Abstract
An interventional pilot study to assess the tolerability and activity of the intralesional injection of rituximab, a chimeric mAb that targets the CD20 antigen, in patients with orbital B‐cell lymphoma. Five patients received four intralesional injections (one injection a week) of rituximab together with ropivicaine 2%. Side‐effects and tumor response were assessed after each injection and during the follow‐up (20 months). Two patients obtained complete remission of the intraorbital lesion. Two patients showed incomplete response after induction therapy and received planned escalating rituximab doses, obtaining regression of subjective symptoms. One patient did not achieve tumor regression after the first injection and underwent systemic treatment. This small exploratory study suggests that intralesional rituximab is a well‐tolerated treatment for patients with primary ocular adnexal lymphoma. These preliminary findings suggest that intralesional rituximab is a well‐tolerated strategy in anterior intraorbital lesion localization of lymphoma. (Cancer Sci 2011; 102: 1565–1567)
Several studies suggest that extranodal marginal zone lymphoma( 1 , 2 ) of mucosa‐associated lymphoid tissue (MALT) is the most common type of primary ocular adnexal lymphoma (OAL). Mucosa‐associated lymphoid tissue lymphoma is an indolent B‐cell non‐Hodgkin’s lymphoma (NHL) characterized by mitotic division of the marginal zone cells within the reactive follicles.( 3 ) It is most commonly seen in the elderly, most frequently affects the stomach, and normally follows an indolent course. It usually arises at sites of chronic inflammation secondary to persistent infections or autoimmune disorders.( 4 ) Ocular MALT lymphoma has been shown to be related to the Chlamydia psittaci infection( 5 ) but results from several studies are conflicting.( 3 , 6 ) The accepted criteria for the diagnosis of MALT are strong staining for CD20, with CD10, CD23, and BCL6 negativity. In order to assess the disease and to define the optimal treatment the following criteria should be considered: (i) histopathological subtype; (ii) extent of the disease (in the ocular region and systemically); and (iii) impact of the lymphoma on the eye and visual function. Thus, the treatment decision varies from patient to patient and uniform guidelines for the treatment of ophthalmic lymphoma have yet to be established.
It is now well accepted that localized proliferation of primary OAL can be controlled using radiotherapy.( 7 , 8 ) Chemotherapy alone or in combination with radiotherapy is considered to be standard therapy for patients with extraorbital involvement.( 9 ) However, there are significant adverse effects of the current treatment strategy.
Recent evidence shows that systemic treatment using the monoclonal anti‐CD20 antibody rituximab seems to be an effective, safe alternative for localized CD20+ OAL and for intraocular diffuse large B‐cell lymphoma.( 10 ) However, systemic treatment with rituximab is expensive and not without side‐effects. Intralesional injection of rituximab has also been reported to be effective and well tolerated in cutaneous B‐cell lymphoma( 11 , 12 ) and, recently, in conjunctival lymphomas.( 13 ) The effect of local injection of rituximab in deeper localizations of OAL, lacrimal gland, and orbital, has never been investigated in published reports.
The aim of this pilot study was to evaluate the toxicity and efficacy of intraorbital injection of rituximab for treatment of patients affected by primary localized CD20 positive orbital and lacrimal gland NHL.
Materials and Methods
Patients older than 18 years affected by symptomatic primary localized CD20 positive OAL, with orbital and lacrimal gland localizations, that had not previously been treated with chemotherapy or immunotherapy, were eligible. Patients with clinical, echographical, and radiological suspicion of OAL underwent biopsy and immunohistochemical staining for B‐cell lymphoma (comprehensive of CD20, CD5, CD10, k/l etc.) was carried out. We enrolled five patients (median age, 66 years; range, 54–77 years; three males and two females). Complete staging including full blood cell count, peripheral blood smears, biochemical parameters, bone marrow biopsy, PET and computed tomography scans of head, neck, chest, abdomen, and pelvis areas, were carried out to exclude systemic spread. The lesion was localized in superotemporal anterior orbit and lachrymal gland in two patients, in anterior orbital spaces with eyelid involvement in one patient, in posterior‐medium orbit in another patient, and in one case all superior orbital spaces were occupied by the lesion. Clinical information relating to each OAL is given in Table 1.
Table 1.
