Abstract
The present study aimed to describe the distribution and features of the SYT–SSX fusion gene in Chinese patients with synovial sarcoma (SS), and to analyze the prognostic value of SYT–SSX fusion type and clinicopathological parameters for tumor‐related death, recurrence, and metastasis in SS. SYT–SSX1 and SYT–SSX2 fusion transcripts were tested by reverse transcription–polymerase chain reaction in 141 formalin‐fixed, paraffin‐embedded SS. The prognostic implication of SYT–SSX fusion type and clinicopathological parameters were analyzed by univariate and multivariate survival analyses. SYT–SSX1 and SYT–SSX2 were detected in 50 (34.5%) and 91 (64.5%) tumors, respectively. SYT–SSX1 (risk ratio [RR] = 2.032, P = 0.004), larger tumor size (RR = 1.859, P = 0.008), and aggressive Fédération Nationale des Centers de Lutte Contre le Cancer grade (RR = 2.094, P = 0.001) were adverse predictors for disease‐specific survival. However, SYT–SSX fusion type was not associated with local recurrence‐free survival (P = 0.216). Patients with larger tumors (RR = 2.071, P = 0.005) and those who received marginal excision (RR = 2.556, P = 0.005) had poor local recurrence‐free survival. Besides, SYT–SSX1 (RR = 1.859, P = 0.037), older age (RR = 1.799, P = 0.040), and aggressive International Union Against Cancer stage (RR = 3.690, P < 0.001) proved to be adverse prognostic factors for metastasis‐free survival. In conclusion, compared to SYT–SSX1, SYT–SSX2 was more frequent in Chinese patients with SS. Moreover, SYT–SSX1 was an adverse predictor for disease‐specific survival and metastasis‐free survival, but had no relation to local recurrence‐free survival. In addition, histological grade and tumor size were also important prognostic factors for SS. (Cancer Sci 2009; 100: 1018–1025)
Synovial sarcoma (SS) accounts for 5–10% of soft tissue sarcomas.( 1 ) It can occur at any age, but is most commonly seen in young adults.( 2 ) Above 80% of SS arise in deep soft tissue of the extremities, especially around the knee.( 3 ) However, it does not arise from or differentiate toward the synovium.( 3 ) Histologically, SS is either of biphasic or monophasic subtype. The former is composed of varying proportions of epithelial and spindle cells, and the latter predominantly contains spindle cells.( 3 ) Cytogenetically, SS is characterized by the translocation t(X;18) (p11.2; q11.2).( 3 , 4 ) This translocation almost always represents the fusion of SYT with either SSX1 or SSX2.( 5 , 6 , 7 )
SYT–SSX1 and SYT–SSX2 appear to be mutually exclusive gene fusions in SS, and the fusion type is concordant in primary tumors and metastases and constant over the course of the disease.( 8 ) Accordingly, the value of SYT–SSX for diagnosis and prognosis is worth studying in SS. SYT–SSX has been recognized as a specific diagnostic marker for SS;( 9 ) however, the prognostic implication of SYT–SSX fusion type is still controversial. Moreover, there might be geographic differences in the frequency of two SYT–SSX fusion types in SS.( 10 ) For Asians, Takenaka et al. most recently reported that SYT–SSX fusion type was not a significant prognostic factor for patients with localized SS in Japan.( 11 ) There have been few reports about the prognostic value of SYT–SSX fusion type in SS for Chinese patients. Therefore, we are ready to describe the distribution and features of SYT–SSX fusion genes in 141 Chinese patients with SS and analyze the prognostic implication of the fusion type, so that people can know SYT–SSX fusion genes and their role in SS in China. Combining the data from different countries in the world, people can recognize the value of SYT–SSX in SS more objectively.
Although SS has been traditionally regarded as a highly malignant tumor, its prognosis varies greatly depending on the number of patients evaluated or clinicopathological features taken into consideration. At present, investigations into the prognosis of SS are often performed in relatively small‐series or include only parts of clinicopathological factors. In addition, up to 50% of SS recur, and approximately 40% of SS metastasize,( 3 ) but there are a few studies about the prognostic factors for local recurrence and metastasis of patients with SS. For these reasons, we analyzed the associations of SYT–SSX fusion type as well as clinicopathological parameters with disease‐specific survival (DSS), local recurrence‐free survival (LRFS), and metastasis‐free survival (MFS), in order to find some helpful prognostic factors for tumor‐related death, recurrence, and metastasis of SS.
