Abstract
The Wilms’ tumor 1 (WT1) gene is overexpressed in leukemia and various types of solid tumor, such as lung and colorectal cancer, and plays an oncogenic role in their tumorigenesis. Recent studies have demonstrated the potential of WT1‐targeting cancer immunotherapy in clinical settings. As expression of WT1 protein in tumor cells is a prerequisite for WT1‐targeting immunotherapy, immunohistochemical methods to detect WT1 protein with high sensitivity and specificity are required. In the present study, we developed a rabbit polyclonal antibody (WT1‐R) against the 9‐mer WT1 235 peptide, which is used for vaccination. The specificity of WT1‐R was confirmed by immunoprecipitation, western blotting analysis, and competitive enzyme‐linked immunosorbent assay. Immunocytochemistry showed the same reactivity against five cell lines (K562, Daudi, HT‐180, SW480, and PC‐14), whereas levels of WT1 mRNA expression determined by real‐time qPCR (RT‐PCR) analysis were not equivalent. Next, we examined the reactivity of WT1‐R in tissue samples compared with a previously developed anti‐WT1 antibody, 6F‐H2. WT1‐R showed greater sensitivity for detecting WT1 protein expression in samples from four different breast cancer patients than 6F‐H2 antibody. The discrepancy in WT1 expression between these methods suggested that immunohistochemical detection of WT1 peptide may be advantageous for predicting the efficacy of WT1 vaccine compared to RT‐PCR, and the highly sensitive WT1 antibody, WT1‐R, may be useful to detect WT1 protein in tumors.
(Cancer Sci 2010; 101: 1089–1092)
Wilms’ tumor 1 (WT1) mRNA is expressed at high levels in hematological malignancies and various cancers, while normal tissue shows only low levels of its expression.( 1 , 2 , 3 , 4 , 5 , 6 ) The specific overexpression of WT1 in malignant cells makes it an attractive potential target for immunotherapy, including WT1‐targeting vaccine therapy.( 7 , 8 , 9 , 10 , 11 ) A peptide has already been developed as a vaccine and its clinical efficacy has been evaluated.( 12 , 13 )
Precise determination of WT1 protein expression in tumors would be useful to predict the efficacy of WT1 vaccine. Although the real‐time quantitative PCR (RT‐PCR) method is commonly used to measure WT1 expression, it is not a direct method to evaluate expression of the target of WT1 vaccine, which is a small part of the WT1 protein. In this regard, immunohistochemical analysis using antibodies that recognize peptide sequences in WT1 protein corresponding to the peptide target of WT1 vaccine may be better correlated with efficacy of the vaccine compared to the RT‐PCR method. In addition, immunohistochemical analysis is sometimes preferred to RT‐PCR as most solid tumors are diagnosed by histopathological analysis of paraffin‐embedded tissues. Immunohistochemical analysis has a number of benefits for estimating the efficacy of WT1 vaccine, but no antibodies for this purpose are commercially available.( 14 , 15 , 16 , 17 , 18 , 19 ) Here, we developed an antibody that is specific for a peptide corresponding to the region recognized by the WT1 vaccine and examined its specificity and reactivity.
Materials and Methods
Primary antibodies. A peptide corresponding to the wild‐type human WT1 (WT1 235 peptide, CMTWNQMNL) was synthesized( 11 ) and coupled to bovine thymoglobulin as a carrier protein. This complex of peptide and protein was used as an immunogen and used to immunize three rabbits four times every 2 weeks. The sera from all rabbits showed a high anti‐WT1 peptide antibody titer 1 week after the last immunization (Immuno‐Biological Laboratories, Gunma, Japan). A sufficient amount of blood volume was collected from the rabbits 9 weeks after the last immunization, and the serum samples were purified using Thiol Sepharose 4B (Amersham Pharmacia Biotech, Piscataway, NJ, USA) coupled with antigen peptide. This purified solution was used as anti‐WT1 antibody (WT1‐R). In addition, mouse monoclonal antibodies to WT1 (clone 6F‐H2; Dako Cytomation, Carpinteria, CA, USA) and to glutathione S‐transferase (GST; clone 40B3; BioPortfolio, Dorset, UK) were also used for comparison.
Purification of recombinant WT1. GST‐WT1 and GST‐delWT1 were produced according to the methods described previously. (20) Briefly, full‐length WT1 and part of the WT1 gene corresponding to amino acids 180–324 were ligated into the pGEX‐5X‐3 vector (GE Healthcare, Buckinghamshire, UK) and transfected into E. coli. After collection of bacterial cells and extraction of the protein, GST‐WT and GST‐delWT1 protein were purified and used for western blotting analysis.
