Abstract
Stage IV marginal zone B‐cell lymphomas (MZL) are detected in more than 25% of lymphoma patients. In this study, we conducted retrospective analyses of specific cases of stage IV MZL in order to assess their clinical features, as well as the treatments and prognoses of these cases. A total of 94 patients with histological diagnosis of stage IV‐MZL from 17 different institutions in Korea were included. Multiple‐mucosa‐associated lymphoid tissue (MALT)‐organs‐involved MZL (M‐MZL) was detected in 34 patients (36.2%). Bone‐marrow‐involved stage IV MZL (BM‐MZL) was detected in 33 patients (35.1%). Median time to progression (TTP) was 2.4 years (95% CI, 1.9–2.9). Five‐ and 10‐year overall survival rates were 84.5% and 79.8%, respectively. Patients with lymph node involvement in stage IV MZL appeared to have worse prognoses in TTP (P = 0.015). Thirty‐one patients were treated with a regimen including rituximab (CTx‐R[+]), and 31 with a regimen that did not include rituximab (CTx‐R[−]). The CTx‐R(+) group showed better responses than the CTx‐R(−) group (83.9%versus 54.8%, P = 0.026). However, no differences in TTP duration were detected (P = 0.113). Stage IV MZL tend to follow an indolent disease course. Therefore, lymph node involvement is a more valuable prognostic factor for TTP. Rituximab appears to contribute to better responses, but not in cases of TTP. (Cancer Sci 2010; 101: 2443–2447)
Marginal zone B‐cell lymphoma (MZL) is a distinct subgroup of non‐Hodgkin’s lymphoma (NHL), and is typically characterized by an indolent clinical course – characterized by recurrent relapses and a long survival duration.( 1 , 2 , 3 ) Advanced stage and nodal involvement are generally regarded as the worse prognostic factors in MZL. Marginal zone B‐cell lymphoma of the mucosa‐associated lymphoid tissue (MALT) type has been shown to be responsible for approximately 7–8% of all NHL. In Korea, MZL accounts for 17% of all B‐cell lymphomas, and is the second most frequent histological subtype, after diffuse large B‐cell lymphoma.( 4 )
The cancer staging system is used to decide the treatment modality, and also used for prognostic predictions. The Ann Arbor staging system is commonly used for Hodgkin’s lymphoma patients. It has also been used for the treatment of NHL, including MZL.( 5 , 6 ) The Ann Arbor staging system Stage IV indicates diffuse or disseminated involvement of one or more extra‐lymphatic organs, including any involvement of the liver or bone marrow.
Even though the Ann Arbor staging system is broadly used and no substitute method currently exists, the MZL staging system has proven insufficient in terms of treatment selection, and has also proven difficult to adapt for accurate staging, primarily as the result of its tendency towards multiple MALT site involvement.( 7 , 8 , 9 , 10 ) Stage IV disease was detected in more than one‐quarter of MZL patients.( 2 , 7 , 8 , 9 , 10 ) According to prior analysis, in a group of patients with disseminated disease, the presence of multiple MALT organ localizations without bone marrow or nodal disease appeared to be associated with better prognoses in terms of overall survival duration, even though that patient group was technically categorized as Ann Arbor stage IV MZL.( 7 )
However, it is important to note that the comparison group contained a relatively small number of patients, and that the treatment modalities were too diverse for accurate evaluation.
The clinical efficacy of rituximab, a chimeric monoclonal antibody targeted against the B‐cell‐specific antigen CD20, has been demonstrated in follicular lymphoma (FL), diffuse large B‐cell lymphoma (DLBCL) and mantle cell lymphoma (MCL).( 11 , 12 , 13 ) The CD20 antigen is expressed on the surfaces of neoplastic cells in virtually all MZL; therefore, there is a reasonable chance that rituximab may be active in advanced stage MZL. Nevertheless, no information regarding the use of rituximab in these lymphomas is currently available.
Therefore, in the present study, we conducted retrospective analyses of the clinical features and treatment outcomes of specific stage IV MZL patients, and identified their clinical courses, prognoses and the role of rituximab therein.
