Abstract
Attenuated Salmonella typhimurium possess the ability to stimulate innate immune responses and preferentially allocate within the solid tumor. These two main characteristics make attenuated Salmonella one of the most attractive vehicles for development of vaccine and also targeted cancer therapies. However, location of Salmonella prevents the process of antigen presentation. Salmonella Type III secretion system can be utilized to circumvent this problem because this system secretes the protein it encoded outside the cells. Heat shock protein 70 (Hsp70) is referred to as an “immunochaperone” for its capacity to elicit tumor‐specific adaptive immune responses in the form of Hsp70‐TAA (tumor associated antigen) complex. Hsp70 facilitates the cross‐presentation of exogenous antigens through its receptor on antigen‐presenting cells and therefore activates an antigen‐specific cytotoxic T lymphocyte (CTL) response, which can directly contribute to potent anti‐tumor immunity. Here, we designed a novel therapeutic vaccine utilizing the type III secretion system and Hsp70 to deliver and present the tumor‐specific antigen. This live recombinant bacteria vaccine, when administrated orally, successfully broke the immune tolerance, induced a specific CTL response against tumor cells, and therefore revealed protective and therapeutic effects against generation and growth of B16F10 melanoma in C57BL/6J mice. (Cancer Sci 2010; 101: 2621–2628)
Melanoma is one of the most immunogenic of all tumors and also one of the most difficult diseases to treat. In recent years, several immunotherapeutic strategies including interferon‐α therapy,( 1 ) interleukin‐2 in combination with other cytokines( 2 , 3 ) or chemotherapeutic agents,( 4 , 5 , 6 ) adoptive immunotherapy using transduced tumor‐specific lymphocytes( 7 , 8 ) and CTLA‐4 blockage therapy( 9 ) have been tried in order to defeat melanoma. Melanoma‐specific antigen such as TRP2( 10 ) and its peptide( 10 , 11 , 12 ) successfully stimulates tumor‐specific cytotoxic T lymphocyte (CTL) responses.( 13 ) However, most of these vaccines were failed to evoke sufficient anti‐tumor immunity in clinical trials. Therapeutic vaccine is a promising method to simultaneously realize prevention and treatment against tumor. To develop an efficient therapeutic vaccine, the key issue is to establish a platform for antigen delivery and presentation. Live bacteria, especially the avirulent strains of Salmonella species, as an ideal vehicle for antigen delivery are advantageous as follows: (i) their heterogeneous surface antigen provides ideal adjuvant for immunological responses; (ii) when orally administrated, they can be taken up by the antigen‐presenting cells (APCs) (e.g. macrophages and dendritic cells) in the gut; (iii) Salmonella vaccine vectors are able to induce antigen‐specific CTL responses( 14 , 15 ) and activation of Natural Killer T (NKT) cells;( 16 ) (iv) Salmonella possess tumor‐targeting properties;( 17 ) and (v) they are easy to manipulate genetically and suit large‐scale production, therefore attenuated Salmonella provide an ideal candidate for holding tumor therapeutic vaccines.
However, the intracellular location of Salmonella limits their potential for exogenous antigen delivery, thereby hampering the use of a Salmonella vaccine against viruses, intracellular bacteria and tumors.( 18 , 19 ) The Salmonella type III secretion system, encoded by Salmonella pathogenicity island (SPI), overcomes this limitation by directly delivering the heterologous proteins into the cytosol of APCs and presenting them to CD8 T cells.( 15 , 20 ) The Salmonella type III secretion system consists of structural, regulatory and secreted elements specialized for the delivery of bacterial proteins into eukaryotic host cells.( 21 ) By fusing immunodominant CTL epitopes with type‐III‐secreted bacterial proteins, the recombinant bacteria vaccine primes antigen‐specific CD8 T cells against a variety of pathogens including influenza,( 19 ) lymphocytic choriomeningitis,( 15 ) listeriosis,( 22 ) simian immunodeficiency viruses( 23 ) and fibrosarcoma.( 24 , 25 ) The ability of heat shock protein 70 (Hsp70) to chaperone a myriad of peptides and receptor‐mediated endocytosis of the associated peptides into APCs are two main properties that make Hsp70 the ideal adjuvant for tumor‐related antigen presentation. The interaction between Hsp70 and APC facilitates cross‐presentation of the exogenous antigen into the MHC class I presenting pathway, therefore markedly increasing the specific immune response against tumor.( 26 )
Here, for the first time, we used the attenuated S. typhimurium strain SL3261 to deliver the melanoma antigen TRP2 fused with type III secretion proteins SopE and Hsp70 in order to facilitate antigen presentation from the bacteria to the APCs and enhance the T cell responses. Effective protective and therapeutic immunity against B16F10 melanoma was observed in the SL/SopE‐Hsp‐TAA vaccine‐treated C57BL/6J mice.
Materials and Methods
Bacterial strains and cell lines. The auxotrophic S. typhimurium strain LB5000 and the attenuated S. typhimurium strain SL3261 were used to host vaccine plasmids as previously described.( 27 ) The B16F10 murine melanoma cell line, HEK293 human embryonic kidney cell line and YAC‐1 murine moloney virus‐transformed lymphoma cell line were stored in our laboratory. The B16F10 cells and YAC‐1 cells were cultured in complete RPMI 1640 media supplemented with 10% fetal bovine serum. The HEK293 cells were cultured in complete Dulbecco’s modified Eagle’s media supplemented with 10% fetal bovine serum.
Reagents. The antibodies used in FACS analysis were purchased from Ebiosciences (San Diego, CA, USA). H‐2Kb/H‐2Db monoclonal antibody was purchased from BD PharMingen (San Diego, CA, USA). Anti‐TRP2 mAb was purchased from Abcam (Cambridge, UK). The medium and fetal bovine serum used in the cell culture were purchased from Invitrogen (Carlsbad, CA, USA). CytoTox 96 Non‐Radioactive Cytotoxicity Assay kit was purchased from Promega (Madison, WI, USA).
