Abstract
Progressive multifocal leukoencephalopathy (PML) is a fatal demyelinating disease that results from an oligodendrocyte infection caused by JC virus. The JC virus early promoter directs cell-specific expression of the viral replication factor large T antigen, and thus transcriptional regulation constitutes a major mechanism of glial tropism in PML. We have previously demonstrated that T antigen controls the JC virus basal promoter in a glial cell-specific manner, since T antigen repressed the JC virus and simian virus 40 (SV40) early promoters in glioma cells but induced strong activation of the JC virus early promoter in nonglial cells. To further analyze these findings, T antigen and nuclear extracts from glial and nonglial cells were used to examine DNase I footprints on the proximal promoter. T-antigen binding to site II was more extensive than expected based on sequence homology with SV40, and nuclear proteins protected several regions of the proximal promoter in a cell-specific manner. Multiple Sp1 binding domains were identified. Site-directed mutagenesis revealed that T-antigen-mediated activation required a TATA box sequence, a pentanucleotide repeat immediately upstream of the TATA box, and an Sp1 binding site downstream of the TATA box. When footprints were obtained with mutant promoters which blocked T-antigen-induced transactivation, no change in T-antigen binding was observed. These results suggest that T antigen activates the JC virus basal promoter in nonglial cells by interaction with the transcription initiation complex.
The human polyomavirus JC virus is the etiologic agent of progressive multifocal leukoencephalopathy (PML). JC virus selectively destroys oligodendrocytes, leading to multiple areas of demyelination and attendant loss of brain function (3, 31). Once a rare condition, PML is no longer infrequent, occurring in 5% of individuals with AIDS (4). JC virus infection exists in a persistent state in kidney tissue and peripheral blood lymphocytes throughout the life of healthy individuals. In the setting of immunodeficiency, the virus infects and destroys oligodendrocytes, producing patches of myelin loss in subcortical white matter (22). Thus, the neuropathological features suggest that reactivated JC virus infection is specific for glial cells.
The JC virus early promoter directs cell-specific expression of the large T antigen, which is required for viral replication, and thus transcriptional regulation constitutes a major mechanism of glial tropism of PML (15). The MH1 form of the JC virus promoter was directly isolated from the brain of a PML patient. Its sequence diverges somewhat from that of the original Mad-1 promoter (11); however, both MH1 and Mad-1 proximal promoters have sequence identity from the initiating codon for T antigen up to a pentanucleotide site just upstream of the viral TATA sequence. The pentanucleotide repeat sequence in MH1 varies by a single base from the perfect repeats seen in the Mad-1 variant of the promoter. This sequence conservation implies that this region could be important for cell specificity.
In a transient transfection analysis, the MH1 JC virus early promoter activated 30- to 40-fold more transcription in U87MG glioma cells than in HeLa cells (12). Deletion analysis showed that the tandem repeats of the enhancer region activated gene expression in both glial and nonglial cells, suggesting that the upstream enhancer region is more important for promoter strength than for cell specificity. Furthermore, the proximal promoter region alone directed 19-fold more activity in glial cells than in nonglial cells (13, 19). Thus, the JC virus basal promoter region is able to direct glial cell-specific gene expression.
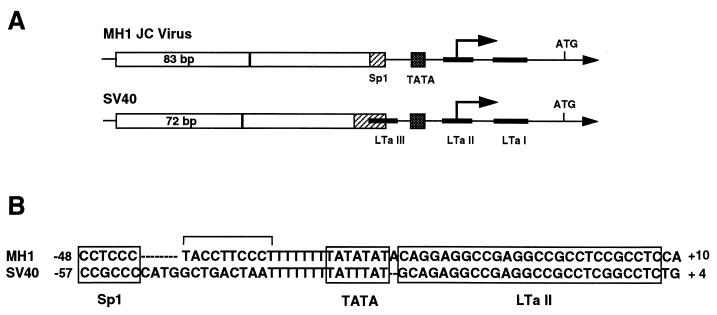
The proximal region of the JC virus promoter contains two sequence homologies with the T-antigen binding sites present in simian virus 40 (SV40) (Fig. 1). In SV40, T antigen represses its own expression by binding to three sites (LTa I, II, and III) (25). To evaluate regulation of the JC virus proximal promoter, we coexpressed JC virus T antigen with the MH1luc reporter in U87MG glioma cells. Increasing levels of T antigen produced the anticipated four- to fivefold repression of the JC virus and SV40 early promoters. In HeLa cells, by comparison, T antigen produced extremely strong transcriptional activation of the JC virus promoter, whereas the SV40 promoter was still repressed fivefold (13). Analysis of deletion mutants showed that T-antigen activation in nonglial cells required only the basal promoter region. Thus, T antigen produced glial cell-specific, divergent regulation of the JC virus basal promoter.
FIG. 1.
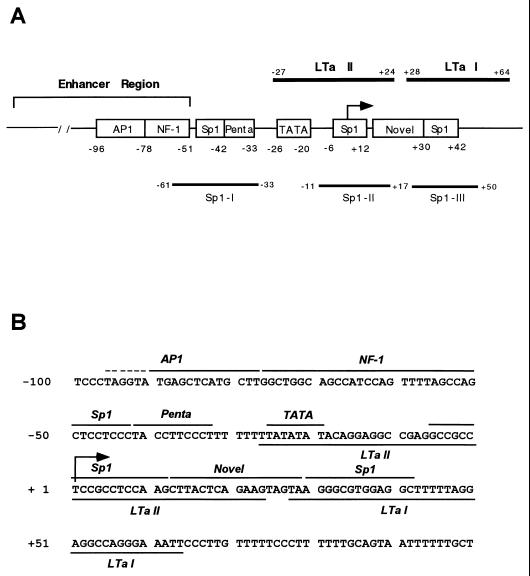
(A) Schematic of the MH-1 JC virus and SV40 early promoters. Open boxes indicate direct tandem repeats of the indicated number of base pairs, dotted boxes represent TATA homologies, and striped boxes represent Sp1 binding sites upstream of the TATA sequence. The SV40 promoter contains three binding sites for the viral protein large T antigen (black boxes), whereas the JC virus early promoter contains only sites LTa I and II, based on sequence homology with SV40 promoter. (B) Comparison of basal promoter region DNA sequences revealing differences between JC virus and SV40 in the region between the Sp1 binding sites and the second T-antigen binding site.
