Abstract
Our previous study showed that exogenous human mesenchymal stem cells (hMSCs) targeted established osteosarcoma and promoted its growth and pulmonary metastasis in vivo. As a follow‐up, the present study aimed to investigate how hMSCs would interact with Saos‐2 through autocrine/paracrine communication. The results showed that co‐injection of hMSCs with Saos‐2 into the proximal tibia of nude mice could promote tumor growth and progression. In vitro, the proliferation of Saos‐2 and hMSCs was promoted by each other’s conditioned medium, in which interleukin‐6 (IL‐6) played an important role. Osteogenic differentiation of hMSCs could be inhibited by conditioned medium of Saos‐2, in which IL‐6 was also involved. Furthermore, decreased IL‐6 secretion by hMSCs during its osteogenesis and increased IL‐6 secretion in response to conditioned medium of Saos‐2 were observed. Based on these data, we suggest that there was a positive feedback loop of IL‐6 in the interaction between hMSCs and Saos‐2. (Cancer Sci 2010; 101: 2554–2560)
The origins of invasive and metastatic phenotypes of carcinoma cells are areas of intense research interest. Although some present models indicate these phenotypes as cell‐autonomous alterations characteristic of the genomes of cancer cells, alternative views propose that growth and metastatic traits of tumor are acquired through exposure of epithelial cancer cells to paracrine signals that are secreted by mesenchymal cell types within the tumor‐associated stroma. Although several lines of evidence indicate the contributions of stromal cells to primary tumor growth,( 1 ) direct experimental demonstration of the influence of these various cells on the growth and metastatic abilities of cancer cells has been difficult to obtain. This is due, to some extent, to the complexity of the mesenchymal cell types that are recruited into the stroma, and to the elusive nature of the putative paracrine signals that are exchanged between the mesenchymal and epithelial compartments of a tumor.
Mesenchymal stem cells (MSCs) in bone marrow are capable of differentiating into osteoblasts, adipocytes, and chondrocytes. In addition, these cells are stromal cells, which are structural components of bone marrow that support ex vivo culture of hematopoesis by providing extracellular matrix components, cytokines and growth factors.( 2 , 3 , 4 , 5 ) Therefore, MSCs have considerable therapeutic potential in several disease processes, including cardiovascular disease,( 6 ) as well as in the treatment of human malignancies.( 7 , 8 , 9 , 10 , 11 ) Recently, the relationship between MSCs and tumors has been highlighted by some interesting results. Hung et al. ( 12 ) showed that MSCs could target microscopic tumors, subsequently proliferate and differentiate, then contribute to the formation of a significant portion of tumor stroma in human colon cancer. Khakoo et al. ( 13 ) provided evidence that human MSCs (hMSCs) exerted potent antitumorigenic effects in a model of Kaposi’s sarcoma. The results from an elegant study by Karnoub et al. ( 14 ) showed that MSCs within tumor stroma promoted breast cancer metastasis.
Osteosarcoma (OS) is the second most common primary malignant bone tumor. Poor prognosis occurs in most patients due to lack of specific clinical symptoms in the early stages of the disease. More importantly, more than half of patients treated by surgery alone develop metastases within 6 months, and more than 80% develop recurrent disease within 2 years of diagnosis without adjuvant chemotherapy.( 15 ) Although integrated multimodal therapies have achieved great improvements in overall survival, the mechanisms underlying the development, progression, and metastasis of OS remain elusive.
Our previous study( 16 ) showed that exogenous hMSCs targeted the OS site and promoted its growth and pulmonary metastasis in nude mice. As a follow‐up, the present report is aimed at elaborating how hMSCs would interact with Saos‐2 after its recruitment to the tumor site. We established an animal model by co‐injecting Saos‐2 and hMSCs into the proximal tibia of nude mice. The tumor volume and pathological manifestations were examined. Then we established a “cell–cell interaction through autocrine/paracrine” model in vitro to investigate the interaction between hMSCs and Saos‐2.
Materials and Methods
Cell cultures, GFP labeling, and conditioned medium (CM) preparation. Human MSCs were grown in α‐MEM (Gibco, Grand Island, NY, USA) supplemented with 10% FBS (Hyclone, Tauranga, New Zealand) and antibiotics (penicillin 100 U/mL, streptomycin 100 μg/mL; Hyclone, Logan, UT, USA) in a humidified atmosphere at 37°C with 5% CO2. Passage 3 hMSCs were incubated with adenovirus–GFP with 150 MOI at 37°C in 5% CO2 overnight.