Vaccine vectors. The vaccine vector were constructed under the control of SopE promoter (pSopE)( 24 ) and encoded a chimeric protein consisting of the first 100 amino acids of SopE (containing its secretion and translocation signals),( 28 ) fused to Hsp70 and the 153rd amino acid to 417th amino acid of TRP2 antigen (containing three immunodominant epitopes).( 11 , 12 , 29 ) The other four control vectors used in these studies were identical to the vaccine vector except that they expressed just SopE or TRP2, or Hsp70 fused with SopE, or TRP2 fused with Hsp70, or TRP2 fused with SopE only (Fig. 1A). The vaccine and control vectors were introduced into the attenuated S. typhimurium strain SL3261 as previously described,( 30 ) thereby generating SL/pSOPE, SL/pTRP2, SL/pHSPTRP2, SL/pSOPE‐HSP, SL/pSOPE‐TRP2 or SL/pSOPE‐HSPTRP2, respectively.
Figure 1.
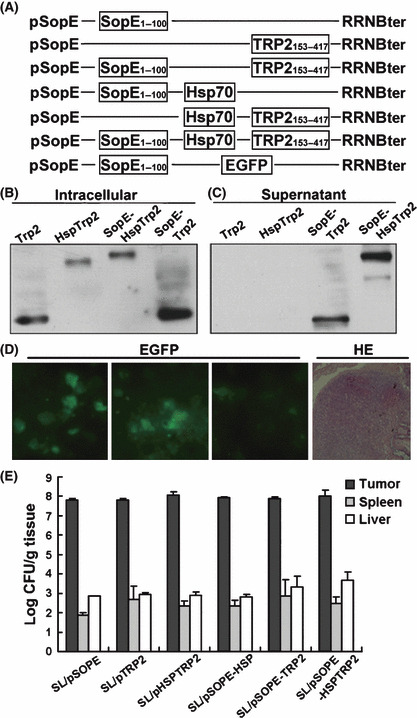
Construction and delivery of the recombinant SL3261 vaccine. (A) The vaccine vector SOPE‐HSPTRP2 was constructed under the control of SopE promoter (pSopE) and the coding sequence of murine melanoma‐specific antigen TRP2153–417 was fused with the type III secretion protein SopE1–100 and Hsp70. Five control vaccine plasmids were constructed in the same way except they expressed either SopE, TRP2, HspTRP2, SopE‐Hsp or SopE‐TRP2 instead. pSopE‐enhanced green fluorescent protein (EGFP) was also constructed to track the location of the orally administered SL3261 within the vaccinated mice. (B,C) Antigen delivery by SL3261 and secretion mediated by SopE was detected by western blotting of the cell lysate and cultural supernatant using anti‐TRP2 mAbs. (D) SL/pSopE‐EGFP, which served as a reporter vaccine, was also orally administrated to the mouse and fresh specimens of its small intestine were removed for analysis after being washed with PBS. The frozen sections were stained with HE (right), and then the EGFP signal in the Peyer’s patches of C57BL/6J mice was captured by LEICA DC500 (LEICA CAMERA, Hesse, Germany) (left). (E) After oral administration, the bacteria in the tumors, livers and spleens were calculated. The recombinant SL3261 vaccines were found to be preferentially accumulated in the solid tumor tissues. The columns represent the mean amounts of bacteria for four mice and the bars represent the SD.
Infections. The HEK293 cells were infected with 2.5 × 107 colony‐forming unit (cfu) different SL3261 strains for 1 h at multiplicity of infection 50. The cells were subsequently washed three times with phosphate‐buffered saline (PBS) and replenished with complete medium supplemented with 100 U/mL penicillin and 100 U/mL streptomycin to kill the extracellular bacteria. After further culture for 4 h, the antigen proteins inside the HEK293 cells or transferred in the cultural supernatant were analyzed by western blot using the anti‐TRP2 mAb.
Mice and tumors. Male C57BL/6J mice were purchased from the Shanghai SLAC Laboratory Animal Co. Ltd (Shanghai, China), and used at 6–8 weeks old. All procedures were performed according to the ethical use of animals. Tumor was established by subcutaneous inoculation with B16F10 melanoma cells into the right flanks of mice.
Bacteria accumulation. When the tumors had reached a mean volume of approximately 80 cm3, mice were fed with 5 × 107 cfu of recombinant vaccine bacteria. Three days after bacteria administration, four mice in each group were killed and their tumors, livers and spleens were excised, weighed and homogenized in 2 mL of ice‐cold sterile PBS. The bacteria were quantified by colonies of homogenates plating on the lysogeny broth medium agar plates containing kanamycin when required.
Oral vaccination and tumor challenge. The C57BL/6J mice were divided into seven groups (n = 8) and immunized thrice at 10‐day intervals by gavage with 5 × 107 cfu of either SL/pSOPE, SL/pTRP2, SL/pHSPTRP2, SL/pSOPE‐HSP, SL/pSOPE‐TRP2 or SL/pSOPE‐HSPTRP2 suspended in 1 mL PBS or 1 mL PBS as a control group. All mice were challenged s.c. into the right flank with 5 × 105 B16F10 cells 7 days after the last immunization. In therapeutic settings, C57BL/6J mice were also divided into seven groups (n = 8) and inoculated s.c. with 5 × 105 B16F10 cells. Seven days after inoculation, the mice were subjected to four oral treatments with 5 × 107 cfu of bacteria at 5‐day intervals. Mice were then examined daily for tumor growth after the challenge or the inoculation, and killed when tumor volume reached 3000 mm3. SL3261 accumulation in the tumors was collected when the mice were killed, and then subjected to 16s rDNA sequencing and PCR analysis for the gene they encoded using the specific primers.
Cytotoxicity assay. Splenocytes were isolated from the vaccinated mice 7 days after B16F10 cell challenge as effecters. Cytotoxicity was assessed by CytoTox 96 Non‐Radioactive Cytotoxicity Assay kit (Promega) using B16F10 cells as targets. The inhibition experiments were performed with 10 μg/mL anti‐mouse MHC class I H‐2Kb/H‐2Db monoclonal antibody. The cytotoxicity of natural killer (NK) cells was estimated the same way by targeting the sensitive cell line YAC‐1.
