Abstract
A recent study reported on mutations in the active site of the isocitrate dehydrogenase 1 (IDH1) gene in several types of gliomas. All mutations detected resulted in an amino acid exchange at position 132. We analyzed the genomic region spanning wild‐type R132 of IDH1 by direct sequencing in 125 glial tumors. A total of 39 IDH1 mutations were observed. Mutations of the IDH2 gene, homologous to IDH1, were often detected in gliomas without IDH1 mutations. In the present study, R172 mutation of the IDH2 gene was detected in one anaplastic astrocytoma. IDH1 or IDH2 mutations were frequently in oligodendrogliomas (67%), anaplastic astrocytomas (62%), anaplastic oligoastrocytomas (75%), anaplastic oligodendrogliomas (50%), secondary glioblastomas (67%), gangliogliomas (38%), and anaplastic gangliogliomas (60%). Primary glioblastomas were characterized by a low frequency of mutations (5%) at amino acid position 132 of IDH1. Mutations of the IDH1 or IDH2 genes were significantly associated with improved outcome in patients with anaplastic astrocytomas. Our data suggest that IDH1 or IDH2 mutation plays a role in early tumor progression of several types of glioma and might arise from a common glial precursor. The infrequency of IDH1 mutation in primary glioblastomas revealed that these subtypes are genetically distinct entities from other glial tumors. (Cancer Sci 2009; 100: 1996–1998)
Gliomas are the most common primary brain tumors and are grouped into four grades according to the World Health Organization (WHO) criteria.( 1 ) This group of tumors includes specific histological subtypes; the most common are astrocytomas, oligodendrogliomas, and ependymomas. Glioblastomas (GBM; WHO grade IV), the most malignant glioma, may manifest rapidly de novo (primary GBM), or develop slowly from low‐grade diffuse or anaplastic astrocytoma (secondary GBM).( 2 )
Recent genomewide mutational analysis revealed somatic mutations of cytosolic NADP+‐dependent isocitrate dehydrogenase (IDH1) in approximately 12% of GBM.( 3 ) Isocitrate dehydrogenase catalyzes the oxidative decarboxylation of isocitrate to α‐ketoglutarate thereby leading to NADPH production.( 4 ) Mutations affected the amino acid arginine at position 132 of the amino acid sequence, which is evolutionarily highly conserved, and is located in the binding site of isocitrate.( 5 ) In the vast majority of the cases, wild‐type arginine at position 132 was replaced by histidine (R132H).
The IDH2 gene is homologous to IDH1, which uses NADP+ as an electron receptor. Gliomas without IDH1 mutations were often found to have mutations at the analogous amino acid (R172) of the IDH2 gene.( 6 ) Both IDH1 and IDH2 mutations reduced the enzymatic activity of the encoded protein.( 6 )
In recent analyses from North American and European groups, IDH1 mutations were more frequent in secondary GBM than primary GBM.( 6 , 7 , 8 ) Similarly, high frequencies of IDH1 mutations were found in diffuse (WHO grade II) or anaplastic astrocytomas (WHO grade III), oligodendrogliomas (WHO grade II), and anaplastic oligodendrogliomas (WHO grade III).( 6 , 7 , 8 , 9 ) On the other hand, IDH2 mutations were only found in WHO grade II or III gliomas without IDH1 mutation.( 6 ) However, IDH1 mutation was rarely found in other cancer types, except for a few cases.( 10 , 11 , 12 ) A recent paper showed that patients with this mutation had a better outcome than those with the wild‐type IDH1 gene.( 6 )
In the present study, to evaluate the prognostic significance of IDH mutations in Japanese glioma patients, we screened a total of 125 gliomas for the mutational hot spot of the IDH1 gene. Furthermore, exon 4 of the IDH2 gene was sequenced in all WHO grade II and III gliomas without an R132 IDH1 mutation.
