]
- 25. Goldsmith EJ, Cobb MH, Chang CI. Structure of MAPKs. Methods Mol Biol 2004; 250: 127–44. [DOI] [PubMed] [Google Scholar]
- 26. Johnson GL, Lapadat R. Mitogen‐activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002; 298: 1911–2. [DOI] [PubMed] [Google Scholar]
- 27. Molnár A, Theodoras AM, Zon LI, Kyriakis JM. Cdc42Hs, but not Rac1, inhibits serum‐stimulated cell cycle progression at G1/S through a mechanism requiring p38/RK. J Biol Chem 1997; 272: 13229–35. [DOI] [PubMed] [Google Scholar]
- 28. Casanovas O, Miró F, Estanyol JM, Itarte E, Agell N, Bachs O. Osmotic stress regulates the stability of cyclin D1 in a p38SAPK2‐dependent manner. J Biol Chem 2000; 275: 35091–7. [DOI] [PubMed] [Google Scholar]
- 29. Kimura Y, Okuda H. Resveratrol isolated from Polygonum cuspidatum root prevents tumor growth and metastasis to lung and tumor‐induced neovascularization in Lewis lung carcinoma‐bearing mice. J Nutr 2001; 131: 1844–9. [DOI] [PubMed] [Google Scholar]
- 30. Tseng SH, Lin SM, Chen JC et al. Resveratrol suppresses the angiogenesis and tumor growth of gliomas in rats. Clin Cancer Res 2004; 10: 2190–202. [DOI] [PubMed] [Google Scholar]
- 31. Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct alpha v integrins. Science 1995; 270: 1500–2. [DOI] [PubMed] [Google Scholar]
- 32. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86: 353–64. [DOI] [PubMed] [Google Scholar]
- 33. Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis 2000; 21: 505–15. [DOI] [PubMed] [Google Scholar]


