Abstract
Programmed cell death 4 (PDCD4) is a newly identified tumor suppressor that can inhibit activator protein (AP)‐1 activation and protein translation. Our previous studies indicate that lost or reduced PDCD4 expression is associated with the progression of ovarian carcinoma. However, direct evidence that PDCD4 inhibits malignant phenotype of human cancer cells is limited. In the present study, we found that PDCD4 expression in ovarian cancer cell lines (SKOV3, 3AO, and CAOV3) inhibited significantly their proliferation and cell cycle progression, and induced apoptosis. More importantly, up‐regulation of PDCD4 expression decreased the colony‐forming capacity of ovarian cancer cells in vitro and tumorigenic capacity in mice. These results demonstrate that PDCD4 can suppress the malignant phenotype of ovarian cancer cells, and may represent a novel therapeutic target for the treatment of ovarian cancer. (Cancer Sci 2009)
Programmed cell death 4 (PDCD4) is an apoptosis‐related gene which was first cloned from a human glioma cDNA library in 1997.( 1 ) It is also called MA‐3 in mice, DUG in rats, and H731 in humans.( 2 , 3 , 4 ) Subsequent studies demonstrated that PDCD4 was able to inhibit tumor promoter‐induced transformation in the mouse JB6 model system.( 5 ) In addition, further work showed that PDCD4‐deficient mice developed spontaneous tumors of the lymphoid origin,( 6 ) and PDCD4 transgenic mice showed significant resistance to tumor induction.( 7 ) Therefore, PDCD4 is regarded as a novel tumor suppressor gene. It has been reported that PDCD4 can suppress protein translation by directly interacting with the eukaryotic initiation factor (eIF) 4 A to inhibit the formation of translation–initiation complex( 8 , 9 , 10 ) or suppress the transcriptional activity of AP‐1 by inhibiting the expression of mitogen‐activated protein kinase kinase kinase kinase 1 (MAP4K1) to block the activation of c‐Jun.( 11 , 12 ) However, down‐regulation of PDCD4 can activate β‐catenin/Tcf and AP‐1‐dependent transcription, and promote the invasion of colon carcinoma cells.( 13 )
Recently, lost or reduced PDCD4 expression has been found in several kinds of human cancers, such as lung cancer,( 14 ) colorectal cancer,( 15 , 16 ) and glioma.( 17 ) Our previous study showed that PDCD4 expression was found to be lost or significantly lower in serous cystadenocarcinomas compared with that in normal ovaries and serous cystadenomas. The loss or reduction of PDCD4 expression in serous cystadenocarcinomas was significantly associated with higher pathological grade and poorer disease‐specific survival of patients.( 18 ) These observations have suggested that PDCD4 is a molecule related to tumorigenesis. However, direct evidence that PDCD4 inhibits malignant phenotype of human cancer cells is limited.
To further investigate the potential roles of PDCD4 in ovarian carcinoma, in this study, we overexpressed PDCD4 in human ovarian cancer cell lines and studied the effect of PDCD4 on the proliferation, cell cycle, apoptosis, and tumorigenesis of ovarian cancer cells in vitro and in vivo.
Materials and Methods
Cell culture and transfection. The human ovarian cancer cell line SKOV3 was purchased from the Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai, China), 3AO was purchased from the Shandong Academy of Medical Sciences (Jinan, China), and CAOV3 was purchased from the China Center for Type Culture Collection (Wuhan, China). Transfection of SKOV3, 3AO, and CAOV3 cells with plasmids (pDsRed2‐N1 and pDsRed2‐N1‐PDCD4) carrying red fluorescent protein gene was performed using Lipofectamine 2000 according to the manufacturer's protocols (Invitrogen, Carlsbad, CA, USA). Fourty‐eight hours later, RT‐PCR and western blot were used for the detection of PDCD4 expression in the transfected cells. To acquire stable PDCD4 expression clones, MOCK or PDCD4‐transfected SKOV3 cells were cultured in a ratio of 1:10 in culture medium containing 300 µg/mL G418 after 24 h of transfection. Several weeks later, the G418‐resistant cell clones, designated as SKOV3‐MOCK and SKOV3‐PDCD4 were collected. In order to verify effectiveness of transfection, red fluorescence in SKOV3‐PDCD4 and SKOV3‐MOCK was observed under fluorescent microscope.
