Abstract
Monosodium urate (MSU) crystals have been reported to evoke specific cell immunity and to work as an adjuvant in a mouse model. The crystals also have another unique characteristic to bind with positively charged proteins, which could help to deliver some antigens into human dendritic cells (DC). We focused on the application of MSU crystals as not only an adjuvant but also as a carrier of positively charged antigenic protein to induce human cytotoxic T cells (CTL) efficiently in vitro. We selected human leukocyte antigen (HLA)‐A2 expressing the multiple myeloma IM‐9 cell line and its product idiotype (Id) protein as one of the best pairs of target cells and positively charged tumor‐specific antigen, respectively. Following the sensitization of DC derived from HLA‐A2‐positive volunteers pulsed with tumor‐specific monoclonal immunoglobulin G‐Fab fragments (IM‐9 Fab) attached to MSU crystals, the DC‐stimulated CD8+ T cells killed significantly more target cells (40.1 ± 1.7%) than those stimulated by DC pulsed with MSU crystals alone (6.2 ± 8.6%, P < 0.01) or IM‐9 Fab alone (4.7 ± 8.1%, P < 0.01). These cytotoxic effects of the DC‐stimulated CD8+ cells were reduced when MSU crystals were precoated with fetal bovine serum. In addition, we confirmed that MSU crystals facilitated human DC to express the maturation marker, CD83 and deliver (Fab′)2, attaching to the crystals by flow cytometer analysis. MSU crystals have distinct advantages of a protein carrier binding with positively charged proteins and delivering antigenic protein into DC, as well as an adjuvant promoting DC maturation and inducing CTL. (Cancer Sci 2008; 99: 2268–2273)
Tumor‐specific immunotherapy can open the way for an alternative approach for patients with hematological malignancies. Studies have shown that cell therapy using sensitized dendritic cells (DC) resulted in good responses in follicular lymphoma, in which tumor‐producing idiotype protein (Id) was used as the specific antigen.( 1 , 2 ) Multiple myeloma seems to be another promising candidate for cell immunotherapy using idiotype proteins, because the monoclonal immunoglobulin secreted into serum by myeloma cells is easily obtained for use as a tumor‐specific antigen. Administration of DC pulsed with Id proteins has been clinically performed in a few studies, however, the results were disappointing because the response cases were very limited.( 3 , 4 , 5 ) The reason why the ideal T‐cell‐mediated immune responses could not be achieved is the functional deficiency of circulating DC( 6 , 7 ) or lower immunogenicity of autologous Id proteins. For successful tumor‐specific immunotherapy, it is necessary to develop an efficient method for DC to recognize tumor‐specific Id proteins and then become mature and activated.
Although monosodium urate monohydrate (MSU) is famous as the causative crystals that induce gouty arthritis, its action as a key substance in local immunoreactions has only been studied in recent years. MSU released from locally damaged cells works as an endogenous danger signal to antigen‐presenting cells.( 8 ) Depending on uric acid concentration, s.c. administered MSU crystals exhibit adjuvant activities to reduce tumor burden in vivo.( 9 ) In addition, MSU crystals have shown another unique characteristic as they bind a lot of serum( 10 ) or cell‐surface proteins due to their negative charge. Among positively charged proteins, immunoglobulin G (IgG) is known to attach tightly to MSU crystals via its Fab portion.( 11 ) These findings demonstrated that MSU crystals might work as an idiotype protein carrier in humans. However, studies using a viable cell system are limited in animal models.
Thus, we investigated the potential of MSU crystals to bind with an Id protein produced by a human myeloma cell line. Using the resulting complex, we then investigated whether cytotoxic T lymphocytes (CTL) targeting myeloma cells could be efficiently induced. The results suggest that binding MSU crystals with Id protein efficiently promoted human DC to recognize the antigen and induce cytotoxic T cells against myeloma cells. Hence, MSU crystals have the potential to function as an ideal antigen carrier and adjuvant.
Materials and Methods
MSU crystals. MSU crystals were prepared based on the method of McCarty et al.( 12 ) To 336 mg of uric acid (Nacalai Tesque, Kyoto, Japan), 80 mL distilled water and 0.080 mg sodium hydroxide were added. After stirring and boiling to completely dissolve uric acid, the solution was allowed to cool naturally to 45°C and was sterilized by filtration (Stericup‐GP Filter Unit, Millipore Japan, Tokyo, Japan). The filtrate was promptly poured into sterile 1.5‐mL microtubes and stored at 4°C for at least 5 days to precipitate MSU crystals.
The obtained MSU crystals were crushed in a sterile manner immediately prior to binding with idiotype protein to facilitate being engulfed by DC. MSU crystals were subjected to four 30‐s ultrasonic disintegration rounds at 200 W using a closed‐type ultrasonic cell crusher (Bioruptor UCW200TM, Toso Denki, Yokohama, Japan). After centrifugation, MSU crystals were washed with phosphate‐buffered saline (Sigma‐Aldrich, St. Louis, MO, USA) and precipitated in the same solution to prepare an idiotype protein carrier.