Human Saos‐2 was purchased from the Chinese Academy of Sciences (Shanghai, China) and grown in α‐MEM supplemented with 10% FBS and antibiotics in 37°C humidified atmosphere with 5% CO2.
Conditioned medium was prepared as previously described.( 17 ) Human MSCs (20 000 cells/cm2), Saos‐2 (20 000 cells/cm2), and 293T (20 000 cells/cm2) were seeded and cultured for 6 h. The cells were then further cultured with serum‐free medium for 24 h. The medium was collected, filtered through a 0.22 μm filter, and stored at −20°C until use.
Tumor xenografts in nude mice. Study protocols involving mice were approved by the Animal Ethics Committee of Shanghai Jiaotong University School of Medicine (Shanghai, China). Thirty‐eight BALB/c male nude mice (5 weeks of age) were purchased from the Chinese Academy of Sciences, and were divided into three groups: the hMSCs group (n = 11); the Saos‐2 group (n = 13); and the hMSCs + Saos‐2 group (n = 14). Fifty microliters of suspension of hMSCs (2 × 108 cells/mL), Saos‐2 (2 × 108 cells/mL), or hMSCs + Saos‐2 with 1:1 ratio (2 × 108 cells/mL) was injected into the right proximal tibia of the mice.( 18 ) After 8 weeks of injections, the mice were killed by excess pentobarbital. In situ tumor and blood were harvested for alkaline phosphatase (ALP) quantification assay,( 19 ) radiographic examination, and pathological analysis.
After injection, the volume of OS was measured at 1‐week intervals until the animal was killed. The volume of tumor was calculated by using the following equation, as reported previously:( 18 ) volume = 0.2618 × L × W × (L + W), where W was an average of the distance at the proximal tibia at the level of the knee joint in the anterior–posterior and medial–lateral planes, and L was the distance from the most distal extent of the distal tumor margin to the proximal tumor margin.
MTT assay. The MTT assay was done according to the manufacturer’s instructions (Sigma‐Aldrich, St Louis, MO, USA). Briefly, 1 × 103 cells were seeded in a 96‐well plate in 150 μL cell culture medium. At each indicated time point, MTT solution was added to each well and the plates were incubated for 3–4 h; subsequently, DMSO (Sigma‐Aldrich) was added to the wells for 5 min. The plates were then read at 570 nm using an automated plate reader (Perkin‐Elmer, Waltham, MA, USA).
Osteogenic differentiation assay of MSCs. To induce osteogenic differentiation, hMSCs were treated with 10 nM dexamethasone, 10 mM β‐glycerophosphate, 50 μg/mL ascorbate phosphate, and 10 nM 1,25‐dihydroxyvitamin D3.
The mRNA expression of osteopontin (OPN) and osteocalcin (OC) was detected by real‐time PCR at day 21 after osteogenic induction, as previously reported.( 20 ) Briefly, total RNA of cells was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. After reverse transcription reaction, real‐time PCR was carried out by an ABI 7900HT system (Applied Biosystems, Carlsbad, CA, USA) using SYBR Premix Ex Taq (Takara, Dalian, China) according to the manufacturer’s instructions. We used GAPDH as the internal control. The primer sequences used for this analysis were: OPN, 5′‐CTCCATTGACTCGAACGACTC‐3′ (forward) and 5′‐CAGGTCTGCGAAACTTCTTAGAT‐3′ (reverse); OC, 5′‐CACTCCTCGCCCTATTGGC‐3′ (forward) and 5′‐CCCTCCTGCTTGGACACAAAG‐3′ (reverse); and GAPDH, 5′‐ATGGGGAAGGTGAAGGTCG‐3′ (forward) and 5′‐GGGGTCATTGATGGCAACAATA‐3′ (reverse).
Detection of cytokines and growth factors in cell‐free supernatant. Seventy‐nine different cytokines and growth factors in CM were screened using Human Cytokine Array V (RayBiotech, Norcross, GA, USA) according to the manufacturer’s instructions. The signal intensities of the cytokine spots were quantified using the HD‐2001 Chip Reader (Shanghai HealthDigit, Shanghai, China). For each spot, the net signal intensity was determined by subtracting the background level from the total raw signal intensity.