Semi‐quantitative RT‐PCR. Total RNA was extracted using a modified TRIzol one‐step extraction method (Sangon Biotech, Shanghai, China). RNA concentrations were determined based on the absorbance at 260 nm. Total RNAs (3 µg) were reversely transcribed to cDNA using the Reverse‐Transcribe Kit (Promega, Madison, WI, USA). cDNA (1 µL) was amplified by PCR using PDCD4 specific primers (sense 5′‐CCA, AAG, AAA, GGT, GGT, GCA‐3′, and antisense 5′‐TGA, GGT, ACT, TCC, AGT, TCC‐3′). The PCR mixture was denatured at 94°C for 2 min then followed by 35 cycles of 95°C for 1 min 30 s, 66°C for 1 min 30 s, and 72°C for 1 min 30 s with an extension cycle at 72°C for 5 min. Amplified cDNAs were analyzed by 2% agarose gel electrophoresis. Human β‐actin primer was as a positive control. RT‐PCR was performed at least three times for each sample.
SDS‐PAGE and western blot. Proteins were extracted using a modified TRIzol one‐step extraction method. The protein extract was dissolved in a loading buffer (1 mM Tris Cl, 3% SDS, 60% glycerol, 75 mM DTT). Protein concentration of the homogenized lysates was measured using a Bradford kit (Bio‐Rad, Hercules, CA, USA), and equal amounts (25 µg) of protein were separated on SDS‐PAGE and transferred onto nitrocellulose membranes. Membranes were then blocked with 5% skim milk in Tris Buffered Saline + Tween (TBST) containing 0.1% Tween 20 for 1 h, and immunoblotting was done by incubating the membranes at 4°C overnight with rabbit antihuman PDCD4 antibody (1:10 000) or human β‐actin antibody (1:1000) followed by secondary antibodies (goat antirabbit IgG) conjugated with peroxidase for 3 h at room temperature. After washing, the protein bands were visualized using the enhanced chemiluminescence method according to the manufacturer's instructions (ECL; Amersham Biosciences, Buckinghamshire, UK). Western blot was performed at least three times for each sample.
Cell proliferation assay. SKOV3 and stably transfected cells (SKOV3‐MOCK and SKOV3‐PDCD4) were plated in 48‐well plates at a density of 3000 cells per well. After 24, 48, 72, 96, 120, and 144 h, the cells were collected and resuspended in PBS. 3AO and CAOV3 cells were transfected transiently in 24‐well plates. After 24, 48, and 72 h of transfection, the cells were also resuspended in PBS. The number of live cells was determined by light microscopy. Each cell was repeated in four wells and the experiment was repeated three times.
Colony formation assay. SKOV3 and stable cell lines were cultured in six‐well plates at a density of 1500 cells per well for 1–2 weeks. 3AO cells were transfected transiently in 24‐well plates. Twenty‐four hours later, transfected cells were reseeded in six‐well plates with a density of 2 × 104 cells per well in the presence of G418 (300 µg/mL) for 1–2 weeks. The cells were fixed with 20% methanol and stained with 1% crystal violet. Colonies that consisted of >50 cells were counted and calculated as a percentage of that of the control group. Each cell was repeated in two wells and the experiment was repeated three times.
Cell cycle analysis. SKOV3, 3AO, and CAOV3 cells were transfected transiently in 24‐well plates. After 48 h, cells were collected and washed once with PBS. The cells were resuspended in PBS containing 100 µg/mL propidium iodide (Jingmei Biotech, Shenzhen, China), and DNA content was evaluated by flow cytometry using the Beckman Coulter Cytomics FC 500 (Beckman Coulter, Fullerton, CA, USA). The experiment was repeated three times.
Detection of apoptosis. Cells were grown on coverlips at a density of 1.25 × 104 cells per well in 24‐well plates. After incubation for 24 h, nuclei of SKOV3, SKOV3‐MOCK, and SKOV3‐PDCD4 were stained with Hoechst staining (Jingmei Biotech) according to manufacturer's protocols and observed under fluorescent microscope. The experiment was repeated three times.
Animal studies. Twenty 6–8‐week‐old female BALB/c nude mice (SLAC, Shanghai, China) were randomly assigned to three groups: the SKOV3 group (n = 4), SKOV3‐MOCK group (n = 8), and SKOV3‐PDCD4 group (n = 8). They were injected subcutaneously in the left flank with 5 × 106 SKOV3, SKOV3‐MOCK, and SKOV3‐PDCD4 cells (prepared in PBS at a density of 5 × 107/mL), respectively. After one week, palpable tumors developed in the SKOV3 group and SKOV3‐MOCK group, which were measured three times a week. Tumor volume was assessed and calculated as follows: length × width × width × 0.4. All mice were sacrificed 4 weeks after initial ovarian cancer cell inoculation and tumors were removed for weight analysis. Then these tumors were fixed in 4% formalin and processed for paraffin embedding. Sections were cut at 5‐µm thickness in a microtome, and PDCD4 expression in tumors was detected by immunohistochemistry. The protocol was approved by the Animal Care and Utilization Committee of Shandong University. This study was also conducted in full compliance with the guidelines for the welfare of animals in experimental neoplasia.