Immunoprecipitation. Aliquots of 25 μg of GST‐WT1 or GST‐delWT1 and GST protein were incubated with 3 μg of purified anti‐WT1 polyclonal antibody at 4°C overnight, and the immune complexes were collected by incubation with protein G‐Sepharose beads at 4°C for 1 h. The beads were washed with TNE buffer (10 mm Tris‐HCl [pH 7.5], 0.1 m NaCl, 1 mm EDTA), and the proteins were eluted with sodium dodecyl sulfate (SDS) sample buffer.
Western blotting analysis. Denatured proteins were fractionated by SDS–polyacrylamide gel electrophoresis and subsequently blotted onto polyvinylidene difluoride membranes. The filters were blocked with PBS containing 3.3% nonfat milk, 1% bovine serum albumin, and 0.05% NaN3 at 37°C for 2 h, and then incubated overnight with the primary antibody. After binding of relevant peroxidase‐conjugated secondary antibodies, the filters were developed with ECL (GE Healthcare).
Competitive enzyme‐linked immunosorbent assay (ELISA). Ninety‐six‐well plates were coated with GST‐WT1 protein (50 ng/well) at 4°C overnight, and then blocked with 0.1% casein in PBS at 4°C overnight. Serial dilutions of WT1 peptide were incubated with anti‐WT1 antibody (WT1‐R) at a concentration of 3 μg/mL at 4°C overnight. The mixtures were added to GST‐WT1‐coated plates and incubated at 37°C for 30 min. After incubation with peroxidase‐conjugated antirabbit IgG, absorbance was determined at 492 nm with an ELISA reader.
Real‐time quantitative‐PCR (RT‐PCR). For RT‐PCR, total RNA was extracted from each cell line and reverse‐transcribed using a Transcriptor First Strand cDNA Synthesis kit (Roche Diagnostics, Tokyo, Japan). Quantitative PCR was performed using a LightCycler (Roche Diagnostics) with LightCycler Fast Start DNA Master SYBR Green I (Roche Diagnostics) according to the manufacturer’s protocol. The primers used for PCR were as follows. Human GAPDH forward: 5′‐TGAACGGGAAGCT‐CACTGG‐3′, reverse: 5′‐TCCACCACCCTGTTGCTGTA‐3′; Human WT‐1 forward: 5′‐CCAGGCTGCAATAAGAGATA‐3′, reverse: 5′‐TCTTTTGAGCTGGTCTGAA‐3′. PCR for human GAPDH was performed for 40 cycles consisting of 95°C for 10 s, 60°C for 10s, and 72°C for 12 s. Human WT‐1 was amplified by 40 cycles of 95°C for 10 s, 62°C for 10 s, and 72°C for 5 s.
Immunohistochemistry. For immunohistochemical staining, cells were fixed in PBS with 10% formaldehyde and then these fixed cells were embedded in agar blocks and sections were then cut using a microtome for immunostaining. Breast cancer and normal gastric mucosa specimens were obtained with informed consent (Pathology Institute Corporation, Toyama, Japan), and antigen retrieval was performed after deparaffinization of the slides by heating the sections in Tris‐buffered saline, pH 9.0, in a water bath at 95°C for 40 min. Sections were allowed to cool to room temperature and endogenous peroxidase activity was blocked with 3% H2O2. The sections were then incubated with WT1‐R or 6F‐H2 as the primary antibody at a dilution of 1:500 (WT1‐R) or 1:50 (6F‐H2) for 30 min, followed by detection using the Dako EnVision/Polymer System (Dako, Ely, Cambridgeshire, UK). Sections were lightly counterstained with hematoxylin.