Materials and Methods
Tumor samples. We examined a total of 125 glioma patients, diagnosed and treated at Tohoku University Hospital. Resected specimens were quick‐frozen in liquid nitrogen and kept at –80°C until nucleic acid extraction. The Ethics Committee of Tohoku University Hospital approved this study. Informed consent for use of their tissues was obtained from all study subjects. The series included two diffuse astrocytomas WHO grade II (DA), 21 anaplastic astrocytomas WHO grade III (AA), 58 primary GBM WHO grade IV (prGBM), three secondary GBM WHO grade IV (secGBM), eight oligodendrogliomas WHO grade II (O), 14 anaplastic oligodendrogliomas WHO grade III (AO), four anaplastic oligoastrocytomas WHO grade III (AOA), eight gangliogliomas WHO grade II (GG), and five anaplastic gangliogliomas (AG) WHO grade III. prGBM and secGBM were defined according to Scherer.( 13 ) Clinical details, including the patient's age at the time of diagnosis, sex, preoperative Karnofsky performance score (KPS) score, extent of resection, adjuvant therapy at recurrence, and the recorded date of disease progression or death were noted.
PCR amplification. Genomic DNA was isolated as previously described.( 14 ) Exon 4 of the IDH1 gene including codon 132 was amplified as described previously.( 5 ) A fragment of 219 bp in length spanning the catalytic domain of IDH2 including codon 172 was amplified using 100 ng each of the sense primer 5′‐CAAGCTGAAGAAGATGTGGAA‐3′ and antisense primer 5′‐CAGAGACAAGAGGATGGCTA‐3′. PCR using standard buffer conditions, 100 ng of DNA, and Ex‐Taq HS DNA Polymerase (Takara Bio, Shiga, Japan) employed 30 cycles with denaturing at 95°C for 30 s, annealing at 56°C for 30 s, and extension at 72°C for 40 s in a total volume of 50 µL.
Direct sequencing for IDH1 and IDH2 mutations. The PCR products were purified using the high pure PCR product purification kit (Roche, Basel, Switzerland). All sequence reactions were carried out using the GenomeLab DTCS quick‐start kit (Beckman Coulter, Fullerton, CA, USA). The reactions were carried out in an automated DNA analyzer (CEQ 8000; Beckman Coulter).
Statistical analyses. All statistical analyses were done with SPSS for Windows (SPSS, Chicago IL, USA). The Mann–Whitney test was used to compare data acquired in each group for the patient age. The overall survival was defined as the time between the first surgery and death. Survival distributions were estimated by Kaplan–Meier analysis and compared among patient subsets using log‐rank tests.
Results and Discussion
IDH mutations in gliomas. Altogether, 125 tumors were analyzed and 39 mutations in IDH1 were detected. All mutations were heterozyous with one wild‐type allele being present. Only codon 132 of IDH1 was affected by mutations and all mutations were of the R132H type. In addition, the mutation of IDH2 at codon 172 was detected in one AA without IDH1 mutation. The type of mutation was R172S (AGG to AGT). Frequencies of mutations are listed in Table 1 and the sequence alterations leading to the R132H are shown in Figure 1. Frequent mutations of IDH1 or IDH2 were observed in AA (62%), secGBM (67%), O (67%), AO (50%), AOA (75%), GG (38%), and in AG (60%). Only a few mutations occurred in prGBM (5%). Despite no mutations in DA, we could not draw any conclusion because there were only two cases.
Table 1.
Isocitrate dehydrogenase 1 (IDH1) and IDH2 mutations in 125 gliomas
| Diagnosis | n | IDH1 No. tumors | IDH2 No. tumors | Combined Mut (%) |
|---|---|---|---|---|
| Diffuse astrocytoma | 2 | 0 | 0 | 0 |
| Oligodendroglioma | 9 | 6 | 0 | 67 |
| Anaplastic astrocytoma | 21 | 12 | 1 | 62 |
| Anaplastic oligoastrocytoma | 4 | 3 | 0 | 75 |
| Anaplastic oligodendroglioma | 14 | 7 | 0 | 50 |
| Primary glioblastoma | 59 | 3 | ND | 5 |
| Secondary glioblastoma | 3 | 2 | ND | 67 |
| Ganglioglioma | 8 | 3 | 0 | 38 |
| Anaplastic ganglioglioma | 5 | 3 | 0 | 60 |
Mut (%), percentage of tumors with mutation; ND, not done.