Patients and Methods
Patients and data. Eligible patients for this retrospective analysis were initially diagnosed with MZL in accordance with REAL/WHO classification criteria. Unified case report forms were provided to the participating institutions. The collected data included age, gender, performance status, Ann Arbor staging system classification, location of primary involvement, presence of B symptoms, hemoglobin, hepatitis serology, initial date of diagnosis and treatment modality used. We also obtained data regarding the time to relapse, relationship with the primary and relapsing sites, salvage treatment modality, and response and survival rates of salvage treatment. The overall response was defined according to Cheson criteria.( 14 ) Because our study involved stage IV MZL, all of the patients recruited into this study had to have been diagnosed as stage IV using the Ann Arbor staging system.
We subdivided the stage IV cases into three discrete groups: (i) multiple (≥2)‐MALT‐organs‐involved MZL (M‐MZL); (ii) bone‐marrow‐involved MZL (BM‐MZL); and (iii) liver‐involved MZL (L‐MZL). All cases in which multiple lesions arose in one MALT organ (e.g. multiple nodular lesions in the lung, both ocular involved lesions, or multiple lesions in the colon) were excluded from this study.
Retrospective data collection of patients with stage IV‐MZL was approved by the local ethical committee.
Histology. The diagnosis of marginal zone B cell lymphoma was based on the characteristic histological findings established in the WHO classifications, as well as the results of immunohistochemical staining for CD20 and CD3. When it proved difficult to exclude other low‐grade B cell lymphomas, immunohistochemical studies for CD5, CD10, CD23, cyclin D1, BCL6 and Ki‐67 were conducted. Because MZL is commonly accompanied by reactive hyperplasia, gene rearrangement studies for the IgH gene were conducted via PCR analysis in an attempt to exclude any benign hyperplasias.
Statistical analysis. Categorical variables in the two groups were compared using χ2 tests or Fisher’s exact tests. P‐values of less than 0.05 were considered statistically significant, and all P‐values corresponded to two‐sided significance tests. Overall survival (OS) and time to progression (TTP) were estimated using the Kaplan–Meier product‐limit method. The TTP was calculated from the date on which treatment began after relapse to the date on which disease progression was recognized or the date of the final follow‐up visit. The OS was measured from the date of relapse to the date of death, or the date of the final follow‐up visit. Survival rates were compared for statistical differences using log‐rank analysis, and a Cox regression model was used for multivariate analysis at a P‐value of <0.05 in the univariate (by log rank test) analysis of OS and TTP. All data were analyzed with SPSS software (Version 16.0, Chicago, IL, USA).
Results
Initial patient characteristics. Between February 1996 and June 2009, a total of 94 patients with histological diagnosis of stage IV MZL from 17 different institutions in Korea were included. The clinical presentations of the patients initially diagnosed with MZL are shown in Table 1.
Table 1.
Patient characteristics
| N = 94 | % | |
|---|---|---|
| Gender | ||
| Male | 60 | 63.8 |
| Female | 34 | 36.2 |
| Age | ||
| Median | 59 (12–82) | |
| ≥60 | 45 | 47.9 |
| <60 | 49 | 52.1 |
| LDH (n = 84)† | ||
| Normal | 53 | 63.1 |
| ≥Normal | 31 | 36.9 |
| Hemoglobin (n = 83)† | ||
| ≥12 g/dL | 48 | 57.8 |
| <12 g/dL | 35 | 42.2 |
| ALC (n = 83)† | ||
| ≥1.0 × 109/L | 62 | 74.7 |
| <1.0 × 109/L | 21 | 25.3 |
| PS (ECOG) | ||
| 0–1 | 86 | 91.5 |
| 2 | 8 | 8.5 |
| B symptom | ||
| A | 77 | 81.9 |
| B | 17 | 18.1 |
| IPI (n = 87)† | ||
| Low | 9 | 10.6 |
| Low‐intermediate | 38 | 43.7 |
| High‐intermediate | 30 | 34.5 |
| High | 10 | 11.5 |
| MZLPI | ||
| Intermediate | 59 | 62.8 |
| High | 35 | 37.2 |
†Number of available patient’s data. ALC, absolute lymphocyte count; IPI, International Prognostic Index; LDH, lactic dehydrogenase; MZLPI, Marginal Zone B‐cell Lymphoma Prognostic Index; PS (ECOG), performance status (Eastern Cooperative Oncology Group).