Results
Anti‐WT1 polyclonal antibody WT1‐R binds specifically to GST‐WT1 protein and 9‐mer peptide corresponding to WT1 vaccine antigen. To assess antibody reactivity, WT1‐R was serially diluted and its binding to plates coated with GST‐WT1 protein and GST protein was examined. WT1‐R bound strongly to GST‐WT1‐coated plates, whereas no binding was observed on GST‐coated plates (Fig. 1A). To evaluate the specificity of WT1‐R antibody, we performed competitive ELISA with WT1 peptide. As shown in Figure 1(B), antibody binding to GST‐WT1 was markedly decreased by preincubation with WT1 peptide compared to control (PBS). Next, we examined antibody binding by immunoprecipitation and Western blotting analysis. As shown in Figure 1(C), GST‐WT1 protein was immunoprecipitated by WT1‐R and anti‐GST antibodies and detected by anti‐GST antibody (lanes 4 and 6). Figure 1(D) shows the results of western blotting analysis using WT1‐R as the primary antibody. In this experiment, we used GST‐delWT1 protein, which included the amino acid sequence targeted by WT1‐R. Purified GST‐delWT1 protein (lane 2) was detected by WT1‐R, whereas WT1‐R did not react with GST protein (lane 1). GST and GST‐delWT1 protein were immunoprecipitated with control (rabbit IgG), anti‐GST, or anti‐GST antibody before western blotting, and WT1‐R reacted only with GST‐delWT1 immunoprecipitated by WT1‐R (lane 6) and anti‐GST antibody (lane 8). These bands disappeared when the antibodies were preincubated with 9‐mer WT1‐peptide before immunoprecipitation (lane 9). These results indicated that WT1‐R antibody could detect WT1 protein and the binding was specific for part of WT1 protein corresponding to the 9‐mer peptide used for WT1 vaccine.
Figure 1.
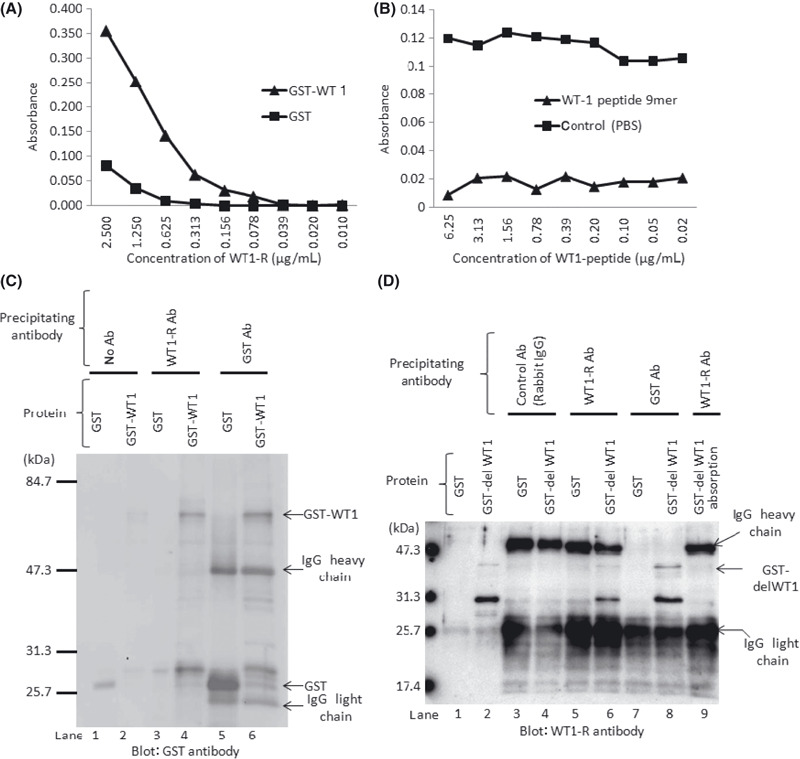
Reactivity of WT1‐R antibody against 9‐mer peptide region of Wilms’ tumor 1 (WT1) protein targeted by WT1 vaccine. (A) Dilution curves of WT1‐R. Plates coated with WT1‐GST or GST protein were incubated with serial dilutions of WT1‐R antibody and the binding of WT1‐R antibody to plates was determined. (B) Results of competitive ELISA. Serially diluted WT1 peptide was incubated with or without 3 mg/mL of WT1‐R antibody, and this mixture was then added to GST‐WT1‐coated plates. (C,D) Western blotting analysis with GST or WT1‐R antibody. Proteins were immunoprecipitated with the antibodies indicated above before electrophoresis.
Immunohistochemical analysis of WT1‐R antibody. Immuno‐histochemical analysis of five cell lines (K562, Daudi, HT‐1080, SW480, and PC‐14) was performed and the results were compared with those of RT‐PCR. As shown in Figure 2, K562 (WT1 copy number/GAPDH copy number: 0.048), Daudi (0.061), and SW480 (0.042) showed about 5–6‐fold higher levels of expression than HT‐1080 (0.01), and the expression level in PC‐14 was almost 1000‐fold lower (0.000052) than in the other lines. These results indicated that the expression was not equivalent in these cell lines although they expressed WT1. On the other hand, in contrast to the difference in WT1 mRNA expression between cell lines, immunohistochemical analysis with WT1‐R showed almost the same binding intensity in these cell lines (Fig. 2).