Immunohistochemistry. The tumor tissues from nude mice were cut at 4–6 µm and transferred to slides. The tissues were deparaffinized in xylene and rehydrated through alcohol gradient. The slides were washed three times with PBS, blocked for endogenous peroxidase activity, preincubated with goat serum, and then incubated with anti‐PDCD4 antibody (1:600) for 1 h at room temperature in a humid chamber. The sections were washed three times for 3 min at room temperature with PBS and then visualized with the Rabbit IgG avidin–biotin–peroxidase complex and DAB Peroxidase Substrate kits (Maixin, Fuzhou, China). Immunohistochemistry was performed twice for each sample.
Statistical analysis. SPSS 10.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis. Student's t‐test was used to evaluate the statistical significance of live cells number, colony number, tumor volume, and tumor weight between the SKOV3‐PDCD4 group and corresponding controls. P‐values less than 0.05 were considered statistically significant.
Results
Overexpression of PDCD4 in ovarian cancer cells. Our previous studies demonstrated that loss of PDCD4 expression occurred only in malignant ovarian tumors and PDCD4 expression status was significantly associated with the high grade of tumors and poor prognosis. To provide direct evidence that PDCD4 inhibits proliferation of ovarian cancer cells, we introduced recombinant pDsRed2‐N1 plasmids that do or do not carry the full‐length PDCD4 cDNA into ovarian cancer cells SKOV3, 3AO, and CAOV3, which express only low levels of endogenous PDCD4. Under fluorescent microscope, there was no red fluorescence in untransfected SKOV3 cells; however, obvious red fluorescence was observed in SKOV3‐MOCK and SKOV3‐PDCD4 cells (Fig. 1a). The results from RT‐PCR and western blot analysis showed that the cells transfected with PDCD4 expression plasmid had stronger PDCD4 expression than the control cells (Fig. 1b,c). These results indicate that the recombinant pDsRed2‐N1‐PDCD4 plasmid gives rise to PDCD4 expression in ovarian cancer cells.
Figure 1.

Overexpression of programmed cell death 4 (PDCD4) in ovarian cancer cells. (a) Fluorescent images of SKOV3 cells after transfection with PDCD4 expression plasmid (PDCD4) or empty vector (MOCK). There was no fluorescence in untransfected SKOV3 cells. (b) PDCD4 mRNA expression in SKOV3, 3AO, and CAOV3 cells was examined by RT‐PCR after stable or transient transfection with PDCD4 plasmid (PDCD4) or empty vector (MOCK). Obvious PDCD4 expression was found in PDCD4‐transfected cells compared with corresponding controls. (c) PDCD4 protein expression in SKOV3, 3AO, and CAOV3 cells was examined by western blot after stable or transient transfection with PDCD4 plasmid (PDCD4) or empty vector (MOCK). The bands of interest were further analyzed by densitometer. Data were normalized to β‐actin. Up‐regulation of PDCD4 expression was found in PDCD4‐transfected cells compared with corresponding controls.
Overexpression of PDCD4 in ovarian cancer cells inhibited their proliferation and prevented their colony‐forming capacity. After transfection, the effect of PDCD4 on the cell growth of ovarian cancer cells was further investigated. The result showed that SKOV3‐PDCD4 cells grew significantly slower than control cells (SKOV3 and SKOV3‐MOCK) (P < 0.001) (Fig. 2a). Additionally, the colony number of SKOV3‐PDCD4 cells was decreased about 50% compared with that of SKOV3‐MOCK cells (P < 0.01) (Fig. 2b). The results were further confirmed in the other two cell lines 3AO and CAOV3 (Fig. 2a,b). The 3AO and CAOV3 cells transfected with PDCD4 expression plasmid grew significantly slower than control cells and their colony‐forming capacity also decreased. This indicates that overexpression of PDCD4 in ovarian cancer cells significantly inhibits their proliferation and colony‐forming capacity.
Figure 2.
