Abstract
The chemotactic cytokines called chemokines are a superfamily of small secreted cytokines that were initially characterized through their ability to prompt the migration of leukocytes. Attention has been focused on the chemokine receptors expressed on cancer cells because cancer cell migration and metastasis show similarities to leukocyte trafficking. CXC chemokine receptor 4 (CXCR4) was first investigated as a chemokine receptor that is associated with lung metastasis of breast cancers. Recently, CXCR4 was reported to be a key molecule in the formation of peritoneal carcinomatosis in gastric cancer. In the present review, we highlight current knowledge about the role of CXCR4 in cancer metastases. In contrast to chemokine receptors expressed on cancer cells, little is known about the roles of cancer cell‐derived chemokines. Cancer tissue consists of both cancer cells and various stromal cells, and leukocytes that infiltrate into cancer are of particular importance in cancer progression. Although colorectal cancer invasion is regulated by the chemokine CCL9‐induced infiltration of immature myeloid cells into cancer, high‐level expression of cancer cell‐derived chemokine CXCL16 increases infiltrating CD8+ and CD4+ T cells into cancer tissues, and correlates with a good prognosis. We discuss the conflicting biological effects of cancer cell‐derived chemokines on cancer progression, using CCL9 and CXCL16 as examples. (Cancer Sci 2007; 98: 1652–1658)
Chemokines are a family of small (8–14 kDa), mostly basic, heparin‐binding cytokines that primarily induce directed migration of various types of leukocytes through interactions with a group of seven transmembrane G protein‐coupled receptors (GPCR). GPCR mediate biological effects such as cell migration. That is, normal rapid leukocyte trafficking is controlled strictly by chemokines and their receptors.( 1 , 2 ) To date, over 50 chemokines and 20 chemokine receptors have been identified, and are grouped into four categories (C, CC, CXC, and CX3C) based on the location of the main cysteine residues near the N termini of these proteins.
Leukocyte trafficking and, to a lesser degree, cancer metastasis have regular rules called organ selectivity. The cancer metastatic process can be divided into several migration steps. First, cancer cells are released from the primary cancer to the surrounding tissues, enter the vascular or lymphatic circulation, and are transported through it. Then, the cells become arrested in the capillary bed of a distant organ and extravasate from the circulation to organ parenchyma. However, although cancer migration from the primary site to distant organs is essential to establish metastasis, we know very little about the molecular mechanisms that regulate cancer cell migration.
It is now thought that chemokines play a significant role in organ‐selective cancer metastasis, because cancer cell migration and metastasis share many similarities with leukocyte trafficking.( 3 , 4 ) Several chemokine receptors are regarded as molecules related to cancer metastasis.( 5 , 6 , 7 , 8 , 9 )
In the present review, we will discuss two topics in solid cancer metastasis and progression (Fig. 1). CXC chemokine receptor 4 (CXCR4) is the most common chemokine receptor that has been demonstrated to be overexpressed in human cancers. More than 23 different human malignancies, including breast cancer, ovarian cancer, melanoma, and prostate cancer, express CXCR4.( 10 ) The focus of the first part of this review is the biology of chemokine receptors (Fig. 1a), especially CXCR4, which is expressed on cancer cells, and the therapeutic strategies against CXCR4 in cancer metastasis.
Figure 1.
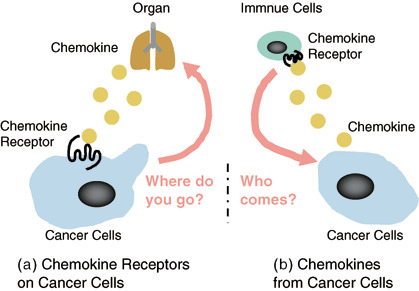
Schematic illustrate of this review. (a) Chemokine receptors expressed on cancer cells in cancer metastasis. (b) Chemokines derived from cancer cells in cancer progression.
Although many findings from both basic and clinical studies have demonstrated an association between chemokine receptors and cancer metastasis, relatively little is known about the role of chemokines secreted directly by cancer cells. Cancer tissue is composed of not only cancer cells but also cancer‐associated stromal cells.( 11 ) Chemokines are secreted, constitutively or inducibly, from various tissues under normal and pathological conditions. In the second part of this review we will therefore discuss which chemokines derived from cancer cells are the main driving force of the infiltration by various cells, especially lymphocytes, into cancer tissues and what potential these cancer cell‐derived chemokines may have for regulating the progression of cancer (Fig. 1b).
Chemokine receptors expressed on cancer cells in cancer metastasis
CXCR4 and its ligand CXCL12. Hematopoietic cells proliferate and differentiate into mature hematopoietic cells in the parenchyma of bone marrow. CXCL12 (also called stromal‐derived factor‐1α) was originally cloned from a murine bone marrow stromal cell line and was identified as a stimulating factor of pre‐B‐cell growth. Constitutive production of CXCL12 from marrow stromal cells is a major source of this protein and CXCL12 is a highly efficient chemoattractant for lymphocytes, monocytes, and CD34+ hematopoietic precursor cells expressing its receptor, CXCR4.( 1 , 2 ) CXCR4 has received much attention because it is regarded as a co‐receptor for infection with T‐tropic (X4) HIV virus.( 12 ) Moreover, the critical roles of CXCR4 in the development of embryos has been revealed by gene knockout studies. The CXCR4 −/– phenotype is lethal in mice owing to defects of cardiac, central nervous system, and hematopoietic stem‐cell homing.( 13 )
CXCR4 in cancer metastasis and poor prognosis. Muller et al. reported the landmark finding that the chemokine receptors CXCR4 and CC chemokine receptor (CCR) 7 are highly expressed in human breast cancer cells, and their respective ligands CXCL12 and CCL21 (also called secondary lymphoid tissue chemokine) play critical roles in determining the metastatic destination of breast cancer.( 5 )
Among all chemokine receptors, CXCR4 is of particular importance in the metastatic behavior and destination of solid cancers.( 5 , 8 , 9 ) Table 1 summarizes the correlations between CXCR4 expression and metastatic behavior in various types of solid cancer cells.( 14 , 15 ) CXCR4 is expressed on various types of cancer cells: breast,( 5 , 16 ) prostate,( 17 ) lung,( 18 ) ovarian,( 19 , 20 ) pancreatic,( 21 ) melanoma,( 22 ) neuroblastoma,( 23 ) esophageal,( 24 ) colorectal,( 25 ) osteosarcoma,( 26 ) and renal.( 27 ) The CXCL12–CXCR4 axis is implicated in the bone metastasis of prostate cancer( 17 ) and non‐small‐cell lung cancer cells, particularly in their dissemination into the pleural space.( 18 ) In ovarian cancer, melanoma and neuroblastoma, CXCL12 directs not only cell migration but also multiple other biological functions, such as the induction of cell adhesion, matrix metalloproteinases, and antiapoptosis.( 19 , 20 ) However, there had been no reports about the role of the CXCR4 in metastatic behaviors of gastric cancer.( 8 )
Table 1.
