Abstract
During the late phase of adenovirus infection, viral mRNA is efficiently transported from the nucleus to the cytoplasm while most cellular mRNA species are retained in the nucleus. Two viral proteins, E1B-55 kDa and E4orf6, are both necessary for these effects. The E4orf6 protein of adenovirus type 5 binds and relocalizes E1B-55 kDa, and the complex of the two proteins was previously shown to shuttle continuously between the nucleus and cytoplasm. Nucleocytoplasmic transport of the complex is achieved by a nuclear export signal (NES) within E4orf6. Mutation of this signal sequence severely reduces the ability of the E1B-55 kDa–E4orf6 complex to leave the nucleus. Here, we examined the role of functional domains within E4orf6 during virus infection. E4orf6 or mutants derived from it were transiently expressed, followed by infection with recombinant adenovirus lacking the E4 region and determination of virus yield. An arginine-rich putative alpha helix near the carboxy terminus of E4orf6 contributes to E1B-55 kDa binding and relocalization as well as to the synthesis of viral DNA, mRNA, and proteins. Further mutational analysis revealed that mutation of the NES within E4orf6 considerably reduces its ability to support virus production. The same effect was observed when nuclear export was blocked with a competitor. Further, a functional NES within E4orf6 contributed to the efficiency of late virus protein synthesis and viral DNA replication, as well as total and cytoplasmic accumulation of viral late mRNA. Our data support the view that NES-mediated nucleocytoplasmic shuttling strongly enhances most, if not all, intracellular activities of E4orf6 during the late phase of adenovirus infection.
A number of viruses that replicate in the nucleus of the cell have devised specialized mechanisms that ensure efficient export of viral RNA from the nucleus to the cytoplasm. Such viruses include the human immunodeficiency virus (HIV) and other lentiviruses (9, 10, 37), simple retroviruses (6), influenza virus (43), hepatitis B virus (23, 27), and adenovirus. In the late phase of infection with adenovirus type 5, viral mRNA is efficiently transported to the cytoplasm but, simultaneously, most cellular mRNA molecules are retained in the nucleus of the cell (1, 3, 46).
Two proteins encoded by adenovirus type 5 are known to be required for the modulation of mRNA transport: E1B-55 kDa and E4orf6 (E4-34 kDa) (1, 18, 46, 56). These proteins form a specific complex within infected (54) and transiently transfected (17) cells. The E4orf3 protein of adenovirus type 5 also colocalizes with E1B-55 kDa. However, E1B-55 kDa preferentially associates with E4orf6 when all three proteins are present in an infected or transfected cell (33, 34). When E1B-55 kDa is expressed by transient or stable transfection, it is mostly localized in cytoplasmic clusters (64, 65). However, in adenovirus-infected cells, several localization patterns were identified in addition to this cytoplasmic location (45). Most notably, E1B-55 kDa colocalizes with E4orf6 in the periphery of viral replication centers within the nucleus. It was therefore suggested that the two proteins may be directly involved in the transport of viral mRNA from these replication centers to the cytoplasm (45). Upon transient coexpression of E1B-55 kDa with E4orf6, both proteins are evenly distributed over the nucleus, arguing that E4orf6 relocalizes E1B-55 kDa from the cytoplasm to the nucleus (17). However, even though the steady-state localization of both proteins is almost exclusively nuclear under these circumstances, the complex of E1B-55 kDa and E4orf6 continuously shuttles between the nucleus and cytoplasm, as has been shown by heterokaryon assays (8). While the two proteins can exit the nucleus when expressed together, neither of them is able to undergo nuclear export in the absence of the other. Nuclear export is driven by a leucine-rich nuclear export signal (NES) within E4orf6. E1B-55 kDa apparently inactivates a nuclear retention signal upon association with E4orf6, resulting in nuclear export of the two proteins. Given the fact that both proteins are required to modulate mRNA transport during adenovirus infection, along with the finding that they undergo nuclear export, it is tempting to speculate that a functional NES might be contributing to the efficient export of viral mRNA, thus supporting virus replication. To test this hypothesis, we assayed whether or not the export function of E4orf6 contributes to the efficiency of virus production and, if so, what stages of the infectious cycle depend on E4orf6 export. Our results suggest that nuclear export of E4orf6 contributes to several steps in virus production, including DNA replication, mRNA accumulation, and a shift in distribution of late viral mRNA from the nucleus to the cytoplasm.
MATERIALS AND METHODS
Cell culture.
HeLa cells were obtained from the American Type Culture Collection and maintained in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS).
Plasmids.
Expression plasmids for E4orf6 and E1B-55 kDa as well as rex chimeras have been described previously (8). All E4orf6 mutants were generated by site-directed mutagenesis (Quikchange; Stratagene). Thereby, the coding sequences for the amino acids to be deleted were replaced by the sequence GGCGCC to generate the mutants E4orf6Δ13-21, E4orf6Δ22-31, E4orf6Δ225-232, and E4orf6Δ264-272. The sequences of the corresponding oligonucleotides employed for mutagenesis were GGCATGACACTAGGCGCCCGCACTCCGTACAG, CTCGGTTGTCTCGGGGCGCCTTTGAGACAGAAACC, GTGGTGCTGTGCTGCGGCGCCATCAGGGTGCGCTGC, and GCCATGTTGTATTCCGGCGCCTTTATTCGCGCGCTG, respectively. The plasmids pGemE4orf6 and pGemE4orf6Δ225-232 were generated by excising the E4orf6 coding regions from the expression plasmids described above with BamHI and ligating them into the vector pGem7Zf(+) (Promega) (previously linearized with the same enzyme) in an orientation that allows the transcription of RNA in the sense direction from the T7 promoter.
Transient transfections.
For electroporation, 0.4 ml of a HeLa cell suspension in DMEM–10% FCS (4 × 106 cells/ml) was subjected to a pulse of 230 V in a cuvette with 0.4-cm gap width, with the resistance set to 200 Ω and the capacitance set to 950 μF. Then, 0.2 ml of the suspension was seeded on a 3.5-cm-diameter cell culture dish in 2 ml of DMEM–10% FCS. Transfections with Superfect (Qiagen) were done as recommended by the manufacturer.
Immunofluorescence.
Cells were seeded on plastic slides (Nunc) suitable for microscopy and then infected as described below or transfected with Superfect. Transfected or infected cells were fixed with paraformaldehyde (4% in phosphate-buffered saline [PBS]; 15 min), permeabilized with Triton X-100 (0.2% in PBS; 25 min), and incubated with antibody as described previously (7). The E2A-72 kDa DNA binding protein was stained with a mouse monoclonal antibody (clone B8-6; gift from T. Shenk). To detect E4orf6, the murine monoclonal antibody RSA3 (45) was used. Hemagglutinin (HA)-tagged E1B-55 kDa was stained with a polyclonal rabbit antibody to the HA tag (Santa Cruz Biotechnology). Primary mouse antibodies were visualized by secondary antibodies coupled to fluorescein isothiocyanate (Jackson Laboratory). Primary rabbit antibodies were detected by a Texas Red-labeled secondary antibody (Jackson Laboratory). Prior to being mounted (Fluoprep mounting medium; bioMérieux), the cell nuclei were briefly stained with 4′,6′-diamidino-2-phenylindole (DAPI).
Immunoprecipitation.
E4orf6 and E4orf6Δ225-232 were prepared with pGemE4orf6 and pGemE4orf6Δ225-232, respectively, by transcription and translation in vitro with T7 RNA polymerase and rabbit reticulocyte lysate (Promega). The reticulocyte lysate was incubated with lysates from HeLa cells or 293 cells (the latter constitutively expressing E1B-55 kDa) and immunoprecipitated with an antibody against E1B-55 kDa (clone 2A6 [55]), as described previously (51). The precipitated proteins were resolved on a 10% polyacrylamide gel, followed by autoradiography.