In this study, we analyzed this cell-specific regulation by DNase I footprinting, using T antigen and glial and nonglial nuclear extracts. In addition, by site-directed mutagenesis, we identified three sequences which are critical for T-antigen activation. T-antigen binding was not affected by these mutations. The results suggest that the initiation complex that forms on the JC virus early promoter participates in cell-specific transcription.
MATERIALS AND METHODS
Cell culture and transient transfection assays.
U87MG glioma and HeLa cell lines were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (HyClone), streptomycin, and penicillin.
Transfection was performed by a standard calcium phosphate method. Cells (2 × 105 in 60-mm-diameter dishes) were transfected with 4 μg of the reporter construct, 1 μg of pSV40-CAT, various amounts of the effector plasmid, and pUC19 plasmid to a total of 10 μg of DNA. pRSV β-gal and pSV40-CAT produced identical normalizing activity. Plasmids used for transient transfection assays were prepared by using Qiagen (Santa Clarita, Calif.) columns. After 48 h, cells were harvested and luciferase assay was performed as previously described (13). To correct for differences in transfection efficiencies among different DNA precipitates, luciferase activity was normalized to that of chloramphenicol acetyltransferase determined by a standard two-phase assay.
Plasmids.
The pMH1long-luc reporter construct contains the 408-bp upstream sequence of the JC virus large T-antigen gene fused to the firefly luciferase gene (12). Base substitutions in the proximal promoter region of the JC virus were generated in the context of the 408-bp upstream sequence, using a QuickChange PCR-based site-directed mutagenesis kit (Stratagene) according to the manufacturer's procedure. The following oligonucleotides were used in the mutagenesis procedure with plasmid pMH1long-luc as the template: 5′-GCTCCTCCCTACAGTCCCTTTTTTT-3′ and 5′-AAAAAAAAGGGACTGTAGGGAGGAG-3′ for the mutant of the first repeat of pentanucleotides (mPent1), 5′-CCTCCCTACCTTTTCTTTTTTTTA-3′ and 5′-ATAAAAAAAAGAAAAGGTAGGGAG-3′ for the mutant of the second repeat of pentanucleotides (mPent2), 5′-TTTTTATATATCCAGGAGGCCGAGG-3′ and 5′-GCCTCGGCCTCCTGGATATATAAAA-3′ for a TATA mutant (mTATA2), 5′-CGAGGCCGCCTCTTTTTCCAAGCTTA-3′ and 5′-GTAAGCTTGGAAAAAGAGGCGGCCTC-3′ for the Sp1-II mutant, and 5′-CCTCCAAGCTTATGACACGGTAGTAAGGG-3′ and 5′-GCCCTTACTACCGTGTCATAAGCTTGGAG-3′ for the novel-sequence mutant. The first set of primers represents coding-strand sequences of the promoter containing the desired mutations (underlined bases), and the second set of primers represents the corresponding noncoding-strand sequences. Constructs with correct mutations were screened by restriction enzyme digestion and sequencing analysis. mPent3 contained irrelevant DNA sequence between Sp1-I site and the polythymidine tract (5′-CCCTCCTCATGCAGGTTTTTTTT). mTATA1 and mTATA3 plasmids contained mutated TATA sequences with changes to that of SV40 (5′-TTTATTTATACAC-3′) and irrelevant sequence (5′-TTTAGCGTCACA-3′), respectively (13).
pJC-T, which expresses full-length JC virus large T antigen under the control of the cytomegalovirus promoter, has been described previously (13) and was used as an effector plasmid.
Preparation of nuclear extracts and DNase I footprinting.
Nuclear extracts were prepared from U87MG human glioma and HeLa cells as described by Dignam et al. (6). The pellet was resuspended in buffer D (20 mM HEPES [pH 7.9], 20% glycerol, 0.1 M KCl, 0.2 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol [DTT]) and dialyzed against the same buffer. The extracts were quick-frozen in liquid N2; aliquots were stored at −70°C and used within 3 months of extraction. Baculovirus-expressed SV40 T antigen was purchased from Molecular Biology Resources (Milwaukee, Wis.), and JC virus T antigen was obtained from Richard J. Frisque (Pennsylvania State University). Recombinant Sp1 protein was purchased from Promega (Madison, Wis.). The JC virus early promoter fragment was prepared by PCR and used as a probe in the DNase I footprinting experiment. For the coding-strand probe, primer 5′-CAGTTTTTAGCCAGCTCTGCT-3′, representing the coding-strand sequence from bp −146 to −127 of the JC virus promoter gene, was labeled by [γ-32P]ATP, using polynucleotide kinase. This was used in PCR, together with an unlabeled oligonucleotide, 5′-GCTTTTTGCAGCAAAAAATTACTGC-3′, representing the noncoding nucleotides from bp +84 to +109 of the JC virus promoter. The noncoding strand probe was prepared by using a labeled oligonucleotide, 5′-GCTTTTTGCAGCAAAAAATTACTGC-3′, representing the noncoding nucleotide sequence from +84 to +109 bp, together with the unlabeled primer 5′-CAGTTTTTAGCCAGCTCTGCT-3′, which represents the coding sequence from bp −146 to −127. The noncoding strand of the SV40 virus promoter was also synthesized by PCR using a labeled primer, 5′-GGCGTCTTCCATTTTACCAAC-3′, representing the noncoding nucleotide sequences encompassing the start codon of the luciferase gene from plasmid pA3PLUC together with the unlabeled primer 5′-AATTAGTCAGCAACCAGGTG-3′, which represents the coding sequence from bp −204 to −185. Using pMH1long-luc or pSV40-luc plasmid DNA (13) as the template, PCR was performed with denaturation, annealing, and DNA synthesis steps at 94°C (0.5 min), 55°C (1 min), and 72°C (1 min), respectively, for a total of 30 cycles. The appropriate end-labeled probe was isolated from a 4% polyacrylamide gel.