Figure 2.

Comparison between immunohistochemical staining with WT1‐R and real‐time quantitative PCR (RT‐PCR) to detect Wilms’ tumor 1 (WT1) expression in five cell lines (Daudi, PC‐14, K562, HT1080, and SW480). RT‐PCR results are shown beneath the photographs. Magnification, ×400.
Next, we compared the reactivity of WT1‐R with that of 6F‐H2 antibody in normal gastric mucosa tissue and breast cancer tissue samples obtained from four different patients (Fig. 3). In normal gastric mucosa, plasma cells showed nonspecific staining with 6F‐H2 antibody, but were completely negative for staining by WT1‐R with no nonspecific binding (Fig. 3A). In breast cancer tissues, immunostaining with 6F‐H2 showed weak positive reactivity in breast cancer samples, while staining with WT1‐R was clearly positive in both the nucleus and cytoplasm of breast cancer cells and the staining intensity was significantly higher with WT1‐R than in 6F‐H2 (Fig. 3B). These observations indicated that WT1‐R antibody is more sensitive for detection of WT1 protein in breast cancer than 6F‐H2 antibody.
Figure 3.

Immunohistochemical analysis of normal gastric mucosa (A) and breast cancer tissue samples from four different patients (B). In (B), the upper and lower photographs show the results of immunohistochemical staining with 6F‐H2 and with WT1‐R, respectively. Magnification, ×120.
Discussion
RT‐PCR is widely employed for determination of WT1 expression.( 11 , 21 ) However, the results of RT‐PCR are not always correlated with protein expression( 22 ) and this method is not suitable for tumors that consist of cells of various types, including malignant and non‐malignant cells, because the results are dependent on the proportion of malignant to normal cells. Therefore, immunohistochemical analysis of WT1 expression in solid tumors may be a better option than RT‐PCR. In the present study, the results of immunohistochemical analysis of WT1 expression were not correlated with those of RT‐PCR. The level of WT1 mRNA transcript expression in PC‐14 was very weak, but protein expression level was almost the same as in the other cell lines. This observation suggested that WT1 mRNA expression is not equivalent to its protein expression. A discrepancy between WT1 mRNA and protein expression was reported previously in childhood leukemia.( 22 ) These results suggested that immunohistochemical analysis is important to predict the efficacy of WT1 vaccines. The WT1‐R antibody developed in the present study shows sensitivity for detection of WT1 protein and may be useful for immunohistochemical analysis.
Currently, there is no standard method for immunohistochemical analysis of WT1 because of a lack of appropriate antibodies. It has already been reported that staining results with 6F‐H2 and another antibody against WT1 showed marked differences in some types of tumor.( 21 ) The WT1‐R antibody developed in the present study showed high sensitivity for detection of WT1 protein in breast cancer samples compared with 6F‐H2 antibody, and may be appropriate for immunohistochemical analysis of WT1. Further studies of the sensitivity and specificity of WT1‐R antibody in various types of cancer are required.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgment
We thank Y. Tani (Pathology Institute Corporation Ltd., Toyama, Japan) for pathology specimens and performing the immunohistochemical analyses.