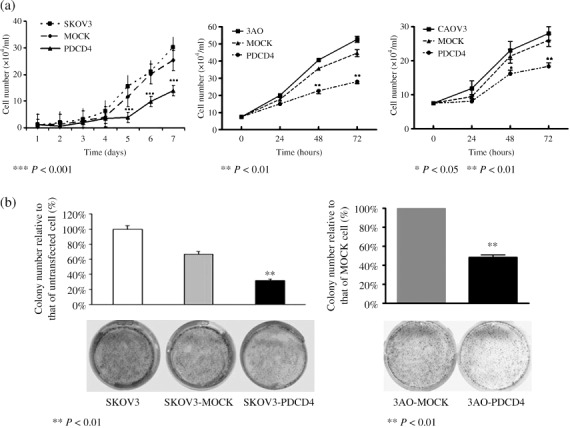
The effect of programmed cell death 4 (PDCD4) overexpression in ovarian cancer cells on cell proliferation and colony formation. (a) The growth curves of SKOV3, 3AO, and CAOV3 cells transfected with PDCD4 plasmid or empty vector stably or transiently. Cell numbers in PDCD4‐transfected cells were significantly reduced compared with corresponding controls. (b) Overexpression of PDCD4 in PDCD4‐transfected cells reduced their colony‐forming capacity compared with corresponding controls.
PDCD4 overexpression in ovarian cancer cells decreased their tumorigenic capacity and prolonged the tumor latency in nude mice. To determine the effect of PDCD4 on the proliferation of SKOV3 in vivo, we inoculated SKOV3, SKOV3‐MOCK, and SKOV3‐PDCD4 cells into BALB/C nude mice. All mice in the SKOV3 group (n = 4) and SKOV3‐MOCK group (n = 8) developed palpable tumors at 6 or 7 days after inoculation which rapidly grew further. By contrast, none of the mice in the SKOV3‐PDCD4 group (n = 8) showed any obvious sign of tumor formation within 3 weeks. Three weeks later, only small tumors were found in two mice (2/8). The overall mean tumor volume in the SKOV3‐PDCD4 group was much smaller than that in SKOV3‐MOCK or SKOV3 group (0.023 ± 0.004 cm3 vs 0.792 ± 0.203 cm3 or 0.824 ± 0.416 cm3, respectively, P < 0.01) (Fig. 3a). Consistent with these results, the mean tumor weight (0.0075 ± 0.0096 g) in the SKOV3‐PDCD4 group was also significantly lower than that of the SKOV3‐MOCK group (0.193 ± 0.079 g, P < 0.05) or the SKOV3 group (0.235 ± 0.139 g) (P < 0.05) (Fig. 3b). In addition, at necropsy, multiple tumor nodules were found in the SKOV3 group and SKOV3‐MOCK group but not in the SKOV3‐PDCD4 group (Fig. 3c). Moreover, the results from immunohistochemistry showed that there was obvious PDCD4 expression in the tumors of the SKOV3‐PDCD4 group compared with those of the SKOV3 or SKOV3‐MOCK groups (Fig. 3d). These results indicate that overexpression of PDCD4 in the SKOV3 cell line decreases the tumorigenic capacity and delays tumor growth in vivo.
Figure 3.