Sixty males and 34 females were included as participants in this study. The median patient age was 59 years (range, 12–82 years). The majority of patients (91.5%) showed good performance status (ECOG 0 or 1). B symptoms were noted in 17 patients. Among the available data, 57.8% of patients had hemoglobin counts above 12 g/dL, and 74.7% had absolute lymphocyte counts (ALC) in excess of 1.0 × 109/L. 54.3% of patients (47 of 84) were assigned to the low or low‐intermediate risk groups in accordance with the International Prognostic Index (IPI). 62.8% (59 of 94) were in the intermediate‐risk group according to the Marginal Zone Lymphoma Prognostic Index (MZLPI).( 15 )
Pattern of stage IV. The patterns of diagnosis of stage IV MZL are shown in Table 2.
Table 2.
Pattern of stage IV marginal zone B‐cell lymphoma
| N = 94 | % | |
|---|---|---|
| Multiple MALT organs† | ||
| Alone | 9 | 9.6 |
| + LN | 25 | 26.6 |
| BM | ||
| + Single MALT organ‡ ± LN | 16 | 17.0 |
| + LN | 17 | 18.1 |
| Liver | ||
| + Single MALT organ§ | 1 | 1.1 |
| + LN | 3 | 3.2 |
| Combined | ||
| BM + Multiple MALT organs† ± LN | 17 | 18.1 |
| Liver + Multiple MALT organs† + LN | 4 | 4.3 |
| BM + Liver ± LN | 2 | 2.1 |
†The most frequent sites of involvement were: gastrointestinal tract, 25 (45.5%); lung, 22 (40%); Waldeyer’s ring, 15 (27.3%); and ocular, 14 (25.5%). ‡Ocular, 5; lung, 4; salivary gland and tonsil, 2; thyroid, breast and hard palate, 1. §lung, 1. BM, bone marrow; LN, lymph node; MALT, mucosa‐associated lymphoid tissue.
Multiple‐MALT organ‐involved MZL was detected in 55 patients (58.5%). Among them, 25 patients presented with LN enlargement. Only nine patients presented without LN enlargement when multiple‐MALT organs were involved. However, the other 21 patients showed combined BM or liver involvement. The numbers of involved multiple‐MALT organs (two, three or four) were 38 (69.1%), 13 (23.6%) and four (7.3%), respectively. The most frequent sites of involvement were the gastrointestinal tract (45.5%), followed by the lung (40%), Waldeyer’s ring (27.3%) and tonsil (25.5%).
Bone‐marrow‐involved stage IV MZL was detected in 33 patients (35.1%), in which nodal involvement without any MALT organ‐involved MZL was noted in 17 patients (18.1%).
In the L‐MZL group, there was only one patient with “Liver + single MALT organ” and three with “Liver + LN involvement.” However, four patients fell into the category “Liver + Multiple MALT organs + LN”.
One‐quarter of patients (24.5%) fulfilled the multiple criteria for stage IV.
Response of treatments and efficacy of rituximab. A total of 92 patients were treated, and two were lost to follow up immediately after diagnosis. Three were observed until progression in a watchful wait status. The other patients began their initial treatments within 1 month of tissue diagnosis. Among 89 patients, 83 received chemotherapy‐based treatments. With the exception of three patients whose responses were not evaluated, complete response or partial response was achieved in 63 patients (75.9%). In the cases of M‐MZL involving nearby organs or operable stage IV MZL, local treatment of radiotherapy or combined operation were conducted in six patients. A complete response was achieved in all six of these patients (Table 3).
Table 3.