The labeled probes of 30,000 cpm were incubated with the above recombinant proteins or nuclear extracts in 40 μl of binding buffer for 25 min at room temperature. After incubation, DNase I digestion was performed with freshly diluted DNase I in 1× binding buffer (20 mM HEPES [pH 7.9], 2 mM MgCl2, 50 mM NaCl, 1 mM DTT, 0.1 mM EDTA, 10% glycerol). Two micrograms of poly(dI-dC) was incubated in the reaction as a nonspecific competitor. The amount of DNase I was adjusted empirically for each protein to produce an even pattern of partially cleaved products. The DNase I reaction was stopped by adding 100 μl of stop buffer (50 mM Tris [pH 8.0], 1% sodium dodecyl sulfate, 10 mM EDTA [pH 8.0], 0.4 mg of proteinase K per ml, 100 mM NaCl). Then samples were extracted twice with phenol-chloroform, and the DNA was precipitated with 3 volumes of ethanol. The DNA pellet was dried and resuspended in sequencing stop buffer (0.05% xylene cyanol, 0.05% bromophenol blue, 10 mM Na2EDTA, 90% deionized formamide) and incubated at 95°C for 3 min. Then an aliquot of sample was loaded onto a 6% polyacrylamide–8 M urea sequencing gel. The location of each band was determined by Maxam-Gilbert sequencing reactions of the labeled probes.
EMSA.
Sense and antisense oligonucleotides corresponding to the sequences of Sp1-II 5′-CAGGAGGCCGAGGCCGCCTCCGCCTCCAAGCTTACT-3′ and 5′-GAGTAAGCTTGGAGGCGGAGGCGGCCTCGGCCTCCT-3′ and Sp1-III 5′-CAGAAGTAGTAAGGGCGTGGAGGCTTTTTAG-3′ and 5′-CCTAAAAAGCCTCCACGCCCTTACTACTTCT-3′ were synthesized (Gene Link, Inc., Thornwood, N.Y.). These oligonucleotides were annealed, gel purified, 32P labeled by T4 DNA kinase, and used as probes. Electrophoretic mobility shift assay (EMSA) and antibody coincubation experiments were performed with 50,000 cpm of labeled probe (approximately 0.05 to 0.1 ng) and nuclear extracts (30 μg) in a final volume of 20 μl of 12.5% glycerol, 12.5 mM HEPES (pH 7.9), 4 mM Tris-HCl (pH 7.9), 60 mM KCl, 1 mM EDTA, and 1 mM DTT with 1 μg of poly(dI-dC) as described previously (16). For supershift assay, antibody was coincubated with the nuclear extract mix for 30 min on ice prior to addition of the radiolabeled probe. Antibodies against Sp1 and p53 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.).
RESULTS
A T-antigen binding site in the JC virus promoter shows extended protection.
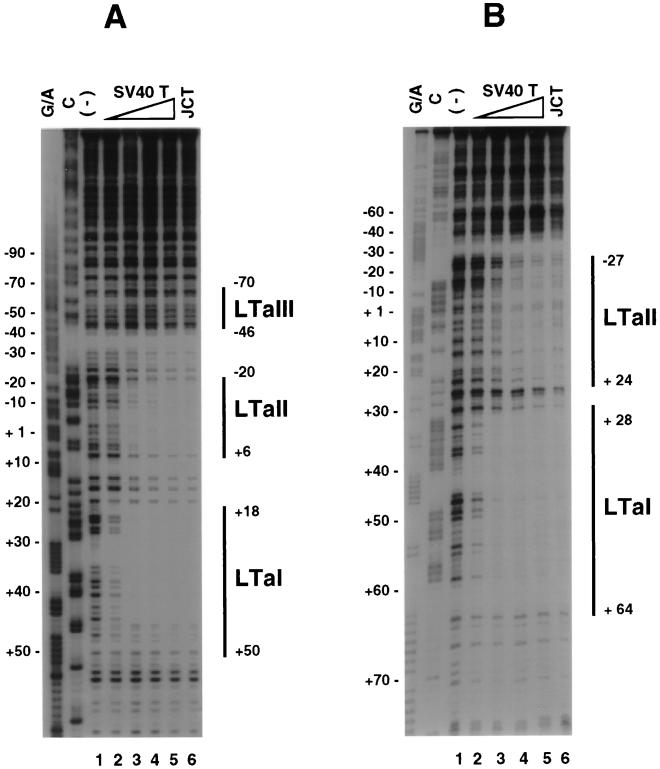
We have previously shown that T antigen regulates the JC virus promoter in a manner divergent from the SV40 early promoter, despite highly similar sequences of the T-antigen binding sites in the two viruses (13). To further study the interaction of T antigen with the JC virus basal promoter region, DNase I footprinting analysis was performed with the JC virus early promoter (Fig. 2B) and SV40 early promoter (Fig. 2A) as probes. SV40 T antigen was used because its promoter regulation was identical to that for JC virus T antigen (13) and it exhibits a high degree of amino acid homology with JC virus T antigen (72%) (8). Indeed, using the limited amount of JC virus T antigen available, we found that the two large T antigens bind in identical manners to the two promoters (Fig. 2).
FIG. 2.
T-antigen binding to the SV40 and JC virus promoter regions. The noncoding strands of the SV40 and JC virus were labeled and used as probes for DNase I footprinting. (A) Footprinting of the SV40 promoter by large T antigen. Maxam-Gilbert sequencing reaction mixtures (lanes G/A and C) were used to localize protein binding. The labeled probe was digested with DNase I in the absence (lane 1) or presence of 0.1 (lane 2), 0.6 (lane 3), 1.3 (lane 4), or 4.0 (lane 5) μg of SV40 T antigen (SV40 T) and 1.5 μg of JC virus T antigen (JCT) (lane 6). T-antigen binding domains (LTa I, LTa II, and LTa III) are indicated by solid lines. (B) Footprinting of the JC virus promoter by T antigen. Protein amounts used are the same as for panel A. Two T-antigen binding domains (LTa I and LTa II) were identified; the protection of LTa II was much more extensive than expected.