Expression of CXC chemokine receptor 4 in cancer metastasis
| Cancer cell type | Site of metastasis | Reference no. |
|---|---|---|
| Breast | Lung, lymph node | 5, 16 |
| Prostate | Bone | 17 |
| Non‐small‐cell lung cancer | Pleural space | 18 |
| Ovarian | Peritoneum | 19, 20 |
| Pancreas | Liver, lung | 21 |
| Melanoma | Lymph node | 22 |
| Neuroblastoma | Bone, bone marrow | 23 |
| Esophageal | Lymph nodes, bone marrow | 24 |
| Colorectal | Liver | 25 |
| Osteosarcoma | Lung | 26 |
| Renal | Adrenal glands, bone | 27 |
| Gastric | Peritoneum | 8 |
Death of patients with advanced gastric carcinoma is frequently caused by peritoneal carcinomatosis, often associated with malignant ascites,( 28 , 29 , 30 ) and no reliable effective treatment is available for this condition. The 5‐year survival rate of patients with peritoneal carcinomatosis is only 2%, even in patients with intraperitoneal cancer cells without macroscopic peritoneal carcinomatosis.( 31 ) To design a new and effective treatment for peritoneal carcinomatosis, it is important to understand the molecular mechanisms that promote the development of this condition.
We recently reported the roles of CXCR4 in peritoneal carcinomatosis of gastric cancer.( 8 ) In a screen of chemokine receptors in human gastric carcinoma cell lines, characteristic expression patterns were found: cells derived from malignant ascites and pleural effusion selectively expressed CXCR4 mRNA, especially NUGC4 cells, which were established from malignant ascites. To clarify the potency of CXCR4 for the promotion of peritoneal carcinomatosis by gastric carcinoma, we extended our investigations to human clinical samples. In peritoneal carcinomatosis, CXCL12 was strongly expressed on peritoneal mesothelial cells, and a higher level of CXCL12 was detected in malignant ascites fluid of patients with peritoneal carcinomatosis of gastric cancer than in normal fluids in the peritoneal cavity. Most importantly, CXCR4 expression in primary tumors of patients with advanced gastric carcinomas was significantly correlated with the occurrence of peritoneal carcinomatosis. In conclusion, our results suggest that the expression of CXCR4 in biopsy specimens from primary gastric tumors may be useful for preoperative evaluation of risks for the occurrence of peritoneal carcinomatosis. Evaluation of CXCL12 levels in normal intraoperative fluids of the abdominal cavity in patients with advanced gastric carcinomas may also be useful as a predictive molecular marker for the risk of peritoneal carcinomatosis.
Moreover, high‐level expression of CXCR4 is a predictor of poor prognosis in lung cancer,( 32 ) melanoma,( 22 ) pancreatic,( 33 ) ovarian,( 34 ) colorectal,( 25 ) and breast cancer.( 5 , 16 , 35 ) CXCR4 expression is observed in approximately 75% of biopsy specimens of invasive ductal carcinoma,( 17 ) and high‐level expression of CXCR4 is correlated with decreased overall survival of patients in breast cancer.( 35 ) In prostate cancer, approximately 80% of patients with untreated cancer respond to androgen withdrawal therapy;( 36 ) however, disease recurrence occurs frequently after progression to a hormone‐refractory status, in which androgen‐independent growth of the cancer is observed.
We recently reported that the human prostate cancer cell line DU‐145 specifically expresses CXCR4 at high levels compared with DU‐145/AR (DU‐145 cells expressing androgen receptor [AR]). DU‐145 showed vigorous migratory responses to CXCL12. In contrast, CXCL12 did not affect the migration of DU‐145/AR cells. These results indicate that expression of AR may downregulate the migratory responses of human prostate cancer cells via chemokines and their receptor systems.( 9 ) Furthermore, CXCR4 expression in prostate cancer patients treated with androgen withdrawal therapy was investigated by immunohistochemical staining. CXCR4 was detected in most of the biopsy specimens from patients with metastatic prostate cancer. Patients with strong expression of CXCR4 in tumors had poorer cause‐specific survival than those with weak expression of CXCR4 (Akashi and Koizumi et al. unpublished data, 2007).
CXCR4 as an attractive target for cancer metastasis therapy. The expression and function of CXCR4 expressed on normal and malignant cells are induced by hypoxia inducible factor (HIF) under hypoxic conditions.( 37 ) In addition, high expression of CXCR4 in biopsy specimens from various primary cancers is significantly associated with poor prognosis and the extent of metastasis.( 35 ) Targeting CXCR4, therefore, is now regarded as a novel and efficient strategy for treating human cancer metastases. The therapeutic strategies are mainly classified into two categories: the application of anti‐CXCR4 monoclonal antibody and of specific low‐molecular weight antagonists for CXCR4.