Treatment result of stage IV marginal zone B‐cell lymphoma
| N = 92† | CR (%) | PR (%) | SD (%) | PD (%) | |
|---|---|---|---|---|---|
| Chemotherapy | |||||
| Alone | 62‡ | 30 (50.8) | 13 (22.0) | 10 (16.9) | 6 (10.2) |
| + OP | 9 | 7 (77.8) | 2 (22.2) | ||
| + RT | 8 | 2 (25.0) | 6 (75.0) | ||
| + OP + RT | 4 | 2 (50.0) | 1 (25.0) | 1 (25.0) | |
| RT | 3 | 3 (100) | |||
| OP ± RT | 3 | 3 (100) | |||
| Observation | 3 | 3 (100) | |||
†Two patients: follow up lost after diagnosis. ‡Three patients: follow up lost after chemotherapy without a response evaluation. CR, complete response; OP, operation; PD, progression of disease; PR, partial response; RT, radiotherapy; SD, stable disease.
Chemotherapy‐only treatment without operation or radiotherapy was performed in 62 patients.
Thirty‐one patients were treated with a regimen including rituximab (CTx‐R[+]) and 31 were treated with a regimen that did not include rituximab (CTx‐R[−]). No clinically significant differences in the patients’ characteristics were detected (Table 4). The CTx‐R(+) group was composed principally of rituximab‐cyclophosphamide, vincristine, and prednisone (R‐CVP) (n = 23) and rituximab‐cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP) (n = 6) cases. The median number of cycles of CTx‐R(+) treatment was eight (range, 1–9 cycles). Fifteen CVP patients and 13 CHOP patients were in the CTx‐R(−) group. The median number of cycles of CTx‐R(−) treatment was six (range, 1–12 cycles). The CTx‐R(+) group exhibited better responses than the CTx‐R(−) group (83.9%versus 54.8%, P = 0.026). Free progression or relapse rates at 1 year from the treatment start date were 94%versus 74% (P = 0.013). However, no statistically significant differences in TTP duration were noted between the CTx‐R(+) and CTx‐R(−) groups (2.4 years versus 2.0 years, P = 0.113) (Fig. 1).
Table 4.
Chemotherapy result of stage IV marginal zone B‐cell lymphoma
| Rituximab (−) (N = 31) | Rituximab (+) (N = 31) | P† | |
|---|---|---|---|
| Characteristics | |||
| Male/Female | 16/15 | 21/10 | 0.300 |
| Age <60/≥60 (years) | 14/17 | 17/14 | 0.612 |
| LN involvement −/+ | 5/26 | 9/22 | 0.363 |
| Hb ≥/<12 g/dL | 12/16 | 18/7 | 0.052 |
| ALC ≥/<1.0 × 109/L | 19/9 | 20/5 | 0.365 |
| PS (ECOG) 0–1/2 | 28/3 | 28/3 | 1.000 |
| BM involvement −/+ | 8/23 | 11/17 | 0.403 |
| MM‐MZL −/+ | 9/22 | 11/20 | 0.786 |
| IPI 0–2/3–5 | 25/13 | 13/16 | 0.257 |
| Regimen | |||
| CVP | 15 | 23 | 0.056 |
| CHOP | 13 | 6 | |
| Others | 3‡ | 2§ | |
| Median cycle (range) | 6 (1–12) | 8 (1–9) | |
| Response | |||
| CR | 11 | 19 | 0.026 |
| PR | 6 | 7 | |
| SD | 7 | 3 | |
| PD | 5 | 1 | |
| NE | 2 | 1 | |
†P‐value by χ2 test. ‡Chloambucil, 1; fludarabine, 2.
§Rituximab monotherapy, 1; Zevalin, 1. Bold values denote statistical significance. ALC, absolute lymphocyte count; BM, bone marrow; CHOP, Cyclophosphamide + Doxorubicin + Vincristine + Prednisone; CR, complete response; CVP, Cyclophosphamide + Vincristine + Prednisone; Hb, hemoglobin; IPI, International Prognostic Index; LN, lymph node; (MM)‐MZL, (multiple‐mucosa‐associated lymphoid tissue (MALT)‐organs‐involved)‐marginal zone B‐cell lymphoma; NE, not evaluable; PD, progression of disease; PR, partial response; PS (ECOG), Eastern Cooperative Oncology Group Performance Status; SD, stable disease; −/+, no/yes.