With increasing concentration of T antigen, footprints became more prominent with both promoters (Fig. 2). Three binding domains (LTa I, LTa II, and LTa III) were identified in the SV40 promoter, as previously described (23). For the JC virus promoter, two protected areas, encompassing the regions from bp +28 to +64 (LTa I) and −27 to +24 (LTa II) relative to the early transcription start site, were identified. JC virus LTa II protection was much more extensive than expected, based on sequence homology of the JC virus and SV40 LTa II sites. Thus, according to sequence homology with SV40 LTa II, the expected protection for the JC virus was from bp −18 to +8. Interestingly, the JC virus LTa II footprint extended to cover the TATA box, which is a major site of sequence divergence between JC virus and SV40 basal promoters. T antigen demonstrated a greater affinity for LTa I than for LTa II, as previously shown for the SV40 promoter (Fig. 2) (23).
Sequences in the region of extended LTa II protection are critical for T-antigen-induced transcriptional activation in nonglial cells.
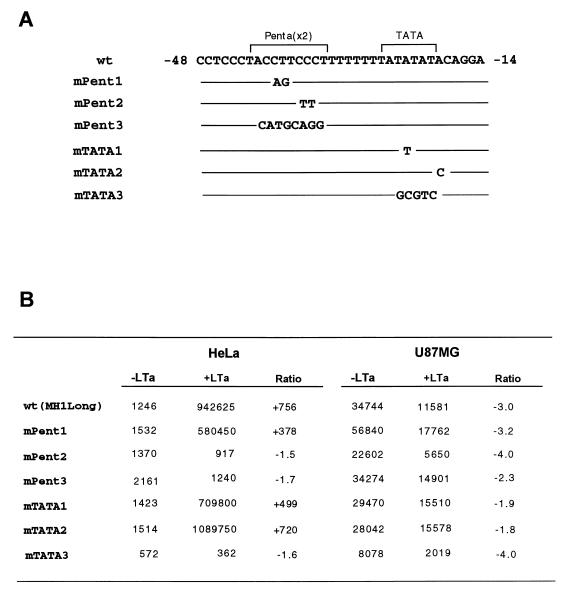
To further investigate cell-specific regulation by T antigen, site-directed mutagenesis was performed in the area of extended T-antigen binding in the LTa II site of MH1 JC virus. Mutations were made within the pentanucleotide repeats and the TATA sequence (Fig. 3A). Figure 3B shows the basal and T-antigen-induced transcription activities for each mutant in HeLa and U87MG glioma cells. Consistent with previous data, the basal transcriptional activity of wild-type pMH1long-luc was 30-fold higher in U87MG cells than in HeLa cells (12). After cotransfection with a T-antigen expression plasmid, the activity of pMH1long-luc increased 756-fold in HeLa cells but decreased 3-fold in U87MG cells. Interestingly, alteration of two specific bases in mPent2 abolished T-antigen-induced transactivation, while the mutation in mPent1 did not affect either basal or T-antigen-induced transactivation. Alteration of the pentanucleotide sequence to an irrelevant sequence (mPent3) also abolished the ability of T antigen to transactivate the promoter.
FIG. 3.
Effects of mutations of the pentanucleotide repeats or TATA box sequences on basal and T-antigen-induced transcriptional activity in U87MG and HeLa cell lines. Human glioma U87MG and HeLa cells were transfected with wild-type (wt) or mutant constructs along with the empty vector (pRcCMV) or effector plasmid (pJC-T). The molar ratio of effector plasmid to reporter plasmid used for transfection was 0.1 in each experiment. In each experiment, stimulation of reporter gene expression by cotransfecting pJC-T is compared to that by cotransfecting pRcCMV (Invitrogen) and is also presented as fold induction (average value from three independent samples). (A) Comparison of nucleotide sequences of the wild-type (wt) and mutated constructs at the pentanucleotide and TATA box area (brackets). Mutated bases are indicated; solid lines represent unchanged sequences. (B) Effects of mutations on the basal and T-antigen-induced transcriptional activity in U87MG and HeLa cells. The values are averages of triplicate samples. The cotransfection experiment was repeated at least twice in each cell line, with nearly identical results.
In HeLa cells, neither the point mutation altering the JC TATA element to the SV40 TATA sequence (TATTTAT in mTATA1) nor a change of the sequence adjacent to the TATA element (TATATATT in mTATA2) affected transcription. However, change of the TATA to an irrelevant sequence (mTATA3) abolished T-antigen-induced transactivation and decreased basal activity roughly twofold. In U87MG cells, the mutations did not alter T-antigen repression; however, the irrelevant sequence mTATA3 reduced the basal activity about fourfold, suggesting that the TATA box sequence is a functional element in the basal promoter. These results suggest an important role for the second pentanucleotide element and TATA sequence for T-antigen-induced transactivation in nonglial cells.
Cell-specific nuclear proteins interact with the JC virus basal promoter.
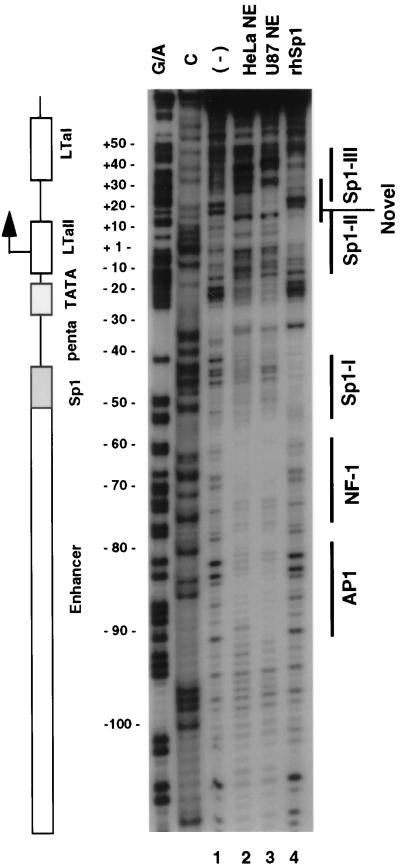
The MH1 JC virus basal promoter is cell specific in the absence of viral proteins (13, 19). Therefore, DNase I footprinting analysis of the basal promoter was performed with nuclear extracts from U87MG and HeLa cells to identify cell-specific binding proteins (Fig. 4). Because Sp1 was known to bind to at least one site in the proximal promoter (11, 12), the footprint of recombinant human Sp1 protein was also obtained.
FIG. 4.