In order to evaluate the ability of the SL/pSOPE‐HSPTRP2 vaccine of presenting the antigen, we performed a cytotoxicity assay using the three TRP2‐derived peptides cross‐linked EL4 cells as targets for the splenocytes from the SL/pSOPE‐HSPTRP2 vaccinated mice.
Flow cytometric analysis. Activation of lymphocytes in the immunized mice was evaluated by double‐colored FACS analysis with FITC‐conjugated anti‐CD8 mAb in combination with phycoerythrin (PE)‐conjugated anti‐CD3 or anti‐CD28 mAbs. Analysis of NK T cell activation was performed by FITC‐labeled anti‐NK‐1.1 Abs and PE‐conjugated DX5 Abs.
Cytokine release assay. The splenocyte cells were cultured together with irradiated B16F10 cells in complete T cell medium for 24 h. The cells were blocked, washed and then stained with FITC‐conjugated anti‐CD4 or anti‐CD8 mAbs. After fixing and permeabilation, the cells were subsequently stained with either PE‐conjugated anti‐IL2 (interleukin 2) or anti‐IFN‐γ (interferon gamma) Abs for two‐colored FACS.
Evaluation of possible side‐effects. Seven days after the third immunization with either SL/pSOPE, SL/pTRP2, SL/pSOPE‐HSP, SL/pHSPTRP2, SL/pSOPE‐TRP2, SL/pSOPE‐HSPTRP2 or PBS, the bodyweight and motor capacity of the mice were evaluated every day. Immunized female C57BL/6J mice were also allowed to cohabitate with immunized males in a 3:1 breeding ratio to evaluate fertility. The days until parturition and number of pups were noted.
Statistical analysis. The statistical significance of differential findings between experimental groups and controls was determined by the student’s t test and considered significant if two‐tailed P < 0.05.
Results
Heterologous secretion and translocation into host cells by the Salmonella type III secretion system. The cultured HEK293 cells were infected with the SL/pSOPE‐HSPTRP2 vaccine, as well as other control vaccines including SL/pTRP2, SL/pHSPTRP2 and SL/pSOPE‐TRP2. The proteins they produced were efficiently delivered into the cells by the invasive bacteria (Fig. 1B), and the SopE chimeric proteins were secreted into cultural supernatants of the infected cells (Fig. 1C). These results indicate that the SopE‐fused tumor antigen can be efficiently transferred from SL3261 to eukaryotic cultured cells through the S. typhimurium type III secretion system. Green fluorescence was observed in the Peyer’s patches of the SL/pSOPE‐EGFP (enhanced green fluorescent protein) vaccinated mice (Fig. 1D). After oral administration, the recombinant SL3261 vaccine can preferentially accumulate within tumor tissue, other than normal tissue like the liver and spleen (Fig. 1E).
Effective antitumor immunity induced by the recombinant S. typhimurium vaccine. The vaccine based on SopE and Hsp70‐fused tumor antigen induced obvious suppression of tumor generation and development. After vaccination with SL/pSOPE‐HSPTRP2 and challenge with B16F10 cells, 75% (six out of eight) of mice in the prophylactic setting showed complete tumor rejection. SL/pSOPE‐TRP2 and other control vaccines showed much less efficiency on tumor rejection (Fig. 2A). As shown in Figure 2B, mice vaccinated with SL/pSOPE‐HSPTRP2 showed conspicuously decreased tumor volume after the B16F10 cell challenge.
Figure 2.
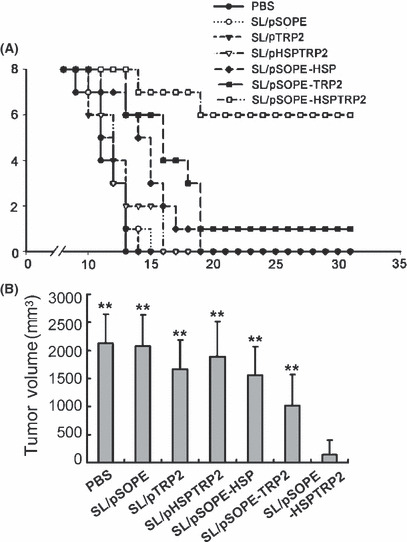
SopE‐based recombinant SL3261 vaccine induced tumor protection. (A) Three vaccinations were injected at 10‐day intervals, and the tumor challenge was performed 7 days after the last immunization. Protective efficiency was demonstrated by the tumor‐free mice in each group (n = 8) vaccinated with either SL/pSOPE, SL/pTRP2, SL/pHSPTRP2, SL/pSOPE‐HSP, SL/pSOPE‐TRP2, SL/pSOPE‐HSPTRP2 or PBS. (B) Columns represent the mean tumor volume for each group estimated on the day 22 after the tumor challenge and the bars represent the SD. **P < 0.01 compared with the SL/pSOPE‐HSPTRP2‐treated group.
The antitumor activity was also observed in the treatment of tumor‐bearing mice with the SL/pSOPE‐TRP2 vaccine. Significant suppression of tumor growth was observed in mice treated with vaccine encoding chimeric antigen fused with SopE, but not in other control groups. After inoculation with B16F10 cells, tumor eradication was observed in five out of eight mice in the SL/pSOPE‐HSPTRP2‐treated mice and in three out of eight mice in the SL/pSOPE‐TRP2‐treated mice (Fig. 3A); however, all mice in the other groups were killed due to extensive tumor within 38 days. The bacteria isolated from the tumors of the vaccine‐treated mice were identified to be SL3261 by 16s rDNA sequencing and the gene they encoded was detected by specific primers (Fig. 3B).
Figure 3.