ELISA and neutralization antibody assay. Human MSCs or Saos‐2 were cultured in complete medium for 24 h, then the complete medium was replaced by serum‐free medium. After 2 h, the CM was harvested, centrifuged to remove particulate matter, and stored at −20°C until analyzed. Quantitation of interleukin‐6 (IL‐6) secreted into the medium was determined using a quantitative sandwich ELISA for human IL‐6 according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA). To impede the activity of IL‐6, IL‐6 neutralization antibody (Peprotech, Rocky Hill, NJ, USA) was added to the CM from Saos‐2 or hMSCs.
siRNA knockdown. The MSCs were transfected with gene‐specific siRNA against IL‐6 or negative scramble control according to manufacturer specification (Thermo Scientific, Austin, TX, USA). After transfection, cells were plated for proliferation assays and RNA harvest.
Statistical analysis. Statistical significance was calculated by Student’s t‐test for two‐sample comparisons and one‐way anova was used for multiple comparisons in SPSS version 16.0 (SPSS, Chicago, IL, USA). Tukey’s test was used to find significant differences in anova. Statistical significance was analyzed on data from at least three independent experiments. P‐values <0.05 were defined as significant. All data are presented as the mean ± SD unless otherwise specified.
Results
Development and progression of OS in vivo with or without hMSCs co‐injection. As shown in our previous study,( 16 ) exogenous hMSCs through the caudal vein could target the OS site and then promote its growth in vivo. We believe that this promotion results from not only the adduction of OS with exogenous hMSCs, but more importantly, the intrinsic interaction between them, which has been strongly confirmed in many other tumors.( 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 ) In the present study, we injected Saos‐2 with or without co‐injection of hMSCs labeled with GFP into the proximal tibia of nude mice to compare the growth and development of tumor. Larger tumor volume was observed from week 3 to week 8 in the group co‐injected with hMSCs, relative to the group without co‐injection (Fig. 1A). Furthermore, statistically significant enhancement of ALP levels was observed in response to co‐injection of hMSCs (Fig. 1B). These results were supported by general and fluorescence observations, radiographic examination, and pathologic analysis of the limb with tumor (Fig. 1C–N), in which hMSCs labeled with GFP were observed to obtain fine fusion with tumor stroma.
Figure 1.
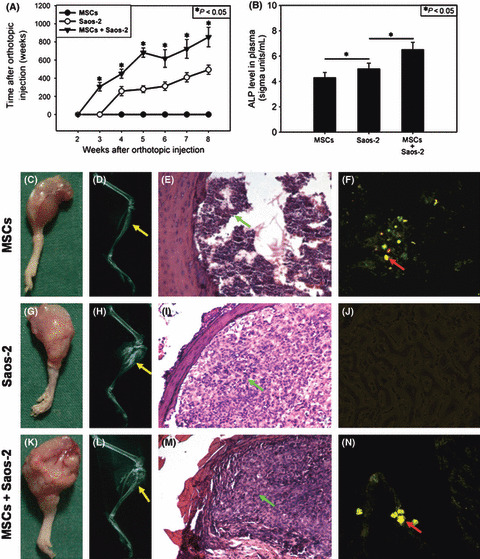
Growth and progression of osteosarcoma (OS) in vivo in response to co‐injection of human mesenchymal stem cells (MSCs). (A) OS volume. *P < 0.05, hMSCs + Saos‐2 versus Saos‐2. (B) Alkaline phosphatase (ALP) in blood. *P < 0.05. (C,G,K) General observation of OS. (D,H,L) X‐ray examination. (E,I,M) Pathological analysis. (F,J,N) Fluorescent photographs. Green arrows, injected cells; red arrows, mean hMSCs labeled with GFP; yellow arrows, site of injection. Results are expressed as the mean ± SD.
Interaction between hMSCs and Saos‐2 through paracrine in vitro. The aforementioned observations indicated that hMSCs and Saos‐2 might supply locally acting cues that interact with each other to promote growth and progression of OS. However, tumor growth in vivo has been proven to be very complicated. So many factors are involved, including not only cell–cell interaction but cell dynamics and the fate of co‐injected hMSCs in the tumor stroma, that it is difficult to investigate which molecule(s) mediated interaction between hMSCs and Saos‐2 through this in vivo animal model. To understand this cross‐talk better, we established a “cell–cell interaction through autocrine/paracrine” model in vitro. The CM of hMSCs (hMSCs‐CM) and Saos‐2 (Saos‐2‐CM) was prepared to treat Saos‐2 and hMSCs, respectively. The CM of 293T cells was used as the control. The results of the MTT assay showed that there was no change in proliferation of hMSCs in response to Saos‐2‐CM during the first 2 days, which was enhanced from day 4 to 8 (Fig. 2A). Accordingly, the proliferation of Saos‐2 in response to hMSCs‐CM showed the same pattern as above (Fig. 2B).
