Abstract
Ultrasound‐mediated gene transfection (sonotransfection) is a promising physical method for gene therapy, especially for cancer gene therapy. To investigate the optimal sonotransfection conditions and to determine whether the optimal transfection rate using sonotransfection is comparable to that of electrotransfection or liposome‐mediated transfection, we sonicated different cancer cell lines (U937, HeLa, PC‐3, Meth A and T‐24) using a 1‐MHz unfocused ultrasound at different intensities, pulse repetition frequencies and exposure times. The ideal ultrasound conditions were noted to be at 1.5 Watt/cm2 pulsed at 0.5 Hz with a duty factor of 50%. The results showed that transfection rate increased with the number of pulses, and peaked between 10 and 15 pulses before it started to decline. Using such optimal conditions, we have shown that sonotransfection is superior to electrotransfection and liposome‐mediated transfection at the fixed conditions used in the present study. These findings suggest that sonotransfection could be a better alternative to other non‐viral methods (e.g. electroporation and liposome‐mediated transfection) of gene transfection, particularly in cancer gene therapy. (Cancer Sci 2006; 97: 1111–1114)
The discovery that most human diseases, such as genetic disorders, metabolic disorders and cancers, are somehow gene‐related( 1 ) has lead to a new concept of therapy − gene therapy. Gene therapy is carried out by introducing recombinant genes into somatic cells to alter the course of a disease process. Several strategies have been designed to introduce functional genes and to allow integration into the nucleus of target cells. Viral‐mediated gene transfer is efficient for the task,( 2 ) but cytotoxicity, cytopathy and antigenicity are among the limiting factors in therapeutic application. Non‐viral methods offer an alternative method for gene transfection. These include electrotransfection and liposome‐mediated transfection. Electrotransfection is a physical method of gene delivery,( 3 , 4 ) whereas liposome‐mediated transfection is considered a chemical method. These two methods are considered to be relatively safer than the viral method.( 3 , 4 , 5 ) However, the inability of these methods to act beyond facilitating cellular uptake of the therapeutic gene leads to a poor transfection rate. The search for a method or combination of methods that could improve the general outcome of therapy remains a big challenge to workers in this field.
The use of ultrasound in therapy( 6 , 7 ) and also in gene transfection( 8 , 9 , 10 , 11 , 12 ) has been investigated both in vitro and in vivo. Poor transfection rates remain a problem, so combinations with other methods are being used to try to improve the outcomes.( 13 , 14 , 15 , 16 , 17 ) An attempt to optimize the conditions ultrasound‐mediated gene transfection (sonotransfection) has been made on skeletal muscle cells, 18 but so far the mechanism remains generally unknown. The leading belief, however, is that ultrasound increases DNA uptake by the cells. Previously, we showed that optimizing apoptosis is possible based on the concept that the bioeffects of ultrasound is mainly due to mechanical damage on the cell membranes.( 7 , 19 , 20 , 21 ) Understanding the membrane damage induced by ultrasound and the physiology of cellular membrane repair has led to an optimal apoptosis induction on cells in vitro using pulse ultrasound.( 22 ) Pulsing, at certain pulse duration, induces membrane damage, and the period of ‘no radiation’ allows the membrane to undergo some repair. This method presumably keeps the cells intact despite the damage that should have been sufficient to induce apoptosis, leading to eventual cell death.
Using pulsed ultrasound, we aimed to determine the optimal conditions under which ultrasound can mediate DNA transfection in different cancer cell lines, and compare such optimized conditions with the transfection efficiencies attained by other non‐viral methods, specifically electrotransfection and liposome‐mediated transfection.
Materials and Methods
Cells and cell culture. Different types of cancer cell lines were grown in vitro. In culturing the cells, U937 (human monohistiocytic lymphoma) and Meth A (murine fibrosarcoma) cells grew as a suspension in a liquid culture medium, whereas HeLa (human epithelial cervix adenocarcinoma), PC‐3 (human epithelial prostate adenocarcinoma) and T‐24 (human epithelial urinary bladder transitional carcinoma) cells grew by attaching to the bottom of treated culture dishes. These cells were obtained from the Human Sciences Research Resources Bank (Human Sciences Foundation, Tokyo, Japan). The cells were maintained in RPMI‐1640 medium (Invitrogen, Groningen, the Netherlands) supplemented with 10% heat‐inactivated fetal bovine serum (Gibco, Carlsbad, CA, USA) at 37°C in humidified air with 5% CO2. The cells used in the experiments were in log phase and cell viability before treatment was over 95%.