DNase I footprinting analysis of the proximal promoter region of JC virus. Nuclear extracts from U87MG and HeLa cells and recombinant human Sp1 were used for DNase I footprinting. The coding strand probe was labeled as described in Materials and Methods. The labeled probe was digested with DNase I in the absence (lane 1) or presence of nuclear extracts prepared from U87MG (lane 2) or HeLa (lane 3) cells or recombinant human Sp1 protein (rhSp1; lane 4). A schematic diagram corresponding to the digested region of the promoter is also presented. Areas of protection were identified by both nuclear extracts in the enhancer region, corresponding to AP1 and NF-1 sites. Areas of protection were noted in the basal promoter region, most of which showed a cell-specific pattern. Three Sp1 binding domains were also identified, as marked by solid lines (Sp1-I, Sp1-II, Sp1-III) at the right.
In the enhancer region, AP1 and NF-1 binding sites were seen as previously reported (Fig. 4) (1, 28). The Sp1 site immediately downstream of the enhancer region is shown to be footprinted modestly by both U87MG and HeLa extracts; however, the patterns of footprinting for the two extracts differed. Consistent with previous observations, recombinant Sp1 protein was shown to protect this site in the footprinting analysis (Fig. 4, lane 4; Fig. 5A, Sp1-I). The pentanucleotide sequence downstream of the Sp1 site was also protected by both nuclear extracts. However, protection by glial extracts was stronger than that by HeLa extracts. The TATA sequence was protected preferentially by U87MG nuclear extracts compared with HeLa extracts. At bp −6 to +12, another domain was protected by both extracts. It includes a sequence, 5′-CCTCCGCCTC-3′ (from bp −2 to +8), which contains a consensus Sp1 binding site (7). This domain was also bound by recombinant Sp1 (Fig. 4, lane 4; Fig. 5A, Sp1-II). An additional cell-specific domain was detected downstream of Sp1-II at bp +12 to +29. This domain did not show sequence homology to known transcription factor binding sites, suggesting that it may represent a novel regulatory element (Fig. 4) (7). A third site was also protected by recombinant Sp1 (Sp1-III [Fig. 4]) and both nuclear extracts. It includes a sequence, 5′-AGGGCGTGG-3′ (from bp +30 to +38) with 80% identity to the consensus Sp1 binding site (7).
FIG. 5.
The proximal JC virus promoter contains multiple protein factor binding sites. (A) Schematic diagram of the upstream regulatory region of the JC virus promoter. AP1 and NF-1 binding sites were identified in the enhancer region. The three Sp1 binding sites identified are marked by open boxes and solid lines. The 5′ proximal region also contains pentanucleotide repeats and TATA box sequences. Downstream of the Sp1-II site, a novel sequence which was predominantly protected by U87MG nuclear extract was identified. T-antigen binding sites are also marked by solid lines. The arrow indicates the transcription start site. (B) Nucleotide sequence of the MH1 JC virus promoter from bp −100 to +100 (11). Nuclear protein binding sites as well as the T-antigen and Sp1 binding sites are represented by solid lines. The dotted line on the AP1 site indicates a sequence bound by only U87MG nuclear extracts. The arrow at +1 denotes the transcription initiation site of the JC virus early gene.
Nearly identical sequences were protected when either the coding or noncoding strand was used in footprinting analysis (Fig. 4 and data not shown). Figure 5 shows a schematic diagram of protein binding to the JC virus promoter which summarizes the footprinting analysis by T antigen, nuclear extracts, and recombinant Sp1 protein. The results indicate that several regions of the basal promoter DNA were protected in a cell-specific manner.
Analysis of Sp1-II, Sp1-III, and the novel protected site.
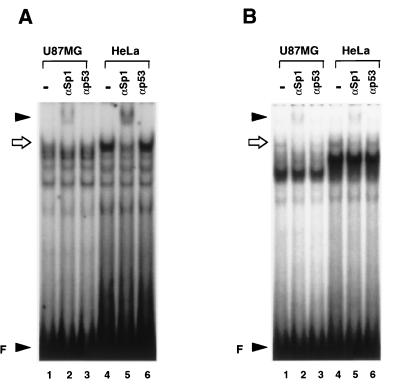
By footprinting analysis with recombinant Sp1 protein, two new Sp1 binding sites were identified (Fig. 4, Sp1-II and Sp1-III). To determine whether Sp1 proteins from nuclear extracts interact with these sites, EMSA was performed (Fig. 6). Nuclear extracts prepared from U87MG and HeLa cells formed cell-specific complexes with oligonucleotides containing the Sp1-II or Sp1-III sequences. These complexes contained at least one DNA-protein complex with similar mobility. In addition, coincubation of U87MG and HeLa nuclear extracts with an Sp1-specific antibody diminished formation of this complex and produced a new supershifted band (Fig. 6), demonstrating that this complex contains Sp1 protein. In a competition assay, a 100-fold excess of unlabeled Sp1-I or Sp1-II oligonucleotide almost completely abolished the complex formation, but the mutant form or the irrelevant oligonucleotide did not affect the complex (data not shown).
FIG. 6.
Sp1-II and Sp1-III are Sp1 binding sites. (A) A 36-bp oligonucleotide containing the Sp1-II sequence was radiolabeled and used as a probe; 30 μg of U87MG or HeLa nuclear extract was coincubated with 0.5 μg of Sp1-specific antibody (lanes 2 and 5) or with 0.5 μg of p53-specific antibody (lanes 3 and 6). A sequence-specific complex was formed by both nuclear extracts. Coincubation with Sp1 antibody but not with p53 antibody diminished formation of a complex marked by the arrow and resulted in formation of a supershifted band (indicated by the arrowhead at the top). Unbound free probe (F) is indicated by the lower arrowhead. (B) A 31-bp oligonucleotide containing the Sp1-III sequence was radiolabeled, and an antibody supershift assay was performed as for panel A. The arrow indicates the DNA-Sp1 complex, and the arrowhead at the top indicates a supershifted band.