Figure 2.
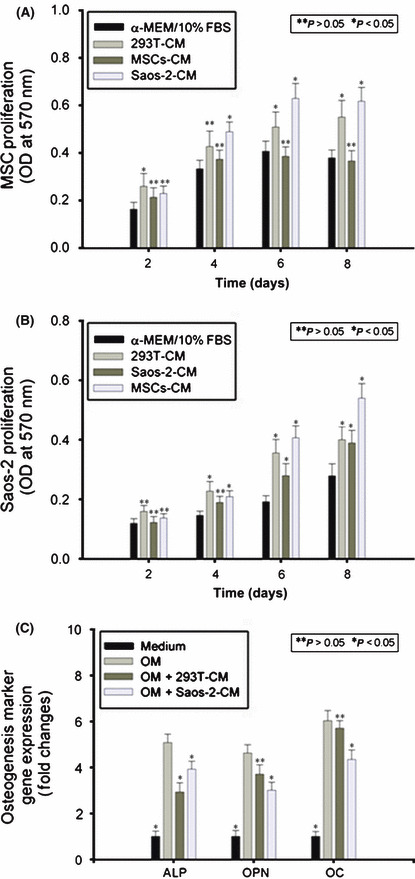
Interaction between human mesenchymal stem cells (hMSCs) and Saos‐2 through paracrine in vitro. Conditioned medium (CM) of hMSCs (hMSCs‐CM), 293T (293T‐CM), and Saos‐2 (Saos‐2‐CM) was prepared and added to the culture medium of Saos‐2 and hMSCs. At the indicated time points, MTT assay was used to monitor hMSCs (A) and Saos‐2 (B) proliferation. Osteogenic induction medium was used to induce hMSCs to undergo osteogenic differentiation, in which Saos‐2‐CM or 293T‐CM was added. Alkaline phosphatase (ALP) quantification assay and real‐time PCR for osteopontin and osteocalcin were carried out (C). Results are expressed as the mean ± SD. *P < 0.05; **P > 0.05.
Then we used osteogenic medium (OM) to induce hMSCs to undergo osteogenic differentiation, in which Saos‐2‐CM or 293T‐CM (as control) was added. The expression levels of ALP, OPN, and OC were investigated. The results showed that the expression levels of ALP, OPN, and OC were downregulated in response to Saos‐2‐CM during osteogenic induction (Fig. 2C).
Screen for some cytokines involved in the interaction between hMSCs and Saos‐2. To determine which molecule(s) might mediate interaction between hMSCs and Saos‐2, the CM of hMSCs and Saos‐2 were screened for the levels of various cytokines, chemokines, and growth factors using Human Cytokine Array V, depicted in Figure 3(A). Of these, high levels of IL‐6 in the CM of both hMSCs and Saos‐2 were observed (Fig. 3B).
Figure 3.
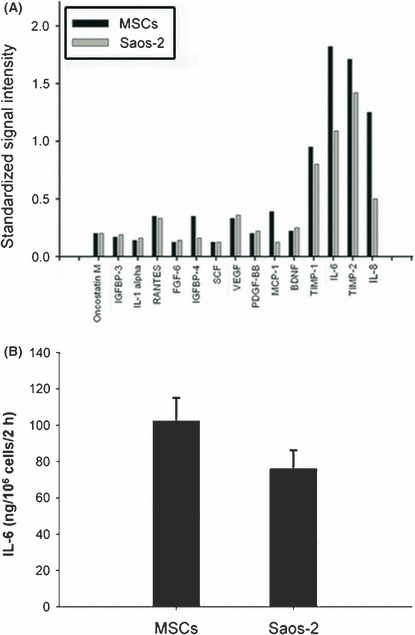
Screen for cytokines involved in the interaction between human mesenchymal stem cells (hMSCs) and Saos‐2. Conditioned medium of hMSCs and Saos‐2 were screened in the present study and plotted for their relative secretion levels (A). The results were expressed as standardized signal intensity. Interleukin‐6 (IL‐6) secretion volume by 106 hMSCs and Saos‐2 for 2 h was assayed (B). Results are expressed as the mean ± SD. BDNF, brain‐derived neurotrophic factor; FGF‐6, fibroblast growth factor; IGFBP, insulin‐like growth factor binding protein; MCP‐1, monocyte chemotactic protein‐1; PDGF‐BB, platelet‐derived growth factor‐BB; RANTES, regulated upon activation, normally T‐expressed, and presumably secreted; SCF, stem cell factor; TIMP, tissue inhibitor of metalloproteinases; VEGF, vascular endothelial growth factor.