Complementation of virus growth.
HeLa cells were transiently transfected with E4orf6 expression plasmids by electroporation. Electroporated cells (800,000) were then seeded in a 3.5-cm-diameter dish for 24 h. Subsequently, the cells were washed with PBS and then overlayed with 800,000 infectious units of the recombinant adenovirus 366, which lacks the entire E4 region, in 500 μl of DMEM without serum. After 3 h of gentle rocking in an incubator, the cells were washed again with PBS and further incubated in DMEM–10% FBS. After another 48-h incubation period, the cells were harvested in PBS and lysed by three cycles of freezing and thawing. To determine the yield of virus, a dilution series of the lysate was made in DMEM and a fresh monolayer of HeLa cells was infected with the dilutions as described above. Twenty-four hours after infection, the cells were fixed and stained with a monoclonal antibody (B8-6) to the E2A-72 kDa DNA binding protein. The cells were stained with DAPI in parallel, and the ratio of infected cells to the total cell count was determined.
When rex-derived export inhibitors were employed, the cells were first seeded in a 3.5-cm-diameter well and then transfected with E4orf6 and export competitor by using Superfect (Qiagen). The transfected cells were infected 24 h later and then processed as described above.
The reason for using two different methods of transfection was as follows. Superfect transfection generally yielded higher expression levels (data not shown). This was desired when studying the effects of the NLSrex export competitor, since these competitors are only effective when expressed at high levels. In contrast, electroporation yielded lower expression levels of E4orf6, reflecting the situation in adenovirus-infected cells. This method was therefore chosen when analyzing the effects of E4orf6 and its mutants in the absence of export competitors.
Assessment of late protein expression.
HeLa cells were transfected and infected with 366 virus as described above. Eighteen hours after infection, the cells were radiolabeled with [35S]Met and [35S]Cys (Promix, Amersham) for 3 h and harvested in 0.6 ml of radioimmunoprecipitation assay (RIPA) buffer (20 mM Tris-HCl [pH 7.6], 150 mM NaCl, 10 mM EDTA, 1% Triton X-100, 1% desoxycholate, 0.1% sodium dodecyl sulfate [SDS]). The viral hexon protein was immunoprecipitated as follows. After preclearing the lysate with protein A-Sepharose (Pharmacia), incubation with a monoclonal antibody (gift from J. Flint) to hexon was allowed to proceed for 3 h. Protein A-Sepharose (10-μl packed volume) was added for 30 min, followed by three washing steps in RIPA buffer and two additional washing steps in SNNTE (50 mM Tris-HCl [pH 7.6], 500 mM NaCl, 5 mM EDTA, 5% sucrose, 1% Nonidet P-40). The precipitated protein was separated on an SDS-polyacrylamide gel, followed by autoradiography.
Detection of virus DNA.
HeLa cells were transfected and infected with 366 virus as described above. At various time points after infection, the cells were harvested and DNA was extracted (Qiagen). The DNA was treated with HindIII and separated on a 0.8% agarose gel, followed by transfer to nylon. The blot was hybridized to a digoxigenin-labeled probe derived from the E1B region of adenovirus type 5, followed by detection with an antidigoxigenin antibody (Roche).
Cell fractionation.
HeLa cells were transfected and infected with 366 virus as described above. At various time points after infection, the cells were scraped off the dish in PBS and pelleted. After resuspension in fractionating buffer (10 mM Tris-HCl [pH 7.6], 150 mM NaCl, 1.5 mM MgCl2, 0.5% Nonidet P-40; 100 μl was used to suspend 800,000 cells), the cells were subjected to vigorous shaking for 5 min, followed by fractionation. The supernatant was used as the cytoplasmic fraction. After one washing step with fractionating buffer, the pellet was defined as the nuclear fraction.
RNase protection assay.
RNA was extracted from total cell pellets or fractions (see above) with Trizol reagent (Life Technologies) and hybridized to a radioactively labeled antisense probe to the viral L5 region (generated with a plasmid obtained from K. Leppard). Hybridization and subsequent RNase digestion were carried out with a premanufactured system (Ambion), followed by denaturing gel electrophoresis and autoradiography. Quantitations were performed with a BioImager system (Fuji).
Immunoblots.
Proteins were separated on SDS–10% polyacrylamide gels and transferred to polyvinylidene difluoride, followed by incubation with antibodies in PBS containing 5% milk powder and 0.5% Tween 20 and chemiluminescent detection (Pierce) of peroxidase-coupled secondary antibody (Jackson Laboratory).
RESULTS
Mapping of a region within E4orf6 that is required for intracellular colocalization with E1B-55 kDa.
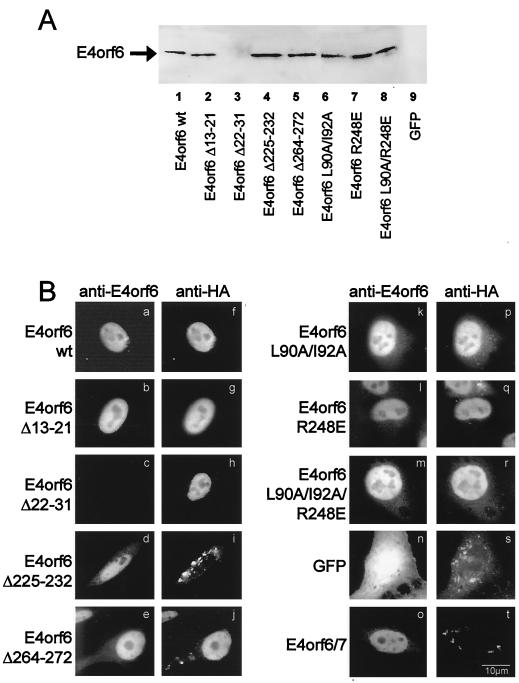
To map the activities of E4orf6, several expression plasmids expressing mutants of E4orf6 were created. In these mutants, small amino acid deletions were introduced at residues that were predicted to be exposed to the surface by a computer-based secondary structure calculation (48). These expression plasmids were transfected into HeLa cells to yield protein amounts comparable to that of wild-type E4orf6, as determined by Western blot analysis (Fig. 1A). Upon deletion of amino acids 22 to 31, the protein was no longer detectable (Fig. 1A, lane 3) with a polyclonal antibody raised against an E4orf6-derived peptide (generous gift from P. Branton) but did efficiently relocalize E1B-55 kDa (see below), arguing that the deleted region is part of the epitope recognized by this antibody. Next, we tested whether these mutants of E4orf6 were still able to relocalize E1B-55 kDa, as was shown previously for wild-type E4orf6 (8, 17). Expression plasmids for E4orf6 and E1B-55 kDa were transiently transfected into HeLa cells, followed by simultaneous immunostaining of the two proteins. All but one of these mutants retained the ability to relocalize E1B-55 kDa to the nucleus (Fig. 1B, a to j). Also, the previously described point mutations that modify the nuclear export properties of E4orf6 (L90A and I92A; R248E; L90A, I92A, and R248E [8]) did not abolish E1B-55 kDa relocalization (Fig. 1B, k to r). Only the deletion of amino acids 225 to 232 eliminated this activity (Fig. 1B, d and i). This deletion removes a putative amphipathic and arginine-rich alpha helix from E4orf6 (44). In parallel, the E4orf6/7 protein failed to colocalize detectably with E1B-55 kDa (Fig. 1B, o and t). E4orf6/7 shares the N-terminal 58 amino acids with E4orf6 but contains a different C-terminal portion. Taken together, these results argue that the carboxy-terminal arginine-rich alpha helix within E4orf6 is required for the relocalization of E1B-55 kDa to the nucleus, confirming recently published data (44).
FIG. 1.