Figure 1.
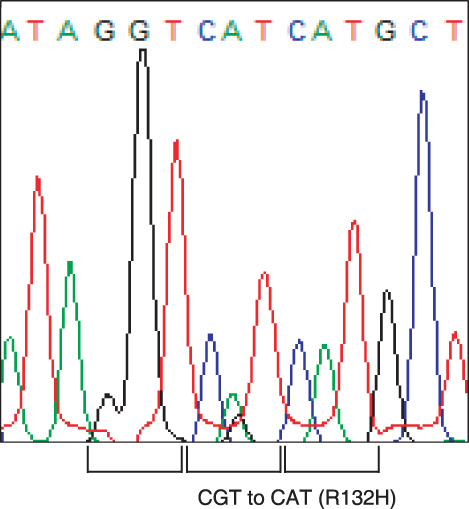
Mutations of the isocitrate dehydrogenase 1 (IDH1) gene in ganglioglioma. Somatic mutations of base pair changes from CGT (Arg) to CAT (His) were detected.
Patients with GBM and IDH1 mutation consisted of three men and two women; their median age at diagnosis was 35 years (range 24–51 years) and those with wild‐type IDH1 consisted of 32 men and 24 women; their median age at diagnosis was 57 years (range 22–76 years). There was a significant difference in the median age (35 vs 57 years, P = 0.0158; Mann–Whitney test). This confirms previous observations.( 6 ) The average ages in patients with DA, O, AO, AOA, AA, GG, and AG did not differ significantly in the groups with and without IDH mutations.
The frequency of mutations in GG (38%) and AG (60%) is noteworthy, although the numbers of tumors included in the present study is too low for a clear conclusion. However, this is the first report to detect IDH1 mutation in gangliogliomas. Cases of gangliogliomas were re‐reviewed by a second pathologist (H.S.) to confirm the diagnosis. Although genetic alterations of this tumor have not been fully characterized, our data suggest that IDH1 mutation is involved in early tumor development of some gangliogliomas.
Patients’ survival and IDH mutation. Patients with a GBM carrying an IDH1 mutation had a median survival of 66 months, which was longer than the 17‐month survival in patients with wild‐type IDH1 (Fig. 2a). However, there was no significant difference because there were too few tumors of GBM with IDH1 mutation (P = 0.1; log‐rank test). Mutations of the IDH1 and IDH2 genes were associated with prolonged overall survival in AA patients; the median overall survival was 50 months for patients with mutation and 22 months for those without mutation (P < 0.001; log‐rank test) (Fig. 2b). These observations are similar to those in a previous report.( 6 ) Differential survival analyses could not be done in patients with DA, O, AO, AOA, GG, or AG because there were too few cases.
Figure 2.
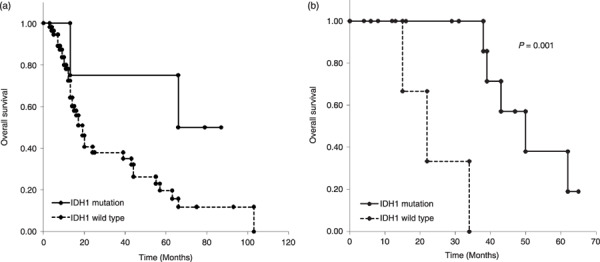
Survival of patients with malignant gliomas with or without isocitrate dehydrogenase 1 (IDH1) gene mutation. (a) Glioblastoma patients: patients with both primary and secondary glioblastomas were included in this analysis. For patients with secondary glioblastomas, survival was calculated from the date of the secondary diagnosis. (b) Anaplastic astrocytoma patients.