Effect of IL‐6 on proliferation of hMSCs and Saos‐2 and osteogenic differentiation of hMSCs. In stem cell biology, IL‐6 family cytokines play an important role in the maintenance of stem cells. Interleukin‐6 enhances proliferation in placenta‐derived MSCs,( 19 ) and is highly expressed in cord blood( 21 ) and bone marrow‐derived MSCs.( 22 ) It is also integral to normal stromal cell function and osteoclast activation.( 23 , 24 ) More importantly, the interaction of hMSCs with Saos‐2 caused a rise in the level of IL‐6 in an in vitro co‐culture model, which implied that IL‐6 might be involved in the interaction between them, at least part (Bian et al., unpublished data, 2007). Therefore, in following experiments, we focussed on assessing whether IL‐6 was involved in interaction between hMSCs and Saos‐2.
We exposed these cells to culture medium containing rhIL‐6 at series concentrations from 5 to 20 ng/mL. The results from MTT showed that 5 and 10 ng/mL IL‐6 could enhance proliferation of hMSCs statistically (Fig. 4A). Likewise, 5, 10, and 20 ng/mL IL‐6 had the same effect on Saos‐2 (Fig. 4B).
Figure 4.
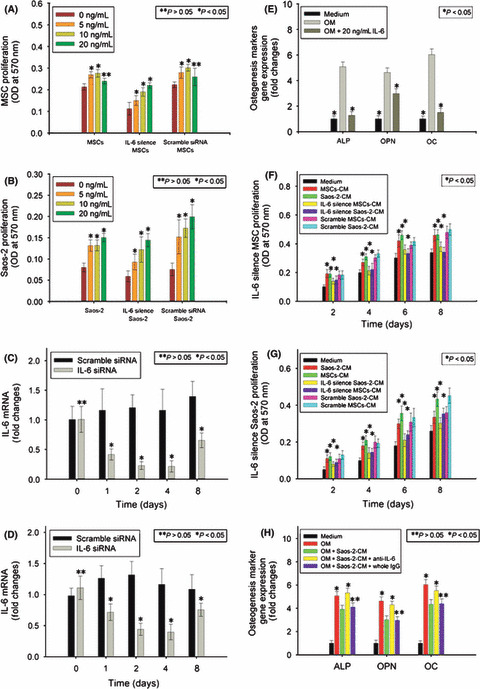
Involvement of interleukin‐6 (IL‐6) in the interaction between human mesenchymal stem cells (hMSCs) and Saos‐2. Human MSCs (A) and Saos‐2 (B) with and without IL‐6 knockdown were exposed to rhIL‐6 from 5 to 20 ng/mL, and MTT assay was used. These two cells were transfected with siRNA against IL‐6. At the indicated time points, real‐time PCR was carried out to assess knockdown of IL‐6 in hMSCs (C) and Saos‐2 (D). Human MSCs were exposed to osteogenic induction medium (OM) containing rhIL‐6 at 20 ng/mL. Alkaline phosphatase (ALP) quantification assay and real‐time PCR were carried out for osteopontin (OPN) and osteocalcin (OC) (E). Conditioned medium (CM) of hMSCs and Saos‐2 with and without IL‐6 knockdown was added to the culture medium of Saos‐2 and hMSCs. At indicated time points, MTT assay was used to monitor hMSC (F) and Saos‐2 (G) proliferation. Osteogenic induction medium was used to induce hMSCs to undergo osteogenic differentiation, in which Saos‐2‐CM in combination with anti‐IL‐6 antibody (whole IgG as control) was added. The ALP quantification assay and real‐time PCR were carried out for OPN and OC (H). Results are expressed as the mean ± SD. *P < 0.05; **P > 0.05.