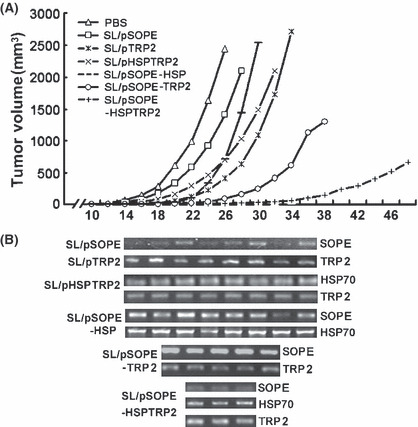
Treatment with the SL3261 vaccine induced tumor suppression. (A) Inoculated mice were divided into six groups (n = 8) and treated respectively with SL/pSOPE‐HSPTRP2 vaccine or other control vaccines or PBS four times at 5‐day intervals and examined. Lines represent the mean tumor volume for eight mice until the day when the first mouse in its own group was killed because of extensive tumor. (B) When the mice were killed, the bacteria that accumulated in the tumor tissue were identified to be SL3261 by 16s rDNA sequencing and the gene encoded was assessed by PCR with gene‐specific primers.
SL3261 vaccine stimulates CTL response. A specific CTL response was induced by SopE‐Hsp70‐mediated antigen presentation. The effecter T cells exhibited significant cell lytic activity against B16F10 cells after SL/pSOPE‐HSPTRP2 immunization and tumor challenge. The observed cytotoxicity was MHC class I antigen‐restricted because it can be inhibited by monoclonal antibody blocking H‐2Kb/H‐2Db (Fig. 4A).
Figure 4.
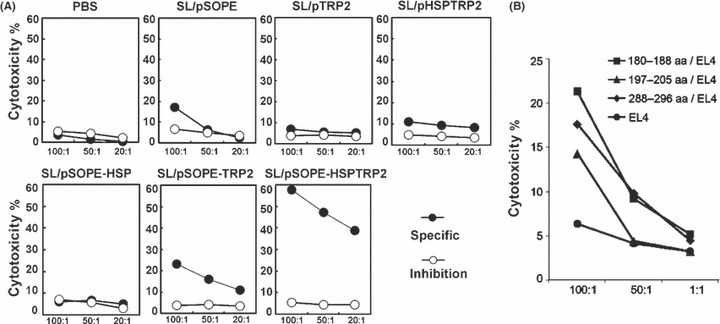
Vaccination with SL/pSOPE‐HSPTRP2 induces specific cytotoxic T lymphocyte (CTL) response. (A) The CTL response was examined by non‐radioactive cytotoxicity assay, using splenocytes collected from the immunized mice as effecter cells and B16F10 cells expressing TRP2 antigen as target cells at different effecter cell/target cell (E/T) ratios. The filled circles (• ) represent lytic activity targeting B16F10 cells, and the empty circles (○) represent the inhibited cytotoxicity via MHC class I mAbs blocking. (B) In order to evaluate the antigen specificity of the CTL response, three peptides of TRP2 were synthesized and cross‐linked onto EL4 cells as target cells, and splenocytes harvested from SL/pSOPE‐HSPTRP2‐treated mice were used as effecter cells.
Murine TRP2153–417 contains three immunodominant CTL epitopes including SVYDFFVWL (180–188 aa), LLGPGRPYR (197–205 aa) and SLDDYNHLV (288–296 aa). The three peptides were synthesized and cross‐linked onto EL4 cells in order to evaluate recognition of the antigen by the CTL. Data showed that all three of the peptides could be targeted by the CTL harvested from the SL/pSOPE‐HSPTRP2‐vaccinated mice (Fig. 4B).
SL/pSOPE‐HSPTRP2 vaccine induced expansion and activation of CD8 T cells. As shown in Figure 5A, expansion of the CD8 T cells was evoked in mice immunized with recombinant SL3261 encoding SopE‐HspTRP2 (Fig. 5B); increased expression of activation marker CD28 was also detected in this group of mice. In contrast, splenocytes isolated from mice immunized with other control vaccine showed far less efficiency in CD8 T cell activation.
Figure 5.
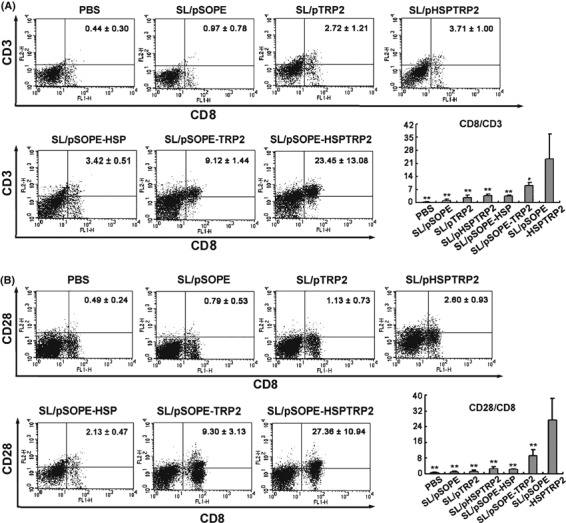
SL/pSOPE‐HSPTRP2 vaccine induced expansion and activation of CD8 T cells. Splenocytes of the vaccinated mice were also subjected to double‐color FACS for CD8 T cell analysis. The expansion and activation of CD8 T cells were performed using CD8 mAb in combination with CD3 or CD28 mAbs. The columns represent the mean for six mice and the bars represent the SD. **P < 0.01 compared with the SL/pSOPE‐HSPTRP2‐treated group. *P < 0.05 compared with the SL/pSOPE‐HSPTRP2‐treated group.
NK cells were activated by SL/pSOPE‐HSPTRP2 vaccine. SopE‐HspTRP2‐based vaccine also induced activation of NK cells. The NK cells harvested from mice vaccinated with SL/pSOPE‐HSPTRP2 showed lytic activity targeting YAC‐1 cells at different effecter‐to‐target ratios (Fig. 6A). In addition, expression of the activation marker of the NK cells was also up‐regulated in SL/pSOPE‐HSPTRP2 vaccine‐treated mice (Fig. 6B).
Figure 6.