Figure 1.
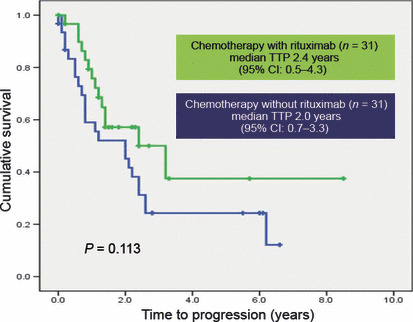
Time to progression (TTP) curves according to chemotherapy with or without rituximab. No statistically significant differences were detected (2.4 years versus 2.0 years, P = 0.113).
Survival and prognostic factor analyses. Median follow‐up duration was 5.8 years (range, 0.5–22.6 years). Median TTP was 2.4 years (95% CI, 1.9–2.9 years) (Fig. 2). No statistically significant differences were noted among the M‐MZL, BM‐MZL, L‐MZL and combined stage IV MZL groups (P = 0.297). However, the cases of LN‐involved stage IV MZL had a worse TTP (P = 0.015, Hazard ratio [95% CI] 2.693 [1.212–5.981]) (Table 5). Cause‐specific OS did not reach the median value. The 5‐year and 10‐year OS rates were 84.5% and 79.8%, respectively. No tested factor influenced the OS duration.
Figure 2.
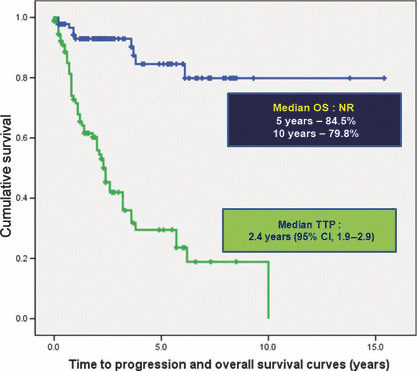
Time to progression (TTP) and overall survival (OS) curves of stage IV marginal zone B‐cell lymphoma. Median TTP was 2.4 years (95% CI, 1.9–2.9 years). The 5‐year and 10‐year OS rates were 84.5% and 79.8%, respectively. NR, did not reach median value.
Table 5.
Prognostic factors of stage IV marginal zone B‐cell lymphoma
| TTP | OS | |||
|---|---|---|---|---|
| P‐value* | Hazard ratio (95% CI) | P‐value* | Hazard ratio (95% CI) | |
| Age ≥60 | 0.227 | 1.398 (0.812–2.406) | 0.607 | 1.388 (0.398–4.833) |
| Gender : male | 0.583 | 0.853 (0.483–1.505) | 0.896 | 1.088 (0.306–3.866) |
| PS (ECOG) ≥ 2 | 0.722 | 1.183 (0.469–2.983) | 0.546 | 0.044 (0.000–1112.6) |
| LDH > Normal | 0.252 | 1.391 (0.791–2.446) | 0.571 | 1.497 (0.371–6.030) |
| B symptoms (+) | 0.054 | 1.903 (0.989–3.659) | 0.575 | 1.580 (0.319–7.836) |
| Hemoglobin <12 | 0.146 | 1.553 (0.857–2.814) | 0.148 | 3.273 (0.656–16.345) |
| ALC <1.0 × 109/L | 0.355 | 1.359 (0.709–2.604) | 0.273 | 2.267 (0.525–9.800) |
| LN involvement (+) | 0.015 | 2.693 (1.212–5.981) | 0.263 | 32.070 (0.074–13925) |
| Multiple MALT organs (+) | 0.055 | 1.746 (0.987–3.088) | 0.642 | 0.745 (0.216–2.577) |
| BM (+) | 0.929 | 1.025 (0.599–1.751) | 0.848 | 1.132 (0.319–4.015) |
| Liver (+) | 0.105 | 0.423 (0.149–1.198) | 0.378 | 0.035 (0.000–60.303) |
| Chemotherapy (+) | 0.455 | 1.477 (0.531–4.112) | 0.511 | 23.6 (0.002–290466) |
*P‐value by Cox regression analysis. Bold values denote statistical significance. ALC, absolute lymphocyte count; BM, bone marrow; LDH, lactic dehydrogenase; LN, lymph node; MALT, mucosa‐associated lymphoid tissue; OS, overall survival; PS (ECOG), Eastern Cooperative Oncology Group Performance Status; TTP, time to progression; (+), yes.