As mentioned above, it was first reported that lung metastasis of human breast cancer is suppressed by the administration of antihuman CXCR4 monoclonal antibody in an SCID mice model.( 5 ) The antibody also inhibits lung metastasis of murine B16 melanoma( 38 ) and bone metastasis of human prostate cancer in a murine model.( 39 )
In addition to anti‐CXCR4 monoclonal antibody, antagonists of CXCR4 are thought to be a promising therapeutic approach for cancer metastasis. The targets for approximately 50% of the current drugs based on this approach are GPCR, and new drugs targeting GPCR continue to be discovered and developed as novel therapeutic targets in several diseases.
AMD3100 (Bicyclam) is very effective against HIV‐1 and HIV‐2 based on its inhibition of virus replication. AMD3100 is the most potent and selective CXCR4 antagonist ever discovered.( 40 ) Administration of AMD3100 suppressed the growth of glioblastoma cells transplanted intracranially into mice and also increased apoptosis of the cells. In addition, the migration and invasion of bladder cancer cells was inhibited by a CXCR4 antagonist (4F‐benzoyl‐TE14011) in vitro,( 41 ) and a single injection of this antagonist significantly reduced the number of pulmonary colonies in an experimental melanoma metastasis model.( 42 ) Another CXCR4 antagonist, TN14003, inhibited the migration and invasion of pancreatic cancer cell lines in vitro ( 43 ) and suppressed breast cancer metastasis in a murine xenograft model.( 44 )
Given the effective suppression of cancer metastasis by specific CXCR4 antagonists in previous reports, we attempted to apply AMD3100 in a xenograft model of NUGC4 cells in nude mice.( 8 ) Interestingly, in AMD3100‐treated mice compared with control mice, marked reduction of the ascitic fluid and inhibition of the growth of disseminated tumors were also observed (Fig. 2). Therefore, CXCR4 antagonists may be useful for the treatment of peritoneal carcinomatosis, an incurable complication of gastric cancers, and especially beneficial for patients with intraperitoneal free cancer cells without macroscopic peritoneal metastasis (Fig. 3).
Figure 2.
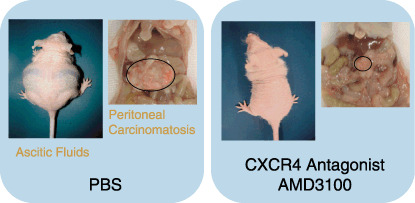
Marked inhibitory effect of the CXC chemokine receptor 4 (CXCR4) antagonist AMD3100 on experimental peritoneal carcinomatosis in gastric cancer. Photographs show representative results of phosphate‐buffered saline (PBS)‐treated mice and AMD3100‐treated mice 40 days after intraperitoneal inoculation of CXCR4‐expressing NUGC4 cells. Treatment with PBS or AMD3100 was carried out daily starting from the day of tumor inoculation. Ascites fluids and omental tumors in the abdominal cavity are shown.
Figure 3.
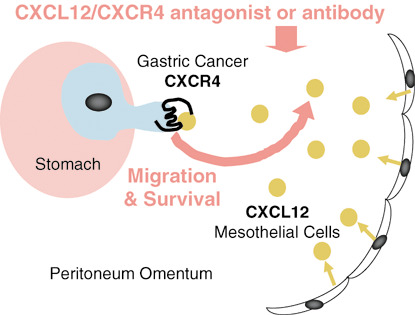
The CXCL12–CXC chemokine receptor 4 (CXCR4) axis plays a pivotal role in peritoneal carcinomatosis of gastric cancer and novel therapeutic strategies targeting CXCR4 for peritoneal carcinomatosis of gastric cancer. The molecular mechanisms that promote the development of peritoneal carcinomatosis. CXCR4‐expressing gastric carcinoma cells are preferentially attracted to the peritoneum cavity where their ligand CXCL12 is produced abundantly. CXCL12 produced by peritoneal mesothelial cells acts on proliferation and survival of CXCR4‐expressing gastric carcinoma in the peritoneal cavity in a paracrine manner. CXCR4 may be a potential therapeutic target for peritoneal carcinomatosis of gastric carcinoma and especially beneficial for patients with i.p.‐free cancer cells without macroscopic peritoneal metastasis.
For future studies on the development of novel combination therapy, including CXCR4 inhibitors for cancer metastasis, it is worthwhile discussing the role of the cooperation between CXCR4 and human epidermal receptor (HER) 2 in the lung metastasis of breast cancers.( 35 ) The role of HER2 in homing to metastatic organs remains unknown, although HER2 enhances cancer metastasis. Li et al. reported that HER2 induced the CXCR4 expression required for the lung metastasis of breast cancer in a mouse model.( 35 ) Moreover, a significant correlation between HER2 and CXCR4 expression was observed and CXCR4 expression was also associated with poor patient overall survival in human breast cancer.
The combination of Trastuzumab (Herceptin) with a taxane is now first‐line therapy used as the standard of care for patients with HER2‐positive metastatic breast cancer. More effective Trastuzumab‐based therapies are now being developed. Novel combinations of Trastuzumab with chemotherapeutic agents such as vinorelbine and gemcitabine are currently under investigation in clinical trials.( 45 ) As anti‐CXCR4 monoclonal antibody and CXCR4 antagonists are attractive therapeutic candidates, these antagonists will be applied as novel combination partners with Trastuzumab in breast cancer.