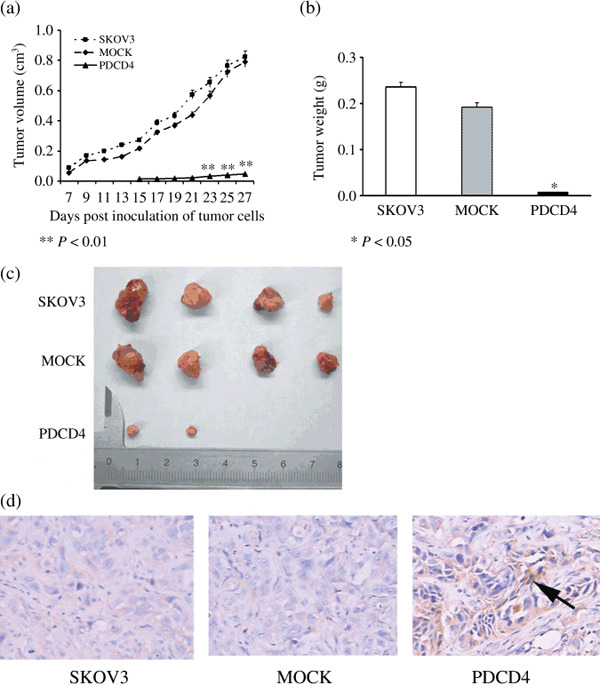
Overexpression of programmed cell death 4 (PDCD4) in ovarian cancer cells inhibited their tumorigenic capacity in nude mice. (a) The growth curves of tumors in SKOV3, SKOV3‐MOCK, and SKOV3‐PDCD4 tumor‐bearing nude mice. The overall mean tumor volume in the SKOV3‐PDCD4 group was much smaller than those in the SKOV3‐MOCK or SKOV3 groups (P < 0.01). (b) Weight of SKOV3, SKOV3‐MOCK, and SKOV3‐PDCD4 tumors. The mean tumor weight in the SKOV3‐PDCD4 group was also significantly lower than that of the SKOV3‐MOCK group or the SKOV3 group (P < 0.05). (c) Representative image of SKOV3, SKOV3‐MOCK, and SKOV3‐PDCD4 tumors. All mice in the SKOV3 and SKOV3‐MOCK groups developed bigger tumors. However, two mice in the SKOV3‐PDCD4 group developed smaller tumors. (d) Expression of PDCD4 in SKOV3, SKOV3‐MOCK, and SKOV3‐PDCD4 tumors detected using immunohistochemistry (× 200). There was obvious PDCD4 expression in tumors of the SKOV3‐PDCD4 group (arrow) compared with those of the SKOV3 and SKOV3‐MOCK groups.
Overexpression of PDCD4 in ovarian cancer cells induced cell cycle arrest and apoptosis. To explore the mechanism whereby PDCD4 inhibits proliferation of ovarian cancer cells, we investigated the effect of PDCD4 on the cell cycle progression and apoptosis of ovarian cancer cells by flow cytometry. As shown in Figure 4(a–c), overexpression of PDCD4 in three ovarian cancer cell lines (SKOV3, 3AO, and CAOV3) induced cell cycle arrest as exemplified by the increased number of cells in the G2‐ or S‐phase. In addition, overexpression of PDCD4 in these three cell lines significantly increased the percentages of hypodiploid cells in sub‐G1 fraction (Fig. 4a–c), indicating that PDCD4 induced apoptosis of ovarian cancer cells. To confirm this data, the effect of PDCD4 on SKOV3 cells was also investigated by Hoechst staining. Morphological analysis with Hoechst staining showed many nuclei with chromatin condensation and the formation of apoptotic bodies in the SKOV3‐PDCD4 cells, but this was very rare in the control cells (Fig. 4d). Taken together, these results indicate that PDCD4 is able to inhibit proliferation of ovarian cancer cells and induce their apoptosis.
Figure 4.
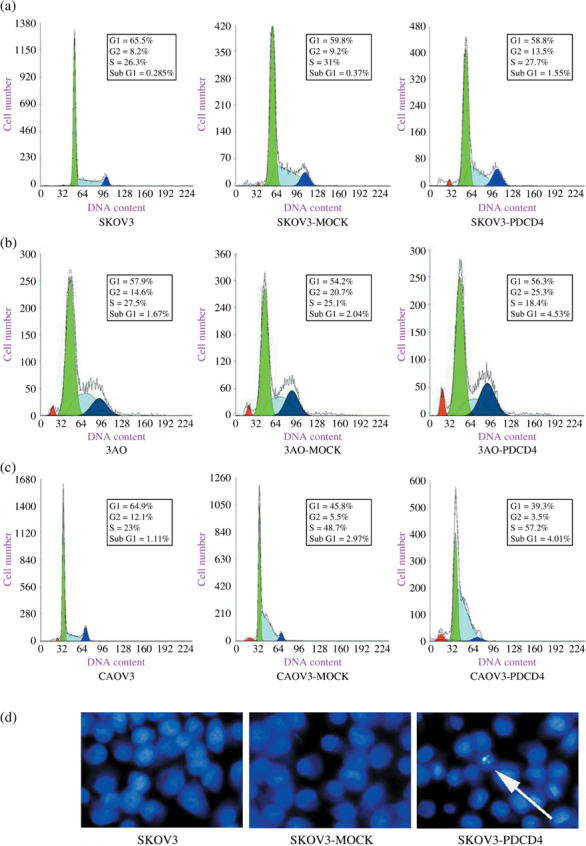
The effect of programmed cell death 4 (PDCD4) overexpression in ovarian cancer cells on cell cycle and apoptosis. (a) PDCD4 overexpression in SKOV3 cells delayed cell cycle progression and induced apoptosis of SKOV3 (representative image). (b) PDCD4 overexpression in 3AO cells delayed cell cycle progression and induced apoptosis of 3AO (representative image). (c) PDCD4 overexpression in CAOV3 cells delayed cell cycle progression and induced apoptosis of CAOV3 (representative image). (d) Overexpression of PDCD4 can induce apoptosis in SKOV3 cells (arrow) compared with corresponding controls (representative image).