Materials and Methods
Patients and materials. The series were composed of 141 SS that were found to be positive for SYT–SSX by reverse transcription (RT)–polymerase chain reaction (PCR) (among them, 140 cases were published previously).( 12 , 13 ) All of them were excision specimens at the Cancer Hospital of Tianjin Medical University from 1973 to 2005. Data collected included patient age at diagnosis, sex, tumor site, tumor size, histological type, histological grade and disease stage, treatment modalities, and follow‐up data. Tumor sites were divided into trunk and extremity (including limb girdles, such as the axillary region, inguinal region, and buttock). Moreover, cases were grouped into superficial and deep tumors according to their location; tumors were deep if they were beneath the superficial fascia. Besides, intrathoracic, intra‐abdominal, retroperitoneal, and pelvic tumors were automatically considered to be deep. Tumor size was defined as the maximum dimension of the resected neoplasm. Histological subtyping was carried out on hematoxylin–eosin‐stained sections using the World Health Organization classification of tumors of soft tissue and bone of 2002.( 3 ) Histological grading was based on the Fédération Nationale des Centers de Lutte Contre le Cancer (FNCLCC) system and assigned to three grades (1, 2, and 3).( 14 ) Disease staging was carried out according to the International Union Against Cancer (UICC)/American Joint Committee on Cancer (AJCC) staging system.( 15 )
RNA extraction and RT‐PCR. RNA was extracted from formalin‐fixed, paraffin‐embedded tumor samples according to the method in a previous article.( 12 ) SYT–SSX1 and SYT–SSX2 transcripts were analyzed by RT‐PCR, as described previously.( 12 ) Briefly, 1 µg RNA was reverse transcribed into cDNA with 5 U reverse transcriptase AMV (Avian myeloblastosis virus), 20 U RNase inhibitor, 50 pmol random primers, and 20 mmol dNTP at 42°C. Then PCR was carried out in a 20‐µL volume system with 1.0 U Taq DNA polymerase, 10× PCR buffer (including 1.5 mmol/L MgCl2), and 4 pmol each of forward primer (SYT, 5′‐CCAGCAGAGGCCTTATGGATA‐3′) and reverse primer (SSX1, 5′‐GTGCAGTTGTTTCCCATCG‐3′; SSX2, 5′‐GCACAGCTCTTTCCCATCA‐3′). The amplification profile of the PCR consisted of 40 cycles of denaturation at 94°C for 50 s, annealing at 58°C for 30 s, and extension at 72°C for 1 min. As positive controls for the integrity of mRNA in each sample, PCR for ubiquitously expressed glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) was carried out (GAPDH‐fw, 5′‐GAAGGTGAAGGTCGGAGTC‐3′; GAPDH‐rv, 5′‐GAAGATGGTGATGGGATTTC‐3′). A negative control (substituting distilled water for template cDNA) was used for each experiment. PCR products were detected by electrophoresis and sequencing.
Statistical analysis. For statistical analysis, the following variables were considered for their prognostic value: age at diagnosis, sex, tumor site, tumor size, histological subtype, FNCLCC grade, UICC stage, SYT–SSX fusion type, surgery modality, radiotherapy, and chemotherapy. Univariate and multivariate analyses were carried out for DSS, LRFS, and MFS. DSS, LRFS, and MFS were defined as the interval from the beginning of treatment to death, to the first recurrence, and to the first metastasis, respectively. Patients who died from causes unrelated to SS were censored at the time of death. Survival curves were computed by the Kaplan–Meier method and compared by the log‐rank test. Two‐tailed P‐values of 0.05 or less were considered statistically significant for prognostic factors. Multivariate analyses based on the stepwise Cox proportional hazards model were used to identify the most significant factors related to outcome. A stepwise forward selection procedure was used, and a significance level of 5% was chosen as the criterion for entering factors in the multivariate model. SPSS statistical software (SPSS, Chicago, IL, USA) was used for the above analyses.
Results
Characteristics of patients and tumors. Among the 141 patients there were 79 men (56.0%) and 62 women (44.0%). The mean age at diagnosis was 37 years, ranging from 4 to 74 years. One hundred tumors (70.9%) were located in extremities, including 33 in upper and 67 in lower extremities. Forty‐one truncal tumors were sited in the shoulder (n = 11), back (n = 7), pelvis (n = 7), chest wall (n = 6), abdominal wall (n = 4), head and neck (n = 3), lung (n = 2), and perineum (n = 1). Furthermore, seven tumors (5.0%) were superficial and 134 (95.0%) were deep. The tumors were <5 cm in 56 patients (39.7%), and ≥5 cm in 85 cases (60.3%). Histologically, 40 biphasic SS (BSS) and 101 monophasic SS (MSS) were recognized. In addition, histological grade 2 was in 67 (47.5%) and grade 3 was in 74 (52.5%) cases. Fifty‐three patients (37.6%) were in stage 1 or 2, and 88 patients (62.4%) were in stage 3 or 4.
Treatment and follow up. Marginal excision, wide local excision, and amputation were carried out in 16 (11.3%), 113 (80.1%), and 12 cases (8.5%), respectively. Thirty‐eight patients (27.0%) received preoperative or postoperative radiotherapy. Chemotherapy was given preoperatively or postoperatively in 81 (57.4%) patients.
The mean follow‐up time was 54 months (range 1–246 months). Sixty patients (42.6%) were alive when the follow up ended at a mean time of 60 months (range 3–246 months). Eighty‐one patients (57.4%) died as a result of their malignancy. The median DSS was 70 months. Local recurrence was observed in 69 patients (48.9%). Among them, nine and three patients had a second and third recurrence, respectively. The mean period from initial surgery to the first local recurrence was 26 months (range 1–128 months). Fifty‐two (36.9%) patients developed metastases, and 10 had multiple concomitant metastatic sites. The metastatic sites included lung (n = 42), bone (n = 7), liver (n = 4), brain (n = 3), vertebrae (n = 2), mediastinum (n = 2), pleura (n = 2), chest wall (n = 2), small intestine (n = 1), and palate (n = 1). Lymph node metastases occurred in nine patients. The mean time interval from the initial surgery to first metastasis was 37 months (range 1–148 months). In addition, synchronous or metachronous local recurrence and metastases were found in 26 patients (18.4%).