Discussion
The Ann Arbor staging classification system is commonly used for the staging of lymphomas.( 5 ) Originally developed for Hodgkin’s disease, this staging scheme was later expanded to include non‐Hodgkin’s lymphoma. Stage IV is defined as more than one extra‐lymphatic organ or tissue, or any involvement of the liver or bone marrow.( 6 ) Staging helps the physician select appropriate treatment options and also facilitates prognosis, or at least estimates the disease outlook and patient survival.
As shown in previous studies, even though the median progression‐free survival duration of advanced stage or disseminated MZL patients was around 2–5 years, more than 80% showed a long duration of survival – a 10‐year OS rate.( 2 , 7 , 10 , 16 ) Our data also demonstrated that stage IV MZL followed an indolent natural course. Through these surveys, several prognostic factors were introduced: LN, advanced stage, hemoglobin, β2‐microglobulin, B symptoms, LDH level and performance status.( 1 , 7 , 15 , 17 , 18 )
In previous studies, different prognoses were assigned to differing varieties of stage IV MZL patients. Patients with multiple‐MALT‐organ‐involved MZL without bone marrow or nodal disease appeared to have better prognoses than those suffering from nodal or BM‐involved stage IV MZLl;( 1 , 7 ) the prognoses of the former group were similar to those of stage I or II MZL patients. Involvement of multiple extranodal sites in MALT lymphoma appears to be biologically distinct from multiple extranodal involvement in other lymphoma types, and such patients may be managed by separately treating each site by either excision or radiotherapy. By way of contrast, cases with disseminated nodal involvement appear to behave more like nodal MZL or disseminated FL.( 1 , 7 )
However, in contrast to previous results, our data showed that LN involvement itself was a poor prognostic factor in stage IV MZL, regardless of the reasons for stage IV multiple MALT organs, BM or liver involvement. The BM and liver are classed as other MALT organs. The reason for the differences between the two observations remains to be clearly determined. However, the fact that we excluded multiple‐site involvement in a single MALT organ from our analysis may be relevant in this regard. In cases of multiple‐site involvement in a single MALT organ, patients can be treated by surgical removal or radiotherapy, if the sites fall within one radiation field or a surgically feasible distance. Additionally, prior to our study of pulmonary MZL, no differences were noted in response rate (RR), TTP and OS, regardless of multiple involvement or single involvement in both lung fields.( 19 ) Thus, we discussed the issue and elected to exclude from our data analysis cases of MZL involving multiple lesions arising in one MALT organ.
In this study, as well as in previous retrospective analyses, the numbers of M‐MZL without LN involvement itself were too small for a definitive elucidation of the relevant clinical characteristics.( 1 ) This may have led to bias, and possibly to errors.
Although our results will require further confirmation and explanation in the future, clear differences were noted in the clinical manifestations and treatment of different variants of stage IV MZL. Organ distribution of MZL differs profoundly according to geographic region. Gastrointestinal tract and ocular adnexa are involved in more than 75% of Korean MZL cases, with a relatively low incidence of BM involvement (∼10%) at relatively younger ages (median, 49 years old).( 2 ) Conversely, in a European study, the skin and salivary glands were the most frequently involved sites of MZL, along with a relatively high incidence of BM involvement (∼30%) at ages over 60.( 1 , 3 , 9 ) Rituximab may also play a role in these differences. Rituximab was more frequently used in our study (50% of chemotherapy) than in previous studies.( 20 ) The poor prognostic effects of BM involvement might be ameliorated by rituximab treatment.