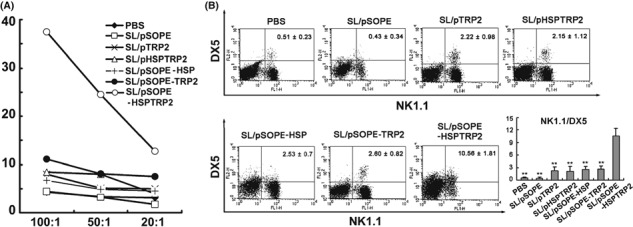
Natural killer (NK) cell cytotoxicity was activated by SopE‐HspTRP2‐based SL3261 vaccine. (A) Cell lytic activity was determined by targeting the YAC‐1 cells, which are sensitive to NK cell cytotoxicity. The dots represent the cytotoxicity of the splenocytes isolated from the vaccinated mice (n = 6) at different E/T ratios. (B) The splenocytes were analyzed by FACS with FITC‐conjugated anti‐NK1.1 Abs and PE‐conjugated anti‐DX5 Abs staining. The columns represent the mean for six mice and the bars represent the SD. **P < 0.01 compared with the SL/pSOPE‐HSPTRP2‐treated group.
Antigen vaccine‐based on SopE‐Hsp70 stimulated secretion of cytokines. Immunization with the recombinant SL3261 encoding SopE‐HspTRP2 chimeric protein resulted in significantly increased release of IL‐2 and IFN‐γ (Fig. 7). The proinflammatory cytokines stimulate the growth, differentiation and survival of antigen‐selected cytotoxic T cells and NK cells.( 31 , 32 )
Figure 7.
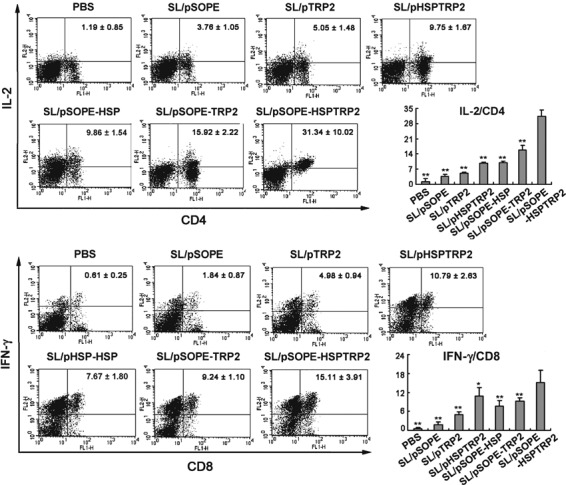
Secreted cytokines were augmented by vaccination with SL/pSOPE‐HSPTRP2. The splenocytes obtained from immunized mice (n = 6) were further cultured for 24 h in complete T cell medium together with irradiated B16F10 cells and then subjected to FITC‐conjugated CD4 or CD8 mAbs staining. After fixing and permeabilizing, the cells were stained with PE‐conjugated interleukin‐2 (IL‐2) or interferon‐gamma (IFN‐γ) Abs for double‐color FACS analysis. The columns represent the mean for six mice and the bars represent the SD. **P < 0.01 compared with the SL/pSOPE‐HSPTRP2‐treated group. *P < 0.05 compared with the SL/pSOPE‐HSPTRP2‐treated group.
Vaccination does not impair weight or fertility of mice. After immunization with SL3261 vaccines, no impact on weight or fertility was observed in the mice in comparison with the mice treated only with PBS (Fig. 8).
Figure 8.
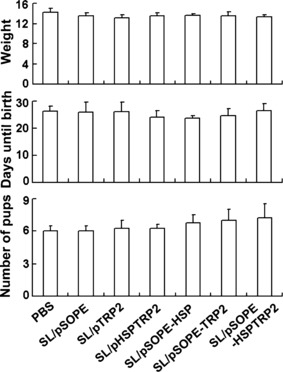
Immunization did not cause side‐effects in mice weight and fertility. The weight of the C57BL/6J mice (n = 4) was evaluated 10 days after the last immunization. Immunized female mice (n = 3) were allowed to cohabitate with males at a 3:1 breeding ratio and days until parturition and number of pups were noted. The columns represent the mean for mice in each group and the bars represent the SD.
Discussion
In order to stimulate the innate immune system and break the immune tolerance, it is essential to establish an efficiency system for antigen delivery and presentation. Attenuated S. typhimurium strain SL3261 contains a mutation in the aromatic amino biosynthetic pathway and was previously reported to be the ideal vector for vaccine development( 22 , 33 ) and cancer gene therapy.( 34 , 35 ) Orally delivered SL3261 as a vaccine vehicle is reported to facilitate the interaction of antigen and APCs, and elicit serial immune responses against virus infection or tumor generation.( 36 , 37 , 38 , 39 , 40 ) After oral administration, we found that the recombinant EGFP encoded by SL3261 were expressed in the Peyer’s patches of the mice. This suggests that the SL3261 vaccine may be ingested by the dendritic cells (DCs) in the gut and the protein may induce the maturation and migration of the DC. Self‐antigen of SL3261 could evoke innate immune, for example, LPS can be recognized by Toll‐like receptors (TLR) and induce immunity by CD14/TLR4/MD2 complex presentation.( 41 ) However, the intracellular location of SL3261 hampers the peptides being presented through the MHC class I restricted pathway. In our designation, this limitation was circumvented by exploiting the translocation capacity of Salmonella type III secreted protein SopE and the cross‐presentation capacity of Hsp‐TAA. SopE delivers heterologous antigens into the cytosol of infected cells and primes MHC class I‐restricted antigen presentation and leads to antigen‐specific CTL responses.( 42 , 43 ) Hsp70 used in our designation plays an important role in antigen presentation. Via Hsp70 receptors on the surface of APCs, such as CD91 or LOX‐1, the antigen fused with Hsp70 can be efficiently taken up by the APCs.( 44 , 45 , 46 , 47 ) Although taking up exogenous antigen usually results in classical MHC class II presentation that can generate CD4 T cell activation, Salmonella mediated phagocytosis and Hsp70 receptor‐mediated endocytosis of the fused TRP2 antigen both result in cross‐presentation of the exogenous antigen via the MHC class I pathway. MHC class I restricted antigen presentation facilitated CD8 T cells, which in turn develops into CTL responses( 48 , 49 ) and contributes to potent anti‐tumor immunity.( 50 ) Furthermore, Hsp70‐TAA can activate CTL responses through regulating the interaction among CD40, TLR‐2, TLR‐4 and cofactor CD14,( 47 , 51 ) or inducing maturation of DC.( 52 ) Immunization with SopE‐HspTRP2 vaccine successfully leads to specific CTL responses against tumor cells. Over 57.7% B16F10 cells were lysed by the effecter T cells collected from the SL/pSOPE‐HSPTRP2‐immunized mice; 23.3% cytotoxicity was observed in the mice vaccinated with the SL/pSOPE‐TRP2 vaccine. As shown, the response was antigen‐dependent and MHC I‐restricted. Recognition of the CTL epitopes of TRP2 by effecter T cells also indicated successful presentation of the TRP2 antigen in our construction. It has been reported that Hsp70 also results in secretion of proinflammatory cytokines by APCs and induction of NK cells through NK receptor CD94.