Clinical information, adverse drug reaction, clinical response, follow‐up, and treatment dosage in five patients with ocular adnexal lymphoma treated with intraorbital injection of rituximab
| Case | Age (years) | Sex | Orbital localization | Adverse drug reaction | Clinical response | Follow‐up | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | 77 | Male | Extraconic superotemporal anterior orbit (lachrymal gland involvement) | Pain at injection site | Stable disease with regression of subjective symptoms | 7 months | 140 mg rituximab (four injections, 5 mg each; 12 injections, 10 mg each) |
| 2 | 61 | Male | Extraconic superotemporal anterior orbit (lachrymal gland involvement) | Fever 5 h after injection | No response | Systemic treatment at end of planned therapy | 140 mg rituximab (four injections, 5 mg each; 12 injections, 10 mg each) |
| 3 | 72 | Male | Extraconic and intraconic superior orbit | None | Stable disease with regression of subjective symptoms | 8 months | 140 mg rituximab (four injections, 5 mg each; 12 injections, 10 mg each) |
| 4 | 54 | Female | Extraconic anterior orbit and eyelid | Pain at injection site | Complete local remission | 7 months Abdominal involvement of lymphoproliferative disease | 100 mg rituximab (four injections, 5 mg each; eight injections, 10 mg each) |
| 5 | 66 | Female | Extraconic medial anterior and medium orbit | None | Complete remission | 20 months | 40 mg rituximab (eight injections, 5 mg each) |
All patients received the planned treatment schedule consisted of intraorbital injection of rituximab (MabThera 100 mg; Roche, Basel, Switzerland) 5 mg once a week for 1 month (four injections/month). The treatment was done in the surgery room, after eyelid iodopovidone disinfection and injection of 2 mL ropivacaine to reduce orbital pain 10 min before rituximab injection. The drug (5 mg/1 mL) was slowly injected using a 27‐gauge needle, paying attention to the pupil size and the onset of exophthalmos. This method can be considered relatively easy and reproducible by an ophtalmologist, not having a greater difficulty than a peri or retrobulbar injection. Patients were then treated with levofloxacin ophthalmic eyedrops, applied four times daily for 7 days, and bandaged. After the first cycle, patients with complete remission underwent a second cycle with the same dosage of rituximab 8 weeks later. In case of incomplete response after induction therapy, patients received a higher dose of rituximab (10 mg/1 mL), for three more cycles. The pattern of peripheral blood lymphocytes was examined monthly throughout the study period. The response was detected by clinical examination, echography and MRI after each cycle of rituximab, and only patients who achieved complete remission stopped the therapy. At the achievement of complete remission or at the end of the study, disease status in all patients was confirmed by PET. Patients who showed progressive disease were withdrawn from the study. All patients provided written signed informed consent indicating that they were aware of the investigational nature and potential risks and benefits of the study, in accordance with the Ethics Committee of the Catholic University of the Sacred Heart (Rome, Italy) and the Declaration of Helsinki. This study was approved by an internal Ethics Committee. Photographs were obtained in selected cases with patients’ permission.
Results
Two patients (cases 4 and 5) obtained complete remission of the intraorbital lesion after three and two cycles, respectively. In case 4, eyelid involvement showed lasting orbital remission until 7 months after treatment, three cycles, when a PET scan showed enhancement of abdominal lymph nodes consistent with systemic relapse of primary disease and was referred to the hematologist. Case 5, with extraconic anterior and medium orbital spaces localization, had a complete response to the first cycle of therapy. A second cycle with the same dosage was given 8 weeks later, and at 20 months of follow‐up was in continuous complete remission detected by clinical echography, PET (Fig. 1), and MRI (Fig. 2).
Figure 1.
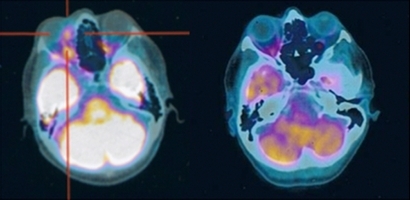
Pretreatment PET scan of patient 5 affected by a left extraconic medial anterior and medium orbital lymphoma (red target) (left panel). Post‐treatment PET scan (at 6 months) shows complete lymphoma remission (right panel).
Figure 2.
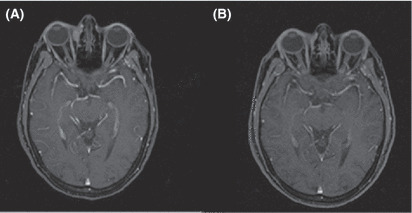
(A) Pretreatment MRI of patient 5 affected by left medial orbital lymphoma localization. (B) Complete lymphoma remission (at 6 months) after intraorbital injection of rituximab.
Two patients (cases 1 and 3) showed incomplete response after induction therapy with 5 mg intraorbital rituximab for four injections. Both patients received planned escalating rituximab dose (10 mg each injection for 12 injections), obtaining regression of subjective symptoms (weeping and pink eye) even in the presence of residual disease evaluated by echographical and PET scans. At 12 months of follow‐up, no signs of disease progression or ocular toxicity were detected.