Chemokines derived from cancer cells in cancer progression
Regulation of leukocyte migration by chemokines secreted from cancer cells. Although cancer tissue consists of various stromal cells, such as leukocytes, fibroblasts, and endothelial cells, we know little about the driving forces for the migration and infiltration of cells into cancer tissue. As with endothelial cells, vascular endothelial growth factor secreted from cancer cells is widely known to be a cytokine that acts as a driving force.( 46 )
However, chemotactic cytokines, chemokines, are representative driving forces of leukocytes in the inflammatory process,( 1 , 2 ) leading us to ask whether or which chemokines secreted from cancer cells are specifically correlated with cancer progression via infiltration of leukocytes into the cancer tissue. Macrophages, lymphocytes, and natural killer cells are the predominant cell types of the immune cells in cancer tissue. In addition, eosinophils, granulocytes, and B cells are present as minor immune cells in some cancers.( 47 )
Leukocyte accumulation in cancer tissue directed by cancer cell‐derived chemokines plays a crucial role in cancer progression and metastasis as chemokine expression has been detected in sarcomas, gliomas, melanomas, and cancers of the breast, lung, esophagus, ovary, and cervix. Cancer cell‐derived chemokines are responsible for the migration and infiltration of various types of leukocytes, including mainly macrophages (tumor‐associated macrophages [TAM]).( 14 )
CCL5 (also called regulation on activation, normal T cell expressed and secreted [RANTES]) and CCL2 (also called monocyte chemoattractant protein [MCP]‐1) are observed commonly in various types of cancers. In breast cancer, lower CCL2 expression correlated with longer relapse‐free survival and decreased TAM,( 48 ) and higher CCL5 expression was associated with an increase in TAM and lymph‐node metastasis.( 49 ) The level of TAM infiltration and invasion was positively correlated with CCL2 expression in esophageal carcinoma.( 50 ) In addition, CX3CL1 (also called Fractalkine) was produced by colorectal cancer, and in contrast to CCL2, CCL5, and TAM, high expression of CX3CL1 was positively correlated with good prognosis and the number of tumor‐infiltrating lymphocytes in colorectal cancer patients.( 51 )
CCL9 and infiltrating immature myeloid cells in colorectal cancer. The adenoma–carcinoma sequence proposed by Fearon and Vogelstein is the most famous principle of colorectal carcinogenesis.( 52 ) The initial genetic change in the development of most colorectal adenoma is thought to be at the adenomatous polyposis (APC) gene locus, and the molecular events associated with these stages are clear: a second hit in the APC gene is sufficient to cause microadenoma development, at least in familial APC.( 53 ) The synergistic genetic mutation of APC and inactivation of transforming growth factor (TGF)‐β family signaling, especially via Sma and MAD‐related protein (SMAD)4, brings about colorectal cancer progression from adenoma.
Kitamura et al. have clearly shown the induction of the progression of adenoma to colorectal cancer by cancer cell‐derived chemokine CCL9 (also called MIP‐1γ) using their excellent technique for construction of transgenic mice.( 54 ) APC single‐mutant mice (Apc +/Δ716) develop only microadenoma;( 55 ) in contrast, cis‐Apc +/Δ716 Smad4+/– mutant mice (cis‐Apc/Smad4) develop intestinal adenocarcinomas with the clinical features of marked invasion and stromal expansion. Interestingly, immature myeloid cells (iMC) expressing CCR1 recruited from the bone marrow were observed in the cancer‐invasion front in cis‐Apc/Smad4 mice. In addition, an increase in the expression of the CCR1 ligand CCL9 was induced by the inactivation of TGF‐β family signaling. To elucidate the role of CCR1‐positive iMC in the cancer‐invasion front, a homozygous CCR1 knockout mutation was made in cis‐Apc/ΔSmad4 mice to generate triple‐mutant mice (cis‐Apc/Smad4 Ccr1 −/–). The lack of CCR1 inhibited the accumulation of CD34+ iMC at the invasion front and, most importantly, prevented cancer invasion.
It may be important to focus on the chemokine receptor CCR1 for infiltrating leukocytes in cancer tissue. CCR1 is shared by several chemokines: CCL3 (also called MIP‐1a), CCL5, CCL7 (also called MCP‐3), and also CCL9. Yang et al. have recently shown the contribution of the CCR1–CCL3 axis to malignant progression of hepatocellular carcinoma.( 56 ) To investigate the roles of CCL3 and CCR1 in hepatocarcinogenesis, CCL3‐ or CCR1‐deficient mice were treated with N‐nitrosodiethylamine (DEN), a known inducer of hepatocellular carcinoma. After DEN treatment, the number of intratumoral Kupffer cells, a rich source of growth factors and matrix metalloproteinases, was decreased markedly in CCR1‐ and CCL3‐deficient mice. Most importantly, foci number and sizes were remarkably reduced and cancer angiogenesis was also markedly diminished in CCR1‐ and CCL3‐deficient mice treated with DEN.
CXCL16 and tumor‐infiltrating lymphocytes in colorectal cancer. Analyses of colorectal cancer specimens by immunohistochemistry have amply demonstrated that higher levels of tumor‐infiltrating lymphocytes can be regarded as a favorable prognostic sign.( 57 ) In particular, tumor‐infiltrating CD8+ T cells are a good prognostic predictor in human colorectal cancer.( 58 )
CXCL16 (also called SR‐PSOX) is a unique CXC chemokine that exists in both a transmembrane form and a soluble form.( 59 ) CXCL16 has been shown to possess multiple biological activities. Soluble CXCL16 induces chemotactic migration of cells expressing its receptor, CXCR6,( 60 ) including CD8+ T cells, CD4+ T cells and natural killer T (NKT) cells,( 61 , 62 , 63 ) whereas cell surface‐anchored CXCL16 can function as a cell‐adhesion molecule for CXCR6‐expressing cells and also as a scavenger receptor for phosphatidylserine and oxidized lipoprotein.( 64 ) Given that the CXCL16 receptor CXCR6 is detected on cytotoxic effector cells with anticancer activity such as CD4+ T cells, CD8+ T cells, and NKT cells cells, high‐level expression of CXCL16 by cancer cells may attract these types of immune cells to colorectal cancer.
We categorized colorectal cancer cases into those with strong expression of CXCL16 and those with weak expression of CXCL16.( 65 ) As shown in Fig. 4a, the numbers of CD8+ T cells and CD4+ T cells were significantly increased in the group with strong CXCL16 expression compared with the weak CXCL16 expression group. The clinicopathological characteristics of colorectal cancer patients were independent of the level of CXCL16 expression. However, the strong CXCL16 expression group had a significantly better prognosis than the weak CXCL16 expression group (Fig. 4b). Interestingly, CXCL16 expression is strongly upregulated in colorectal cancer cells compared to normal colon epithelium in the majority of colorectal cancer cases. In addition, adenoma also expresses CXCL16.