Interesting data came from the proliferation of hMSCs in response to 20 ng/mL IL‐6. It did not enhance the proliferation of hMSCs in a dose‐dependent manner, relative to 5 and 10 ng/mL. Considering the much higher secretion level of IL‐6 by hMSCs, we hypothesized that the high level of IL‐6 secreted by hMSCs, together with the exogenous 20 ng/mL IL‐6, led to a “saturation” in the effect of IL‐6 on the proliferation of hMSCs. To verify this hypothesis, we blocked IL‐6 autocrine of hMSCs (Fig. 4C) and Saos‐2 (Fig. 4D) by siRNA knockdown of IL‐6, then exposed them to exogenous IL‐6 at series concentrations. The dose‐dependent enhancement of hMSCs by exogenous IL‐6 from 5 to 20 ng/mL was recovered following block of IL‐6 autocrine (Fig. 4A), which supported our hypothesis. The same results were observed in Saos‐2 (Fig. 4B).
We then exposed hMSCs to OM containing rhIL‐6 at 20 ng/mL concentration to investigate the effect of IL‐6 on osteogenic differentiation of hMSCs. The results showed that expression levels of ALP, OPN, and OC were downregulated in response to rhIL‐6 during osteogenic induction (Fig. 4E).
Involvement of IL‐6 in interaction between hMSCs and Saos‐2.
Having observed the interaction between hMSCs and Saos‐2 (Fig. 2) and the effect of rhIL‐6 on their proliferation and osteogenic differentiation (Fig. 4A,B,E), we then asked whether IL‐6 was involved in the interaction between them through autocrine/paracrine. We blocked IL‐6 autocrine of hMSCs and Saos‐2 by siRNA knockdown, then exposed them to hMSCs‐CM, Saos‐2‐CM, IL‐6 silenced hMSCs‐CM, and IL‐6 silenced Saos‐2‐CM. The results from MTT assay showed that hMSCs and Saos‐2 could enhance their own proliferation through IL‐6 autocrine. More importantly, IL‐6 played an essential role in enhancement of hMSC proliferation by Saos‐2‐CM, and vice versa (Fig. 4F,G). We then used OM to induce hMSCs to undergo osteogenic differentiation, in which Saos‐2‐CM with or without anti‐IL‐6 antibody was added. The results showed that anti‐IL‐6 antibody rescued inhibition of osteogenic differentiation of hMSCs in response to Saos‐2‐CM, to at least a large extent (Fig. 4H).
Interleukin‐6 secretion by hMSCs during osteogenic differen‐tiation and in response to Saos‐2‐CM. Having observed the inhibition of osteogenic differentiation of hMSCs by IL‐6 (Fig. 4E,H), we supposed that IL‐6 secretion by hMSCs might decrease gradually along with its osteogenic differentiation process. To confirm this hypothesis, we investigated the secretion level of IL‐6 by hMSCs during osteogenesis. As Figure 5(A) shows, IL‐6 secretion decreased eventually at days 7 and 14 after osteogenic differentiation of hMSCs compared with day 0, although not to a statistically significant extent. However, there was a remarkable decrease in IL‐6 secretion at days 21 and 28, compared with day 0.
Figure 5.
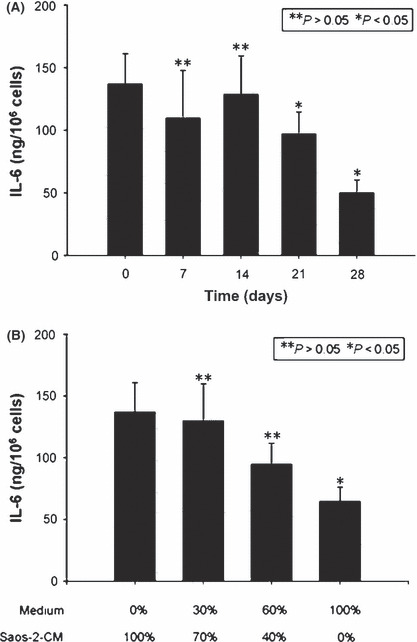
Interleukin‐6 (IL‐6) secretion by human mesenchymal stem cells (hMSCs) during osteogenic differentiation and in response to Saos‐2 conditioned medium (CM). Osteogenic induction medium was used to induce hMSCs to undergo osteogenic differentiation. At the indicated time points, IL‐6 secretion was assayed (A). Human MSCs were exposed to culture medium containing Saos‐2‐CM at gradients from 100% to 0%. Interleukin‐6 secretion of hMSCs was assayed (B). Results are expressed as the mean ± SD. *P < 0.05; **P > 0.05.