The clinical efficacy of rituximab, a chimeric monoclonal antibody targeted toward the B‐cell‐specific antigen CD20, was initially demonstrated in cases of FL, but the use of this antibody has been extended over the last few years to the majority of subtypes of B‐cell CD20 positive non‐Hodgkin’s lymphomas; this has, thus far, generated some encouraging results. In MZL, small numbers of case reports have chronicled the use of rituximab as a single agent or phase II trial combination with chemotherapeutic regimens.( 20 , 21 , 22 , 23 )
In our data, which were focused on stage IV MZL chemotherapy, rituximab in combination with chemotherapy resulted in better responses than conventional chemotherapy alone, as well as lower relapse or progression rates at 1 year. Similar to prior case reports and clinical trials with rituximab, rituximab monotherapy elicited a good clinical response in the initial treatment, but this effect did not persist for longer than 12 months.( 20 ) Therefore, we should consider combinations of rituximab with conventional chemotherapy and maintenance therapy clinical trials using non‐toxic rituximab monotherapy to extend the achieved benefits of CTx‐R(+), hopefully over several years.
According to the results of our survey, Stage IV MZL tends to be an indolent disease, characterized by prolonged survival with frequent relapses. The majority of these cases can be effectively controlled with chemotherapy‐based treatments and prolonged survival can thereby be achieved. Lymph node involvement was found to be a more valuable prognostic factor than M‐MZL or BM‐MZL/L‐MZL for TTP. Rituximab also appears to contribute to better responses, but not in TTP.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- ALC
absolute lymphocyte count
- BM
bone marrow
- CHOP
Cyclophosphamide + Doxorubicin + Vincristine + Prednisone
- CR
complete response
- CVP
Cyclophosphamide + Vincristine + Prednisone
- Hb
hemoglobin
- IPI
International Prognostic Index
- LDH
lactic dehydrogenase
- LN
lymph node
- MALT
mucosa‐associated lymphoid tissue
- (MM)‐MZL
(multiple‐mucosa‐associated lymphoid tissue (MALT)‐organs‐involved)‐marginal zone B‐cell lymphoma
- MZLPI
Marginal Zone B‐cell Lymphoma Prognostic Index
- NE
not evaluable
- OP
operation
- OS
overall survival
- PD
progression of disease
- PR
partial response
- PS
(ECOG), Eastern Cooperative Oncology Group Performance Status
- RT
radiotherapy
- SD
stable disease
- TTP
time to progression
Acknowledgments
This paper was supported by Dong‐A University Research Fund.
References
- 1. Zucca E, Conconi A, Pedrinis E et al. Nongastric marginal zone B‐cell lymphoma of mucosa‐associated lymphoid tissue. Blood 2003; 101: 2489–95. [DOI] [PubMed] [Google Scholar]
- 2. Oh SY, Ryoo BY, Kim WS et al. Nongastric marginal zone B‐cell lymphoma: analysis of 247 cases. Am J Hematol 2007; 82: 446–52. [DOI] [PubMed] [Google Scholar]
- 3. Isaacson PG. Update on MALT lymphomas. Best Pract Res Clin Haematol 2005; 18: 57–68. [DOI] [PubMed] [Google Scholar]
- 4. Ko YH, Kim CW, Park CS et al. REAL classification of malignant lymphomas in the Republic of Korea: incidence of recently recognized entities and changes in clinicopathologic features. Hematolymphoreticular Study Group of the Korean Society of Pathologists. Revised European‐American lymphoma. Cancer 1998; 83: 806–12. [PubMed] [Google Scholar]
- 5. Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res 1971; 31: 1860–1. [PubMed] [Google Scholar]
- 6. Lister TA, Crowther D, Sutcliffe SB et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol 1989; 7: 1630–6. [DOI] [PubMed] [Google Scholar]
- 7. Thieblemont C, Berger F, Dumontet C et al. Mucosa‐associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood 2000; 95: 802–6. [PubMed] [Google Scholar]