Target cells and DC. The myeloma cell line, IM‐9, which produces monoclonal IgG‐κ and expresses human leukocyte antigen (HLA)‐A2, was used as the target cell. Cells were cultured as a suspension in RPMI‐1640 medium (Sigma‐Aldrich) containing 10% heat‐inactivated fetal bovine serum (FBS; Sigma‐Aldrich).
Monocytes from peripheral blood of healthy volunteers were acquired using the RosetteSep Human monocyte Enrichment Cocktail (StemCell Technologies, Vancouver, Canada) according to the manufacturer's protocols. These monocyte‐enriched cells (CD14+ cells, >75%) were further purified using the plastic adherence technique. Monocytes were cultured for 6 days in 10% human AB serum (Sigma‐Aldrich) RPMI‐1640 medium supplemented with 100 ng/mL granulocyte macrophage colony‐stimulating factor (GM‐CSF; Pepro Tech, Rocky Hill, NJ, USA), 100 ng/mL IL‐4 (Pepro Tech EC, London, UK) and 10% human AB serum to yield immature DC.
Human leukocyte antigen type A in IM‐9 cells and healthy volunteers was determined by polymerase chain reaction sequence‐based typing (PCR‐SBT).
Idiotype protein. Monoclonal Fab fragments were obtained from IM‐9 supernatants or sera from patients with IgG‐κ type multiple myeloma. IM‐9 supernatants or sera from patients were applied to a sterile protein G column (Hitrap Protein G 5 mL; GE Healthcare, Tokyo, Japan). IgG combined with protein G was eluted with 0.1 M glycine‐HCL (pH 2.7), according to the manufacturer's protocols. Eluted IgG was incubated at 37°C for 12 h in a mixture of 0.25 mg/mL papain (Merck, Darmstadt, Germany), 0.12 mg/mL cystein and 2 mM ethylenediaminetetraacetic acid sodium (pH 7.4), and after severing IgG into Fab and Fc fragments, 10.8 mM iodoacetamide was added to stop the reaction. The resulting solution was poured onto a Protein‐A column (Hitrap Protein A, 5 mL; Amersham Pharmacia Biotech, Tokyo, Japan) to adsorb Fc fragments and undigested IgG, and Fab fragments (nonadsorbed components) were collected. After centrifugation and concentration (Amicon Ultra‐4 centrifugation type filter unit; Millipore Japan), the resulting solution was dialyzed for 1 h using the drop dialysis method with 0.1 M phosphate‐buffered saline solution (pH 7.4) and a dialyzing membrane (Millipore 0.025 µM VSWP 25 mm; Millipore Japan), and was then sterilized by filtration using a sterilized syringe‐driven filter unit (Millex‐GV, 0.22 mm, PVDF; Millipore Japan) to prepare an idiotype protein solution. Immunofixation electrophoresis was performed in order to confirm purification and positive charge of Fab fragments using a Titan Gel IFE Agarose Set and Titan Gel ImmunoFix Antisera Kit (Helena Laboratories, Saitama, Japan) according to the manufacturer's protocols.
Fluorescein isothiocyanate (FITC)‐labeled murine IgG‐Fab fragments were prepared using FITC‐labeled murine IgG‐F(ab′)2 (Dako Japan, Kyoto, Japan). After mixing 100 µg of MSU crystals with 100 µg FITC‐labeled IgG‐F(ab′)2 or 100 µg FITC‐labeled IgG‐Fab and leaving the mixture to stand at 4°C for 3 h, the resulting MSU crystals were washed three times using diluted hydrochloric acid (pH 6.5–7.0). Transmission images of the MSU crystals were obtained under a light microscope, and fluorescent microscopic images were then taken at an excitation wavelength of 488 nm.
Phagocyte and maturation assay of DC. Phagocytic analysis was performed with a flow cytometer. MSU crystals and R‐phycoerythrin (RPE)‐labeled IgG‐(Fab′)2 (Polyclonal Goat Anti‐Mouse Immunoglobulins/RPE, goat [Fab′]2; Dako Japan) were incubated at 4°C for 3 h. Cationic lipid N‐(2,3‐dioleoyloxy‐1‐propyl)trimethylammonium methyl sulfate (DOTAP; Sigma‐Aldrich) and RPE‐labeled IgG‐(Fab′)2 were used as controls. RPE‐labeled IgG‐(Fab′)2 was used in this study because the fluorescence of digested Fab was insufficient for flow cytometer analysis. After DC (1 × 106/mL) were incubated in the presence of RPE‐labeled (Fab′)2 (200 µg/mL) attached to MSU crystals (200 µg/mL), RPE‐labeled (Fab′)2 (200 µg/mL) conjugated with cationic lipid DOTAP (200 µg/mL) or RPE‐labeled (Fab′)2 alone (200 µg/mL) at 37°C for 60 min, the intensity of RPE‐labeled (Fab′)2 in DC was examined using an EPICS XL Cytometer (Coulter‐Immunotech, Krefeld, Germany). In addition, engulfment of the RPE‐labeled (Fab′)2 attached to MSU crystals was evaluated by fluorescence microscopy at 550 nm.