SYT–SSX transcripts. Fifty (34.5%) tumors were positive for SYT–SSX1, and 91 (64.5%) were positive for SYT–SSX2 (Fig. 1). The correlations between SYT–SSX fusion type and other clinicopathological parameters are listed in Table 1. A strong association of fusion type and histological subtype was observed in this series (P < 0.001). In contrast to major BSS containing the SYT–SSX1 transcript, MSS predominantly expressed SYT–SSX2. Moreover, the rate of chemotherapy in SYT–SSX2‐positive patients was significantly higher than that in SYT–SSX1‐positive cases (P = 0.042). In addition, there were no significant correlations of SYT–SSX fusion type with sex, age at diagnosis, tumor site, tumor size, FNCLCC grade, UICC stage, surgery modality, or radiotherapy (all P > 0.05; Table 1).
Figure 1.
The electrophoresis images of reverse transcription–polymerase chain reaction products of (a) SYT–SSX1 and (b) SYT–SSX2. M, DNA marker; N, negative control; 1–4, formalin‐fixed, paraffin‐embedded synovial sarcoma tissues.
Table 1.
Relationship between SYT–SSX fusion type and clinicopathological parameters
| Clinicopathological parameters | n | SYT–SSX1 | SYT–SSX2 | χ2 | P |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 79 | 32 | 47 | 1.998 | 0.157 |
| Female | 62 | 18 | 44 | ||
| Age (years) | |||||
| <37 | 74 | 24 | 50 | 0.624 | 0.270 |
| ≥37 | 67 | 26 | 41 | ||
| Tumor site | |||||
| Trunk | 41 | 14 | 27 | 0.044 | 0.834 |
| Extremity | 100 | 36 | 64 | ||
| Tumor size (cm) | |||||
| <5 | 56 | 23 | 33 | 1.278 | 0.258 |
| ≥5 | 85 | 27 | 58 | ||
| Histological type | |||||
| MSS | 101 | 18 | 83 | 48.402 | <0.001 |
| BSS | 40 | 32 | 8 | ||
| FNCLCC grade | |||||
| 2 | 67 | 19 | 48 | 2.814 | 0.093 |
| 3 | 74 | 31 | 43 | ||
| UICC stage | |||||
| 1 or 2 | 54 | 14 | 40 | 3.477 | 0.062 |
| 3 or 4 | 87 | 36 | 51 | ||
| Surgery mdality | |||||
| Marginal excision | 16 | 9 | 7 | 3.408 | 0.065 |
| Wide excision and amputation | 125 | 41 | 84 | ||
| Radiotherapy | |||||
| Yes | 38 | 10 | 28 | 1.901 | 0.168 |
| No | 103 | 40 | 63 | ||
| Chemotherapy | |||||
| Yes | 81 | 23 | 58 | 4.153 | 0.042 |
| No | 60 | 27 | 33 | ||
BSS, biphasic synovial sarcoma; FNCLCC, Fédération Nationale des Centers de Lutte Contre le Cancer; MSS, monophasic synovial sarcoma; UICC, International Union Against Cancer.
Survival analysis. The association of clinicopathological variables with DSS, LRFS, and MFS, and the P‐values for log‐rank tests derived from the univariate analysis are shown in Table 2. SYT–SSX fusion type was correlated with DSS (P = 0.007). The survival curve of SYT–SSX2‐positive patients was significantly better than that of SYT–SSX1‐positive patients (Fig. 2a). Furthermore, age at diagnosis (P = 0.027; Fig. 2b), tumor size (P = 0.013; Fig. 2c), FNCLCC grade (P = 0.001; Fig. 2d), and UICC stage (P = 0.003; Fig. 2e) correlated with DSS. However, there was no significant association between DSS and sex, tumor site, histological type, surgery modality, radiotherapy, or chemotherapy (all P > 0.05; Table 2). Multivariate analysis indicated that SYT–SSX1 (risk ratio [RR] = 2.032, P = 0.004), larger tumor size (RR = 1.859, P = 0.008), and aggressive FNCLCC grade (RR = 2.094, P = 0.001) were adverse predictors for DSS (Table 3).
Table 2.