Figure 4.
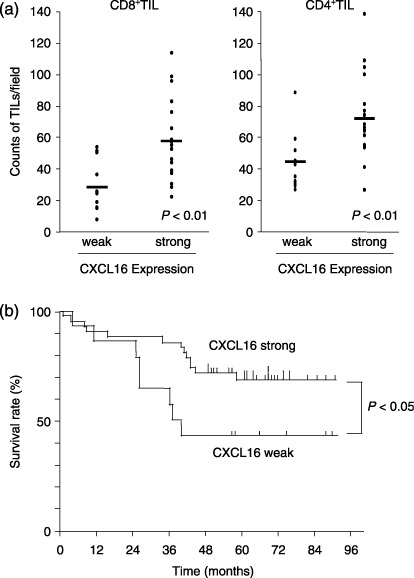
High‐level expression of cancer cell‐derived CXCL16 correlates with increased tumor‐infiltrating lymphocytes and a good prognosis in colorectal cancer. (a) Tumor‐infiltrating lymphocyte (TIL) counts. CD8+ and CD4+ cells were counted in five randomly selected areas at the tumor border for each case. The average TIL counts were plotted and compared between weak and strong CXCL16 expression groups. The number of CD8+ and CD4+ cells was significantly increased in the strong group compared with the weak group. (b) Kaplan–Meier survival curves of 58 colorectal cancer patients. The strong CXCL16 expression group had significantly longer survival than the weak CXCL16 expression group (Log‐rank, P = 0.041).
The transition from adenoma to carcinoma in carcinogenesis for colorectal cancer is induced by multiple genetic alternations that promote the growth of a clonal population of cells. In apparent contrast to the observation of CXCL16 expression in colon adenoma, the adenoma in Apc+/Δ716 mice does not express CCL9, and few CCR1‐positive immature myeloid cells are accumulated at the adenoma. These results suggest that the expression of CXCL16 in colorectal cancer is induced by genetic alternations that occur early in the process of the adenoma–carcinoma sequence, at the stage when normal colon epithelium changes to adenoma, compared to the later induction of CCL9 in colorectal carcinogenesis.
It remains to be seen why colorectal cancer upregulates CXCL16 production during the sequence of carcinogenesis. Upregulation of CXCL16 by colorectal cancer cells could be potentially disadvantageous for their growth. In fact, like the chemokines CCL2, CCL5, and CCL9, several cytokines produced by cancer cells are known to have immunosuppressive potency. TGF‐β produced by cancer cells typically affects CD4 Th subsets, and cancer growth progresses in the immunosuppressed state induced by TGF‐β.( 66 )
Our findings thus imply a novel role for CXCL16 in colorectal cancer progression, namely, upregulation of CXCL16 expression by colorectal cancer cells may represent a self‐check mechanism against cancer progression through a chemokine expressed by cancer cells (Fig. 5).
Figure 5.
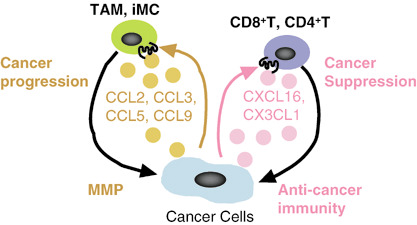
Cancer cell‐derived chemokines: friends or enemies? Cancer cell‐derived chemokines have conflicting aspects: positive or negative regulation of cancer progression. Immature myeloid cells (iMC) are recruited by cancer cell‐derived CCL9, and tumor‐associated macrophages (TAM) by CCL2, CCL3, and CCL5. Consequently, CCL9, CCL2, CCL3, and CCL5 induce the invasion of cancer cell via abundant matrix metalloproteinases produced by infiltrating iMC and TAM in cancer tissue. In contrast, CXCL16 and CX3CL1 inhibit cancer progression. CXCL16 and CX3CL1 guide tumor‐infiltrating lymphocytes (TIL), especially, CD8+ and CD4+ T cells, into cancer tissue. A fraction of TIL attracted by CXCL16 may be directed to cancer cells, leading to a certain degree of anticancer immunity.
Conclusion
Since the time when novel aspects of ‘chemokine receptors and cancer metastasis’ were first discussed by Muller et al.,( 5 ) chemokine receptors expressed on cancer cells have been discovered to play key roles in cancer metastasis (Fig. 1a). Many reports on this subject have been published. Some merely reported the effects of chemokines on cancer cells in vitro; some achieved good therapeutic results using antagonist or neutralizing antibodies in cancer metastasis models in mice in vivo; and moreover, some immunohistochemical analyses of surgical specimens of cancer patients showed a good connection between the in vivo findings above and clinicopathological data. Today, based on analysis of many chemokine receptors, we can be fairly certain that CXCR4 remains an attractive candidate for cancer metastasis therapy. We therefore approach a turning point from ‘the elucidation of biological functions of chemokine receptors in cancer metastasis’ to ‘the development of new therapeutic strategies based on chemokine receptors, especially CXCR4, for cancer metastasis in patients’ (Fig. 3).
As shown in Fig. 1b, we have also discussed the conflicting biological effects of infiltrating leukocytes induced by cancer cell‐derived chemokines, such as CCL9, CCL3, and CXCL16, on cancer progression. The question of which cancer cell‐derived chemokines are friends and which are enemies remains unanswered (Fig. 5). Cancer cell‐derived chemokines increase the infiltrating immune cells in a particular cancer type, and promote or suppress cancer progression according to the potency of the infiltrating cells.