Mutational analysis of E1B-55 kDa relocalization by E4orf6. (A) Plasmids were generated to express E4orf6 with the indicated deletions and mutations. HeLa cells were transfected (Superfect; Qiagen), followed by SDS-polyacrylamide gel electrophoresis and Western blotting detection of E4orf6 with a polyclonal rabbit antibody (generous gift from P. Branton). GFP, green fluorescent protein. (B) E4orf6 and its mutants (400 ng of expression plasmid) were transiently expressed as indicated along with HA-tagged E1B-55 kDa (100 ng of the corresponding expression plasmid), followed by immunodetection of both proteins. E4orf6 and mutants as well as E4orf6/7 are shown in panels a to e and k to o; E1B-55 kDa is shown in panels f to j and p to t. wt, wild type.
Further mapping studies were performed to find the region(s) within E4orf6 required for import into the nucleus. The fragments consisting of amino acids 12 to 32 and 230 to 283 of E4orf6 were each individually capable of mediating the nuclear accumulation of a beta-galactosidase reporter protein (S. Ristea and M. Dobbelstein, unpublished observations). Therefore, both portions of E4orf6 may contain nuclear import signals. Future studies employing double-deletion mutants and in vitro nuclear import assays will be required to further address the mechanism of the E4orf6 protein's nuclear import.
E4orf6 employs amino acids 225 to 232 to associate with E1B-55 kDa in vitro.
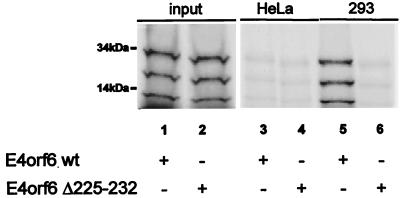
The results described above strongly suggest that amino acids 225 to 232 within E4orf6 are required to allow relocalization of E1B-55 kDa. This seems contrary to a previous study (53) where the interaction of E1B-55 kDa and E4orf6 was analyzed by immunoprecipitation, mapping the region of interaction with E1B-55 kDa exclusively to the amino-terminal portion of E4orf6. To further explore this issue, E4orf6 and E4orf6Δ225-232 were generated by transcription and translation in vitro (Fig. 2, lanes 1 and 2). The proteins were incubated with a lysate from 293 cells that contain E1B-55 kDa (lanes 5 and 6). As a control, parallel experiments were carried out with a lysate from HeLa cells that do not contain adenoviral proteins (lanes 3 and 4). An antibody to E1B-55 kDa was added to the mixtures, and this antibody was then used for immunoprecipitation with protein A-Sepharose beads. The precipitated proteins were separated on an SDS-polyacrylamide gel, followed by autoradiography. In this experiment, considerable amounts of E4orf6 were precipitated in the presence of E1B-55 kDa (lane 5) but not in the absence of E1B-55 kDa (lane 3). In contrast, E4orf6Δ225-232 largely failed to coprecipitate with E1B-55 kDa (lane 6). Thus, residues 225 to 232 within E4orf6 are either necessary for, or at least strongly contribute to, the interaction between E1B-55 kDa and E4orf6.
FIG. 2.
Association of E4orf6 but not E4orf6Δ225-232 with E1B-55 kDa. E4orf6 and its mutant E4orf6Δ225-232 were synthesized and radioactively labeled by transcription and translation in vitro (lanes 1 and 2). The proteins were then incubated with lysates from HeLa cells (lanes 3 and 4) or 293 cells (containing E1B-55 kDa; lanes 5 and 6). After immunoprecipitation with a monoclonal antibody (2A6) against E1B-55 kDa, the precipitated material was separated by SDS-polyacrylamide gel electrophoresis and exposed to a BioImager screen. wt, wild type.
Virus growth supported by E4orf6 and mutants.
Next, we asked how these different mutations within E4orf6 might affect the growth of adenovirus. To test this, an assay that was previously established to assess the role of different E4 proteins in adenovirus infection was used (30).
Expression plasmids for E4orf6 and mutants were transiently transfected into HeLa cells by electroporation. Cells (800,000) were seeded in a 3.5-cm-diameter dish and, 24 h later, infected with the defective adenovirus 366 (lacking the entire E4 region [18]) at a multiplicity of infection of 1. This virus is unable to replicate at low multiplicities of infection (24). However, the presence of transiently expressed E4orf6 enables the virus to replicate and leads to the production of new virus particles. After further incubation for 48 h, the cells were harvested in PBS and lysed by three cycles of freezing and thawing. The lysate was then diluted with DMEM and applied to a fresh monolayer of HeLa cells, and infected cells were subsequently detected with an antibody against the E2A DNA binding protein. Expression of this protein was previously shown not to be reduced by the deletion of the E4 region (38). The amount of cells staining positive for E2A in the assay involving wild-type E4orf6 was arbitrarily set to 100%, and the other values were normalized accordingly.
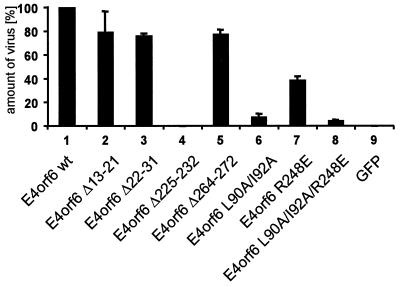
This assay was performed with all the mutants described above. Most mutations resulted in little or no detectable loss in virus production (Fig. 3A). However, the same mutant E4orf6Δ225-232 that no longer relocalized E1B-55 kDa also failed to support virus production (lane 4), arguing that the association of E4orf6 with E1B-55 kDa may considerably contribute to efficient adenovirus production. Most notably, the E4orf6 protein with a mutation in the NES (L90A and I92A) was compromised in its ability to support virus replication (lane 6). While this mutation did not completely abrogate virus replication, it did result in a considerable reduction of virus yield. This led us to hypothesize that the nuclear export function of E4orf6 may affect virus replication. However, this experiment does not exclude the possibility that a different function of E4orf6 that contributes to virus replication might be affected by the mutation involving the changes L90A and I92A simultaneously with the NES function.
FIG. 3.
Synthesis of virus particles upon E4orf6 expression. Expression plasmids for E4orf6 and mutants were transiently transfected into HeLa cells by electroporation. Cells (800,000) were seeded in a 3.5-cm-diameter dish and, 24 h later, infected with the defective adenovirus 366 (lacking the entire E4 region) at a multiplicity of infection of 1. After further incubation for 48 h, the cells were harvested in PBS and lysed by three cycles of freezing and thawing. The lysate was then diluted with DMEM and applied to a fresh monolayer of HeLa cells, and infected cells were subsequently detected with an antibody against the E2A DNA binding protein. The amount of cells staining positive for E2A in the assay involving wild type (wt) E4orf6 was arbitrarily set to 100%, and the other values were normalized accordingly. The error bars represent standard errors of at least three independent experiments. GFP, green fluorescent protein.
Export blockage suppresses E4orf6 function in virus production.
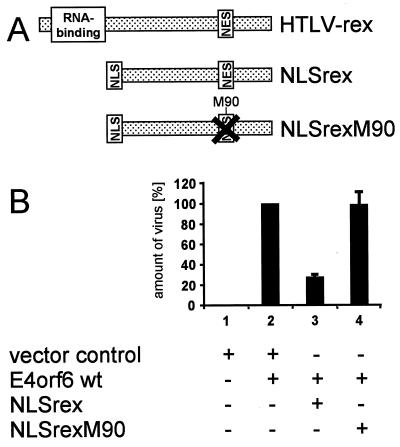
To further evaluate the concept that nuclear export of E4orf6 contributes to virus replication, we used an export competitor (Fig. 4A) that was previously shown to block the activity of leucine-rich NESs (8, 29, 32, 49). This competitor was derived from a fragment of the human T-cell leukemia virus type 1 rex protein that contains a functional NES. This fragment was fused to the nuclear import signal (nuclear localization signal) of simian virus 40 T antigen. The chimeric protein expressed from this construct undergoes rapid nuclear export and import, and it presumably exhausts the activity of one or several components of the nuclear export machinery. As a control, we used a similar construct with a mutation in the NES of the rex fragment. An expression plasmid for beta-galactosidase served as an additional control. These three plasmids were each cotransfected with an expression plasmid for wild-type E4orf6 (Superfect; Qiagen), followed by infection with the E4-less 366 virus and quantitation of newly synthesized virus. Compared to the controls, the export competitor reduced the yield of virus (Fig. 4B; compare lane 1 with lanes 2 and 3). This argues that it is indeed a nuclear export function that contributes to the enhancement of virus production by E4orf6.