The monocyte‐derived immature DC phenotype was examined by flow cytometry analysis. After DC (1 × 106/mL) were cultured with various stimuli for 48 h, CD83 expression intensity was examined by antihuman CD83 antibody (CD83‐PE; Coulter‐Immunotech, Marseille, France) and flow cytometry.
T‐cell proliferation assay. Cell proliferation was assessed with a CellTiter 96 Non‐Radioactive Cell Proliferation Assay (Promega, Madison, WI, USA) according to the manufacturer's protocols. Autologous CD3+ cells from healthy volunteers were acquired by using the RosetteSep Human T cell Enrichment Cocktail (StemCell Technologies). Autologous CD3+ cells (>95% 1 × 106/mL) were stimulated for 5 days with a fixed number of monocyte‐derived DC (responder cells : DC, 10:1) in a final volume of 100 µL in a 96‐well flat‐bottomed plate (Iwaki, Tokyo, Japan) in AIMV medium (Sigma‐Aldrich) containing 10% human AB serum. Stimulation index (SI) was determined by calculating the ratio of formazan levels over background formazan levels (mean of triplicates) in the un‐stimulated CD3+ cells population. Data are expressed as mean ± standard deviation (SD) of three volunteers in triplicate.
Cytotoxicity assay. DC (0.25 × 106/mL) and CD3+ cells (2.5 × 106/mL involved in 30–35% of CD8+ cells) derived from the same volunteer were incubated in a final volume of 2 mL/well AIMV medium containing 10% human AB serum. After 1 week, DC (0.25 × 106/mL) were added as second stimulation. Fresh medium and human AB serum were added to the wells every 4 or 5 days. Following incubation for a week after the second stimulation, CD8+ cells (>97%) were selected as effector cells using MACS (Miltenyi Biotec, Bergisch Gladbach, Germany), and placed in a round‐bottomed microtiter plate (Iwaki) at a concentration of 5 × 104 cells/well. Target cells were added at an effector : target ratio of 5:1 in a final volume of 100 µL/well. Following incubation for 4 h at 37°C in a humid atmosphere with 5% CO2, the plate was centrifuged at 2500 g for 4 min in order to obtain the supernatant for cytotoxicity assay. Cytotoxicity was measured by lactate dehydrogenase release assay using the CytoTox 96 Non‐Radioactive Cytotoxicity Assay (Promega) according to the manufacturer's protocols and as previously described.( 13 , 14 ) Data are expressed as mean ± SD of three independent experiments.
Statistical analysis. Numerical data are uniformly expressed as mean ± SD. Statistical analysis was performed using a two‐tailed Student's t‐test. P ≤ 0.05 was considered significant.
Results
IgG‐Fab and MSU crystal complexes. Purification and positive charge of the Fab fragment were confirmed by immunofixation electrophoresis. Fab fragments made by digestion using papain from IgG of the IM‐9 culture supernatants could be seen either as a monoclonal band in the lane for protein fractionation using sulfosalicylic acid and acetic acid or in the lane fixed using antihuman κ antibody. However, this monoclonal band was absent when fixed using antihuman IgG antibody; thus, it was confirmed that tumor‐derived IgG‐κ Fab fragments were purified. Additionally, the Fab fragments had moved to the positive side from the applied position and were fixed, thus, confirming that they were positively charged (Fig. 1a). Purification and positive charge of the Fab fragments from the sera of multiple myeloma patients were also confirmed by immunofixation electrophoresis (data not shown).
Figure 1.