A number of studies have examined the inhibitory effects of chemokines on cancer growth. As a result, the expression of specific chemokines at the tumor site may attract T cells, NK cells, and dendritic cells bearing relevant chemokine receptors, which may lead to the induction of anticancer immunity. Significant cancer‐suppressive activity was reported for chemokines such as CCL3, CCL21 (also called SLC), CCL27 (also called ILC and CTACK), and CX3CL1 by transducing their genes into a variety of experimental tumors.( 67 , 68 , 69 , 70 , 71 ) The accumulation of these findings may provide additional clues regarding our unsettled questions. In future studies, the significance of immune recognition in the pathogenesis of cancers affected by cancer cell‐derived chemokines will be the subject of intense investigation.
Acknowledgments
We thank Dr Takashi Nakayama and Dr Osamu Yoshie (Department of Microbiology and SORST, Kinki University School of Medicine, Osaka Japan) and Dr Koichi Tsuneyama (Department of Pathology I, Faculty of Medicine, 21st Century COE program, University of Toyama) for helpful advice and for technical support. This study was supported in part by Grant‐in‐Aid for Young Scientists B (nos 15790089 and 18790114), Grant‐in‐Aid for Cancer Research (nos 16022224 and 16023225) and Grant‐in‐Aid for the 21st Century COE Program and for Cooperative Link of Unique Science and Technology for Economy Revitalization from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
References
- 1. Yoshie O, Imai T, Nomiyama H. Novel lymphocyte‐specific CC chemokines and their receptors. J Leukoc Biol 1997; 62: 634–44. [DOI] [PubMed] [Google Scholar]
- 2. Yoshie O. Immune chemokines and their receptors: the key elements in the genesis, homeostasis and function of the immune system. Springer Semin Immunopathol 2000; 22: 371–91. [DOI] [PubMed] [Google Scholar]
- 3. Zlotnik A. Chemokines and cancer. Int J Cancer 2006; 119: 2026–9. [DOI] [PubMed] [Google Scholar]
- 4. Tanaka T, Bai Z, Srinoulprasert Y, Yang BG, Hayasaka H, Miyasaka M. Chemokines in tumor progression and metastasis. Cancer Sci 2005; 96: 317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muller A, Homey B, Soto H et al . Involvement of chemokine receptors in breast cancer metastasis. Nature 2001; 410: 50–6. [DOI] [PubMed] [Google Scholar]
- 6. Koizumi K, Kozawa Y, Ohashi Y et al . CCL21 promotes the migration and adhesion of highly lymph node metastatic human non‐small cell lung cancer Lu‐99 in vitro . Oncol Rep 2007; 17: 1511–16. [PubMed] [Google Scholar]
- 7. Nakamura ES, Koizumi K, Kobayashi M et al . RANKL‐induced CCL22/macrophage‐derived chemokine produced from osteoclasts potentially promotes the bone metastasis of lung cancer expressing its receptor CCR4. Clin Exp Metastasis 2006; 23: 9–18. [DOI] [PubMed] [Google Scholar]
- 8. Yasumoto K, Koizumi K, Kawashima A et al . Role of the CXCL12/CXCR4 axis in peritoneal carcinomatosis of gastric cancer. Cancer Res 2006; 66: 2181–7. [DOI] [PubMed] [Google Scholar]
- 9. Akashi T, Koizumi K, Nagakawa O, Fuse H, Saiki I. Androgen receptor negatively influences the expression of chemokine receptors (CXCR4, CCR1) and ligand‐mediated migration in prostate cancer DU‐145. Oncol Rep 2006; 16: 831–6. [PubMed] [Google Scholar]
- 10. Murakami T, Cardones AR, Hwang ST. Chemokine receptors and melanoma metastasis. J Dermatol Sci 2004; 36: 71–8. [DOI] [PubMed] [Google Scholar]
- 11. Zalatnai A. Molecular aspects of stromal–parenchymal interactions in malignant neoplasms. Curr Mol Med 2006; 6: 685–93. [DOI] [PubMed] [Google Scholar]
- 12. Wegner SA, Ehrenberg PK, Chang G, Dayhoff DE, Sleeker AL, Michael NL. Genomic organization and functional characterization of the chemokine receptor CXCR4, a major entry co‐receptor for human immunodeficiency virus type 1. J Biol Chem 1998; 273: 4754–60. [DOI] [PubMed] [Google Scholar]
- 13. Nagasawa T, Hirota S, Tachibana K et al . Defects of B‐cell lymphopoiesis and bone marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF‐1. Nature 1996; 382: 635–8. [DOI] [PubMed] [Google Scholar]
- 14. Slettenaar VI, Wilson JL. The chemokine network: a target in cancer biology? Adv Drug Deliv Rev 2006; 58: 962–74. [DOI] [PubMed] [Google Scholar]
- 15. Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol 2004; 14: 171–9. [DOI] [PubMed] [Google Scholar]
- 16. Kato M, Kitayama J, Kazama S, Nagawa H. Expression pattern of CXC chemokine receptor‐4 is correlated with lymph node metastasis in human invasive ductal carcinoma. Breast Cancer Res 2003; 5: 144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell‐derived factor‐1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res, 2002; 62: 1832–7. [PubMed] [Google Scholar]
- 18. Oonakahara K, Matsuyama W, Higashimoto I et al . Stromal‐derived factor‐1α/CXCL12–CXCR4 axis is involved in the dissemination of NSCLC cells into pleural space. Am J Respir Cell Mol Biol 2004; 30: 671–7. [DOI] [PubMed] [Google Scholar]
- 19. Scotton CJ, Wilson JL, Scott K et al . Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res 2002; 62: 5930–8. [PubMed] [Google Scholar]