FIG. 4.
Interference of an export competitor with virus production. (A) Schematic depiction of export competitors. The carboxy-terminal portion of the human T-cell leukemia virus type 1 (HTLV-1) rex protein was fused to the nuclear localization signal (NLS) of simian virus 40 T antigen. A mutant of this chimera carrying a small deletion within the rex NES was used as a control. Another control was performed by expressing beta-galactosidase. (B) Cells were transfected with expression plasmids for wild-type (wt) E4orf6 (1 μg) along with beta-galactosidase or the NLSrex chimeric protein or a mutant NLSrex chimera lacking a functional NES (2 μg each; Superfect; Qiagen). After transfection, the function of E4orf6 in virus production was assayed as described in the legend to Fig. 3. The amount of virus obtained in the presence of E4orf6 and beta-galactosidase was set to 100%, and the other results were normalized accordingly.
Residues 225 to 232 and the NES within E4orf6 contribute to viral protein synthesis.
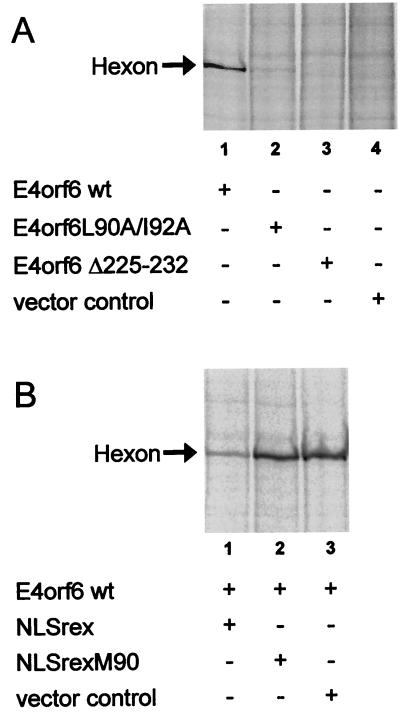
Given the loss in virus production that is induced by removal of the residues 225 to 232 from E4orf6 or a mutation in the NES of E4orf6, we attempted to find out what step in virus replication might be relying on these domains. Therefore, we first determined if the reduction in virus yield is accompanied by a loss in late viral protein synthesis when residues 225 to 232 or the NES within E4orf6 is mutated. To this end, cells were transfected with E4orf6 expression plasmids or the control plasmid. After infection with E4-less 366 virus, the cells were harvested and the amount of hexon protein (a structural protein encoded by the L3 region of the viral genome) was determined by radioactive labeling of the cellular proteins, followed by immunoprecipitation of hexon. As shown in Fig. 5A, E4orf6 supported late protein production (lane 1), whereas a control plasmid did not (lane 4). Notably, a mutation in the NES of E4orf6 (L90A and I92A) led to a reduction in the amount of hexon (lane 2). In the presence of E4orf6Δ225-232, no hexon protein could be detected (lane 3). Similarly, when the rex-derived export competitor NLSrex was coexpressed with E4orf6, hexon accumulation was suppressed compared to that resulting from the transfection of control plasmids (Fig. 5B). Thus, the association of E4orf6 with E1B-55 kDa as well as the NES function of E4orf6 seem to contribute to the efficiency of late virus protein synthesis.
FIG. 5.
Late protein synthesis upon E4orf6 expression. (A) HeLa cells were electroporated with expression plasmids for E4orf6, E4orf6Δ225-232, and the mutant E4orf6L90A/I92A within the NES. The cells were then infected with 366 as described in the legend to Fig. 2 and harvested 24 h after infection. To detect the expression of the viral hexon protein, the cells were metabolically labeled with [35S] Met and [35S] Cys, followed by immunoprecipitation of hexon proteins, SDS-polyacrylamide gel electrophoresis, and autoradiography. wt, wild type. (B) The cells were transfected by using Superfect (Qiagen) with wild-type E4orf6 along with expression plasmids for an export competitor as indicated. Hexon protein was detected by immunoblotting.
The NES within E4orf6 supports viral DNA replication.
Besides the expression of late genes, the replication of viral DNA is a prominent step after entry into the late phase of the infectious cycle. Therefore, we determined whether DNA synthesis is supported by the NES function of E4orf6. To test this, HeLa cells were transfected to express wild-type or mutant E4orf6 and infected with 366 virus. At three different time points, the cells were harvested and viral DNA was detected by Southern blotting. As shown in Fig. 6A, viral DNA did not accumulate in the absence of E4orf6 (lanes 1 to 3) or in the presence of the mutant E4orf6Δ225-232, which does not detectably interact with E1B-55 kDa (lanes 4 to 6). In contrast, both E4orf6 and E4orf6L90A/I92A supported viral DNA synthesis. However, viral DNA accumulated to greater amounts in the presence of wild-type E4orf6 than in the presence of the NES mutant (compare lanes 10 to 12 with 7 to 9). In parallel, the export competitor NLSrex but not its mutant considerably suppressed DNA production when cotransfected with E4orf6 (Fig. 6B). Hence, a functional NES within E4orf6 ultimately leads to enhanced production of viral DNA.
FIG. 6.
Replication of viral DNA upon E4orf6 expression. (A) HeLa cells were electroporated and infected as described in the legend to Fig. 3. At the time points indicated after infection, DNA was isolated, treated with HindIII, separated by agarose electrophoresis, and blotted. A genomic fragment corresponding to the adenovirus E1B region was detected by Southern hybridization. GFP, green fluorescent protein; wt, wild type. (B) The cells were transfected (Superfect) with wild-type E4orf6 along with expression plasmids for the export competitor as indicated. Virus DNA was detected by Southern blotting.
The NES within E4orf6 increases the overall accumulation of viral mRNA.
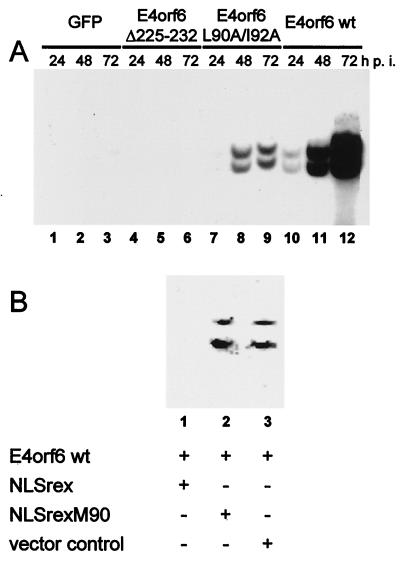
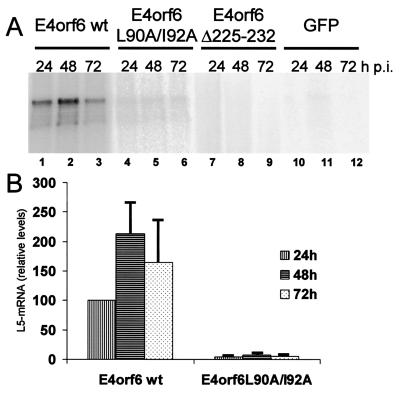
Finally, we tested the effects of E4orf6 and its NES on the accumulation and intracellular distribution of late viral mRNA. To this end, HeLa cells were transfected and infected as described above, followed by an RNase protection assay to quantitate the L5 late mRNA (this RNA species' accumulation and export were previously shown to be most strongly dependent on the expression of E1B-55 kDa protein [35]). Considerably more RNA accumulated in E4orf6-transfected cells than in cells expressing the NES mutant (Fig. 7A; compare lanes 1 to 3 and 4 to 6). Quantitation of the radiation intensities revealed a roughly 50-fold difference between the amounts of RNA that accumulated in the presence of wild-type and NES mutant E4orf6 at 72 h postinfection (Fig. 7B, lanes 3 and 6). When E4orf6Δ225-232 was expressed, no late viral mRNA could be detected (Fig. 7A, lanes 7 to 9), similar to the control with no E4orf6 protein (lanes 10 to 12). These results argue that interaction of E4orf6 with E1B-55 kDa and the NES within E4orf6 plays important roles in the overall accumulation of late viral mRNA.