At the end of planned therapy, only one patient (case 2) with medium and posterior orbital spaces localization, experienced progressive disease and subsequently underwent systemic treatment.
No patients experienced adverse drug reaction, but one patient developed fever 5 h after injection, that lasted only 3 h. Two patients reported pain on the site of the injection after the first intraorbital rituximab treatment. For this reason, we injected 2 mL ropivacaine 10 min before each rituximab treatment in the same orbital zone. Treatment schedules, adverse drug reactions, and clinical outcomes for each patient are presented in Table 1.
Discussion
Rituximab is a mAb that binds the CD20 antigen, persists in circulation for long periods, causes the lysis of B cells by complement‐dependent cytotoxicity and antibody‐dependent cytotoxicity, induces apoptosis of malignant cells, and makes tumor cells more susceptible to chemotherapeutic agents through a synergistic effect.( 14 ) It produces response in approximately 50% of patients with relapsed B‐cell NHL( 15 , 16 ) and approximately 75% of patients with untreated follicular NHL.( 17 ) Rituximab is now an established part of induction and salvage chemotherapy regimens for B‐cell NHL.( 18 , 19 ) The typical schedule is 375 mg/m2 i.v. each week for four doses. Generally, this drug is well tolerated and is not associated with significant myelosuppression, but systemic adverse effects have been reported such as fever (73.0%), nausea (18.9%), thrombocytopenia (18.9%), and hypotension (16.2%).( 20 ) The few published case series show the high activity of rituximab in both newly diagnosed and relapsed OAL, but early recurrence is common, particularly in pre‐treated patients.( 21 ) The treatment is nevertheless very expensive.
Standard treatment of ocular adnexal MALT lymphomas consists of local radiotherapy, although this approach is associated with frequent relapses and complications such as cataract formation, corneal ulceration, and eye dryness at long term follow‐up.( 22 ) These localized complications need to be kept in mind by the treating oncologist.
Intralesional injection of rituximab has also been reported to be effective and well tolerated in cutaneous B‐cell lymphoma( 11 , 12 ) and conjunctival B‐cell lymphoma.( 13 ) In our patients, the intraorbital lesions were well circumscribed and we carried out the intraorbital injections in order to reduce systemic side‐effects and costs. In this report, we confirm that intralesional injection of rituximab in the treatment of primary orbital ocular adnexa CD20+ lymphomas could be considered as an effective choice of therapy with negligible side‐effects and good response rate. It can also be repeated if relapse or progressive disease is detected. The response to intraorbital rituximab injection seems to be useful in anterior intraorbital lesion localization of lymphoma. In cases of medium and posterior intraorbital localization we reported an incomplete response in two patients and did not achieve any response in one patient.
This is the first report of successful treatment of orbital OAL by intraorbital injection of rituximab. However, the long‐term effects are not known. Further studies would assess the optimal dosage of rituximab in relation to the lesion localization, particularly regarding the deep intraconic orbital involvement.
Intraorbital injection with low doses of rituximab in primary ocular adnexal CD20+ lymphoma seems to be a safe and useful therapy with negligible side‐effects compared with chemo/radiotherapy or high doses of rituximab given i.v. In addition, use of intraorbital rituximab does not preclude systemic option therapy. Further studies are warranted to clarify long‐term effects and the relation between orbital localization of primary ocular adnexal lymphoma and optimal local dosage of rituximab.
Disclosure Statement
The authors have no conflict of interest to report.