References
- 1. Oji Y, Miyoshi S, Maeda H et al. Overexpression of the Wilms’ tumor gene WT1 in de novo lung cancers. Int J Cancer 2002; 100: 297–303. [DOI] [PubMed] [Google Scholar]
- 2. Ueda T, Oji Y, Naka N et al. Overexpression of the Wilms’ tumor gene WT1 in human bone and soft‐tissue sarcomas. Cancer Sci 2003; 94: 271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oji Y, Yamamoto H, Nomura M et al. Overexpression of the Wilms’ tumor gene WT1 in colorectal adenocarcinoma. Cancer Sci 2003; 94: 712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oji Y, Yano M, Nakano Y et al. Overexpression of the Wilms’ tumor gene in esophageal cancer. Anticancer Res 2004; 24: 3103–8. [PubMed] [Google Scholar]
- 5. Oji Y, Suzuki T, Nakano Y et al. Overexpression of the Wilms’ tumor gene W T1 in primary astrocytic tumors. Cancer Sci 2004; 95: 822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oji Y, Nakamori S, Fujikawa M et al. Overexpression of the Wilms’ tumor gene WT1 in pancreatic ductal adenocarcinoma. Cancer Sci 2004; 95: 583–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oka Y, Udaka K, Tsuboi A et al. Cancer immunotherapy targeting Wilms’ tumor gene WT1 product. J Immunol 2000; 164: 1873–80. [DOI] [PubMed] [Google Scholar]
- 8. Oka Y, Tsuboi A, Elisseeva OA, Udaka K, Sugiyama H. WT1 as a novel target antigen for cancer immunotherapy. Curr Cancer Drug Targets 2002; 2: 45–54. [DOI] [PubMed] [Google Scholar]
- 9. Nakajima H, Kawasaki K, Oka Y et al. WT1 peptide vaccination combined with BCG‐CWS is more efficient for tumor eradication than WT1 peptide vaccination alone. Cancer Immunol Immunother 2004; 53: 617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oka Y, Tsuboi A, Taguchi T et al. Induction of WT1 (Wilms’ tumor gene)‐specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci U S A 2004; 101: 13885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oka Y, Tsuboi A, Kawakami M et al. Development of WT1 peptide cancer vaccine against hematopoietic malignancies and solid cancers. Curr Med Chem 2006; 13: 2345–52. [DOI] [PubMed] [Google Scholar]
- 12. Morita S, Oka Y, Tsuboi A et al. A phase I/II trial of a WT1 (Wilms’ tumor gene) peptide vaccine in patients with solid malignancy: safety assessment based on the phase I data. Jpn J Clin Oncol 2006; 36: 231–6. [DOI] [PubMed] [Google Scholar]
- 13. Izumoto S, Tsuboi A, Oka Y et al. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg 2008; 108: 963–71. [DOI] [PubMed] [Google Scholar]
- 14. Foster MR, Johnson JE, Olson SJ, Allred DC. Immunohistochemical analysis of nuclear versus cytoplasmic staining of WT1 in malignant mesotheliomas and primary pulmonary adenocarcinomas. Arch Pathol Lab Med 2001; 125: 1316–20. [DOI] [PubMed] [Google Scholar]
- 15. Chen BF, Tzen CY, Liang DC, Liu HC, Huang YW, Fan CC. Immuno‐histochemical expression of Wilms’ tumor 1 protein in nephroblastoma. J Chin Med Assoc 2004; 67: 506–10. [PubMed] [Google Scholar]
- 16. Waldstrøm M, Grove A. Immunohistochemical expression of Wilms tumor gene protein in different histologic subtypes of ovarian carcinomas. Arch Pathol Lab Med 2005; 129: 85–8. [DOI] [PubMed] [Google Scholar]
- 17. Wilsher M, Cheerala B. WT1 as a complementary marker of malignant melanoma: an immunohistochemical study of whole sections. Histopathology 2007; 51: 605–10. [DOI] [PubMed] [Google Scholar]
- 18. Kushitani K, Takeshima Y, Amatya VJ, Furonaka O, Sakatani A, Inai K. Immunohistochemical marker panels for distinguishing between epithelioid mesothelioma and lung adenocarcinoma. Pathol Int 2007; 57: 190–9. [DOI] [PubMed] [Google Scholar]
- 19. Ellison DA, Parham DM, Bridge J, Beckwith JB. Immunohistochemistry of primary malignant neuroepithelial tumors of the kidney: a potential source of confusion? A study of 30 cases from the National Wilms Tumor Study Pathology Center. Hum Pathol 2007; 38: 205–11. [DOI] [PubMed] [Google Scholar]
- 20. Oji Y, Kitamura Y, Kamino E et al. WT1 IgG antibody for early detection of nonsmall cell lung cancer and as its prognostic factor. Int J Cancer 2009; 125: 381–7. [DOI] [PubMed] [Google Scholar]
- 21. Nakatsuka S, Oji Y, Horiuchi T et al. Immunohistochemical detection of WT1 protein in a variety of cancer cells. Mod Pathol 2006; 19: 804–14. [DOI] [PubMed] [Google Scholar]
- 22. Kerst G, Bergold N, Gieseke F et al. WT1 protein expression in childhood acute leukemia. Am J Hematol 2008; 83: 382–6. [DOI] [PubMed] [Google Scholar]