FIG. 7.
Accumulation of late viral mRNA upon E4orf6 expression. (A) HeLa cells were electroporated and infected as described in the legend to Fig. 3. Total RNA was isolated at the time points indicated, and L5 RNA was detected by an RNase protection assay. wt, wild type; GFP, green fluorescent protein. (B) The signals obtained by the RNase protection assay were quantitated with a BioImager (Fuji). The value obtained with wild-type E4orf6 24 h postinfection was set 100%, and the other signal intensities were normalized accordingly. The average signal intensities along with the standard deviations of at least three independent experiments are shown.
The NES within E4orf6 supports the accumulation of viral mRNA in the cytoplasm.
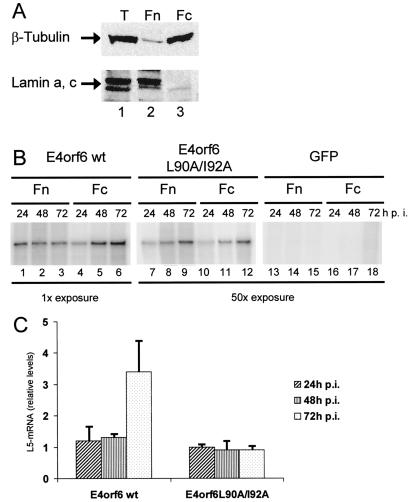
Given the differences in RNA accumulation, we next determined if the intracellular distribution of mRNA might also be affected by the NES function of E4orf6. Therefore, after transfection and infection of HeLa cells, we harvested the cells at different time points, followed by separation into nuclear and cytoplasmic fractions. To verify the accuracy of cell fractionation, cytoplasmic and nuclear proteins were detected by immunoblot analysis in each fraction. While the cytoplasmic β-tubulin protein was detected exclusively in the cytoplasmic fraction, not in the nuclear fraction (Fig. 8A; compare lanes 2 and 3, upper panel), the reverse was true for the nuclear lamin proteins (lamin a and lamin c) (Fig. 8A, lanes 2 and 3, lower panel). This shows that the separation procedure efficiently separates cytoplasmic from nuclear material. The amount of L5 RNA in each fraction was measured by RNase protection assay, and the band intensities were quantified with a BioImager. The values obtained from the cytoplasmic fractions were divided by the values obtained from the corresponding nuclear fractions. The resulting numbers are shown graphically in Fig. 8C. At 72 h after infection, the ratio of cytoplasmic to nuclear RNA was about 4 in E4orf6-transfected cells but only between 1 and 1.5 upon mutation of the NES (Fig. 8B and C). Concerning the intracellular distribution of viral mRNA, the effects of an export competitor such as NLSrex could not be successfully determined (data not shown). This was presumably due to the fact that at late times after transfection and infection (72 h), only small amounts of NLSrex were still expressed, resulting in insufficient blockage of export. Nonetheless, the results presented here argue that the NES function of E4orf6 specifically enhances the cytoplasmic accumulation of late viral mRNA.
FIG. 8.
Nucleocytoplasmic distribution of late viral mRNA upon E4orf6 expression. (A) HeLa cells were separated into cytoplasmic (Fc) and nuclear (Fn) fractions as described in Materials and Methods. The fractions as well as total cell lysate (T) were analyzed by immunoblotting with antibodies against β-tubulin as well as types a and c of the lamin protein. (B) HeLa cells were transfected and infected as described in the legend to Fig. 3. At the time points indicated, the cells were harvested and separated into nuclear and cytoplasmic fractions. In each fraction, L5 RNA was detected by an RNase protection assay. Note that the exposure time chosen after expression of the NES mutant E4orf6 protein was roughly 50 times longer than the exposure after expression of wild-type (wt) E4orf6. GFP, green fluorescent protein. (C) The signals obtained by RNase protection assay were quantitated with a BioImager (Fuji). Then, the ratio between signals derived from the cytoplasmic fraction and the nuclear fraction was calculated in each case. The average ratios along with the standard deviations of at least three independent experiments are shown.
DISCUSSION
Our data show that the ability of E4orf6 to support adenovirus replication cosegregates with two necessary functions: intracellular association with E1B-55 kDa seems to be required for E4orf6-driven virus production, while nuclear export of E4orf6 apparently increases the yield of viral particles. The latter phenomenon correlates with the contribution of E4orf6 and its export signal to late protein synthesis and DNA replication, as well as the accumulation of total and cytoplasmic mRNA. These data support the view that the NES within E4orf6 is of considerable importance for virus replication and may enhance the nucleocytoplasmic transport of late viral mRNA.
Our mapping studies on the relocalization of E1B-55 kDa by E4orf6 reveal that a deletion of residues 225 to 232 within E4orf6 abolishes the protein's ability to relocalize E1B-55 kDa to the nucleus. Accordingly, the E4orf6/7 protein does not detectably colocalize with E1B-55 kDa protein either. E4orf6/7 shares the amino-terminal portion with E4orf6 but differs from it in the C-terminal part, further supporting the concept that the C-terminal portion of E4orf6 is required to relocalize E1B-55 kDa. This observation is in agreement with a recent report (44). Earlier mapping studies that used immunoprecipitation to detect interactions between E1B-55 kDa and E4orf6 (53) came up with a different conclusion. In these studies, only the amino-terminal portion common to E4orf6 and E4orf6/7 was required to detect association with E1B-55 kDa and no gross difference between the binding efficiency of full-length E4orf6 and that of E4orf6/7 was reported. In contrast, our results show that wild-type E4orf6 binds to E1B-55 kDa at least more efficiently than E4orf6Δ225-232. While we cannot fully explain the apparent discrepancy, we suspect that the amino-terminal portion of E4orf6 may have some ability to associate with E1B-55 kDa under nonstringent conditions or in the presence of a large excess of E1B-55 kDa. In any case, our immunofluorescence and immunoprecipitation studies strongly suggest that the C-terminal portion of E4orf6 at least enhances the efficiency of the interaction with E1B-55 kDa.
At first glance, it was unexpected that a mutation of the NES within E4orf6 had multiple effects on the infectious cycle. As an explanation, one could argue that the region of E4orf6 that contains the NES might carry out functions in addition to nuclear export. However, this possibility is rendered unlikely by the fact that expression of an export competitor had effects similar to those of a mutation of the NES. Therefore, we favor a second possibility: a defect in the nuclear export of E4orf6 may result in a defect of mRNA transport. This in turn can be expected to reduce late protein synthesis and the production of virus particles.