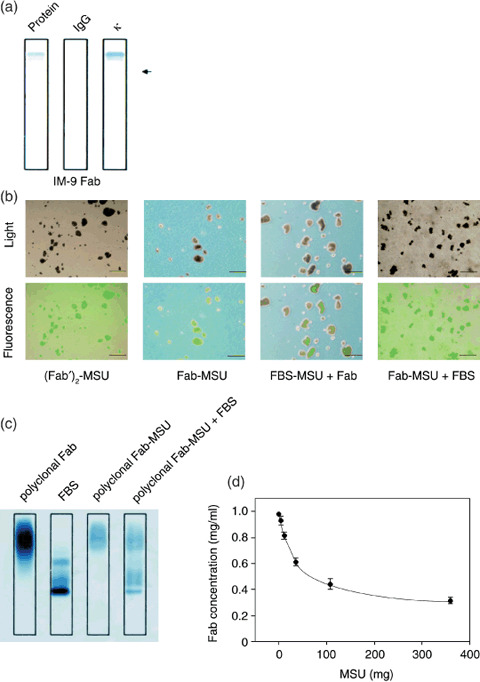
Monosodium urate (MSU) crystals bound with Fab fragments. (A) Purification and positive charge of Fab fragments from IM‐9 supernatants were confirmed using immunofixation electrophoresis; protein fractionation using sulfosalicylic acid and acetic acid (Protein), fixed using antihuman immunoglobulin G antibody (IgG), or fixed using antihuman κ antibody (κ). The arrow shows the position of sample application. (B) Fluorescein isothiocyanate (FITC)‐labeled (Fab′)2 attached to MSU crystals ([Fab′]2‐MSU), FITC‐labeled Fab attached to MSU crystals (Fab‐MSU), fetal bovine serum attached to MSU crystals followed by FITC‐labeled Fab (FBS‐MSU + Fab), or FITC‐labeled Fab attached to MSU crystals followed by FBS (MSU‐Fab + FBS) were examined by light microscopy and fluorescence microscopy. Scale lines, 10 µm. (C) Proteins attached to MSU crystals were investigated by immunofixation electrophoresis. MSU and polyclonal Fab fragments from healthy volunteers were incubated at 4°C for 3 h (polyclonal Fab‐MSU), and then some of polyclonal Fab‐MSU complexes were mixed with 10% FBS for 3 h (polyclonal Fab‐MSU + FBS). After dissolving MSU crystals, the solution was concentrated and applied to immunofixation electrophoresis. (D) Fab concentration in supernatant containing a fixed amount of Fab (100 µg) and various amounts of MSU crystals were measured in order to calculate the amount of attached Fab fragments. Data are expressed as mean ± standard deviation of four independent experiments.
Attachment between MSU and the FITC‐labeled polyclonal Fab fragment of murine IgG (IgG‐Fab) by leaving them to stand at 4°C for 3 h was confirmed by fluorescence microscopy. Untreated MSU crystals were colorless and transparent under fluorescent light (data not shown). MSU crystals mixed with murine FITC‐labeled IgG‐F(ab′)2 ([Fab′]2‐MSU) and murine FITC‐labeled IgG‐Fab (Fab‐MSU) were colorless under the light microscope but appeared green on fluorescence microscopy. The green fluorescence of the MSU crystals was diminished by mixing MSU crystals with FBS for 3 h, followed by the addition of murine FITC‐labeled IgG‐Fab (FBS‐MSU + Fab). On the other hand, the fluorescence was not affected by adding FBS after FITC‐labeled IgG‐Fab and MSU were conjugated (Fab‐MSU + FBS) (Fig. 1b).
The stability of the attachment between the polyclonal Fab fragments and MSU crystals was also confirmed by immunofixation electrophoresis. MSU crystals and polyclonal Fab fragments from healthy volunteers were incubated at 4°C for 3 h (polyclonal Fab‐MSU), and then some of polyclonal Fab‐MSU complexes were mixed with 10% FBS for 3 h (polyclonal Fab‐MSU + FBS). After dissolving MSU crystals, the solution was concentrated and applied to immunofixation electrophoresis. Fab fragments attached to MSU crystals (polyclonal Fab‐MSU) were fixed at the same lane range of control polyclonal Fab (polyclonal Fab) for protein fractionation using sulfosalicylic acid and acetic acid. The Fab fragments remained after 3 h treatment of polyclonal Fab‐MSU complex with FBS despite FBS being partially attached to the crystals (polyclonal Fab‐MSU + FBS) (Fig. 1c).
In order to quantify the attachment between MSU crystals and prepared IgG‐κ Fab fragments, various amounts of MSU crystals were added to phosphate buffer containing a fixed amount of IgG‐κ Fab fragments (100 µg), and this mixture was left to stand at 4°C for 3 h. After centrifugation, the amount of protein in the supernatant was measured in order to calculate the amount of attached IgG‐Fab. MSU quickly bound with up to one‐third the volume of IgG‐κ Fab, but became saturated at more than half the volume of IgG‐κ Fab (Fig. 1d). Based on these results, MSU and IgG‐Fab were mixed in a 1:1 ratio in order to ensure that half the protein was attached to MSU crystals.
Immunoglobulin G‐κ Fab fragments prepared from IM‐9 culture supernatants or from the sera of multiple myeloma patients were attached to MSU crystals by incubating them at 4°C for 3 h (IM‐9 Id‐MSU complex or control Id‐MSU complex, respectively).