Finally, we studied IL‐6 secretion by hMSCs in response to Saos‐2‐CM. Human MSCs were exposed to culture medium containing Saos‐2‐CM at gradients from 100% to 0%. The result showed that IL‐6 secretion by hMSCs was elevated as the dose of Saos‐2‐CM increased (Fig. 5B).
Discussion
In the rapidly expanding field of cancer research, increasing attention has been paid to the relationship between stem cells and tumor cells. A lot of data have been gathered. However, it is because of controversial and even contrary results that there is no definite conclusion concerning whether stem cells promote or inhibit tumorigenesis and development until now.
Mesenchymal stem cells have been shown to contribute to tissue regeneration and to the formation of fibrous scars at the sites of injury.( 25 , 26 ) Tumors are regarded as “wounds that do not heal”.( 27 ) Recent reports indicate that MSCs contribute to tumor growth. When injected i.v. into mice pre‐implanted with tumor‐bearing xenografts such as breast cancer,( 11 ) gliomas,( 8 , 9 ) colon carcinomas,( 12 , 17 ) and melanomas,( 10 ) MSCs were recruited specifically to the developing tumors.
Some cases show that hMSCs home to sites of tumorigenesis, where they inhibit tumor cell function. For example, hMSCs home to sites of Kaposi’s sarcoma, and potently inhibit tumor growth in vivo by downregulating Akt activity in tumor cells that are cultured with hMSCs prior to transplantation in animal tumor models.( 13 ) However, other cases indicate that stem cells could promote tumor cell function. For example, Karnoub et al. ( 14 ) showed that MSCs within stroma promote breast cancer metastasis, in which CCL5 played an essential role. Our previous study( 16 ) showed that exogenous hMSCs targeted the OS site and promoted its growth and pulmonary metastasis in vivo, in which SDF‐1 and CCL5 were involved. Furthermore, the results from the present study showed that co‐injection of hMSCs with Saos‐2 into proximal tibia of nude mice could promote tumor growth and progression in vivo.
The aforementioned controversial results reflect the complexity of interactions between stem cells and tumor cells. Obviously, it is vital to elucidate the molecular mechanisms underlying these interactions, whether MSCs support or inhibit tumor growth. But great difficulties come from direct application of in vivo animal models in investigations into the molecular mechanisms because of intense involvement of many uncertain factors, such as cell dynamics and the fate of MSCs in tumor stroma. So in the current report, we established a cell–cell interaction through autocrine/paracrine model in vitro.
Autocrine/paracrine is one of the most fundemental modes of interaction between MSCs and tumor cells. In the current study, we observed that the proliferation of hMSCs and Saos‐2 was promoted by each other’s CM. Osteogenic and adipogenic differentiation processes of hMSCs could be inhibited by CM of Saos‐2 in vitro. Both hMSCs and Saos‐2 can secrete many cytokines, chemokines, and growth factors. To screen for target cytokines that are likely to mediate interaction between these two cell types, a cytokine array was used, which indicated that IL‐6 might be a potential target.
Interleukin‐6 is a pleiotropic cytokine that plays a significant role in the growth and differentiation, two opposing mechanisms, of cells. Several studies have addressed the role of IL‐6 in tumor cell growth in vitro, but its exact role remains varied and unclear. There are reports indicating IL‐6 as a growth factor in cancer disease for myeloma/plastocytoma, renal cell carcinoma, cervical carcinoma, AIDS Kaposi’s sarcoma‐derived cells, and certain T‐ and B‐cell lymphomas.( 28 , 29 , 30 , 31 , 32 , 33 ) Honma et al. ( 34 ) showed no significant effect on cell proliferation of human breast cancer cell lines (SK‐BR‐3 and MCF‐7) but cell proliferation was significantly increased when IL‐6 and estrone sulfate were simultaneously added to the incubation medium.
In the current study, we found that hMSCs and Saos‐2 could enhance their own proliferation through IL‐6 autocrine. More importantly, IL‐6 played an essential role in enhancement of hMSC proliferation by Saos‐2‐CM, and vice versa. Osteogenic differentiation of hMSCs could be inhibited by CM of Saos‐2, in which IL‐6 was involved. Furthermore, decrease in IL‐6 secretion by hMSCs during its osteogenic differentiation and increase in IL‐6 secretion in response to CM of Saos‐2 were observed. Based on these data, we suggest that there was a positive feedback loop of IL‐6 in interaction between hMSCs and Saos‐2 (Fig. 6).
Figure 6.