Univariate analyses for DSS, LRFS and MFS in 141 synovial sarcomas (P‐values are for log‐rank tests)
| Factor | n | 5‐year DSS (%) | P‐value | 5‐year LRFS (%) | P‐value | 5‐year MFS (%) | P‐value |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 79 | 51.10 | 0.397 | 45.18 | 0.253 | 55.25 | 0.755 |
| Female | 62 | 59.36 | 53.35 | 65.12 | |||
| Age (years) | |||||||
| <37 | 73 | 67.08 | 0.027 | 50.74 | 0.563 | 71.16 | 0.010 |
| ≥37 | 68 | 41.17 | 47.02 | 44.78 | |||
| Tumor site | |||||||
| Trunk | 41 | 47.97 | 0.662 | 67.38 | 0.171 | 52.83 | 0.119 |
| Extremity | 100 | 60.24 | 43.35 | 62.26 | |||
| Tumor size (cm) | |||||||
| <5 | 56 | 72.80 | 0.013 | 66.96 | 0.009 | 67.37 | 0.139 |
| ≥5 | 85 | 40.79 | 35.87 | 53.11 | |||
| Histological type | |||||||
| MSS | 101 | 57.99 | 0.330 | 51.41 | 0.528 | 61.22 | 0.584 |
| BSS | 40 | 49.10 | 43.02 | 55.39 | |||
| FNCLCC grade | |||||||
| Grade 2 | 67 | 69.77 | 0.001 | 54.40 | 0.020 | 75.54 | < 0.001 |
| Grade 3 | 74 | 41.59 | 44.05 | 44.64 | |||
| UICC stage | |||||||
| Stage 1 or 2 | 53 | 70.77 | 0.003 | 56.42 | 0.041 | 82.83 | < 0.001 |
| Stage 3 or 4 | 88 | 44.81 | 44.41 | 43.88 | |||
| SYT–SSX fusion type | |||||||
| SYT–SSX1 | 50 | 40.93 | 0.007 | 37.88 | 0.216 | 43.61 | 0.013 |
| SYT–SSX2 | 91 | 62.15 | 53.82 | 66.37 | |||
| Surgery modality | |||||||
| Marginal excision | 16 | 42.86 | 0.138 | 22.32 | 0.012 | 52.73 | 0.259 |
| Wide excision and amputation | 125 | 56.94 | 52.85 | 60.94 | |||
| Radiotherapy | |||||||
| Yes | 38 | 68.52 | 0.450 | 49.65 | 0.936 | 71.86 | 0.271 |
| No | 103 | 48.25 | 49.62 | 54.02 | |||
| Chemotherapy | |||||||
| Yes | 81 | 52.41 | 0.499 | 44.71 | 0.086 | 56.54 | 0.244 |
| No | 60 | 59.58 | 55.00 | 64.92 | |||
BSS, biphasic synovial sarcoma; DSS, disease‐specific survival; FNCLCC, Fédération Nationale des Centers de Lutte Contre le Cancer; LRFS, local recurrence‐free survival; MFS, metastasis‐free survival; MSS, monophasic synovial sarcoma; UICC, International Union Against Cancer.
Figure 2.
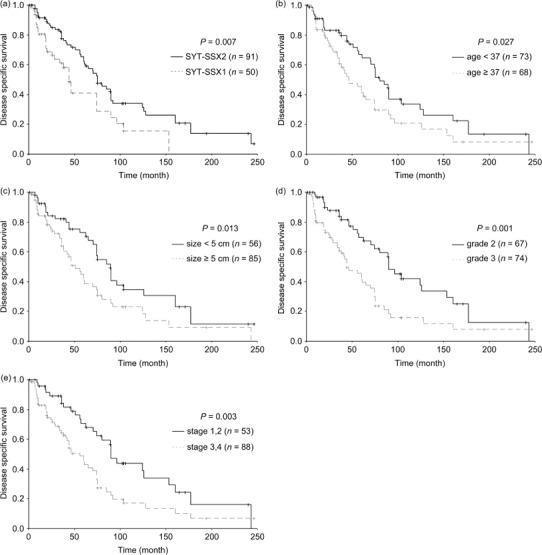
The curves of disease‐specific survival according to (a) SYT–SSX fusion type, (b) age at diagnosis, (c) tumor size, (d) Fédération Nationale des Centers de Lutte Contre le Cancer grade, and (e) International Union Against Cancer stage in 141 patients with synovial sarcoma.
Table 3.
Multivariate analyses for DSS in 141 synovial sarcomas
| Factor | DSS | ||
|---|---|---|---|
| Risk ratio | 95% confidence interval | P‐value | |
| SYT–SSX1/SYT–SSX2 | 2.032 | 1.259–3.278 | 0.004 |
| Tumor size ≥5 cm/<5 cm | 1.859 | 1.175–2.942 | 0.008 |
| FNCLCC grade 3/grade 2 | 2.094 | 1.328–3.301 | 0.001 |
DSS, disease‐specific survival; FNCLCC, Fédération Nationale des Centers de Lutte Contre le Cancer.
As shown in Table 2, SYT–SSX fusion type was not associated with LRFS (P = 0.216; Fig. 3a). Only tumor size (P = 0.009; Fig. 3b), FNCLCC grade (P = 0.020; Fig. 3c), UICC stage (P = 0.041; Fig. 3d), and surgery modality (P = 0.012, Fig. 3e) were proposed to correlate with LRFS by univariate analysis. Tumor size and surgery modality were independent prognostic factors for LRFS (Table 4). The patients with larger tumors (RR = 2.071, P = 0.005) and those who received marginal excision (RR = 2.556, P = 0.005) had a poor LRFS.
Figure 3.