Ultrasound apparatus and intensity measurement. This set‐up used an ultrasound (ITO Ultrasonics, Tokyo, Japan) with 1‐MHz radiation frequency. This device had two settings: the first setting was continuous whereas the other was pulsed with a variable pulse repetition frequency (PRF) ranging from 0.5 to 100 Hz at a fixed duty factor (DF) of 50%. The plane unfocused transducer with a diameter of 3.0 cm was used with 35‐mm culture dishes in the sonication experiments. The ISATA (Spatial Average Temporal Average) values at continuous wave were 0.054, 0.233, 0.634 and 0.865 Watt (W)/cm2 for the intensity readings 0.5, 1.0, 1.5 and 2.0 W/cm2, respectively.
Transfection experiments. The plasmid vector pGL3‐control with the luciferase gene (Promega, Madison, WI, USA) was used as reporter. The plasmids were prepared on a large scale using an endotoxin‐free plasmid preparation kit (EndoFree Plasmid Kit; Qiagen, Valencia, CA, USA) and stored at −20°C until use.
Sonotransfection. For attached cell types (e.g. HeLa), approximately 1 million cells were plated in 35‐mm culture dishes and incubated for 24 h to attain at least 60% confluence. Ten micrograms of expression vector was added to 0.75 mL culture medium containing the cells, incubated for 30 min, then added with 0.75 mL freshly prepared (slightly shaken) medium before sonication. The treated cells were collected by centrifugation and were then cultured overnight in 2.0 mL culture medium before the cells were assayed for luciferase expression.
Electrotransfection. Ten microgram of expression vector was added to 1 mL phosphate‐buffered saline (PBS) containing approximately 1 million cells. The suspension was then transferred to a specially designed cuvette with 0.4 mL gap (Gene Pulser Cuvette, Bio‐Rad Laboratories, Hercules, CA, USA). The cuvette was properly positioned in an exposure chamber before being given an electroshock of 1.2 kV at 25 µF (total exposure time was approximately 0.3 ms) using an electroporator device (Gene Pulser, Bio‐Rad Laboratories). The treated cells were then collected, and 2 mL culture medium was added and incubated overnight before the cells were assayed for luciferase expression.
Liposome‐mediated transfection. Three types of liposomes, formulated by Kikuchi et al. and provided by Daiichi Pharmaceuticals Company (Tokyo, Japan), have been used in previous studies. These three types of liposomes, designated L1, L2 and L3,( 5 , 15 ) basically contain O,O′‐ditetradecanoyl‐N‐(α‐trimethylammonioacetyl) diethanolamine chloride (DC‐6–14), dioleoylphosphatidylethanolamine (DOPE) and/or cholesterol (Chol) in varying ratios. L3 has the highest transfection rate with HeLa cells.( 15 ) In the present study we used L3 in our liposome‐mediated gene transfections.
Approximately 1 million HeLa cells were plated in a 35‐mm polystyrene tissue culture dish (Falcon: Becton Dickinson & Co, Plymouth, England) and incubated for 24 h to attain at least 60% confluence. A 500‐µL sample of liposome solution containing 50 µg of plasmid DNA was incubated for 15 min before being diluted 1/50 with the medium. The medium in the dish containing the cells was then taken out and replaced with 1.5 mL medium containing liposome–DNA complexes. The cells were then collected, lysed and assayed for luciferase expression after 24 h under the usual incubation conditions.
Luciferase assay. The luciferase assay was used to assess ultrasound‐mediated DNA transfection of cells. DNA transfection by electroporation and lipid‐mediated transfection were also done for comparative studies and also on combination treatment experiments.
The plasmid vectors pGL3‐control with the luciferase gene and pEGFP‐N1 with the green fluorescent protein (GFP) gene (Clontech Laboratories, Palo Alto, CA, USA) were used as reporters. The plasmids were prepared on a large scale using an endotoxin‐free plasmid preparation kit (EndoFree Plasmid Kit; Qiagen, Valencia, CA, USA) and stored at −20 C until use.