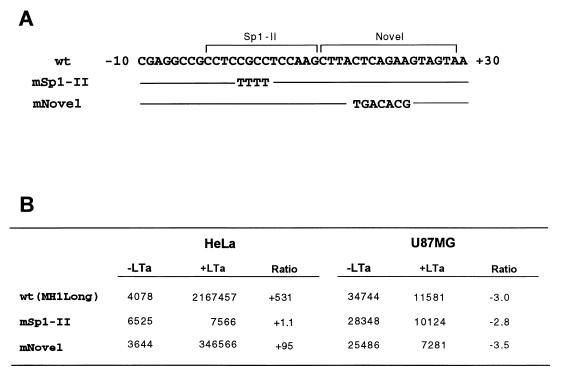
By footprinting analysis with nuclear extracts, three new binding domains were identified downstream of the TATA box (Fig. 4 and 5). To determine whether the Sp1-II and novel sequences were involved in T-antigen-induced transactivation, site-directed mutagenesis was performed (Fig. 7). Mutation of the Sp1-II sequence abolished T-antigen-mediated activation, whereas mutation of the novel sequence reduced induction about fivefold. T-antigen repression in U87MG cells was unchanged for both mutants. In contrast, mutation of the Sp1 site upstream of the pentanucleotide sequence (Sp1-I) did not affect T-antigen-induced transactivation (13).
FIG. 7.
Effects of mutations of Sp1-II and the novel protected site on basal and T-antigen-induced transcriptional activity in U87MG and HeLa cells. Cotransfection was performed as described for Fig. 3. (A) Comparison of nucleotide sequences of the wild-type (wt) and mutated constructs of Sp1-II and novel sites. (B) Effects of mutations on the basal and T-antigen-induced transcriptional activity in U87MG and HeLa cells.
Mutation of DNA motifs important for T-antigen-induced transactivation do not affect T-antigen binding.
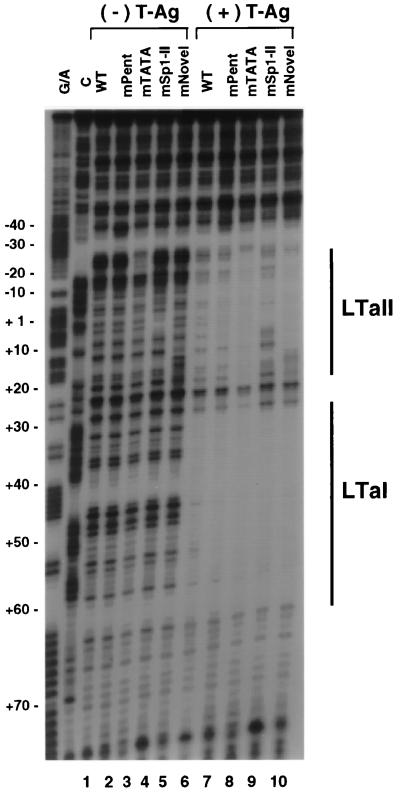
To address whether any of the above mutations affected T-antigen binding, footprinting analysis was performed with mutants of the pentanucleotide sequence (mPent3), TATA element (mTATA3), Sp1-II, and novel sequence. T antigen was incubated with these mutant probes in addition to a wild-type probe, and footprints were compared (Fig. 8). In the absence of T antigen, the DNA digestion patterns of each mutant were slightly modified at the site of mutation. The binding of T antigen to LTa I and LTa II was not significantly changed by the mutation in the pentanucleotide sequence, which had abolished activation (Fig. 8, lane 7). In the case of the irrelevant TATA mutant, protection by T antigen was stronger than that of the wild-type sequence (lane 8). The Sp1-II mutant reduced T-antigen binding moderately (lane 9). These results suggests that T antigen regulates the JC virus promoter largely by protein-protein interactions surrounding the TATA site rather than by direct DNA binding.
FIG. 8.
Effects of mutations on T-antigen binding to the JC virus promoter. The noncoding-strand probes were prepared with wild-type (WT; lanes 1 and 6), pentanucleotide mutant (lanes 2 and 7), TATA mutant (lanes 3 and 8), Sp1 mutant (lanes 4 and 9), and novel-sequence mutant constructs (lane 5 and 10). Each probe was digested with DNase I in the absence (lanes 1 to 5) or presence (lanes 6 to 10) of T antigen (T-Ag). The T-antigen binding sites are marked by solid lines at the right. Only the Sp1 mutant altered T-antigen binding to the promoter.
DISCUSSION
This study characterizes the basal region of the glial cell-specific JC virus early promoter. Two T-antigen binding sites were identified as protected regions in DNase I footprinting analysis using recombinant large T antigen. The LTa II protection was much more extensive than anticipated, based on sequence homology with the SV40 promoter. Nuclear extracts from glial and HeLa cells protected multiple regions of the JC virus basal promoter in a cell-specific manner, and these regions overlap the sites of T-antigen binding. By site-directed mutagenesis, sequences which are critical for T-antigen activation were also identified. These mutants failed to block the binding of T antigen, suggesting that protein-protein interactions, rather than direct DNA binding, are responsible for T-antigen activation.
We previously demonstrated that T antigen repressed JC virus and SV40 early promoters four- to fivefold in U87MG glioma cells (13). In contrast, T antigen induced strong activation of the JC virus early promoter in nonglial cells, whereas the SV40 promoter was repressed. T antigen also activates the JC virus early promoter in other nonglial cells (e.g., Saos and U20S osteosarcoma cells and JEG3 choriocarcinoma cells [data not shown]). The activation of the JC virus early promoter could represent either transactivation or derepression. T antigen activates a larger number of cellular and viral promoters in vitro and in vivo (24, 30). Simple basal promoter regions are sufficient for transactivation, and a wide variety of transcription factor binding sites can cooperate with T antigen in activation (5, 10). T antigen lacks a strong activation domain, and DNA binding is not required. T antigen can interact with TATA binding protein (TBP), can discriminate between TATA sequences for transactivation, and can substitute for TBP-associated factor TAFII250 (5), strongly suggesting a role in transcription initiation. Thus, the divergent regulation of the JC virus early promoter could reflect cell-specific or TATA-specific TFIID complexes. Indeed, the ability of TATA and pentanucleotide mutations to abolish T-antigen induction suggests that regions surrounding the TATA box are crucial for this effect. Also, the differences in footprinting over the TATA region between the glial and HeLa nuclear extracts support this hypothesis.