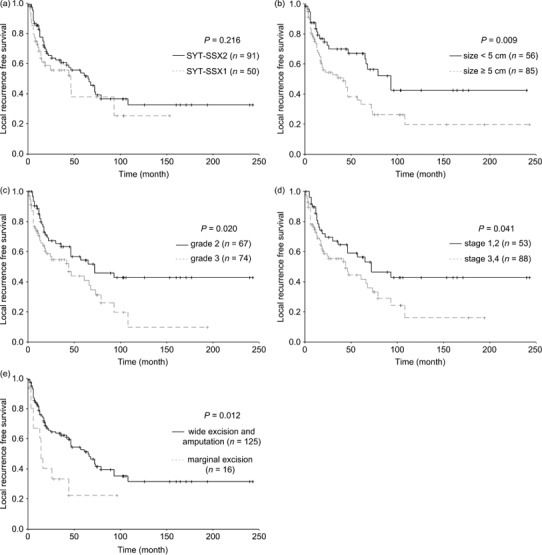
The curves of local recurrence‐free survival according to (a) SYT–SSX fusion type, (b) tumor size, (c) Fédération Nationale des Centers de Lutte Contre le Cancer grade, (d) International Union Against Cancer stage, and (e) surgery modality in 141 patients with synovial sarcoma.
Table 4.
Multivariate analyses for LRFS in 141 synovial sarcomas
| Factor | LRFS | ||
|---|---|---|---|
| Risk ratio | 95% confidence interval | P‐value | |
| Tumor size ≥5 cm/<5 cm | 2.071 | 1.239–3.462 | 0.005 |
| Marginal excision/wide excision and amputation | 2.556 | 1.320–4.947 | 0.005 |
LRFS, local recurrence‐free survival.
Univariate analyses demonstrated that SYT–SSX fusion type (P = 0.013, Fig. 4a), age (P = 0.010; Fig. 4b), FNCLCC grade (P < 0.001; Fig. 4c), and UICC stage (P < 0.001, Fig. 4d) affected MFS (Table 2). In the end, SYT–SSX1 fusion type (RR = 1.859, P = 0.037), older age (RR = 1.799, P = 0.040), and aggressive UICC stage (RR = 3.690, P < 0.001) proved to be adverse predictors for MFS by multivariate analysis (Table 5).
Figure 4.
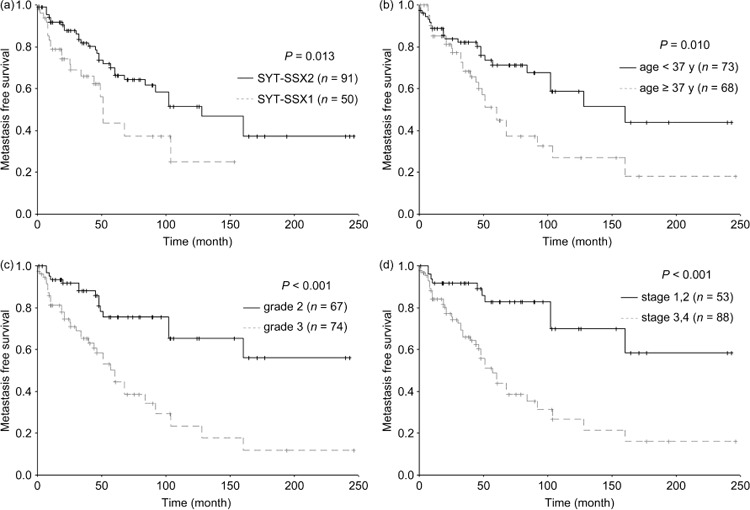
The curves of metastasis‐free survival according to (a) SYT–SSX fusion type, (b) age at diagnosis, (c) Fédération Nationale des Centers de Lutte Contre le Cancer grade, and (d) International Union Against Cancer stage in 141 patients with synovial sarcoma.
Table 5.
Multivariate analyses for MFS in 141 synovial sarcomas
| Factor | MFS | ||
|---|---|---|---|
| Risk ratio | 95% confidence interval | P‐value | |
| SYT–SSX1/SYT–SSX2 | 1.859 | 1.040–3.325 | 0.037 |
| Patient age ≥37/<37 years old | 1.799 | 1.029–3.145 | 0.040 |
| Stage 3 or 4/stage 1 or 2 | 3.690 | 1.833–7.430 | <0.001 |
MFS, metastasis‐free survival.
Discussion
The prognostic implication of SYT–SSX fusion type in SS has been controversial since Kawai et al. reported that patients carrying SYT–SSX1 had a significant reduction of MFS compared with those carrying SYT–SSX2 in a study of 45 cases.( 16 ) Subsequently, SYT–SSX1 was suggested to be an unfavorable independent factor for SS by several other series with relatively small numbers of patients( 17 , 18 , 19 ) and a multi‐institutional retrospective analysis of 243 patients.( 20 ) In contrast, Guillou et al. showed a trend for tumors with SYT–SSX2 to be more aggressive than those with SYT–SSX1, but the difference was not statistically significant.( 9 ) Moreover, Takenaka et al. most recently reported that SYT–SSX fusion type was not a significant prognostic factor for patients with localized SS in Japan.( 11 ) In the present study, SYT–SSX fusion type was an independent adverse predictor for DSS and MFS in patients with SS, but it was not associated with LRFS.