It seems surprising that differences in late protein synthesis and DNA replication were observed as early as 24 h after infection when wild-type E4orf6 was compared with the NES mutant protein, while clear differences in mRNA distribution were only detected at 72 h postinfection. Similarly, the E1B-55 kDa protein was previously shown to affect the cytoplasmic accumulation of viral mRNA only at such late time points (35). A possible explanation for this phenomenon is that the E1B-55 kDa–E4orf6 complex may affect intranuclear mRNA transport and processing steps in addition to the nucleocytoplasmic distribution of mRNA. Such intranuclear transport and/or processing may be influenced by E4orf6 and its NES at far earlier time points than those at which the nucleocytoplasmic distribution is affected. Indeed, an influence of E4orf6 on the processing of viral mRNA was previously reported (41, 42). Any differences in the intranuclear distribution of mRNA would not be detected upon fractionation into nuclear and cytoplasmic fractions. mRNA transport between intranuclear compartments and its dependence on E1B-55 kDa have been proposed previously (35). By analogy, the temporally dissociated expression of Rev-responsive HIV RNA and the HIV Rev protein results in a failure of mRNA export, arguing that Rev may affect intranuclear transport steps in addition to nucleocytoplasmic transport (28). Further, it was recently reported that Rev not only enhances the export of incompletely spliced HIV mRNA but also modulates intracellular processing steps, such as mRNA splicing and polyadenylation (26). A similar scenario may apply to adenovirus and its mRNA transport modulators.
In our experiments, we have blocked the nuclear export activity of E4orf6 either by point mutation or by a chimeric protein that acts as a competitor for rev-mediated export. Another way of blocking rev-like nuclear export consists of the use of the drug leptomycin, which disrupts the interaction between leucine-rich export signals and their receptor, exportin 1 (11, 13, 40, 61). We have evaluated the effects of leptomycin B on virus replication in our experimental system. However, the drug was considerably toxic to HeLa cells, and it could not be applied for the time required for virus replication (18 to 72 h) without nonspecifically suppressing viral growth simply by killing the cells (data not shown). Therefore, we did not base our studies on the effects of leptomycin B. It is certainly advisable to take these toxic effects of the drug into consideration when using it in other experimental systems, and in particular when longer incubations of cells with the drug are required.
While it seems clear that the nuclear export function of E4orf6 is involved in virus replication and cytoplasmic RNA accumulation, the mechanism by which E4orf6, along with E1B-55 kDa, affects mRNA distribution remains to be elucidated. Intriguingly, E1B-55 kDa turned out to have RNA binding activity, leading to the hypothesis that direct contacts between E1B-55 kDa and viral RNA may allow RNA export (22). Possibly, the complex of E1B-55 kDa and E4orf6 binds viral mRNA, followed by nucleocytoplasmic export of the entire protein-RNA compound. Since the tripartite leader region of late viral mRNA was shown to enhance RNA export in infected cells (25), one role of this RNA element may consist of stabilizing such RNA-protein contacts.
However, the idea that the E1B-55 kDa–E4orf6 complex might directly act as a carrier for viral mRNA, like the HIV Rev protein, is almost certainly simplistic. First, the RNA binding activity of E1B-55 kDa could not be shown to be specific for the tripartite leader region common to all late viral mRNA molecules (22). Further, even cellular mRNA sequences, when expressed by virus DNA, were found to undergo nucleocytoplasmic transport with characteristics similar to those of viral mRNA, albeit with lower efficiency than tripartite-leader-containing RNA (25, 62). Most importantly, the model describing the E1B-55 kDa and E4orf6 proteins as RNA carriers does not provide an explanation for the fact that cellular mRNA molecules are retained in the nucleus during adenovirus infection. The simultaneous transport block on cellular mRNA and enhanced export of viral mRNA suggest that the viral proteins may bind a cellular factor that is required for RNA export. Such a factor would then be available for export of viral mRNA but would no longer be at the disposition of cellular mRNA. This concept is even more intriguing given the observation that a modified E1B-55 kDa protein targeted to the nucleus is able to inhibit mRNA export in the yeast S. cerevisiae and may thus inactivate an essential mRNA export factor (36). The existence of an export factor that is targeted by E1B-55 kDa–E4orf6 was previously postulated (8, 45), and a candidate protein was recently identified (14) and termed E1B-AP5. E1B-AP5 is strongly homologous to the hnRNP U protein that is known to be associated with mRNA. It was therefore proposed that the E1B-55 kDa–E4orf6 complex might associate with viral mRNA through E1B-AP5 and block the export of cellular mRNA by removing E1B-AP5 from the sites of cellular mRNA production (14). While this model continues to be attractive, our unpublished observations suggest that other cellular transport factors may be contacted by E1B-55 kDa as well. Thus, it seems conceivable that a number of cellular mRNA transporters can be targeted by the E1B-55 kDa–E4orf6 complex. Future studies are aimed at the identification and characterization of this set of proteins.
Why does the E4orf6 protein support virus growth even in the absence of a functional export signal? Possibly, some other functions of E4orf6 may be retained in the NES mutant, contributing to virus production. Such functions of E4orf6 may be related to the modulation of RNA splicing (4, 39) or the avoidance of concatemer formation between viral genomic DNA molecules (60). Alternatively, the complex of E1B-55 kDa and E4orf6 may support mRNA transport, to some extent at least, between intranuclear compartments even in the absence of a functional NES.
Apparently, there is some overlap in the E4 gene products' ability to support virus replication. While a recombinant virus lacking the entire E4 region is virtually unable to replicate at low multiplicities of infection, the orf3 and orf6 proteins are each individually capable of rescuing the defect to some extent, either when the orf3 or the orf6 gene is placed into the genome of an otherwise E4-less virus (24) or when they are transiently expressed (30). In our experiments, the orf3 proteins showed far weaker activity in supporting virus replication than orf6 proteins (data not shown), in accordance with earlier reports (24, 30). Possibly, E4orf3 can take over some of the functions carried out by E4orf6 during infection, such as the modulation of mRNA splicing (39), but fails to fulfil other functions, such as mediating mRNA transport.
Besides RNA transport, another well-characterized function of E1B-55 kDa and E4orf6 consists of the inactivation and destabilization of the p53 tumor suppressor protein (47, 51, 57, 63). Important questions for future studies will be whether these seemingly different activities are interrelated and, if so, what biochemical mechanism connects them. These questions become even more intriguing given the fact that E1B-55 kDa's role in virus replication apparently depends on the phase in the cell cycle at the time of infection (15).
The cellular p53 antagonist mdm2 also contains a NES and shuttles between the nucleus and cytoplasm. While the NES of mdm2 contributes to the intracellular degradation of p53, at least in some cell types (12, 50, 58), p53 degradation was detected in the presence of E1B-55 kDa and E4orf6 even when the NES of E4orf6 was mutated (51; our unpublished observations). Therefore, for adenovirus proteins, the link between the export function of E1B-55 kDa–E4orf6 and p53 degradation may not simply consist of the ability to carry p53 to the cytoplasm.
Mapping studies on the E1B-55 kDa protein are under way to determine whether or not its function in mRNA transport can be separated from its ability to bind p53. Such mutants might be of interest in designing viruses that selectively infect and destroy tumor cells, such as dl1520 (2), which was later renamed onyx-015 (5). While the growth of such a virus does not necessarily depend on the p53 status in cultured cells (16, 19, 20, 52, 59), it seems to be restricted to tumor cells in the context of the entire organism (21, 31). A further understanding of E1B-55 kDa's different functions and possibly their selective deletion may ultimately help to develop improved versions of such therapeutic viruses, in addition to providing new insights into growth regulation and mRNA transport.
ACKNOWLEDGMENTS
We thank H.-D. Klenk for generous support, C. König and S. Ristea for helpful discussions, T. Shenk for recombinant viruses, P. Branton, J. Flint, and T. Shenk for antibodies, and K. Leppard and C. Caravokyri for plasmids.