Although the biological function of the IDH1 mutation is not fully understood, a recent report showed that IDH1 mutation impairs the enzyme's affinity for its substrate and dominantly inhibits wild‐type IDH1 activity through the formation of catalytically inactive heterodimers.( 15 ) They concluded that IDH1 appears to be a tumor suppressor, and its mutation contributes to tumorigenesis through induction of the Hypoxia‐inducible factor (HIF)‐1 pathway.( 15 )
In summary, mutations of the IDH1 gene were frequently found in several types of grade II and III gliomas, including gangliogliomas, suggesting that IDH1 mutations are very early events in gliomagenesis and may affect common glial precursors. The IDH2 gene mutation was found in only one AA. The infrequency of IDH1 mutation in primary GBM revealed that these subtypes are genetically distinct entities from other glial tumors.
Disclosure Statement
All authors have no conflict and interest to disclose.
Acknowledgments
This work was supported in part by Grants‐in‐Aid for Cancer Research from the Ministry of Health and Welfare in Japan to T.T. We thank Nippon Gene Research Laboratories for direct sequencing analysis.
References
- 1. Kleihues P, Cavenee WK. Pathology and genetics of tumours of the nervous system. In: Kleihues P, Cavenee WK, eds. World Health Organization Classification of Tumours. Lyon: IARC, 2000. [Google Scholar]
- 2. Ohgaki H, Dessen P, Jourde B et al . Genetic pathways to glioblastoma: a population‐based study. Cancer Res 2004; 64: 6892–9. [DOI] [PubMed] [Google Scholar]
- 3. Parsons DW, Jones S, Zhang X et al . An integrated genomic analysis of human glioblastoma multiforme. Science 2008; 321: 1807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koshland DE Jr, Walsh K, LaPorte DC. Sensitivity of metabolic fluxes to covalent control. Curr Top Cell Regul 1985; 27: 13–22. [DOI] [PubMed] [Google Scholar]
- 5. Xu X, Zhao J, Xu Z et al . Structures of human cytosolic NADP‐dependent isocitrate dehydrogenase reveal a novel self‐regulatory mechanism of activity. J Biol Chem 2004; 279: 33 946–57. [DOI] [PubMed] [Google Scholar]
- 6. Yan H, Parsons DW, Jin G et al . IDH1 and IDH2 mutations in gliomas. N Engl J Med 2009; 360: 765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, Von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol 2008; 116: 597–602. [DOI] [PubMed] [Google Scholar]
- 8. Ichimura K, Pearson DM, Kocialkowski S et al . IDH1 mutations are present in the majority of common adult gliomas but are rare in primary glioblastomas. Neuro Oncol 2009. doi 10.1215/15228517-2009-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol 2009; 174: 1149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bleeker FE, Lamba S, Leenstra S et al . IDH1 mutations at residue p.R132 (IDH1) (R132) occur frequently in high‐grade gliomas but not in other solid tumors. Hum Mutat 2009; 30: 7–11. [DOI] [PubMed] [Google Scholar]
- 11. Holdhoff M, Parsons DW, Diaz LA Jr. Mutations of IDH1 and IDH2 are not detected in brain metastases of colorectal cancer. J Neurooncol 2009. doi 10.1007/s11060-009-9855-y. [DOI] [PubMed] [Google Scholar]
- 12. Kang MR, Kim MS, Oh JE et al . Mutational analysis of IDH1 codon 132 in glioblastomas and other common cancers. Int J Cancer 2009; 125: 353–5. [DOI] [PubMed] [Google Scholar]
- 13. Scherer HJ. Cerebral astrocytomas and their derivatives. Am J Cancer 1940; 40: 159–98. [Google Scholar]
- 14. Sonoda Y, Kumabe T, Watanabe M et al . Long‐term survivors of glioblastoma: clinical features and molecular analysis. Acta Neurol 2009. doi 10.1007/s00701-009-0387-1. [DOI] [PubMed] [Google Scholar]
- 15. Zhao S, Lin Y, Xu W et al . Glioma‐derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF‐1alpha. Science 2009; 324: 261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