Effects of MSU on DC. Uptake of Id‐MSU complex was investigated using RPE‐labeled IgG‐(Fab′)2 as Id. The intensity of RPE‐labeled (Fab′)2‐MSU complex ([Fab′]2‐MSU) in DC after 1‐h incubation was compared to RPE‐labeled (Fab′)2 conjugated with DOTAP ([Fab′]2‐DOTAP) or RPE‐labeled‐(Fab′)2 alone ([Fab′]2). The peak intensity of DC pulsed with (Fab′)2‐MSU and (Fab′)2‐DOTAP was clearly on the right side of DC pulsed with (Fab′)2 alone (Fig. 2a). RPE‐labeled (Fab′)2‐MSU complex attached to the DCs in 30 min but was incorporated into DC in 60 min as seen by fluorescence microscopy (Fig. 2b).
Figure 2.
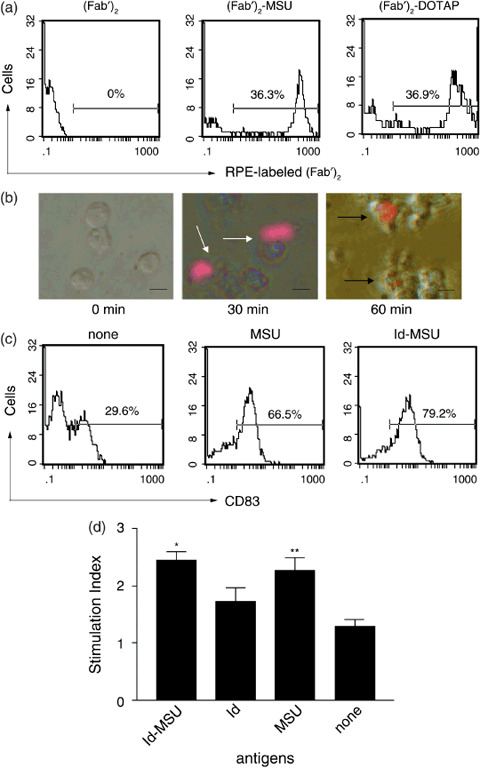
Monosodium urate (MSU) crystals affected dendritic cells (DC). (a) Phagocytotic ability of DC pulsed with R‐phycoerythrin (RPE)‐labeled (Fab′)2‐MSU complex ([Fab′]2‐MSU), RPE‐labeled (Fab′)2 conjugated with N‐(2,3‐dioleoyloxy‐1‐propyl)trimethylammonium methyl sulfate ([Fab′]2‐DOTAP) or RPE‐labeled (Fab′)2 ([Fab′]2) after 1 h incubation was examined by flow cytometry. Results are representative of two similar independent experiments. (b) Engulfment of RPE‐labeled (Fab′)2 attached to MSU crystals was evaluated by fluorescence microscopy. The white arrow shows RPE‐labeled (Fab′)2‐MSU complex attached to DC, while the black arrow shows RPE‐labeled (Fab′)2 was incorporated into DC under fluorescence microscopy. Scale lines, 20 µm. (c) CD83 expression in DC pulsed with MSU crystals, idiotype (Id)‐MSU complex or none for 48 h was examined by flow cytometry. Results are representative of at least two similar experiments. (d) T‐cell proliferation activity of DC pulsed with Id‐MSU complex. Autologous CD3+ cells were co‐cultured with a fixed number of DC (T : DC 10:1) for 5 days. Stimulation index (SI) was calculated as the ratio of formazan levels in the sample over background formazan levels. *P < 0.01 (vs Id alone) **P < 0.01 (vs none). Data are expressed as mean ± standard deviation of three volunteers in triplicate.
Flow cytometry was performed to determine whether MSU crystals induce DC maturation. Before treatment with MSU crystals, monocyte‐derived immature DC were positive for CD86 and class II molecules but negative for CD83 (data not shown). When DC (1 × 106/mL) were co‐cultured with MSU (200 µg/mL) or Id‐MSU complex (Id 200 µg and MSU 200 µg/mL) for 48 h, the peak intensity of CD83 molecules clearly shifted to the right side when compared to DC alone (Fig. 2c). However, the intensity did not change for the CD86 or class II molecules (data not shown).
In order to determine whether MSU crystals facilitate the T‐cell proliferation activity of DC, the proliferative effects of DC on autologous CD3+ cells were investigated. DC pulsed with Id‐MSU complex significantly facilitated the proliferation of autologous CD3+ cells when compared to DC pulsed with Id alone. The SI were 2.4 ± 0.2 and 1.7 ± 0.2, respectively. DC pulsed with MSU alone significantly facilitated the proliferation of autologous CD3+ cells when compared to DC alone. The SI were 2.3 ± 0.2 and 1.3 ± 0.1, respectively. No significant proliferation differences were seen between the autologous CD3+ cells stimulated by the Id‐MSU complex‐pulsed DC and those stimulated by MSU‐pulsed DC (Fig. 2d).