This work was supported by the German Research Foundation and the P. E. Kempkes foundation. M.D. was a recipient of the Stipendium für Infektionsbiologie of the German Cancer Research Center.
REFERENCES
- 1.Babiss L E, Ginsberg H S, Darnell J E., Jr Adenovirus E1B proteins are required for accumulation of late viral mRNA and for effects on cellular mRNA translation and transport. Mol Cell Biol. 1985;5:2552–2558. doi: 10.1128/mcb.5.10.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker D D, Berk A J. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1987;156:107–121. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]
- 3.Beltz G A, Flint S J. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J Mol Biol. 1979;131:353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- 4.Berget S M, Moore C, Sharp P A. Spliced segments at the 5′ terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci USA. 1977;74:3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bischoff J R, Kirn D H, Williams A, Heise C, Horn S, Muna M, Ng L, Nye J A, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 6.Bray M S, Prasad S, Dubay J W, Hunter E, Jeang K T, Rekosh D, Hammarskjold M L. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proc Natl Acad Sci USA. 1994;91:1256–1260. doi: 10.1073/pnas.91.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobbelstein M, Arthur A K, Dehde S, van Zee K, Dickmanns A, Fanning E. Intracistronic complementation reveals a new function of SV40 T antigen that co-operates with Rb and p53 binding to stimulate DNA synthesis in quiescent cells. Oncogene. 1992;7:837–847. [PubMed] [Google Scholar]
- 8.Dobbelstein M, Roth J, Kimberly W T, Levine A J, Shenk T. Nuclear export of the E1B 55-kDa and E4 34-kDa adenoviral oncoproteins mediated by a rev-like signal sequence. EMBO J. 1997;16:4276–4284. doi: 10.1093/emboj/16.14.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer U, Huber J, Boelens W C, Mattaj I W, Lührmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 10.Fischer U, Meyer S, Teufel M, Heckel C, Lührmann R, Rautmann G. Evidence that HIV-1 Rev directly promotes the nuclear export of unspliced RNA. EMBO J. 1994;13:4105–4112. doi: 10.1002/j.1460-2075.1994.tb06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fornerod M, Ohno M, Yoshida M, Mattaj I W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 12.Freedman D A, Levine A J. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol Cell Biol. 1998;18:7288–7293. doi: 10.1128/mcb.18.12.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 14.Gabler S, Schutt H, Groitl P, Wolf H, Shenk T, Dobner T. E1B 55-kilodalton-associated protein: a cellular protein with RNA-binding activity implicated in nucleocytoplasmic transport of adenovirus and cellular mRNAs. J Virol. 1998;72:7960–7971. doi: 10.1128/jvi.72.10.7960-7971.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodrum F D, Ornelles D A. The early region 1B 55-kilodalton oncoprotein of adenovirus relieves growth restrictions imposed on viral replication by the cell cycle. J Virol. 1997;71:548–561. doi: 10.1128/jvi.71.1.548-561.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodrum F D, Ornelles D A. p53 status does not determine outcome of E1B 55-kilodalton mutant adenovirus lytic infection. J Virol. 1998;72:9479–9490. doi: 10.1128/jvi.72.12.9479-9490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodrum F D, Shenk T, Ornelles D A. Adenovirus early region 4 34-kilodalton protein directs the nuclear localization of the early region 1B 55-kilodalton protein in primate cells. J Virol. 1996;70:6323–6335. doi: 10.1128/jvi.70.9.6323-6335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halbert D N, Cutt J R, Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985;56:250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall A R, Dix B R, O'Carroll S J, Braithwaite A W. p53-dependent cell death/apoptosis is required for a productive adenovirus infection. Nat Med. 1998;4:1068–1072. doi: 10.1038/2057. [DOI] [PubMed] [Google Scholar]
- 20.Hay J G, Shapiro N, Sauthoff H, Heitner S, Phupakdi W, Rom W N. Targeting the replication of adenoviral gene therapy vectors to lung cancer cells: the importance of the adenoviral E1b-55kD gene. Hum Gene Ther. 1999;10:579–590. doi: 10.1089/10430349950018652. [DOI] [PubMed] [Google Scholar]
- 21.Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff D D, Kirn D H. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 22.Horridge J J, Leppard K N. RNA-binding activity of the E1B 55-kilodalton protein from human adenovirus type 5. J Virol. 1998;72:9374–9379. doi: 10.1128/jvi.72.11.9374-9379.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J, Liang T J. A novel hepatitis B virus (HBV) genetic element with Rev response element-like properties that is essential for expression of HBV gene products. Mol Cell Biol. 1993;13:7476–7486. doi: 10.1128/mcb.13.12.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang M M, Hearing P. Adenovirus early region 4 encodes two gene products with redundant effects in lytic infection. J Virol. 1989;63:2605–2615. doi: 10.1128/jvi.63.6.2605-2615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang W, Flint S J. The tripartite leader sequence of subgroup C adenovirus major late mRNAs can increase the efficiency of mRNA export. J Virol. 1998;72:225–235. doi: 10.1128/jvi.72.1.225-235.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, Wimler K M, Carmichael G G. Intronless mRNA transport elements may affect multiple steps of pre-mRNA processing. EMBO J. 1999;18:1642–1652. doi: 10.1093/emboj/18.6.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Z-M, Yen T S B. Role of the hepatitis B virus posttranscriptional regulatory element in export of intronless transcripts. Mol Cell Biol. 1995;15:3864–3869. doi: 10.1128/mcb.15.7.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iacampo S, Cochrane A. Human immunodeficiency virus type 1 Rev function requires continued synthesis of its target mRNA. J Virol. 1996;70:8332–8339. doi: 10.1128/jvi.70.12.8332-8339.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katahira J, Ishizaki T, Sakai H, Adachi A, Yamamoto K, Shida H. Effects of translation initiation factor eIF-5A on the functioning of human T-cell leukemia virus type I Rex and human immunodeficiency virus Rev inhibited trans dominantly by a Rex mutant deficient in RNA binding. J Virol. 1995;69:3125–3133. doi: 10.1128/jvi.69.5.3125-3133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ketner G, Bridge E, Virtanen A, Hemström C, Pettersson U. Complementation of adenovirus E4 mutants by transient expression of E4 cDNA and deletion plasmids. Nucleic Acids Res. 1989;17:3037–3048. doi: 10.1093/nar/17.8.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirn D, Hermiston T, McCormick F. ONYX-015: clinical data are encouraging. Nat Med. 1998;4:1341–1342. doi: 10.1038/3902. [DOI] [PubMed] [Google Scholar]
- 32.Kiyokawa T, Umemoto T, Watanabe Y, Matsushita S, Shida H. Two distinct pathways for intronless mRNA expression: one related, the other unrelated to human immunodeficiency virus Rev and human T cell leukemia virus type I Rex functions. Biol Signals. 1997;6:134–142. doi: 10.1159/000109119. [DOI] [PubMed] [Google Scholar]
- 33.König C, Roth J, Dobbelstein M. Adenovirus type 5 E4orf3 protein relieves p53 inhibition by E1B 55-kilodalton protein. J Virol. 1999;73:2253–2262. doi: 10.1128/jvi.73.3.2253-2262.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leppard K N, Everett R D. The adenovirus type 5 E1b 55K and E4 Orf3 proteins associate in infected cells and affect ND10 components. J Gen Virol. 1999;80:997–1008. doi: 10.1099/0022-1317-80-4-997. [DOI] [PubMed] [Google Scholar]