The ratio of SYT–SSX1 : SYT–SSX2 fusion is close to 2:1 in the majority of studies;( 9 , 11 , 20 , 21 ) however, it is up to 1:2 in the present study. Kokovic et al. reported that the ratio of two SYT–SSX transcripts in the Slovenian group was different from that in the Dutch group. They therefore raised the hypothesis that there are possible geographic differences in the frequency of SYT–SSX fusion transcripts in SS.( 10 ) For Asians, there were only two studies in Japan. Inagaki et al. detected SYT–SSX1 in 10 and SYT–SSX2 in nine patients, and found that SYT–SSX1 was correlated with shorter MFS in SS.( 18 ) Takenaka et al. indicated that SYT–SSX fusion type was not a significant prognostic factor in 57 SYT–SSX1‐positive and 34 SYT–SSX2‐positive SS.( 11 ) Although the ratio of SYT–SSX1 : SYT–SSX2 in the present study is different from other major studies, the 5‐year and 10‐year DSS, LRFS, and MFS rates in our research are comparable to others.( 22 , 23 , 24 , 25 ) Therefore, the ratio of SYT–SSX1 : SYT–SSX2 fusion and the prognostic impact of fusion type on the survival of patients with SS needs some international collaboration studies and systematic reviews by meta‐analysis.
Guillou et al. showed that histological grade was the most significant prognostic factor for both DSS and MFS of SS.( 26 ) Nevertheless, Trassard et al. reported that FNCLCC grade was an independent predictor for LRFS, but not for DSS or MFS.( 23 ) In the present study, aggressive histological grade was an independent adverse predictor for DSS. Although histological grade was proposed to correlate with MFS by univariate analysis, its significance was not manifested by multivariate analysis. Multivariate analysis proved that stage was an independent predictor for MFS. The UICC/AJCC staging system was established on four parameters: histological grade, tumor size, lymph node metastasis, and distant metastasis. Therefore, histological grade must be considered when evaluating the prognosis for MFS. Accordingly, the histological grade should be listed when the pathologist diagnoses SS, which contributes to the evaluation of prognosis and selection of suitable therapies for clinical doctors.
Moreover, tumor size < 5 cm was an advantageous factor for both DSS and LRFS in the present study. Although tumor size was shown not to be associated with MFS by univariate and multivariate analyses, it must be considered when staging, which is an independent predictor for MFS. Tumor size has been a classic clinical predictor for the prognosis of patients with SS;( 27 , 28 , 29 , 30 , 31 ) however, the classifications for tumor size were different in some studies, such as 5,( 24 , 28 ) 7,( 26 ) and 8 cm.( 23 ) We choose 5 cm as the cut‐off point because it was used in the UICC/AJCC staging system.( 15 )
Several studies have demonstrated better survival with younger age at diagnosis.( 27 , 28 , 32 , 33 , 34 ) However, Spillane et al. reported that younger age was associated with a poor prognosis.( 24 ) Moreover, Ladanyi et al. proposed that age was not correlated with prognosis of patients with SS in a multi‐institutional analysis,( 20 ) which is similar to some other studies.( 11 , 29 , 35 , 36 , 37 ) As a continuous variable, the prognostic implication of age was analyzed according to different cut‐off points. Trassard et al. showed there was no significant difference of prognosis between patients 33 years of age or younger and those older than 33 years.( 23 ) However, when expressed as a continuous variable, increasing age was an independent adverse prognostic factor for DSS.( 33 ) In the present study, we selected the mean age at diagnosis (37 years) as the cut‐off point, and showed that older age was an adverse predictor for DSS and MFS but had no relation to LRFS. Some authors explain this age‐related unfavorable influence in terms of biological and immunological factors, age‐dependent resistance to adjuvant chemotherapy, and so on.( 38 )
Generally, adequate local excision with postoperative radiotherapy can control local recurrence.( 3 ) In the present study, surgery modality was shown to be an independent predictor for LRFS. The patients who received wide local excision or amputation had a better LRFS compared with those with only marginal excision. However, as previously reported by others,( 23 , 27 , 39 ) we did not find an influence of radiotherapy on DSS, LRFS, or MFS in SS. Furthermore, the role of chemotherapy in the treatment of SS is controversial.( 11 , 23 , 28 , 34 , 35 , 40 , 41 ) In the present study, we failed to observe a significant improvement in patient outcome when chemotherapy was used. In contrast, patients who received chemotherapy tended to have a poor LRFS. Nevertheless, it is best not to draw firm conclusions regarding the efficacy of therapy from our results because the treatment was not randomized and many patients with serious pathogenetic conditions received adjuvant therapy. Future multi‐institutional randomized clinical trials should be conducted in order to clearly know the role of therapy.
Takenaka et al. observed that the majority of SYT–SSX1‐positive tumors were located in the extremities, whereas SYT–SSX2‐positive tumors were equally in the extremities and trunk.( 11 ) We failed to find associations of SYT–SSX fusion type with clinicopathological parameters, except the relationship with histological subtype and chemotherapy. As previously reported,( 16 , 20 , 21 ) major BSS were positive for SYT–SSX1, and SYT–SSX2‐positive tumors were mainly MSS. Because the selection of chemotherapy was not randomized, the relationship between SYT–SSX fusion type and chemotherapy was not convincing and needs further certification.