( 53 ) In this study, secretion of cytokines and activation of NK cells were both observed in SL/pSOPE‐HSPTRP2‐vaccinated mice. The co‐stimulatory signal provided by Salmonella infection then stimulates the production of various interleukins (IL‐2 in particular), which was also observed in our study. The IL‐2/IL‐2R interaction increases the growth, differentiation and survival of antigen‐selected cytotoxic T cells.( 54 ) IL‐2 also plays an essential role in discriminating self and non‐self antigens( 55 ) and inducing the differentiation and proliferation of NK cells.( 56 , 57 ) The increased secretion of IFN‐γ leads to up‐regulation of MHC class I molecules, promotion of Th1 differentiation, and activation of APC, NK cells and cytotoxic T cells.( 58 ) In summary, after oral administration of the SL/pSOPE‐HSPTRP2 vaccine, the bacteria were taken up by macrophages or DC, where the antigen they encoded could be processed. Then the antigens fused with Hsp70 were secreted into the cytosol of the APC with the help of SopE, and Hsp70 facilitated MHC‐I restricted presentation and activated cytotoxic T cells. Besides the CTL responses, NK cell expansion and activation were induced by vaccination. Therefore, the CTL and NK cells were the effecter cells that contributed to the dramatic antitumor immunity we observed in the SL/pSOPE‐HSPTRP2‐vaccinated mice.
The SopE‐HspTRP2 vaccine transferred by SL3261 achieved not only 75% tumor protection, but also induced tumor eradication in five mice (n = 8). Most other vaccines using naked DNA or antigen protein result in little therapeutic effect. The therapeutic efficiency of the SL/pSOPE‐HSPTRP2 vaccine may be attributed mainly to the invasive property of the SL3261 strain. Live S. typhimurium, when accumulated within tumor tissue at an “infected state”, can be recognized by the host immune system and induce recruitment of immune cells.( 59 ) Preferential accumulation of the SL3261 strain in tumor tissue then provides targets for the anti‐Salmonella‐specific cytotoxic T cells( 60 ) and NK cells,( 61 ) so that tumor cells but not normal cells in adjacent tissues are recognized and killed. Large quantities of debris generated from the dead tumor cells can be taken up by endogenous APCs and then stimulate tumor‐specific T cell responses.( 62 ) This may explain the significant tumor therapeutic efficiency we observed.
In this study, we established an antigen delivery platform using the attenuated S. typhimurium strain SL3261 to host vaccine plasmids encoding Hsp70‐TAA fused with Salmonella type III secreted protein SopE. Oral administration of this vaccine stimulates tumor‐specific CTL and NK cell responses and therefore induces dramatic protective and therapeutic antitumor immunity. Our findings provide a promising method for the development of vaccines and targeted drugs against tumor.
Disclosure Statement
The authors have no conflict of interest.
References
- 1. Kirkwood JM, Strawderman MH, Ernstoff MS et al. Interferon alfa‐2b adjuvant therapy of high‐risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996; 14(1): 7–17. [DOI] [PubMed] [Google Scholar]
- 2. Agarwala SS, Glaspy J, O’Day SJ et al. Results from a randomized phase III study comparing combined treatment with histamine dihydrochloride plus interleukin‐2 versus interleukin‐2 alone in patients with metastatic melanoma. J Clin Oncol 2002; 20(1): 125–33. [DOI] [PubMed] [Google Scholar]
- 3. Gollob JA, Veenstra KG, Parker RA et al. Phase I trial of concurrent twice‐weekly recombinant human interleukin‐12 plus low‐dose IL‐2 in patients with melanoma or renal cell carcinoma. J Clin Oncol 2003; 21(13): 2564–73. [DOI] [PubMed] [Google Scholar]
- 4. Eton O, Legha SS, Bedikian AY et al. Sequential biochemotherapy versus chemotherapy for metastatic melanoma: results from a phase III randomized trial. J Clin Oncol 2002; 20(8): 2045–52. [DOI] [PubMed] [Google Scholar]
- 5. Keilholz U, Goey SH, Punt CJ et al. Interferon alfa‐2a and interleukin‐2 with or without cisplatin in metastatic melanoma: a randomized trial of the European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Clin Oncol 1997; 15(7): 2579–88. [DOI] [PubMed] [Google Scholar]
- 6. Rosenberg SA, Yang JC, Schwartzentruber DJ et al. Prospective randomized trial of the treatment of patients with metastatic melanoma using chemotherapy with cisplatin, dacarbazine, and tamoxifen alone or in combination with interleukin‐2 and interferon alfa‐2b. J Clin Oncol 1999; 17(3): 968–75. [DOI] [PubMed] [Google Scholar]
- 7. Zhou J, Shen X, Huang J et al. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol 2005; 175(10): 7046–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Charo J, Finkelstein SE, Grewal N et al. Bcl‐2 overexpression enhances tumor‐specific T‐cell survival. Cancer Res 2005; 65(5): 2001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tivol EA, Borriello F, Schweitzer AN et al. Loss of CTLsA‐4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLsA‐4. Immunity 1995; 3(5): 541–7. [DOI] [PubMed] [Google Scholar]
- 10. Bloom MB, Perry‐Lalley D, Robbins PF et al. Identification of tyrosinase‐related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med 1997; 185(3): 453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parkhurst MR, Fitzgerald EB, Southwood S et al. Identification of a shared HLA‐A*0201‐restricted T‐cell epitope from the melanoma antigen tyrosinase‐related protein 2 (TRP2). Cancer Res 1998; 58(21): 4895–901. [PubMed] [Google Scholar]