At 24 h after treatment, cell lysate was prepared and luciferase expression was assayed with a luciferase assay kit (Promega) using a luminometer (Turner designed luminometer TD‐20/20; Promega). Following the manufacturer's instructions, cells in a culture dish were washed twice with PBS before adding 500 µL of lysis buffer. After a 15‐min incubation at room temperature, the cell lysate was collected and resuspended by pipetting. Twenty microlitres of the supernatant was mixed with 100 µL of luciferase assay reagent and luciferase expression was measured using a luminometer. The luminescence of each sample was counted for 10 s. A portion of the lysate was used to determine the protein concentration with a protein assay kit (Bio‐Rad Laboratories); 20 µL of each lysate was mixed well with 1 mL of the protein assay reagent and placed at room temperature for 5 min before being analyzed using a spectrophotometer (DU60; Beckman Coulter, Fullerton, CA, USA) at 595 nm absorbance. The protein concentration was calculated according to a standard curve plotted using IgG as a standard protein reference. The enzyme activity was expressed in relative light units (RLU)/mg protein where the luminescence count of each sample was divided by its protein content in mg to standardize the luciferase activity in accordance with the protein concentration of the cell lysate.
Measurement of cell viability. The Trypan blue exclusion test was carried out by mixing 200µl of cell suspension with an equal amount of 0.3% Trypan blue solution (Sigma, St Louis, MO, USA) in PBS. After 5 min incubation at room temperature, the number of cells excluding Trypan blue (unstained) was counted using a Burker Turk hemocytometer (EKDS, Tokyo, Japan) to estimate the number of viable cells immediately after sonication.
Fluorescence microscopy and flow cytometry. Using pEGFP‐N1, HeLa cells were sonicated with a varying number of pulses. Twenty‐four hours after sonotransfection, visualization of GFP expression was carried out using a fluorescence microscope (Nikon Eclipse TE 300; Nikon Corporation, Tokyo, Japan) using a fluorescein‐isothiocyanate (FITC) filter with a digital camera (Hamamatsu Photonics K. K., Hamamatsu, Japan). The digital camera was connected directly to a computer to capture visualized images as computer files.
To determine the transfection rate, the cells were harvested by trypsinization, washed with PBS and suspended in a PBS solution before flow cytometry (Epics XL; Beckman Coulter) was carried out to count the number of cells emitting GFP (or FITC) signals. The values were normalized by deducting the reading values of the control (unsonicated sample that was also treated with the GFP gene). The transfection rate of each sample was calculated as the percentage of cells with FITC signals above that of the control cells, divided by the total number of cells counted.
Results
After several experiments using the 1‐MHz ultrasound set‐up in the present study, significant transfection was observed at the following parameter ranges: intensity, 1.3–2.0 W/cm2; PRF, 0.5–10 Hz at 50% DF; and exposure time, 5–60 s. The best data was noted to be at 1.5 W/cm2 and at 0.5 Hz PRF, at which pulses could be easily monitored and counted. At 1.5 W/cm2, DNA transfection efficiency in different cell lines was investigated using a different number of pulses. The HeLa cell line, which is known to have good transfection efficiencies using other methods such as electroporation and lipid‐mediated transfection, was used in most of the experiments. Another cell line that is efficient in transfection (PC‐3) and three other cell lines that are poor in transfection (U937, Meth A and T‐24) were also used. The results were then compared. The ideal ultrasound conditions were 1.0 MHz, 1.5 W/cm2, pulsed at 0.5 Hz with 50% DF. Using these conditions, the results showed that the transfection rate increased with the number of pulses (1 s/pulse) and peaked between 10 and 15 pulses before it started to decline (Fig. 1A,B). Light microscopy with Trypan blue staining also showed that cell killing increased with increasing number of pulses (Fig. 1C). Careful examination of the data show that at 10 pulses, cell killing was either comparable with or better than the other methods. Loss of viability for HeLa cells with sonotransfection, liposome‐mediated transfection and electrotransfection was 23.1 ± 7.4, 48.2 ± 8.9 and 28.2 ± 11.7. (% ± SD), respectively.
Figure 1.