Cytotoxic activity of effector cells against target cells. In order to clarify the cytotoxic ability of effector cells generated by DC pulsed with the IM‐9 Id‐MSU complex, a cytotoxicity assay was conducted. The cytotoxicity of the effector cells stimulated with DC collected from HLA‐A2 volunteers and pulsed with the IM‐9 Id‐MSU complex was significantly greater when compared to effector cells stimulated with DC alone (Fig. 3a) (40.1 ± 1.7% and 3.6 ± 3.5%, respectively). No significant cytotoxicity was seen in the controls: effector cells stimulated by DC pulsed with control Id‐MSU complex, DC pulsed with MSU and DC pulsed with Id (Fig. 3a) (cytotoxicity: 5.9 ± 2.1%, 6.2 ± 8.6% and 4.7 ± 8.1%, respectively). The cytotoxic effects of effector cells stimulated by DC pulsed with the IM‐9 Id‐MSU complex was reduced from 40.1 ± 1.7% to 4.3 ± 1.0% when MSU crystals were incubated in 10% FBS solution 3 h before mixing with Fab fragment (Fig. 3b), and was reduced from 40.1 ± 1.7% to 6.6 ± 6.9% when effector cells were stimulated by DC collected from HLA‐A2‐negative volunteers and pulsed with the IM‐9 Id‐MSU complex (Fig. 3c).
Figure 3.
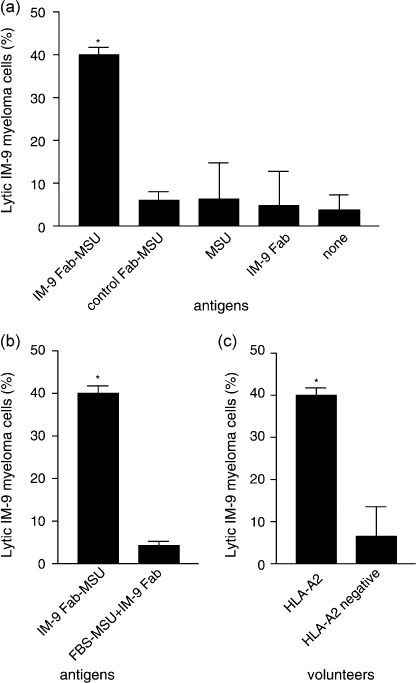
Cytotoxic T lymphocytes (CTL) were induced by dendritic cells (DC) pulsed with idiotype–monosodium urate (Id‐MSU) complex. Effector cells were stimulated by DC for 2 weeks. Cytotoxicity was measured by lactate dehydrogenase release assay. (a) Cytotoxicity of effector cells from human leukocyte antigen (HLA)‐A2‐positive volunteers against target cells generated by DC pulsed with IM‐9 Id‐MSU complex was compared to control antigens; control Id‐MSU complex, MSU crystals alone, Id alone, or nothing. *P < 0.01 (vs all other controls). (b) Cytotoxicity of effector cells educated by IM‐9 Id‐MSU complex‐pulsed DC was compared to those generated by MSU bathed in 10% fetal bovine serum solution for 3 h before mixing with Id to block MSU binding with Id. *P < 0.01. (C) Cytotoxicity of effector cells from HLA‐A2‐positive volunteers was compared to that of HLA‐A2‐negative volunteers against target cells. *P < 0.01. Data are expressed as mean ± standard deviation of three independent experiments.
Discussion
In the present study, monoclonal IgG‐Fab protein derived from IM‐9 (multiple myeloma cell line) cells was attached to MSU crystals and induced allogeneic CD8+ cells targeting IM‐9 cells. The cytotoxicity against IM‐9 cells of CD8+ cells induced by DC pulsed with Id‐MSU complex was significantly higher than that of CD8+ cells induced by DC pulsed with monoclonal IgG‐Fab alone. In addition, high levels of cytotoxicity against the target cells were not observed in CD8+ cells derived from HLA‐A unmatched volunteers. These findings suggest that the binding of MSU crystals with the IgG idiotype protein induced CTL against myeloma cells under HLA‐A restriction.
To efficiently induce CTL, it was necessary for IgG‐Fab protein to attach MSU crystals. MSU crystals reportedly bind with many proteins as well as IgG due to electrostatic conditions. The present study using immunofixation electrophoresis and fluorescence microscopy confirmed that Fab fragments were positively charged and attached to MSU crystals. The stability of Fab‐MSU complex after treatment with FBS was confirmed by using fluorescence microscopy and immunofixation electrophoresis. MSU crystals have the potential to tightly bind with Fab fragments. DC pulsed with Id‐MSU complex induced highly cytotoxic CD8+ cells, but DC pulsed with MSU crystals that were first soaked in FBS and then treated with Fab did not induce effective CTL. The reason for this may be that Fab is not likely to be engulfed by DC. As MSU crystals are easily engulfed by DC, antigen protein is readily engulfed when attached to MSU crystals. The importance of binding between MSU crystals and antigenic tumor protein may be demonstrated in a mouse model showing that injection of the MSU crystals near the implanted tumor reduced the tumor burden.( 9 ) MSU crystals may have an adjuvant effect on binding with antigenic proteins from the dying tumor cells to stimulate antigen‐presenting cells and induce CTL.