- 12. Sun Y, Song M, Stevanovic S et al. Identification of a new HLA‐A(*)0201‐restricted T‐cell epitope from the tyrosinase‐related protein 2 (TRP2) melanoma antigen. Int J Cancer 2000; 87(3): 399–404. [DOI] [PubMed] [Google Scholar]
- 13. Zhang H, Wang W, Li Q et al. Fusion protein of ATPase domain of Hsc70 with TRP2 acting as a tumor vaccine against B16 melanoma. Immunol Lett 2006; 105(2): 167–73. [DOI] [PubMed] [Google Scholar]
- 14. Wijburg OL, Van Rooijen N, Strugnell RA. Induction of CD8+ T lymphocytes by Salmonella typhimurium is independent of Salmonella pathogenicity island 1‐mediated host cell death. J Immunol 2002; 169(6): 3275–83. [DOI] [PubMed] [Google Scholar]
- 15. Shams H, Poblete F, Russmann H et al. Induction of specific CD8+ memory T cells and long lasting protection following immunization with Salmonella typhimurium expressing a lymphocytic choriomeningitis MHC class I‐restricted epitope. Vaccine 2001; 20(3–4): 577–85. [DOI] [PubMed] [Google Scholar]
- 16. Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol 2007; 5(6): 405–17. [DOI] [PubMed] [Google Scholar]
- 17. Low KB, Ittensohn M, Le T et al. Lipid A mutant Salmonella with suppressed virulence and TNFalpha induction retain tumor‐targeting in vivo. Nat Biotechnol 1999; 17(1): 37–41. [DOI] [PubMed] [Google Scholar]
- 18. Schaible UE, Collins HL, Kaufmann SH. Confrontation between intracellular bacteria and the immune system. Adv Immunol 1999; 71: 267–377. [DOI] [PubMed] [Google Scholar]
- 19. Russmann H, Shams H, Poblete F et al. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science 1998; 281(5376): 565–8. [DOI] [PubMed] [Google Scholar]
- 20. Sevil Domenech VE, Panthel K, Winter SE et al. Heterologous prime‐boost immunizations with different Salmonella serovars for enhanced antigen‐specific CD8 T‐cell induction. Vaccine 2008; 26(15): 1879–86. [DOI] [PubMed] [Google Scholar]
- 21. Galan JE, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 1999; 284(5418): 1322–8. [DOI] [PubMed] [Google Scholar]
- 22. Berchtold C, Panthel K, Jellbauer S et al. Superior protective immunity against murine listeriosis by combined vaccination with CpG DNA and recombinant Salmonella enterica serovar typhimurium . Infect Immun 2009; 77(12): 5501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Evans DT, Chen LM, Gillis J et al. Mucosal priming of simian immunodeficiency virus‐specific cytotoxic T‐lymphocyte responses in rhesus macaques by the Salmonella type III secretion antigen delivery system. J Virol 2003; 77(4): 2400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nishikawa H, Sato E, Briones G et al. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J Clin Invest 2006; 116(7): 1946–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Panthel K, Meinel KM, Sevil Domenech VE et al. Prophylactic anti‐tumor immunity against a murine fibrosarcoma triggered by the Salmonella type III secretion system. Microbes Infect 2006; 8(9–10): 2539–46. [DOI] [PubMed] [Google Scholar]
- 26. Javid B, MacAry PA, Oehlmann W et al. Peptides complexed with the protein HSP70 generate efficient human cytolytic T‐lymphocyte responses. Biochem Soc Trans 2004; 32(Pt 4): 622–5. [DOI] [PubMed] [Google Scholar]
- 27. Fu W, Lan H, Liang S et al. Suicide gene/prodrug therapy using salmonella‐mediated delivery of Escherichia coli purine nucleoside phosphorylase gene and 6‐methoxypurine 2′‐deoxyriboside in murine mammary carcinoma 4T1 model. Cancer Sci 2008; 99(6): 1172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee SH, Galan JE. Salmonella type III secretion‐associated chaperones confer secretion‐pathway specificity. Mol Microbiol 2004; 51(2): 483–95. [DOI] [PubMed] [Google Scholar]
- 29. Wang RF, Johnston SL, Southwood S et al. Recognition of an antigenic peptide derived from tyrosinase‐related protein‐2 by CTLs in the context of HLA‐A31 and ‐A33. J Immunol 1998; 160(2): 890–7. [PubMed] [Google Scholar]
- 30. Fu W, Chu L, Han X et al. Synergistic antitumoral effects of human telomerase reverse transcriptase‐mediated dual‐apoptosis‐related gene vector delivered by orally attenuated Salmonella enterica Serovar Typhimurium in murine tumor models. J Gene Med 2008; 10(6): 690–701. [DOI] [PubMed] [Google Scholar]
- 31. Beadling C, Johnson KW, Smith KA. Isolation of interleukin 2‐induced immediate‐early genes. Proc Natl Acad Sci U S A 1993; 90(7): 2719–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beadling C, Smith KA. DNA array analysis of interleukin‐2‐regulated immediate/early genes. Med Immunol 2002; 1(1): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoiseth SK, Stocker BA. Aromatic‐dependent Salmonella typhimurium are non‐virulent and effective as live vaccines. Nature 1981; 291(5812): 238–9. [DOI] [PubMed] [Google Scholar]
- 34. Chen G, Wei DP, Jia LJ et al. Oral delivery of tumor‐targeting Salmonella exhibits promising therapeutic efficacy and low toxicity. Cancer Sci 2009; 100(12): 2437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jia LJ, Wei DP, Sun QM et al. Oral delivery of tumor‐targeting Salmonella for cancer therapy in murine tumor models. Cancer Sci 2007; 98(7): 1107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cancellieri V, Fara GM. Demonstration of specific IgA in human feces after immunization with live Ty21a Salmonella typhi vaccine. J Infect Dis 1985; 151(3): 482–4. [DOI] [PubMed] [Google Scholar]