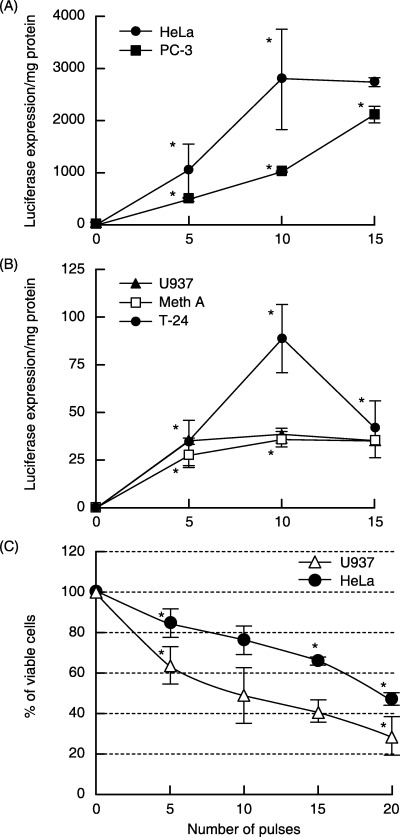
Using ultrasound, the optimal sonotransfection conditions were 1.5 W/cm2 (0.634 W/cm2), duty factor of 50%, and pulse repetition frequency of 0.5 Hz (1 s/pulse). Transfection experiments were carried out by counting the number of pulses (or bursts). Different cell lines were sonicated after incubating the cells with plasmid vector pGL3‐control with the luciferase gene as reporter. (A) Two cell lines known to be efficient in transfection (HeLa and PC‐3) and (B) three other cells types showing poor transfection efficiency (U937, Meth A and T‐24) were used in the sonotransfection experiments. (C) The loss of cell viability after sonication. Asterisks indicate statistical significance vs the preceding point (Student's unpaired t‐test, P < 0.05).
To evaluate the level of efficiency of sonotransfection, we compared the results with other transfection methods. We used HeLa cells as they are representative of transfection‐efficient cells, and carried out liposome‐mediated transfection using the most efficient of the liposome series used previously.( 5 , 15 ) Electroporation was also carried out on this cell line. Sonotransfection (2711.8 ± 91.8, mean ± SD) was shown to be superior to electrotransfection (141.6 ± 154.9) and liposome‐mediated transfection (1062.0 ± 88.8) (Fig. 2A). Sonotransfection (38.3 ± 3.8 and 36.2 ± 4.0) also showed superior results over electrotransfection (5.5 ± 0.6 and 16.5 ± 0.6) and over liposome‐mediated transfection (4.9 ± 1.5 and 19.6 ± 1.8) using the U937 and Meth A cell lines, respectively (Fig. 2B). All data in the evaluation of transfection efficiency are expressed as luciferase expression/mg protein in a sample.
Figure 2.
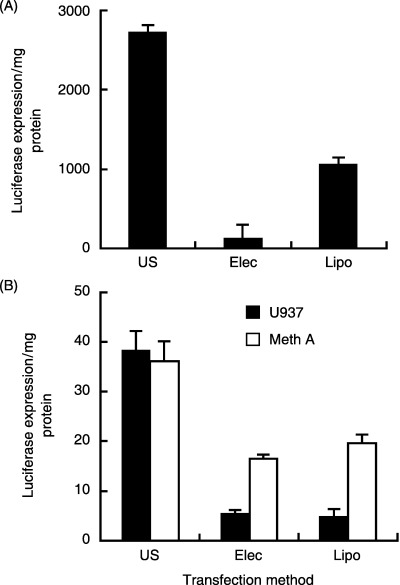
Sonotransfection compared with other methods. (A) Using HeLa cells, which had the highest transfection rate among the cell lines used, transfection efficiencies were compared for the different methods. (B) Showing the most similar and lowest levels of transfection among the five cell lines, U937 and Meth A cells were subjected to electrotransfection and the data compared with that of sonotransfection. US, sonotransfection; Elec, electrotransfection; Lipo, liposome‐mediated transfection. Using the Student's unpaired t‐test at P < 0.05, sonotransfection consistently showed significantly higher values than the other methods (Elec and Lipo).
To further verify the above findings, we used the GFP gene in the sonotransfection of HeLa cells. The highest transfection rate (16.1 ± 7.4%) was attained at 10 pulses (Fig. 3). The fluorescence micrographs of cells expressing GFP 24 h after sonication also support the findings.
Figure 3.