References
- 1. Coupland SE, Hummel M, Stein H. Ocular adnexal lymphomas: five case presentations and a review of the literature. Surv Ophthalmol 2002; 47: 470–90. [DOI] [PubMed] [Google Scholar]
- 2. Bardenstein DS. Ocular adnexal lymphoma: classification, clinical disease, and molecular biology. Ophthalmol Clin North Am 2005. 18: 187–97. [DOI] [PubMed] [Google Scholar]
- 3. Daibata M, Nemoto Y, Togitani K et al. Absence of Chlamydia psittaci in ocular adnexal lymphoma from Japanese patients. Br J Haematol 2006; 132: 651–2. [DOI] [PubMed] [Google Scholar]
- 4. Pelstring RJ, Zellmer RB, Sulak LE. Hodgkin’s disease in association with human immunodeficiency virus infection. Cancer 1991; 67: 1865–73. [DOI] [PubMed] [Google Scholar]
- 5. Ferreri AJ, Guidoboni M, Ponzoni M et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst 2004; 96: 586–94. [DOI] [PubMed] [Google Scholar]
- 6. Mulder MM, Heddema ER, Pannekoek Y et al. No evidence for an association of ocular adnexal lymphoma with Chlamydia psittaci in a cohort of patients from the Netherlands. Leuk Res 2006; 30: 1305–7. [DOI] [PubMed] [Google Scholar]
- 7. Tsang RW, Gospodarowicz MK, Pintilie M et al. Localized mucosa‐associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol 2003; 21: 4157–64. [DOI] [PubMed] [Google Scholar]
- 8. Uno T, Isobe K, Shikama N et al. Radiotherapy for extranodal, marginal zone, B‐cell lymphoma of mucosa‐associated lymphoid tissue originating in the ocular adnexa: a multiinstitutional, retrospective review of 50 patients. Cancer 2003; 98: 865–71. [DOI] [PubMed] [Google Scholar]
- 9. Coupland SE, Heimann H, Bechrakis NE. Primary intraocular lymphoma: a review of the clinical, histopathological and molecular biological features. Graefes Arch Clin Exp Ophthalmol 2004; 242: 901–13. Epub 2004 October 29. [DOI] [PubMed] [Google Scholar]
- 10. Rubenstein JL, Fridlyand J, Abrey L et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol 2007; 25: 1350–6. [DOI] [PubMed] [Google Scholar]
- 11. Kerl K, Prins C, Saurat JH et al. Intralesional and intravenous treatment of cutaneous B‐cell lymphomas with the monoclonal anti‐CD20 antibody rituximab: report and follow‐up of eight cases. Br J Dermatol 2006; 155: 1197–200. [DOI] [PubMed] [Google Scholar]
- 12. Roguedas AM, Watier H, Paintaud G et al. Intralesional therapy with anti‐CD20 monoclonal antibody rituximab: local and systemic efficacy in primary cutaneous B‐cell lymphoma. Br J Dermatol 2005; 152: 541–4. [DOI] [PubMed] [Google Scholar]
- 13. Ferreri AJ, Govi S, Colucci A et al. Intralesional rituximab a new therapeutic approach for patients with conjunctival lymphomas. Ophthalmology 2011; 118: 24–8. [DOI] [PubMed] [Google Scholar]
- 14. Kimby E. Tolerability and safety of Rituximab (Mabthera). Cancer Treat Rev 2005; 31: 456–73. [DOI] [PubMed] [Google Scholar]
- 15. Tobinai K, Kobayashi Y, Narabayashi M et al. Feasibility and pharmacokinetic study of a chimeric anti‐CD20 monoclonal antibody (IDEC‐C2B8, rituximab) in relapsed B‐cell lymphoma. The IDEC‐C2B8 Study Group. Ann Oncol 1998; 9: 527–34. [DOI] [PubMed] [Google Scholar]
- 16. Pride MW, Shuey S, Grillo‐Lopez A et al. Enhancement of cell‐mediated immunity in melanoma patients immunized with murine anti‐idiotypic monoclonal antibodies (MELIMMUNE) that mimic the high molecular weight proteoglycan antigen. Clin Cancer Res 1998; 4: 2363–70. [PubMed] [Google Scholar]
- 17. Witzig TE, Vukov AM, Habermann TM et al. Rituximab therapy for patients with newly diagnosed, advanced‐stage, follicular grade I non‐Hodgkin’s lymphoma: a phase II trial in the North Central Cancer Treatment Group. J Clin Oncol 2005; 23: 1103–8. [DOI] [PubMed] [Google Scholar]
- 18. Habermann TM, Weller EA, Morrison VA et al. Rituximab‐CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B‐cell lymphoma. J Clin Oncol 2006; 24: 3121–7. [DOI] [PubMed] [Google Scholar]
- 19. Coiffier B. Rituximab in combination with CHOP improves survival in elderly patients with aggressive non‐Hodgkin’s lymphoma. Semin Oncol 2002; 29 (Suppl 6): 18–22. [DOI] [PubMed] [Google Scholar]
- 20. Maloney DG, Grillo‐López AJ, White CA et al. IDEC‐C2B8 (Rituximab) anti‐CD20 monoclonal antibody therapy in patients with relapsed low‐grade non‐Hodgkin’s lymphoma. Blood 1997; 90: 2188–95. [PubMed] [Google Scholar]
- 21. Nückel H, Meller D, Steuhl KP et al. Anti‐CD20 monoclonal antibody therapy in relapsed MALT lymphoma of the conjunctiva. Eur J Haematol 2004; 73: 258–62. [DOI] [PubMed] [Google Scholar]
- 22. Liao SL, Kao SC, Hou PK, Chen MS. Results of radiotherapy for orbital and adnexal lymphoma. Orbit 2002; 21: 117–23. [DOI] [PubMed] [Google Scholar]