In summary, we observed that SYT–SSX2 was more frequent than SYT–SSX1 in Chinese patients with SS, and confirmed the association of SYT–SSX fusion type with histological subtype. Most importantly, we demonstrated the prognostic value of the fusion type for DSS and MFS. In addition, histological grade and tumor size were also indicated to be important for the prognosis of SS. However, further studies, especially international collaborations and randomized clinical trials, should be carried out in order to find the exact prognostic factors for patients with SS.
Acknowledgment
The authors thank the National Natural Science foundation of China for financial support (grant number 30572097).
References
- 1. Kransdorf MJ. Malignant soft tissue tumors in a large referral population: distribution of diagnoses by age, sex, and location. AJR Am J Roentgenol 1995; 164: 129–34. [DOI] [PubMed] [Google Scholar]
- 2. Enzinger FM, Weiss SW. Soft Tissue Tumors, 3rd edn. St Louis, MO: Mosby, 1995. [Google Scholar]
- 3. Fletcher CDM, Unni KK, Mertens F. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press, 2002. [Google Scholar]
- 4. Mitelman Database of chromosome aberrations in cancer (Website on the internet). 2002. Available from URL: http://cgapncinihgov/Chromosomes/Mitelman .
- 5. Ladanyi M. Fusions of the SYT and SSX genes in synovial sarcoma. Oncogene 2001; 20: 5755–62. [DOI] [PubMed] [Google Scholar]
- 6. Sandberg AA, Bridge JA. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: synovial sarcoma. Cancer Genet Cytogenet 2002; 133: 1–23. [DOI] [PubMed] [Google Scholar]
- 7. Dos Santos NR, De Bruijn DR, Geurts van Kessel A. Molecular mechanisms underlying human synovial sarcoma development. Genes Chromosom Cancer 2001; 30: 1–14. [DOI] [PubMed] [Google Scholar]
- 8. Panagopoulos I, Mertens F, Isaksson M et al . Clinical impact of molecular and cytogenetic findings in synovial sarcoma. Genes Chromosomes Cancer 2001; 31: 362–72. [DOI] [PubMed] [Google Scholar]
- 9. Guillou L, Coindre J, Gallagher G et al . Detection of the synovial sarcoma translocation t(X;18) (SYT–SSX) in paraffin‐embedded tissues using reverse transcriptase–polymerase chain reaction: a reliable and powerful diagnostic tool for pathologists. A molecular analysis of 221 mesenchymal tumors fixed in different fixatives. Hum Pathol 2001; 32: 105–12. [DOI] [PubMed] [Google Scholar]
- 10. Kokovic I, Bracko M, Golouh R et al . Are there geographical differences in the frequency of SYT–SSX1 and SYT–SSX2 chimeric transcripts in synovial sarcoma? Cancer Detect Prev 2004; 28: 294–301. [DOI] [PubMed] [Google Scholar]
- 11. Takenaka S, Ueda T, Naka N et al . Prognostic implication of SYT–SSX fusion type in synovial sarcoma: a multi‐institutional retrospective analysis in Japan. Oncol Rep 2008; 19: 467–76. [PubMed] [Google Scholar]
- 12. Sun B, Sun Y, Wang J et al . The diagnostic value of SYT–SSX detected by reverse transcriptase–polymerase chain reaction (RT‐PCR) and fluorescence in situ hybridization (FISH) for synovial sarcoma: a review and prospective study of 255 cases. Cancer Sci 2008; 99: 1355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun B, Sun Y, Wang J et al . Extent, relationship and prognostic significance of apoptosis and cell proliferation in synovial sarcoma. Eur J Cancer Prev 2006; 15: 258–65. [DOI] [PubMed] [Google Scholar]
- 14. Trojani M, Contesso G, Coindre JM et al . Soft tissue sarcomas of adults: study of pathological and prognostic variables and definition of a histological grading system. Int J Cancer 1984; 33: 37–42. [DOI] [PubMed] [Google Scholar]
- 15. Sobin LH, Wittekind CH. International Union against Cancer: TNM Classification of Malignant Tumours, 6th edn. New York: Wiley, 2002. [Google Scholar]
- 16. Kawai A, Woodruff J, Healey JH et al . SYT–SSX gene fusion as a determinant of morphology and prognosis in synovial sarcoma. N Engl J Med 1998; 338: 153–60. [DOI] [PubMed] [Google Scholar]
- 17. Nilsson G, Skytting B, Xie Y et al . The SYT–SSX1 variant of synovial sarcoma is associated with a high rate of tumor cell proliferation and poor clinical outcome. Cancer Res 1999; 59: 3180–4. [PubMed] [Google Scholar]
- 18. Inagaki H, Nagasaka T, Otsuka T et al . Association of SYT–SSX fusion types with proliferative activity and prognosis in synovial sarcoma. Mod Pathol 2000; 13: 482–8. [DOI] [PubMed] [Google Scholar]