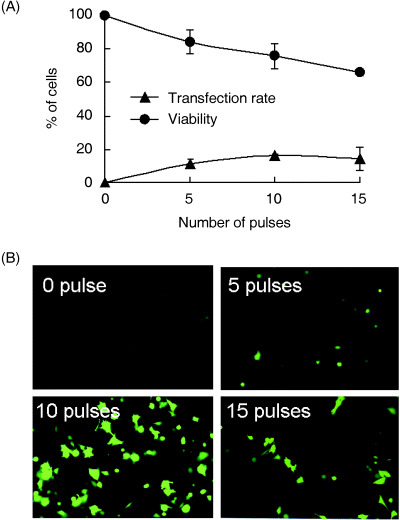
Cell viability, transfection rate and green fluorescent protein (GFP) sonotransfection. (A) Using HeLa cells, relative transfection rate and loss of cell viability by flow cytometry and Trypan blue staining, respectively. (B) Fluorescence microscopic observation of HeLa cells 24 h following GFP gene sonotransfection using a 1‐MHz ultrasound, 0.5‐Hz pulse repetition frequency, 50% duty factor and at different number of pulses.
Discussion
The present study shows that sonotransfection can be optimized. Furthermore, our data suggests that such optimal sonotransfection is comparably more efficient in transfection than the two other non‐viral methods − the electroporation and liposome‐mediated methods − at least in vitro.
Electroporation, which is a more established physical method, has been considered as the most efficient non‐viral strategy for gene delivery and is also being highlighted for its perceived low cost, safety and ease of use.( 4 ) Studies have shown that this physical non‐viral transfection method is particularly beneficial when applied locally in vivo for the desired localized transfection to attain a local or systemic effect of gene therapy. All of these advantageous features and characteristic modes of application for electroporation are essentially present with sonotransfection. Furthermore, our finding that sonotransfection has a superior rate of transfection compared with electroporation suggests that ultrasound could be a better alternative as a non‐viral method of transfection.
Liposome‐mediated transfection, however, remains under challenge as a method to introduce genes. As a chemical method, local application is not practical, whereas toxicity is still a big issue for systemic application. Research has been carried out with liposome‐mediated transfection, aiming to raise its transfection efficiency without increasing the effective concentration by combining it with other methods.( 15 ) Although it has been shown to be better than electroporation as a non‐viral method, even with enhanced efficiency our data show that the efficiency of liposome‐mediated transfection remains comparably lower than sonotransfection.
Five different cell types were used in the experiments. Four were of human origin (U937, HeLa, PC‐3 and T‐24 cells) and vary as to transfection efficiencies with different methods. The fifth cell line used was of murine origin (Meth A cells). Our data showed that in all of the cell types used, sonotransfection seemed to achieve better results than the other methods. In vivo, it is expected that different ultrasound conditions are needed to attain such in vitro effects. Inclusion of the Meth A cell line in our experiments prepared us for a possible in vivo experiment using cells that can be inoculated to experimental animals such as rats or mice. If, however, such in vivo conditions fail, ex vivo treatment could be another option. Such ex vivo treatment has been suggested previously, and may be particularly useful for leukemia treatment.( 7 )
In summary, our data show that optimal sonotransfection conditions can mediate gene transfection better than the other non‐viral methods. These findings suggest that sonotransfection offers great potential for local treatment or in ex vivo treatment in cancer gene therapy.
Acknowledgments
This study was supported in part by a Grant in Aid for Scientific Research on Priority Areas (12217049 and 16500328) from the Ministry of Education, Culture, Sports, Sciences and Technology, and also in part by the 21st Century COE Program of the Toyama Medical and Pharmaceutical University, Japan.