It is critical for inducing antigen‐specific CD8+ cells to deliver antigen into DC efficiently. Previously, liposomes comprising cationic lipids,( 15 , 16 ) keyhole limpet hemocyanin (KLH),( 17 , 18 ) ovalbumin (OVA) and bovine serum albumin (BSA) have been used for this purpose. The present flow cytometer study demonstrated that Id proteins attached to MSU crystals are easily captured by DC as much as those conjugated with DOTAP. In contrast to DOTAP, DC pulsed with Id‐MSU complex for 48 h exhibited the proliferation of T cells; therefore, the present technique is clinically useful due to the lack of cytotoxicity toward DC.
Following antigen engulfment, DC are necessary to facilitate maturation and activation. Studies have shown that a combination of pro‐inflammatory stimuli, such as tumor necrosis factor‐α, interleukin (IL)‐6, IL‐1β and prostaglandin E2,( 19 ) a combination of OK‐432 and prostaglandin E2,( 20 ) or the active component of OK‐432,( 21 ) help DC to mature. MSU crystals have also been reported to induce the maturation of DC in a mouse model.( 8 ) Based on the finding that DC treated with MSU crystals increased the expression of CD83 antigen and promoted the proliferation of autologous CD3+ T cells, we verified here that MSU crystals induce the maturation of human DC for the first time.
The present study clearly demonstrated that MSU crystals induced CTL. However, it is controversial whether DC activated by MSU crystals always induce CTL following the process of cross‐presentation.( 8 , 22 , 23 , 24 ) Ma et al. showed that uric acid crystals promote DC to produce IL‐12 and enhance the T‐helper (Th)1 immune response in mice.( 23 ) Meanwhile, Behrens et al. showed that crystalline uric acid blocked tumor growth by inducing antibodies, which depended on Th2 CD4+ cells but not CD8+ lymphocytes in mice.( 24 ) Because we have not investigated the detailed mechanism whereby MSU leads DC toward the cross‐presentation of CTL, further study will be needed to clarify the effect of MSU crystals on T cells.
It is difficult to activate DC and induce CTL derived from patients with multiple myloma.( 6 , 7 ) Because the results of the present study lead us expect that MSU crystals help dysfunctional DC to induce autologous CTL, we are planning to investigate the effect of the MSU crystals on DC derived from myeloma patients in vitro in our next study. However, the project will be unable to include a cytotoxicity assay because of difficulty in obtaining enough primary myeloma cells. Very recently, Chesi et al. reported an innovative in vivo animal model that mimics human multiple myeloma,( 25 , 26 ) which may be useful in clarifying the ability of MSU crystals to activate DC in an autologous setting in vivo.
In conclusion, binding of MSU crystals with the Id protein efficiently promoted DC to recognize the antigen and induce cytotoxic T cells against myeloma cells. MSU crystals have the potential to function as an ideal antigen carrier and adjuvant. Using the Id‐MSU complex, we anticipate a bright future for immunotherapy against multiple myeloma.
Acknowledgments
We would like to thank Ms Hiromi Sakurada for technical assistance and Ms Konomi Sugimoto for secretarial assistance.
References
- 1. Hsu FJ, Benike C, Fagnoni F et al . Vaccination of patients with B‐cell lymphoma using autologous antigen‐pulsed dendritic cells. Nat Med 1996; 2: 52–8. [DOI] [PubMed] [Google Scholar]
- 2. Timmerman JM, Czerwinski DK, Davis TA et al . Idiotype‐pulsed dendritic cell vaccination for B‐cell lymphoma: clinical and immune responses in 35 patients. Blood 2002; 99: 1517–26. [DOI] [PubMed] [Google Scholar]
- 3. Reichardt VL, Okada CY, Liso A et al . Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma – a feasibility study. Blood 1999; 93: 2411–19. [PubMed] [Google Scholar]
- 4. Abdalla AO, Hansson L, Eriksson I et al . Idiotype protein vaccination in combination with adjuvant cytokines in patients with multiple myeloma – evaluation of T‐cell responses by different read‐out systems. Haematologica 2007; 92: 110–14. [DOI] [PubMed] [Google Scholar]
- 5. Titzer S, Christensen O, Manzke O et al . Vaccination of multiple myeloma patients with idiotype‐pulsed dendritic cells: immunological and clinical aspects. Br J Haematol 2000; 108: 805–16. [DOI] [PubMed] [Google Scholar]