- 20. Scotton CJ, Wilson JL, Milliken D, Stamp G, Balkwill FR. Epithelial cancer cell migration: a role for chemokine receptors? Cancer Res 2001; 61: 4961–5. [PubMed] [Google Scholar]
- 21. Saur D, Seidler B, Schneider G et al . CXCR4 expression increases liver and lung metastasis in a mouse model of pancreatic cancer. Gastroenterology 2005; 129: 1237–50. [DOI] [PubMed] [Google Scholar]
- 22. Scala S, Ottaiano A, Ascierto PA et al . Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res 2005; 11: 1835–41. [DOI] [PubMed] [Google Scholar]
- 23. Russell HV, Hicks J, Okcu MF, Nuchtern JG. CXCR4 expression in neuroblastoma primary tumors is associated with clinical presentation of bone and bone marrow metastases. J Pediatr Surg 2004; 39: 1506–11. [DOI] [PubMed] [Google Scholar]
- 24. Kaifi JT, Yekebas EF, Schurr P et al . Tumor‐cell homing to lymph nodes and bone marrow and CXCR4 expression in esophageal cancer. J Natl Cancer Inst 2005; 97: 1840–7. [DOI] [PubMed] [Google Scholar]
- 25. Kim J, Takeuchi H, Lam ST et al . Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol 2005; 23: 2744–53. [DOI] [PubMed] [Google Scholar]
- 26. Oda Y, Yamamoto H, Tamiya S et al . CXCR4 and VEGF expression in the primary site and the metastatic site of human osteosarcoma: analysis within a group of patients, all of whom developed lung metastasis. Mod Pathol 2006; 19: 738–45. [DOI] [PubMed] [Google Scholar]
- 27. Pan J, Mestas J, Burdick MD et al . Stromal derived factor‐1 (SDF‐1/CXCL12) and CXCR4 in renal cell carcinoma metastasis. Mol Cancer 2006; 3: 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Duarte I, Llanos O. Patterns of metastases in intestinal and diffuse types of carcinoma of the stomach. Hum Pathol 1981; 12: 237–42. [DOI] [PubMed] [Google Scholar]
- 29. Maehara Y, Moriguchi S, Kakeji Y et al . Pertinent risk factors and gastric carcinoma with synchronous peritoneal dissemination or liver metastasis. Surgery 1991; 110: 820–3. [PubMed] [Google Scholar]
- 30. Takahashi I, Matsusaka T, Onohara T et al . Clinicopathological features of long‐term survivors of scirrhous gastric cancer. Hepatogastroenterology 2000; 47: 1485–8. [PubMed] [Google Scholar]
- 31. Bando E, Yonemura Y, Takeshita Y et al . Intraoperative lavage for cytological examination in 1297 patients with gastric carcinoma. Am J Surg 1999; 178: 256–62. [DOI] [PubMed] [Google Scholar]
- 32. Spano JP, Andre F, Morat L et al . Chemokine receptor CXCR4 and early‐stage non‐small cell lung cancer: pattern of expression and correlation with outcome. Ann Oncol 2004; 15: 613–17. [DOI] [PubMed] [Google Scholar]
- 33. Koshiba T, Hosotani R, Miyamoto Y, Ida J, Tsuji S, Nakajima S. Expression of stromal cell‐derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res 2000; 6: 3530–5. [PubMed] [Google Scholar]
- 34. Jiang YP, Wu XH, Shi B, Wu WX, Yin GR. Expression of chemokine CXCL12 and its receptor CXCR4 in human epithelial ovarian cancer: an independent prognostic factor for tumor progression. Gynecol Oncol 2006; 103: 226–33. [DOI] [PubMed] [Google Scholar]
- 35. Li YM, Pan Y, Wei Y et al . Upregulation of CXCR4 is essential for HER2‐mediated tumor metastasis. Cancer Cell 2004; 6: 459–69. [DOI] [PubMed] [Google Scholar]
- 36. Debruyne F. Hormonal therapy of prostate cancer. Semin Urol Oncol 2002; 20 (3 Suppl 1): 4–9. [DOI] [PubMed] [Google Scholar]
- 37. Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood 2006; 107: 1761–7. [DOI] [PubMed] [Google Scholar]
- 38. Murakami T, Maki W, Cardones AR et al . Expression of CXC chemokine receptor‐4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res 2002; 62: 7328–34. [PubMed] [Google Scholar]
- 39. Sun YX, Schneider A, Jung Y et al . Skeletal localization and neutralization of the SDF‐1 (CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo . J Bone Miner Res 2005; 20: 318–29. [DOI] [PubMed] [Google Scholar]
- 40. De Clercq E. Potential clinical applications of the CXCR4 antagonist Bicyclam AMD3100. Mini Rev Med Chem 2005; 5: 805–24. [DOI] [PubMed] [Google Scholar]
- 41. Retz M, Sidhu SS, Lehmann J, Tamamura H, Fujii N, Basbaum C. New HIV‐drug inhibits in vitro bladder cancer migration and invasion. Eur Urol 2005; 48: 1025–30. [DOI] [PubMed] [Google Scholar]
- 42. Takenaga H, Tamamura K, Hiramatsu N et al . A single treatment with microcapsules containing a CXCR4 antagonist suppresses pulmonary metastasis of murine melanoma. Biochem Biophys Res Commun 2004; 320: 226–32. [DOI] [PubMed] [Google Scholar]
- 43. Mori R, Doi M, Koizumi ET et al . CXCR4 antagonist inhibits stromal cell‐derived factor 1‐induced migration and invasion of human pancreatic cancer. Mol. Cancer Ther 2004; 3: 29–37. [PubMed] [Google Scholar]
- 44. Liang Z, Wu T, Lou H et al . Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Res 2004; 64: 4302–8. [DOI] [PubMed] [Google Scholar]
- 45. Jackisch C. HER‐2‐positive metastatic breast cancer: optimizing trastuzumab‐based therapy. Oncologist 2006; 11 (Suppl 1): 34–41. [DOI] [PubMed] [Google Scholar]
- 46. Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev 1992; 13: 18–32. [DOI] [PubMed] [Google Scholar]
- 47. Vicari AP, Caux C. Chemokines in cancer. Cytokine Growth Factor Rev 2002; 13: 143–54. [DOI] [PubMed] [Google Scholar]