The results could also be explained by a derepression mechanism, as observed in the cellular hsp70 promoter (17). The adenovirus protein E1A activates the hsp70 promoter in a TATA sequence-specific manner by dissociating a 19-kDa repressor, Dr1, from TBP. Similarly, a repressor could bind the JC virus basal promoter and inhibit T-antigen expression in nonglial cells. In this model, transcriptional activity would increase through derepression by T antigen. Cell-specific methylation in the GC-rich basal promoter region, as discussed below, constitutes another potential mechanism for T-antigen regulation (27). Our results do not permit a clear distinction between activation and derepression, although the fact that none of the mutations produced a significant change in the basal expression in HeLa cells might argue for the former.
By footprinting analysis, two T-antigen binding sites were identified on the JC virus early promoter (Fig. 2). LTa I sites were protected similarly in JC virus and SV40 promoters, as expected from sequence homologies. However, JC virus LTa II protection was much larger than expected. Intriguingly, the more extensive JC virus LTa II footprint covers the basal promoter region that has been implicated in glial cell-specific expression, and the upstream portion of the footprint covers the TATA sequences.
Several investigators have demonstrated the lack of a glial cell-specific DNase I footprint on the enhancer region of the Mad-1 JC virus early promoter (1, 2, 29). In the enhancer region, NF-1 and AP1 binding sites were identified, but no differences in binding pattern from glial and HeLa extracts were found, except that the 5′ marginal protection of the AP1 site by U87MG nuclear extracts is more extensive than in HeLa extract (Fig. 4). Although the binding patterns of the two extracts for the NF-1 site appear similar, alternative forms of NF-1 might bind to this site as reported previously (18, 28).
By comparison, there is cell-specific protein binding to the proximal JC virus promoter (Fig. 4). Immediately downstream of the enhancer region, a site which was weakly protected by both nuclear extracts but in slightly different manners was identified. Recombinant human Sp1 protein strongly protected this site, consistent with our previous data which demonstrated that this is functional Sp1 binding site (11, 12). Downstream of this site, two additional areas of protection were identified by recombinant Sp1 protein. Within these areas, two Sp1 binding sites were identified by consensus sequence (7), and antibody supershift analysis indicated that all three sites are Sp1 binding sites (Fig. 6). All three Sp1 motifs are near the TATA box. Sp1 is known to be a ubiquitous transcription factor that controls numerous cellular and viral genes, including housekeeping, signal pathway-induced, and tissue-specific genes (14). Based on its frequent occurrence in CpG islands, one possible mechanism underlying transcriptional control by Sp1 may be its role in maintaining methylation-free CpG islands in active genes (9, 21). In vitro methylation of the MH1 JC virus early promoter leads to very strong repression of transcription after transfection into glial cells (data not shown). Thus, cell-specific methylation is another potential mechanism regulating JC virus early gene expression.
The pentanucleotide repeats (5′-TACCTTCCCT) immediately upstream of the TATA sequence were protected by both U87MG and HeLa nuclear extracts, with the U87MG footprint giving stronger protection. Because this region differs in sequence from the SV40 basal promoter, it was considered to be a potential binding site for a transcriptional repressor. Although several proteins that bind to this sequence have been identified, their relevance to glial cell specificity remains unclear (20, 26). In the present study, mutation of this pentanucleotide sequence did not affect basal transcriptional activity in either HeLa or U87MG cells. However, it clearly plays an important role in T-antigen-induced activation.
The TATA box was more strongly protected by U87MG nuclear extracts than by HeLa extracts, and the pattern of protection was slightly different as well. This finding was consistent with the data from the mutational analysis (Fig. 3), in which the irrelevant TATA mutant reduced baseline expression more strongly in U87MG cells than in HeLa cells. Thus, the JC virus promoter is TATA dependent in each cell type, but with significant differences. Moreover, mutation of TATA sequence abolished T-antigen activation. Thus, the TATA box appears to be a crucial element not only for basal transcription but also for T-antigen-mediated transcription of the JC virus promoter.
A strong footprint was found downstream of the second Sp1 site, straddling the junction between LTa I and LTa II, with U87MG nuclear extracts. This sequence does not show homology to any known transcription factor binding site (7). To investigate the functional importance of this novel sequence, site-directed mutagenesis was performed. The mutation produced a fivefold reduction in T-antigen activation but did not cause any change in basal promoter activity.
In summary, site-directed mutagenesis reveals several DNA motifs which are critical for T-antigen activation. In particular, the DNA sequences surrounding the TATA box were found to be very important. Interestingly, alteration of two specific bases residing within the second pentanucleotide repeat abolished T-antigen-induced activation. The change of TATA or the second Sp1 into an irrelevant sequence also abolished activation. The binding of T antigen was not significantly altered by mutations (Fig. 8). These results suggest that the transcription initiation complex that forms on the JC virus early promoter controls cell-specific transcription. The mutations may change the binding of the cognate protein factors rather than the binding of T antigen itself. These findings are in sharp contrast to the steric hindrance model for T-antigen repression of the SV40 or JC virus promoter (23).
ACKNOWLEDGMENTS
This work was supported by NIH grant NS35735 to J.W.H.
We thank Richard Frisque for his generous gift of the JC virus T antigen.