- 37. Viret JF, Favre D, Wegmuller B et al. Mucosal and systemic immune responses in humans after primary and booster immunizations with orally administered invasive and noninvasive live attenuated bacteria. Infect Immun 1999; 67(7): 3680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nencioni L, Villa L, De Magistris MT et al. Cellular immunity against Salmonella typhi after live oral vaccine. Adv Exp Med Biol 1987; 216B: 1669–75. [PubMed] [Google Scholar]
- 39. Ashby D, Leduc I, Lauzon W et al. Attenuated Salmonella typhimurium SL3261 as a vaccine vector for recombinant antigen in rabbits. J Immunol Methods 2005; 2: 153–64. [DOI] [PubMed] [Google Scholar]
- 40. Luria‐Perez R, Cedillo‐Barron L, Santos‐Argumedo L et al. A fusogenic peptide expressed on the surface of Salmonella enterica elicits CTLs responses to a dengue virus epitope. Vaccine 2007; 25(27): 5071–85. [DOI] [PubMed] [Google Scholar]
- 41. Stewart I, Schluter PJ, Shaw GR. Cyanobacterial lipopolysaccharides and human health – a review. Environ Health 2006; 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kotton CN, Lankowski AJ, Scott N et al. Safety and immunogenicity of attenuated Salmonella enterica serovar Typhimurium delivering an HIV‐1 Gag antigen via the Salmonella Type III secretion system. Vaccine 2006; 24(37–39): 6216–24. [DOI] [PubMed] [Google Scholar]
- 43. Konjufca V, Wanda SY, Jenkins MC et al. A recombinant attenuated Salmonella enterica serovar Typhimurium vaccine encoding Eimeria acervulina antigen offers protection against E. acervulina challenge. Infect Immun 2006; 74(12): 6785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chu CT, Pizzo SV. Receptor‐mediated antigen delivery into macrophages. Complexing antigen to alpha 2‐macroglobulin enhances presentation to T cells. J Immunol 1993; 150(1): 48–58. [PubMed] [Google Scholar]
- 45. Binder RJ, Vatner R, Srivastava P. The heat‐shock protein receptors: some answers and more questions. Tissue Antigens 2004; 64(4): 442–51. [DOI] [PubMed] [Google Scholar]
- 46. Delneste Y, Magistrelli G, Gauchat J et al. Involvement of LOX‐1 in dendritic cell‐mediated antigen cross‐presentation. Immunity 2002; 17(3): 353–62. [DOI] [PubMed] [Google Scholar]
- 47. Takemoto S, Nishikawa M, Takakura Y. Pharmacokinetic and tissue distribution mechanism of mouse recombinant heat shock protein 70 in mice. Pharm Res 2005; 22(3): 419–26. [DOI] [PubMed] [Google Scholar]
- 48. Singh‐Jasuja H, Hilf N, Arnold‐Schild D et al. The role of heat shock proteins and their receptors in the activation of the immune system. Biol Chem 2001; 382(4): 629–36. [DOI] [PubMed] [Google Scholar]
- 49. Li Z, Menoret A, Srivastava P. Roles of heat‐shock proteins in antigen presentation and cross‐presentation. Curr Opin Immunol 2002; 14(1): 45–51. [DOI] [PubMed] [Google Scholar]
- 50. Blachere NE, Li Z, Chandawarkar RY et al. Heat shock protein‐peptide complexes, reconstituted in vitro, elicit peptide‐specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med 1997; 186(8): 1315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Basu S, Binder RJ, Ramalingam T et al. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 2001; 14(3): 303–13. [DOI] [PubMed] [Google Scholar]
- 52. Somersan S, Larsson M, Fonteneau JF et al. Primary tumor tissue lysates are enriched in heat shock proteins and induce the maturation of human dendritic cells. J Immunol 2001; 167(9): 4844–52. [DOI] [PubMed] [Google Scholar]
- 53. Gross C, Hansch D, Gastpar R et al. Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94. Biol Chem 2003; 384(2): 267–79. [DOI] [PubMed] [Google Scholar]
- 54. Stern JB, Smith KA. Interleukin‐2 induction of T‐cell G1 progression and c‐myb expression. Science 1986; 233(4760): 203–6. [DOI] [PubMed] [Google Scholar]
- 55. Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med 1998; 188(2): 287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Waldmann TA. The biology of interleukin‐2 and interleukin‐15: implications for cancer therapy and vaccine design. Nat Rev Immunol 2006; 6(8): 595–601. [DOI] [PubMed] [Google Scholar]
- 57. Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin‐15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol 1999; 17: 19–49. [DOI] [PubMed] [Google Scholar]
- 58. Schoenborn JR, Wilson CB. Regulation of interferon‐gamma during innate and adaptive immune responses. Adv Immunol 2007; 96: 41–101. [DOI] [PubMed] [Google Scholar]
- 59. Curcio C, Di Carlo E, Clynes R et al. Nonredundant roles of antibody, cytokines, and perforin in the eradication of established Her‐2/neu carcinomas. J Clin Invest 2003; 111(8): 1161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Avogadri F, Martinoli C, Petrovska L et al. Cancer immunotherapy based on killing of Salmonella‐infected tumor cells. Cancer Res 2005; 65(9): 3920–7. [DOI] [PubMed] [Google Scholar]
- 61. Miranda D, Puente J, Blanco L et al. Lysis of Salmonella typhi intracellularly infected U937 cells by human natural killer cells: effect of protein kinase inhibitors. Am J Ther 2003; 10(1): 32–9. [DOI] [PubMed] [Google Scholar]
- 62. Ribas A, Timmerman JM, Butterfield LH et al. Determinant spreading and tumor responses after peptide‐based cancer immunotherapy. Trends Immunol 2003; 24(2): 58–61. [DOI] [PubMed] [Google Scholar]