- 35.Leppard K N, Shenk T. The adenovirus E1B 55 kd protein influences mRNA transport via an intranuclear effect on RNA metabolism. EMBO J. 1989;8:2329–2336. doi: 10.1002/j.1460-2075.1989.tb08360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang S, Hitomi M, Tartakoff A M. Adenoviral E1B-55kDa protein inhibits yeast mRNA export and perturbs nuclear structure. Proc Natl Acad Sci USA. 1995;92:7372–7375. doi: 10.1073/pnas.92.16.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malim M H, Hauber J, Le S Y, Maizel J V, Cullen B R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989;338:254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- 38.Medghalchi S, Padmanabhan R, Ketner G. Early region 4 modulates adenovirus DNA replication by two genetically separable mechanisms. Virology. 1997;236:8–17. doi: 10.1006/viro.1997.8737. [DOI] [PubMed] [Google Scholar]
- 39.Mühlemann O, Kreivi J P, Akusjärvi G. Enhanced splicing of nonconsensus 3′ splice sites late during adenovirus infection. J Virol. 1995;69:7324–7327. doi: 10.1128/jvi.69.11.7324-7327.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishi K, Yoshida M, Fujiwara D, Nishikawa M, Horinouchi S, Beppu T. Leptomycin B targets a regulatory cascade of crm1, a fission yeast nuclear protein, involved in control of higher order chromosome structure and gene expression. J Biol Chem. 1994;269:6320–6324. [PubMed] [Google Scholar]
- 41.Nordqvist K, Ohman K, Akusjarvi G. Human adenovirus encodes two proteins which have opposite effects on accumulation of alternatively spliced mRNAs. Mol Cell Biol. 1994;14:437–445. doi: 10.1128/mcb.14.1.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Öhman K, Nordqvist K, Akusjärvi G. Two adenovirus proteins with redundant activities in virus growth facilitates tripartite leader mRNA accumulation. Virology. 1993;194:50–58. doi: 10.1006/viro.1993.1234. [DOI] [PubMed] [Google Scholar]
- 43.O'Neill R E, Talon J, Palese P. The influenza virus NEP (NS2 protein) mediates the nuclear export of viral ribonucleoproteins. EMBO J. 1998;17:288–296. doi: 10.1093/emboj/17.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orlando J S, Ornelles D A. An arginine-faced amphipathic alpha helix is required for adenovirus type 5 e4orf6 protein function. J Virol. 1999;73:4600–4610. doi: 10.1128/jvi.73.6.4600-4610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ornelles D A, Shenk T. Localization of the adenovirus early region 1B 55-kilodalton protein during lytic infection: association with nuclear viral inclusions requires the early region 4 34-kilodalton protein. J Virol. 1991;65:424–429. doi: 10.1128/jvi.65.1.424-429.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pilder S, Moore M, Logan J, Shenk T. The adenovirus E1B-55K transforming polypeptide modulates transport or cytoplasmic stabilization of viral and host cell mRNAs. Mol Cell Biol. 1986;6:470–476. doi: 10.1128/mcb.6.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Querido E, Marcellus R C, Lai A, Charbonneau R, Teodoro J G, Ketner G, Branton P E. Regulation of p53 levels by the E1B 55-kilodalton protein and E4orf6 in adenovirus-infected cells. J Virol. 1997;71:3788–3798. doi: 10.1128/jvi.71.5.3788-3798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rost B, Sander C. Prediction of protein structure at better than 70% accuracy. J Mol Biol. 1993;232:584–599. doi: 10.1006/jmbi.1993.1413. [DOI] [PubMed] [Google Scholar]
- 49.Roth J, Dobbelstein M. Export of hepatitis B virus RNA on a Rev-like pathway: inhibition by the regenerating liver inhibitory factor IκB α. J Virol. 1997;71:8933–8939. doi: 10.1128/jvi.71.11.8933-8939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roth J, Dobbelstein M, Freedman D A, Shenk T, Levine A J. Nucleo-cytoplasmic shuttling of the hdm2 oncoprotein regulates the levels of the p53 protein via a pathway used by the human immunodeficiency virus rev protein. EMBO J. 1998;17:554–564. doi: 10.1093/emboj/17.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roth J, König C, Wienzek S, Weigel S, Ristea S, Dobbelstein M. Inactivation of p53 but not p73 by adenovirus type 5 E1B 55-kilodalton and E4 34-kilodalton oncoproteins. J Virol. 1998;72:8510–8516. doi: 10.1128/jvi.72.11.8510-8516.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rothmann T, Hengstermann A, Whitaker N J, Scheffner M, zur Hausen H. Replication of ONYX-015, a potential anticancer adenovirus, is independent of p53 status in tumor cells. J Virol. 1998;72:9470–9478. doi: 10.1128/jvi.72.12.9470-9478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rubenwolf S, Schütt H, Nevels M, Wolf H, Dobner T. Structural analysis of the adenovirus type 5 E1B 55-kilodalton–E4orf6 protein complex. J Virol. 1997;71:1115–1123. doi: 10.1128/jvi.71.2.1115-1123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarnow P, Hearing P, Anderson C W, Halbert D N, Shenk T, Levine A J. Adenovirus early region 1B 58,000-dalton tumor antigen is physically associated with an early region 4 25,000-dalton protein in productively infected cells. J Virol. 1984;49:692–700. doi: 10.1128/jvi.49.3.692-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarnow P, Sullivan C A, Levine A J. A monoclonal antibody detecting the adenovirus type 5-E1b-58Kd tumor antigen: characterization of the E1b-58Kd tumor antigen in adenovirus-infected and -transformed cells. Virology. 1982;120:510–517. doi: 10.1016/0042-6822(82)90054-x. [DOI] [PubMed] [Google Scholar]
- 56.Shenk T. Adenoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2111–2148. [Google Scholar]
- 57.Steegenga W T, Riteco N, Jochemsen A G, Fallaux F J, Bos J L. The large E1B protein together with the E4orf6 protein target p53 for active degradation in adenovirus infected cells. Oncogene. 1998;16:349–357. doi: 10.1038/sj.onc.1201540. [DOI] [PubMed] [Google Scholar]
- 58.Tao W, Levine A J. Nucleocytoplasmic shuttling of oncoprotein Hdm2 is required for Hdm2-mediated degradation of p53. Proc Natl Acad Sci USA. 1999;96:3077–3080. doi: 10.1073/pnas.96.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turnell A S, Grand R J, Gallimore P H. The replicative capacities of large E1B-null group A and group C adenoviruses are independent of host cell p53 status. J Virol. 1999;73:2074–2083. doi: 10.1128/jvi.73.3.2074-2083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiden M D, Ginsberg H S. Deletion of the E4 region of the genome produces adenovirus DNA concatemers. Proc Natl Acad Sci USA. 1994;91:153–157. doi: 10.1073/pnas.91.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolff B, Sanglier J J, Wang Y. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem Biol. 1997;4:139–147. doi: 10.1016/s1074-5521(97)90257-x. [DOI] [PubMed] [Google Scholar]
- 62.Yang U C, Huang W, Flint S J. mRNA export correlates with activation of transcription in human subgroup C adenovirus-infected cells. J Virol. 1996;70:4071–4080. doi: 10.1128/jvi.70.6.4071-4080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yew P R, Berk A J. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature. 1992;357:82–85. doi: 10.1038/357082a0. [DOI] [PubMed] [Google Scholar]
- 64.Zantema A, Fransen J A, Davis-Olivier A, Ramaekers F C, Vooijs G P, DeLeys B, van der Eb A J. Localization of the E1B proteins of adenovirus 5 in transformed cells, as revealed by interaction with monoclonal antibodies. Virology. 1985;142:44–58. doi: 10.1016/0042-6822(85)90421-0. [DOI] [PubMed] [Google Scholar]
- 65.Zantema A, Schrier P I, Davis-Olivier A, van Laar T, Vaessen R T, van der Eb A. Adenovirus serotype determines association and localization of the large E1B tumor antigen with cellular tumor antigen p53 in transformed cells. Mol Cell Biol. 1985;5:3084–3091. doi: 10.1128/mcb.5.11.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]