References
- 1. Zondervan KT, Cardon LR. The complex interplay among factors that influence allelic association. Nat Rev Genet 2004; 5: 89–100. [DOI] [PubMed] [Google Scholar]
- 2. Wilson DR. Viral‐mediated gene transfer for cancer treatment. Curr Pharm Biotechnol 2002; 3: 151–64. [DOI] [PubMed] [Google Scholar]
- 3. Bloquel C, Fabre E, Bureau MF, Scherman D. Plasmid DNA electrotransfer for intracellular and secreted protein expression: new methodological developments and applications. J Gene Med 2004; 6 (Suppl. 1): S11–23. [DOI] [PubMed] [Google Scholar]
- 4. Uesato M, Gunji Y, Tomonaga T et al. Synergistic antitumor effect of antiangiogenic factor genes on colon 26 produced by low‐voltage electroporation. Cancer Gene Ther 2004; 11: 625–32. [DOI] [PubMed] [Google Scholar]
- 5. Kikuchi A, Aoki Y, Sugaya S et al. Development of novel cationic liposomes for efficient gene transfer into peritoneal disseminated tumor. Hum Gene Ther 1999; 10: 947–55. [DOI] [PubMed] [Google Scholar]
- 6. Feril LB Jr, Umemura KT, Tachibana S, Manalo K, Riesz AH. Sound waves and antineoplastic drugs: the possibility of an enhanced combined anticancer therapy. J Med Ultrasonics 2002; 29: 173–87. [DOI] [PubMed] [Google Scholar]
- 7. Feril LB Jr, Kondo T. Biological effects of low intensity ultrasound: the mechanism involved, and its implications on therapy and on biosafety of ultrasound. J Radiat Res (Tokyo) 2004; 45: 479–89. [DOI] [PubMed] [Google Scholar]
- 8. Nozaki T, Ogawa R, Feril LB, Kagiya G, Fuse H, Kondo T. Enhancement of ultrasound‐mediated gene transfection by membrane modification. J Gene Med 2003; 5: 1046–55. [DOI] [PubMed] [Google Scholar]
- 9. Lawrie A, Brisken AF, Francis SE et al. Ultrasound‐enhanced transgene expression in vascular cells is not dependent upon cavitation‐induced free radicals. Ultrasound Med Biol 2003; 29: 1453–61. [DOI] [PubMed] [Google Scholar]
- 10. Newman CM, Lawrie A, Brisken AF, Cumberland DC. Ultrasound gene therapy: on the road from concept to reality. Echocardiography 2001; 18: 339–47. [DOI] [PubMed] [Google Scholar]
- 11. Ogawa R, Kagiya G, Feril LB et al. Ultrasound mediated intravesical transfection enhanced by treatment with lidocaine or heat. J Urol 2004; 172: 1469–73. [DOI] [PubMed] [Google Scholar]
- 12. Taniyama Y, Tachibana K, Hiraoka K et al. Development of safe and efficient novel nonviral gene transfer using ultrasound: enhancement of transfection efficiency of naked plasmid DNA in skeletal muscle. Gene Ther 2002; 9: 372–80. [DOI] [PubMed] [Google Scholar]
- 13. Teupe C, Richter S, Fisslthaler B et al. Vascular gene transfer of phosphomimetic endothelial nitric oxide synthase (S1177D) using ultrasound‐enhanced destruction of plasmid‐loaded microbubbles improves vasoreactivity. Circulation 2002; 105: 1104–9. [DOI] [PubMed] [Google Scholar]
- 14. Anwer K, Kao G, Proctor B et al. Ultrasound enhancement of cationic lipid‐mediated gene transfer to primary tumors following systemic administration. Gene Ther 2000; 7: 1833–9. [DOI] [PubMed] [Google Scholar]
- 15. Feril LB Jr, Ogawa R, Kobayashi H, Kikuchi H, Kondo T. Ultrasound enhances liposome‐mediated gene transfection. Ultrason Sonochem 2005; 12: 489–93. [DOI] [PubMed] [Google Scholar]
- 16. Wang WD, Chen ZT, Li R, Li DZ, Duan YZ, Cao ZH. Enhanced efficacy of radiation‐induced gene therapy in mice bearing lung adenocarcinoma xenografts using hypoxia responsive elements. Cancer Sci 2005; 96: 918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshida T, Ohnami S, Aoki K. Development of gene therapy to target pancreatic cancer. Cancer Sci 2004; 95: 283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feril LB Jr, Kondo T, Takaya K, Riesz P. Enhanced ultrasound‐induced apoptosis and cell lysis by a hypotonic medium. Int J Radiat Biol 2004; 80: 165–75. [DOI] [PubMed] [Google Scholar]
- 19. Liang HD, Lu QL, Xue SA et al. Optimisation of ultrasound‐mediated gene transfer (sonoporation) in skeletal muscle cells. Ultrasound Med Biol 2004; 30: 1523–9. [DOI] [PubMed] [Google Scholar]
- 20. Feril LB Jr, Tsuda Y, Kondo T et al. Ultrasound‐induced killing of monocytic U937 cells enhanced by 2,2′‐azobis (2‐amidinopropane) dihydrochloride. Cancer Sci 2004; 95: 181–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prentice PCA, Dholakia K, Prausnitz M, Campbell P. Membrane disruption by optically controlled microbubble cavitation. Nat Phys 2005; 1: 107–10. [Google Scholar]
- 22. Feril LB Jr, Kondo T, Cui ZG et al. Apoptosis induced by the sonomechanical effects of low intensity pulsed ultrasound in a human leukemia cell line. Cancer Lett 2005; 221: 145–52. [DOI] [PubMed] [Google Scholar]