Discussion
Epithelial ovarian carcinoma is the third‐leading common gynecologic cancer and number one cause of gynecologic cancer death in women.( 19 ) Our previous studies have shown that loss or reduction of PDCD4 expression occurs in serous cystadenocarcinomas and correlates with tumor progression and prognosis. However, the effect of PDCD4 on the malignant phenotype of ovarian cancer is not clear. In the present study, we provide direct evidence demonstrating that overexpression of PDCD4 inhibits the malignant phenotype of ovarian cancer cells.
The malignant nature of ovarian cancers arises from their rapid proliferation. It has been reported that PDCD4 could inhibit growth of endocrine tumor cells,( 20 ) and S6 kinase 1‐, beta‐transducin repeat‐containing protein (βTRCP)‐mediated degradation of PDCD4 promoted protein translation and cell growth.( 21 ) We also found that overexpression of PDCD4 in ovarian cancer cells could effectively inhibit their proliferation in vitro, which was in accordance with those findings. Clonogenic assay is a method to evaluate the proliferative ability and tumorigenicity of single cell in vitro.( 22 ) In this study, we demonstrated that up‐regulation of PDCD4 expression could result in a significant reduction in the colony number of ovarian cancer cells using plate clonogenic assay. This phenomenon was also found in two glioma cell lines, U251 and U87.( 23 ) These results demonstrated overexpression of PDCD4 in tumor cells could prevent their colony‐forming ability in vitro.
To confirm this effect in vivo, we injected subcutaneously the SKOV3 cells carrying the PDCD4 gene into the nude mice. The result showed that all mice in control groups developed palpable tumors 6 or 7 days later. However, only 25% (2/8) of mice in the SKOV3‐PDCD4 group developed smaller tumors 3 weeks later. It has been reported that two of four mice injected with CAOV3‐pDOR‐p16 cells failed to form tumors, and the other two mice developed tumors 7–14 days later than mice of the control group.( 24 ) This indicates that overexpression of PDCD4 could decrease the tumorigenic capacity of SKOV3 and increase tumor latency in nude mice. Our results demonstrate that PDCD4 can also suppress the malignant phenotype of ovarian cancer cells in vivo. However, there is a significant difference between tumor growth in vitro and in vivo. The discrepancy of cell growth in vitro and that in vivo may be related to host defense or factors affecting tumor cell growth in vivo such as angiogenesis. Indeed, it has been reported that overexpression of PDCD4 can inhibit tumor angiogenesis by down‐regulating expression of vascular endothelial growth factor and fibroblast growth factor‐2 in vivo.( 25 ) Taken together, these results indicate that PDCD4 is a human tumor suppressor which may represent a new and useful molecular target for ovarian cancer therapy.
To date, the mechanism of proliferation inhibition induced by PDCD4 remains unclear. Cell cycle inhibition and apoptosis induction may be responsible. Jansen et al. reported that PDCD4 may enhance geldanamycin‐induced G2‐M arrest in UO‐31 cells expressing low levels of Rb protein.( 26 ) Our results showed that restoration of PDCD4 expression could induce S‐phase arrest in U251 glioma cells.( 23 ) In addition, it has been reported that PDCD4 can induce apoptosis of the human breast cancer cell line T‐47D and human hepatocellular carcinoma–derived cell line Huh7 in vitro.( 27 , 28 ) Similarly, aerosol delivery of urocanic acid‐modified chitosan/programmed cell death 4 complex can enhance apoptosis of murine lung cancer cells and control their cell cycle progression in K‐ras null mice or AP‐1 luciferase reporter mice.( 25 , 29 ) Consistent with these reports, we showed in this study that PDCD4 expression in three ovarian cancer cell lines resulted in cell cycle arrest. Our results from flow cytometry and Hoechst staining also showed that PDCD4 could induce apoptosis of these ovarian cancer cell lines. These results suggest that the inhibitory effect of PDCD4 on ovarian cancer cells may be related to both cell cycle arrest and apoptosis. However, overexpression of PDCD4 in colon carcinoma cell line RKO cells did not induce apoptosis or alter their cell cycle progression.( 12 ) A possible explanation for the discrepancies in different types of human cancers is that the function of PDCD4 might be cell‐type specific. Depending on the cell types studied, the inhibitory properties of PDCD4 may be mediated by several different mechanisms, including promotion of apoptosis and inhibition of the cell cycle, angiogenesis, invasion, and tumorigenesis.( 30 ) Further investigation is required to elucidate the mechanism of the inhibitory effect of PDCD4 in various cancer cells.