- 6. Brown RD, Pope B, Murray A et al . Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up‐regulate CD80 (B7‐1) expression after huCD40LT stimulation because of inhibition by transforming growth factor‐beta1 and interleukin‐10. Blood 2001; 98: 2992–8. [DOI] [PubMed] [Google Scholar]
- 7. Ratta M, Fagnoni F, Curti A et al . Dendritic cells are functionally defective in multiple myeloma: the role of interleukin‐6. Blood 2002; 100: 230–7. [DOI] [PubMed] [Google Scholar]
- 8. Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 2003; 425: 516–21. [DOI] [PubMed] [Google Scholar]
- 9. Hu DE, Moore AM, Thomsen LL, Brindle KM. Uric acid promotes tumor immune rejection. Cancer Res 2004; 64: 5059–62. [DOI] [PubMed] [Google Scholar]
- 10. Terkeltaub R, Tenner AJ, Kozin F, Ginsberg MH. Plasma protein binding by monosodium urate crystals. Analysis by two‐dimensional gel electrophoresis. Arthritis Rheum 1983; 26: 775–83. [DOI] [PubMed] [Google Scholar]
- 11. Kozin F, McCarty DJ. Molecular orientation of immunoglobulin G adsorbed to microcrystalline monosodium urate monohydrate. J Laboratory Clin Med 1980; 95: 49–58. [PubMed] [Google Scholar]
- 12. McCarty DJ Jr, Faires JS. A comparison of the duration of local anti‐inflammatory effect of several adrenocorticosteroid esters – a bioassay technique. Curr Ther Res Clin Exp 1963; 5: 284–90. [PubMed] [Google Scholar]
- 13. Marten A, Renoth S, von Lilienfeld‐Toal M et al . Enhanced lytic activity of cytokine‐induced killer cells against multiple myeloma cells after co‐culture with idiotype‐pulsed dendritic cells. Haematologica 2001; 86: 1029–37. [PubMed] [Google Scholar]
- 14. Hayashi T, Hideshima T, Akiyama M et al . Ex vivo induction of multiple myeloma‐specific cytotoxic T lymphocytes. Blood 2003; 102: 1435–42. [DOI] [PubMed] [Google Scholar]
- 15. Santin AD, Hermonat PL, Ravaggi A et al . Induction of human papillomavirus‐specific CD4(+) and CD8(+) lymphocytes by E7‐pulsed autologous dendritic cells in patients with human papillomavirus type 16‐ and 18‐positive cervical cancer. J Virol 1999; 73: 5402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiriva‐Internati M, Wang Z, Salati E, Bumm K, Barlogie B, Lim SH. Sperm protein 17 (Sp17) is a suitable target for immunotherapy of multiple myeloma. Blood 2002; 100: 961–5. [DOI] [PubMed] [Google Scholar]
- 17. Tacken PJ, de Vries IJ, Gijzen K et al . Effective induction of naive and recall T‐cell responses by targeting antigen to human dendritic cells via a humanized anti‐DC‐SIGN antibody. Blood 2005; 106: 1278–85. [DOI] [PubMed] [Google Scholar]
- 18. Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T‐dependent response. J Immunol 1998; 161: 1313–19. [PubMed] [Google Scholar]
- 19. Mehlhop E, Villamide LA, Frank I et al . Enhanced in vitro stimulation of rhesus macaque dendritic cells for activation of SIV‐specific T cell responses. J Immunol Meth 2002; 260: 219–34. [DOI] [PubMed] [Google Scholar]
- 20. Sato M, Takayama T, Tanaka H et al . Generation of mature dendritic cells fully capable of T helper type 1 polarization using OK‐432 combined with prostaglandin E (2). Cancer Sci 2003; 94: 1091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okamoto M, Furuichi S, Nishioka Y et al . Expression of toll‐like receptor 4 on dendritic cells is significant for anticancer effect of dendritic cell‐based immunotherapy in combination with an active component of OK‐432, a streptococcal preparation. Cancer Res 2004; 64: 5461–70. [DOI] [PubMed] [Google Scholar]
- 22. Shi Y, Galusha SA, Rock KL. Cutting edge: elimination of an endogenous adjuvant reduces the activation of CD8 T lymphocytes to transplanted cells and in an autoimmune diabetes model. J Immunol 2006; 176: 3905–8. [DOI] [PubMed] [Google Scholar]
- 23. Ma XJ, Tian DY, Xu D et al . Uric acid enhances T cell immune responses to hepatitis B surface antigen‐pulsed‐dendritic cells in mice. World J Gastroenterol 2007; 13: 1060–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Behrens MD, Wagner WM, Krco CJ et al . The endogenous danger signal, crystalline uric acid, signals for enhanced antibody immunity. Blood 2008; 111: 1472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuehl WM. Modeling multiple myeloma by AID‐dependent conditional activation of MYC. Cancer Cell 2008; 13: 85–7. [DOI] [PubMed] [Google Scholar]
- 26. Chesi M, Robbiani DF, Sebag M et al . AID‐dependent activation of a MYC transgene induces multiple myeloma in a conditional mouse model of post‐germinal center malignancies. Cancer Cell 2008; 13: 167–80. [DOI] [PMC free article] [PubMed] [Google Scholar]