- 19. Mezzelani A, Mariani L, Tamborini E et al . SYT–SSX fusion genes and prognosis in synovial sarcoma. Br J Cancer 2001; 85: 1535–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ladanyi M, Antonescu CR, Leung DH et al . Impact of SYT–SSX fusion type on the clinical behavior of synovial sarcoma: a multi‐institutional retrospective study of 243 patients. Cancer Res 2002; 62: 135–40. [PubMed] [Google Scholar]
- 21. Antonescu CR, Kawai A, Leung DH et al . Strong association of SYT–SSX fusion type and morphologic epithelial differentiation in synovial sarcoma. Diagn Mol Pathol 2000; 9: 1–8. [DOI] [PubMed] [Google Scholar]
- 22. Lewis JJ, Antonescu CR, Leung DH et al . Synovial sarcoma: a multivariate analysis of prognostic factors in 112 patients with primary localized tumors of the extremity. J Clin Oncol 2000; 18: 2087–94. [DOI] [PubMed] [Google Scholar]
- 23. Trassard M, Le Doussal V, Hacene K et al . Prognostic factors in localized primary synovial sarcoma: a multicenter study of 128 adult patients. J Clin Oncol 2001; 19: 525–34. [DOI] [PubMed] [Google Scholar]
- 24. Spillane AJ, A’Hern R, Judson IR et al . Synovial sarcoma: a clinicopathologic, staging, and prognostic assessment. J Clin Oncol 2000; 18: 3794–803. [DOI] [PubMed] [Google Scholar]
- 25. Ferrari A, Gronchi A, Casanova M et al . Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer 2004; 101: 627–34. [DOI] [PubMed] [Google Scholar]
- 26. Guillou L, Benhattar J, Bonichon F et al . Histologic grade, but not SYT–SSX fusion type, is an important prognostic factor in patients with synovial sarcoma: a multicenter, retrospective analysis. J Clin Oncol 2004; 22: 4040–50. [DOI] [PubMed] [Google Scholar]
- 27. Oda Y, Hashimoto H, Takeshita S et al . The prognostic value of immunohistochemical staining for proliferating cell nuclear antigen in synovial sarcoma. Cancer 1993; 72: 478–85. [DOI] [PubMed] [Google Scholar]
- 28. Bergh P, Meis‐Kindblom JM, Gherlinzoni F et al . Synovial sarcoma: identification of low and high risk groups. Cancer 1999; 85: 2596–607. [DOI] [PubMed] [Google Scholar]
- 29. Singer S, Baldini EH, Demetri GD et al . Synovial sarcoma: prognostic significance of tumour size, margin of resection and mitotic activity for survival. J Clin Oncol 1996; 14: 1201–8. [DOI] [PubMed] [Google Scholar]
- 30. Okcu MF, Despa S, Choroszy M et al . Synovial sarcoma in children and adolescents: thirty three years of experience with multimodal therapy. Med Pediatr Oncol 2001; 37: 90–6. [DOI] [PubMed] [Google Scholar]
- 31. Deshmukh R, Mankin HJ, Singer S. Synovial sarcoma: the importance of size and location for survival. Clin Orthop 2004; 419: 155–61. [PubMed] [Google Scholar]
- 32. Raney RB. Synovial sarcoma in young people: background, prognostic factors, and therapeutic questions. J Pediatr Hematol Oncol 2005; 27: 207–11. [DOI] [PubMed] [Google Scholar]
- 33. Wright PH, Sim FH, Soule EH et al . Synovial sarcoma. J Bone Joint Surg [Am] 1982; 64: 112–22. [PubMed] [Google Scholar]
- 34. Mullen JR, Zagars GK. Synovial sarcoma outcome following conservation surgery and radiotherapy. Radiother Oncol 1994; 33: 23–30. [DOI] [PubMed] [Google Scholar]
- 35. Brodsky JT, Burt ME, Hadju SI et al . Synovial sarcoma. Clinicopathological features, treatment and prognosis. Cancer 1992; 70: 484–9. [DOI] [PubMed] [Google Scholar]
- 36. Rooser BO, Willen H, Hugoson A et al . Prognostic factors in synovial sarcoma. Cancer 1989; 63: 2182–5. [DOI] [PubMed] [Google Scholar]
- 37. Golouh R, Vuzevski V, Bracko M et al . Synovial sarcoma: a clinicopathological study of 36 cases. J Surg Oncol 1990; 45: 20–8. [DOI] [PubMed] [Google Scholar]
- 38. Cohen HJ. Biology of aging as related to cancer. Cancer 1994; 74: 2092–100. [DOI] [PubMed] [Google Scholar]
- 39. Choong PFM, Pritchard DJ, Sim FH et al . Long‐term survival in high grade soft tissue sarcoma: prognostic factors in synovial sarcoma. Int J Oncol 1995; 7: 161–9. [DOI] [PubMed] [Google Scholar]
- 40. Kampe CE, Rosen G, Eilber F et al . Synovial sarcoma: a study of intensive chemotherapy in 14 patients with localized disease. Cancer 1993; 72: 2161–9. [DOI] [PubMed] [Google Scholar]
- 41. Brecht IB, Ferrari A, Int‐Veen C et al . Grossly‐resected synovial sarcoma treated by the German and Italian Pediatric Soft Tissue Sarcoma Cooperative Groups: discussion on the role of adjuvant therapies. Pediatr Blood Cancer 2006; 46: 11–17. [DOI] [PubMed] [Google Scholar]