In conclusion, we have demonstrated that overexpression of PDCD4 in ovarian cancer cells not only inhibits the proliferation and colony‐forming ability of tumor cells in vitro, but also decreases their tumorigenic capacity and delays tumor growth in nude mice. This indicates that PDCD4 may represent an important therapeutic target for the treatment of ovarian cancer.
Acknowledgments
We thank Dr Ozaki (Department of Internal Medicine, Saga Medical School, Saga University, Saga, Japan) for kindly providing the PDCD4 expression plasmids and Dr N.H. Colburn (National Institutes of Health, USA) for the anti‐PDCD4 antibody. This work was supported by grants from the Natural Science Foundation of China (No. 30628015), National “973” program (No. 2006CB503803), Doctor Funds of Shandong Province (No. 2006BS03064), and Post‐doctor Funds of China (No. 20070411096).
References
- 1. Matsuhashi S, Yoshinaga H, Yatsuki H, Tsugita A, Hori K. Isolation of a novel gene from a human cell line with Pr‐28 Mab which recognizes a nuclear antigen involved in the cell cycle. Res Commun Biochem Cell Mol Biol 1997; 1: 109–20. [Google Scholar]
- 2. Shibahara K, Asano M, Ishida Y, Aoki T, Koike T, Honjo T. Isolation of a novel mouse gene MA‐3 that is induced upon programmed cell death. Gene 1995; 166: 297–301. [DOI] [PubMed] [Google Scholar]
- 3. Goke A, Goke R, Knolle A et al . DUG is a novel homologue of translation initiation factor 4G that binds eIF4A. Biochem Biophys Res Commun 2002; 297: 78–82. [DOI] [PubMed] [Google Scholar]
- 4. Yoshinaga H, Matsuhashi S, Fujiyama C, Masaki Z. Novel human PDCD4 (H731) gene expressed in proliferative cells is expressed in the small duct epithelial cells of the breast as revealed by an anti‐H731 antibody. Pathol Int 1999; 49: 1067–77. [DOI] [PubMed] [Google Scholar]
- 5. Cmarik JL, Min H, Hegamyer G et al . Differentially expressed protein Pdcd4 inhibits tumor promoter‐induced neoplastic transformation. Proc Natl Acad Sci USA 1999; 96: 14037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hilliard A, Hilliard B, Zheng SJ et al . Translational regulation of autoimmune inflammation and lymphoma genesis by programmed cell death 4. J Immunol 2006; 177: 8095–102. [DOI] [PubMed] [Google Scholar]
- 7. Jansen AP, Camalier CE, Colburn NH. Epidermal expression of the translation inhibitor programmed cell death 4 suppresses tumorigenesis. Cancer Res 2005; 65: 6034–41. [DOI] [PubMed] [Google Scholar]
- 8. Yang HS, Jansen AP, Komar AA et al . The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol 2003; 23: 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waters LC, Veverka V, Böhm M et al . Structure of the C‐terminal MA‐3 domain of the tumour suppressor protein Pdcd4 and characterization of its interaction with eIF4A. Oncogene 2007; 26: 4941–50. [DOI] [PubMed] [Google Scholar]
- 10. Yang HS, Cho MH, Zakowicz H et al . A novel function of the MA‐3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A. Mol Cell Biol 2004; 24: 3894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang HS, Jansen AP, Nair R et al . A novel transformation suppressor, Pdcd4, inhibits AP‐1 transactivation but not NF‐kappaB or ODC transactivation. Oncogene 2001; 20: 669–76. [DOI] [PubMed] [Google Scholar]
- 12. Yang HS, Matthews CP, Clair T et al . Tumorigenesis suppressor Pdcd4 down‐regulates mitogen‐activated protein kinase kinase kinase kinase 1 expression to suppress colon carcinoma cell invasion. Mol Cell Biol 2006; 26: 1297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Q, Sun Z, Yang HS. Downregulation of tumor suppressor Pdcd4 promotes invasion and activates both beta‐catenin/Tcf and AP‐1‐dependent transcription in colon carcinoma cells. Oncogene 2008; 27: 1527–35. [DOI] [PubMed] [Google Scholar]