- 48. Ueno T, Toi M, Saji H et al . Significance of macrophage chemoattractant protein‐1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res 2000; 6: 3282–9. [PubMed] [Google Scholar]
- 49. Luboshits G, Shina S, Kaplan O et al . Elevated expression of the CC chemokine regulated on activation, normal T cell expressed and secreted (RANTES) in advanced breast carcinoma. Cancer Res 1999; 59: 4681–7. [PubMed] [Google Scholar]
- 50. Ueno T, Toi M, Saji H et al . Monocyte chemoattractant protein‐1 expression correlates with macrophage infiltration and tumor vascularity in human esophageal squamous cell carcinomas. Int J Cancer 2002; 102: 220–4. [DOI] [PubMed] [Google Scholar]
- 51. Ohta M, Tanaka F, Yamaguchi H, Sadanaga N, Inoue H, Mori M. The high expression of fractalkine results in a better prognosis for colorectal cancer patients. Int J Oncol 2005; 26: 41–7. [PubMed] [Google Scholar]
- 52. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990; 61: 759–67. [DOI] [PubMed] [Google Scholar]
- 53. Lamlum H, Papadopoulou A, Ilyas M et al . APC mutations are sufficient for the growth of early colorectal adenomas. Proc Natl Acad Sci USA 2000; 97: 2225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kitamura T, Kometani K, Hashida H et al . SMAD4‐deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet 2007; 39: 467–75. [DOI] [PubMed] [Google Scholar]
- 55. Oshima H, Oshima M, Kobayashi M, Tsutsumi M, Taketo MM. Morphological and molecular processes of polyp formation in ApcΔ716 knockout mice. Cancer Res 1997; 57: 644–9. [PubMed] [Google Scholar]
- 56. Yang X, Lu P, Fujii C. Essential contribution of a chemokine, CCL3, and its receptor, CCR1, to hepatocellular carcinoma progression. Int J Cancer 2006; 118: 1869–76. [DOI] [PubMed] [Google Scholar]
- 57. Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour‐infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol 1997; 182: 318–24. [DOI] [PubMed] [Google Scholar]
- 58. Naito Y, Saito K, Shiiba K et al . CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 1998; 58: 3491–4. [PubMed] [Google Scholar]
- 59. Shimaoka T, Kume N, Minami M et al . Molecular cloning of a novel scavenger receptor for oxidized low density lipoprotein, SR‐PSOX, on macrophages. J Biol Chem 2000; 275: 40663–6. [DOI] [PubMed] [Google Scholar]
- 60. Liao F, Alkhatib G, Peden KW, Sharma G, Berger EA, Farber JM. STRL33, A novel chemokine receptor‐like protein, functions as a fusion cofactor for both macrophage‐tropic and T cell line‐tropic HIV‐1. J Exp Med 1997; 185: 2015–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wilbanks A, Zondlo SC, Murphy K et al . Expression cloning of the STRL33/BONZO/TYMSTR ligand reveals elements of CC, CXC, and CX3C chemokines. J Immunol 2001; 166: 5145–54. [DOI] [PubMed] [Google Scholar]
- 62. Hase K, Murakami T, Takatsu H et al . The membrane‐bound chemokine CXCL16 expressed on follicle‐associated epithelium and M cells mediates lympho‐epithelial interaction in GALT. J Immunol 2006; 176: 43–51. [DOI] [PubMed] [Google Scholar]
- 63. Thomas SY, Hou R, Boyson JE et al . CD1d‐restricted NKT cells express a chemokine receptor profile indicative of Th1‐type inflammatory homing cells. J Immunol 2003; 171: 2571–80. [DOI] [PubMed] [Google Scholar]
- 64. Shimaoka T, Nakayama T, Fukumoto N et al . Cell surface‐anchored SR‐PSOX/CXC chemokine ligand 16 mediates firm adhesion of CXC chemokine receptor 6‐expressing cells. J Leukoc Biol 2004; 75: 267–74. [DOI] [PubMed] [Google Scholar]
- 65. Hojo S, Koizumi K, Tsuneyama K et al . High‐level expression of the chemokine CXCL16 by tumor cells correlates with a good prognosis and increased tumor‐infiltrating lymphocytes in colorectal cancer. Cancer Res 2007; 67: 4725–31. [DOI] [PubMed] [Google Scholar]
- 66. Tada T, Ohzeki S, Utsumi K et al . Transforming growth factor‐β‐induced inhibition of T cell function. Susceptibility difference in T cells of various phenotypes and functions and its relevance to immunosuppression in the tumor‐bearing state. J Immunol 1991; 146: 1077–82. [PubMed] [Google Scholar]
- 67. Van Deventer HW, Serody JS, McKinnon KP, Clements C, Brickey WJ, Ting JP. Transfection of macrophage inflammatory protein 1α into B16, F10 melanoma cells inhibits growth of pulmonary metastases but not subcutaneous tumors. J Immunol 2002; 169: 1634–9. [DOI] [PubMed] [Google Scholar]
- 68. Vicari AP, Ait‐Yahia S, Chemin K, Mueller A, Zlotnik A, Caux C. Antitumor effects of the mouse chemokine 6Ckine/SLC through angiostatic and immunological mechanisms. J Immunol 2000; 165: 1992–2000. [DOI] [PubMed] [Google Scholar]
- 69. Gao JQ, Tsuda Y, Katayama K et al . Antitumor effect by interleukin‐11 receptor α‐locus chemokine/CCL27, introduced into tumor cells through a recombinant adenovirus vector. Cancer Res 2003; 63: 4420–5. [PubMed] [Google Scholar]
- 70. Guo J, Chen T, Wang B et al . Chemoattraction, adhesion and activation of natural killer cells are involved in the antitumor immune response induced by fractalkine/CX3CL1. Immunol Lett 2003; 89: 1–7. [DOI] [PubMed] [Google Scholar]
- 71. Xin H, Kikuchi T, Andarini S et al . Antitumor immune response by CX3CL1 fractalkine gene transfer depends on both NK and T cells. Eur J Immunol 2005; 35: 1371–80. [DOI] [PubMed] [Google Scholar]