REFERENCES
- 1.Amemiya K, Traub R, Durham L, Major E O. Adjacent nuclear factor-1 and activator protein binding sites in the enhancer of the neurotropic JC virus. J Biol Chem. 1992;267:14204–14211. [PubMed] [Google Scholar]
- 2.Amemiya K, Traub R, Durham L, Major E O. Interaction of a nuclear factor-1-like protein with the regulatory region of the human polyomavirus JC virus. J Biol Chem. 1989;264:7025–7032. [PubMed] [Google Scholar]
- 3.Astrom K-E, Mancall E L, Richardson E P. Progressive multifocal leukoencephalopathy. Brain. 1958;81:93–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- 4.Bacellar H, Munoz A, Miller E N, Cohen B A, Besley D, Selnes O A, Becker J T, McArthur J C. Temporal trends in the incidence of HIV-1-related neurological diseases. Neurology. 1994;44:1892–1900. doi: 10.1212/wnl.44.10.1892. [DOI] [PubMed] [Google Scholar]
- 5.Damania B, Alwine J C. TAF-like function of SV40 large T antigen. Genes Dev. 1996;10:1369–1381. doi: 10.1101/gad.10.11.1369. [DOI] [PubMed] [Google Scholar]
- 6.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faisst S, Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992;20:3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frisque R J, Bream G L, Canella M T. Human polyomavirus JC virus genome. J Virol. 1984;51:458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graff J R, Herman J G, Myohanen S, Baylin S B, Vertino P M. Mapping patterns of CpG island methylation in normal and neoplastic cell implicates both upstream and downstream regions in de novo methylation. J Biol Chem. 1997;272:22322–22329. doi: 10.1074/jbc.272.35.22322. [DOI] [PubMed] [Google Scholar]
- 10.Gruda M, Alwine J C. Simian virus 40 (SV40) T-antigen transcriptional activation mediated through the Oct/SPH region of the SV40 late promoter. J Virol. 1991;65:3553–3558. doi: 10.1128/jvi.65.7.3553-3558.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henson J, Saffer J, Furneaux H. The transcriptional factor Sp1 binds to the JC virus promoter and is selectively expressed in glial cells in human brain. Ann Neurol. 1992;32:72–77. doi: 10.1002/ana.410320112. [DOI] [PubMed] [Google Scholar]
- 12.Henson J W. Regulation of the glial-specific JC virus early promoter by the transcription factor Sp1. J Biol Chem. 1994;269:1046–1050. [PubMed] [Google Scholar]
- 13.Henson J W, Schnitker B L, Lee T-S, McAllister J. Cell-specific activation of the glial-specific JC virus early promoter by large T antigen. J Biol Chem. 1995;270:13240–13245. doi: 10.1074/jbc.270.22.13240. [DOI] [PubMed] [Google Scholar]
- 14.Kadonaga J T, Carner K R, Masiarz FR, Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987;51:1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- 15.Kenny S, Natarajan V, Strike D, Jhoury G, Salzman N P. JC virus enhancer-promoter active in human brain cells. Science. 1984;226:1337–1339. doi: 10.1126/science.6095453. [DOI] [PubMed] [Google Scholar]
- 16.Kim H S, Seo H, Yang C, Brunet J-F, Kim K S. Noradrenergic-specific transcription of the dopamine β-hydroxylase gene requires synergy of multiple cis-acting elements including at least two Phox2a-binding sites. J Neurosci. 1998;18:8247–8260. doi: 10.1523/JNEUROSCI.18-20-08247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraus V B, Inostroza J A, Yeung K, Reinberg D, Nevins J R. Interaction of the Dr1 inhibitory factor with the TATA binding protein is disrupted by adenovirus E1A. Proc Natl Acad Sci USA. 1994;91:6279–6282. doi: 10.1073/pnas.91.14.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krebs C J, Dey B, Kumar K. The cerebellum-enriched form of nuclear factor I is functionally different from ubiquitous nuclear factor I in glial-specific promoter regulation. J Neurochem. 1996;66:1354–1361. doi: 10.1046/j.1471-4159.1996.66041354.x. [DOI] [PubMed] [Google Scholar]
- 19.Krebs C J, McAvoy M T, Kumar G. The JC virus minimal core promoter is glial cell specific in vivo. J Virol. 1995;69:2434–2442. doi: 10.1128/jvi.69.4.2434-2442.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu M, Kumar K U, Pater M M, Pater A. Identification and characterization of a JC virus pentanucleotide repeat element binding protein: cellular nucleic acid binding protein. Virus Res. 1998;58:73–82. doi: 10.1016/s0168-1702(98)00108-7. [DOI] [PubMed] [Google Scholar]
- 21.Macleod D, Charlton J, Mullins J, Bird A P. Sp1 sites in the mouse aprt gene promoter are required to prevent methylation of the CpG island. Genes Dev. 1994;8:2282–2292. doi: 10.1101/gad.8.19.2282. [DOI] [PubMed] [Google Scholar]
- 22.Major E O, Amemiya K, Tornatore C S, Houff S A, Berger J R. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992;5:49–73. doi: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers R M, Rio D C, Robbins A K, Tjian R. SV40 gene expression is modulated by the cooperative binding of T antigen to DNA. Cell. 1981;25:373–384. doi: 10.1016/0092-8674(81)90056-8. [DOI] [PubMed] [Google Scholar]
- 24.Rice P W, Cole C N. Efficient transcriptional activation of many simple modular promoters by simian virus 40 large T antigen. J Virol. 1993;76:6689–6697. doi: 10.1128/jvi.67.11.6689-6697.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rio D C, Tjian R. SV40 T antigen binding site mutations that affect autoregulation. Cell. 1983;32:1227–1240. doi: 10.1016/0092-8674(83)90305-7. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A K, Kumar G. A 53 kDa protein binds to the negative regulatory region of the JC virus early promoter. FEBS Lett. 1991;281:272–274. doi: 10.1016/0014-5793(91)80409-v. [DOI] [PubMed] [Google Scholar]
- 27.Slack A, Cervoni N, Pinard M, Szyf M. DNA methyltransferase is a downstream effector of cellular transformation triggered by simian virus 40 large T antigen. J Biol Chem. 1999;274:10105–10112. doi: 10.1074/jbc.274.15.10105. [DOI] [PubMed] [Google Scholar]
- 28.Sumner C, Shinohara T, Durham L, Traub R, Major E O, Amemiya K. Expression of multiple classes of the nuclear factor-1 family in the developing brain: differential expression of two classes of NF-1 genes. J Neurovirol. 1996;2:87–100. doi: 10.3109/13550289609146542. [DOI] [PubMed] [Google Scholar]
- 29.Tada H, Lashgari M, Rappaport J, Khalili K. Cell type-specific expression of JC virus early promoter is determined by positive and negative regulation. J Virol. 1989;63:463–466. doi: 10.1128/jvi.63.1.463-466.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor I A C, Solomon W, Weiner B M, Paucha P, Bradley M, Kingston R E. Stimulation of the human heat shock 70 promoter in vitro by simian virus 40 large T antigen. J Biol Chem. 1989;264:16160–16164. [PubMed] [Google Scholar]
- 31.Zu Rhein G M, Chou S-M. Particles resembling papova viruses in human cerebral demyelinating disease. Science. 1965;148:1477–1479. doi: 10.1126/science.148.3676.1477. [DOI] [PubMed] [Google Scholar]