- 14. Chen Y, Knösel T, Kristiansen G et al . Loss of PDCD4 expression in human lung cancer correlates with tumour progression and prognosis. J Pathol 2003; 200: 640–6. [DOI] [PubMed] [Google Scholar]
- 15. Lee S, Bang S, Song K, Lee I. Differential expression in normal–adenoma‐carcinoma sequence suggests complex molecular carcinogenesis in colon. Oncol Rep 2006; 16: 747–54. [PubMed] [Google Scholar]
- 16. Mudduluru G, Medved F, Grobholz R et al . Loss of programmed cell death 4 expression marks adenoma‐carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer 2007; 110: 1697–707. [DOI] [PubMed] [Google Scholar]
- 17. Gao F, Zhang P, Zhou C et al . Frequent loss of PDCD4 expression in human glioma: possible role in the tumorigenesis of glioma. Oncol Rep 2007; 17: 123–8. [PubMed] [Google Scholar]
- 18. Wang X, Wei Z, Gao F et al . Expression and prognostic significance of PDCD4 in human epithelial ovarian carcinoma. Anticancer Res 2008; 28: 2991–6. [PubMed] [Google Scholar]
- 19. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin 2007; 57: 43–66. [DOI] [PubMed] [Google Scholar]
- 20. Göke R, Gregel C, Göke A, Arnold R, Schmidt H, Lankat‐Buttgereit B. Programmed cell death protein 4 (PDCD4) acts as a tumor suppressor in neuroendocrine tumor cells. Ann N Y Acad Sci 2004; 1014: 220–1. [DOI] [PubMed] [Google Scholar]
- 21. Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1‐ and betaTRCP‐mediated degradation of PDCD4 promotes protein translation and cell growth. Science 2006; 314: 467–71. [DOI] [PubMed] [Google Scholar]
- 22. Hoffman RM. In vitro sensitivity assays in cancer: a review, analysis, and prognosis. J Clin Lab Anal 1991; 5: 133–43. [DOI] [PubMed] [Google Scholar]
- 23. Gao F, Wang X, Zhu F et al . PDCD4 gene silencing in gliomas is associated with 5′CpG island methylation and unfavorable prognosis. J Cell Mol Medical 2008. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang M, Wei J, Zhang JL. Replacement of the p16 gene in human ovarian cancer cells. Chinese Med J 2001; 114: 857–9. [PubMed] [Google Scholar]
- 25. Jin H, Kim TH, Hwang SK et al . Aerosol delivery of urocanic acid‐modified chitosan/programmed cell death 4 complex regulated apoptosis, cell cycle, and angiogenesis in lungs of K‐ras null mice. Mol Cancer Ther 2006; 5: 1041–9. [DOI] [PubMed] [Google Scholar]
- 26. Jansen AP, Camalier CE, Stark C, Colburn NH. Characterization of programmed cell death 4 in multiple human cancers reveals a novel enhancer of drug sensitivity. Mol Cancer Ther 2004; 3: 103–10. [PubMed] [Google Scholar]
- 27. Afonja O, Juste D, Das S, Matsuhashi S, Samuels HH. Induction of PDCD4 tumor suppressor gene expression by RAR agonists, antiestrogen and HER‐2/neu antagonist in breast cancer cells. Evidence for a role in apoptosis. Oncogene 2004; 23: 8135–45. [DOI] [PubMed] [Google Scholar]
- 28. Zhang H, Ozaki I, Mizuta T et al . Involvement of programmed cell death 4 in transforming growth factor‐beta1‐induced apoptosis in human hepatocellular carcinoma. Oncogene 2006; 25: 6101–12. [DOI] [PubMed] [Google Scholar]
- 29. Hwang SK, Jin H, Kwon JT et al . Aerosol‐delivered programmed cell death 4 enhanced apoptosis, controlled cell cycle and suppressed AP‐1 activity in the lungs of AP‐1 luciferase reporter mice. Gene Ther 2007; 14: 1353–61. [DOI] [PubMed] [Google Scholar]
- 30. Lankat‐Buttgereit B, Lenschen B, Schmidt H, Göke R. The action of Pdcd4 may be cell type specific: evidence that reduction of dUTPase levels might contribute to its tumor suppressor activity in Bon‐1 cells. Apoptosis 2008; 13: 157–64. [DOI] [PubMed] [Google Scholar]