- 8. Raderer M, Wohrer S, Streubel B et al. Assessment of disease dissemination in gastric compared with extragastric mucosa‐associated lymphoid tissue lymphoma using extensive staging: a single‐center experience. J Clin Oncol 2006; 24: 3136–41. [DOI] [PubMed] [Google Scholar]
- 9. Kalpadakis C, Pangalis GA, Vassilakopoulos TP et al. Non‐gastric extra‐nodal marginal zone lymphomas – a single centre experience on 76 patients. Leuk Lymphoma 2008; 49: 2308–15. [DOI] [PubMed] [Google Scholar]
- 10. Arcaini L, Burcheri S, Rossi A et al. Nongastric marginal‐zone B‐cell MALT lymphoma: prognostic value of disease dissemination. Oncologist 2006; 11: 285–91. [DOI] [PubMed] [Google Scholar]
- 11. Marcus R, Imrie K, Solal‐Celigny P et al. Phase III study of R‐CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol 2008; 26: 4579–86. [DOI] [PubMed] [Google Scholar]
- 12. Feugier P, Van Hoof A, Sebban C et al. Long‐term results of the R‐CHOP study in the treatment of elderly patients with diffuse large B‐cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 2005; 23: 4117–26. [DOI] [PubMed] [Google Scholar]
- 13. Gressin R, Caulet‐Maugendre S, Deconinck E et al. Evaluation of the (R)VAD+C regimen for the treatment of newly diagnosed Mantle Cell Lymphoma. Combined results of two prospective phase II trials from the French GOELAMS group. Haematologica 2010; 95: 258–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheson BD, Horning SJ, Coiffier B et al. Report of an international workshop to standardize response criteria for non‐Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999; 17: 1244. [DOI] [PubMed] [Google Scholar]
- 15. Oh SY, Kwon HC, Kim WS et al. Nongastric marginal zone B‐cell lymphoma: a prognostic model from a retrospective multicenter study. Cancer Lett 2007; 258: 90–7. [DOI] [PubMed] [Google Scholar]
- 16. De Boer JP, Hiddink RF, Raderer M et al. Dissemination patterns in non‐gastric MALT lymphoma. Haematologica 2008; 93: 201–6. [DOI] [PubMed] [Google Scholar]
- 17. Mazloom A, Medeiros LJ, McLaughlin PW et al. Marginal zone lymphomas: factors that affect the final outcome. Cancer 2010. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18. Nathwani BN, Anderson JR, Armitage JO et al. Marginal zone B‐cell lymphoma: a clinical comparison of nodal and mucosa‐associated lymphoid tissue types. Non‐Hodgkin’s Lymphoma Classification Project. J Clin Oncol 1999; 17: 2486–92. [DOI] [PubMed] [Google Scholar]
- 19. Oh SY, Kim WS, Kim JS et al. Pulmonary marginal zone B‐cell lymphoma of MALT type – what is a prognostic factor and which is the optimal treatment, operation, or chemotherapy?: Consortium for Improving Survival of Lymphoma (CISL) study. Ann Hematol 2010; 89: 563–8. [DOI] [PubMed] [Google Scholar]
- 20. Conconi A, Martinelli G, Thieblemont C et al. Clinical activity of rituximab in extranodal marginal zone B‐cell lymphoma of MALT type. Blood 2003; 102: 2741–5. [DOI] [PubMed] [Google Scholar]
- 21. Salar A, Domingo‐Domenech E, Estany C et al. Combination therapy with rituximab and intravenous or oral fludarabine in the first‐line, systemic treatment of patients with extranodal marginal zone B‐cell lymphoma of the mucosa‐associated lymphoid tissue type. Cancer 2009; 115: 5210–7. [DOI] [PubMed] [Google Scholar]
- 22. Martinelli G, Laszlo D, Ferreri AJ et al. Clinical activity of rituximab in gastric marginal zone non‐Hodgkin’s lymphoma resistant to or not eligible for anti‐Helicobacter pylori therapy. J Clin Oncol 2005; 23: 1979–83. [DOI] [PubMed] [Google Scholar]
- 23. Jager G, Neumeister P, Brezinschek R et al. Treatment of extranodal marginal zone B‐cell lymphoma of mucosa‐associated lymphoid tissue type with cladribine: a phase II study. J Clin Oncol 2002; 20: 3872–7. [DOI] [PubMed] [Google Scholar]