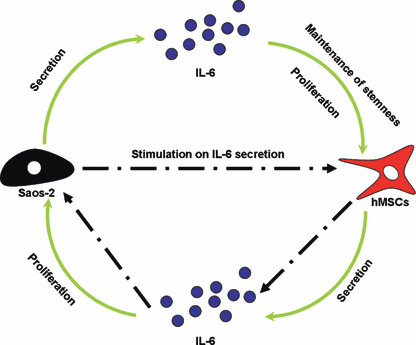
Positive feedback loop of interleukin‐6 (IL‐6) in interaction between human mesenchymal stem cells (hMSCs) and Saos‐2 based on data in the current study.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This research was supported by grants from the Shanghai Science and Technology Development Fund (10410711100), the Scientific Research and Innovation of Shanghai Municipal Education Commission (11YZ46), New Century Excellent Talents in University (NCET‐06‐0401), Key Disciplines of Shanghai Municipal Education Commission (J50206), and the Shanghai Key Laboratory of Orthopaedic Implants (08DZ2230300).
References
- 1. Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer 2001; 1: 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Austin TW, Solar GP, Ziegler FC, Liem L, Matthews W. A role for the Wnt gene family in hematopoiesis: expansion of multilineage progenitor cells. Blood 1997; 89: 3624–35. [PubMed] [Google Scholar]
- 3. Dexter TM, Spooncer E, Schofield R, Lord BI, Simmons P. Haemopoietic stem cells and the problem of self‐renewal. Blood Cells 1984; 10: 315–39. [PubMed] [Google Scholar]
- 4. Van Den Berg DJ, Sharma AK, Bruno E, Hoffman R. Role of members of the Wnt gene family in human hematopoiesis. Blood 1998; 92: 3189–202. [PubMed] [Google Scholar]
- 5. Willert K, Brown JD, Danenberg E et al. Wnt proteins are lipid‐modified and can act as stem cell growth factors. Nature 2003; 423: 448–52. [DOI] [PubMed] [Google Scholar]
- 6. Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res 2004; 95: 9–20. [DOI] [PubMed] [Google Scholar]
- 7. Komarova S, Kawakami Y, Stoff‐Khalili MA, Curiel DT, Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther 2006; 5: 755–66. [DOI] [PubMed] [Google Scholar]
- 8. Nakamizo A, Marini F, Amano T et al. Human bone marrow‐derived mesenchymal stem cells in the treatment of gliomas. Cancer Res 2005; 65: 3307–18. [DOI] [PubMed] [Google Scholar]
- 9. Nakamura K, Ito Y, Kawano Y et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther 2004; 11: 1155–64. [DOI] [PubMed] [Google Scholar]
- 10. Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow‐derived mesenchymal stem cells as vehicles for interferon‐beta delivery into tumors. Cancer Res 2002; 62: 3603–8. [PubMed] [Google Scholar]
- 11. Studeny M, Marini FC, Dembinski JL et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted‐delivery vehicles for anticancer agents. J Natl Cancer Inst 2004; 96: 1593–603. [DOI] [PubMed] [Google Scholar]
- 12. Hung SC, Deng WP, Yang WK et al. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin Cancer Res 2005; 11: 7749–56. [DOI] [PubMed] [Google Scholar]
- 13. Khakoo AY, Pati S, Anderson SA et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med 2006; 203: 1235–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karnoub AE, Dash AB, Vo AP et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007; 449: 557–63. [DOI] [PubMed] [Google Scholar]
- 15. Meyers PA, Gorlick R. Osteosarcoma. Pediatr Clin North Am 1997; 44: 973–89. [DOI] [PubMed] [Google Scholar]
- 16. Grishko V, Xu M, Ho R et al. Effects of hyaluronic acid on mitochondrial function and mitochondria‐driven apoptosis following oxidative stress in human chondrocytes. J Biol Chem 2009; 284: 9132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Menon LG, Picinich S, Koneru R et al. Differential gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. Stem Cells 2007; 25: 520–8. [DOI] [PubMed] [Google Scholar]
- 18. Luu HH, Kang Q, Park JK et al. An orthotopic model of human osteosarcoma growth and spontaneous pulmonary metastasis. Clin Exp Metastasis 2005; 22: 319–29. [DOI] [PubMed] [Google Scholar]
- 19. Marsh JL, Buckwalter J, Gelberman R et al. Articular fractures: does an anatomic reduction really change the result? J Bone Joint Surg Am 2002; 84‐A: 1259–71. [PubMed] [Google Scholar]
- 20. Sobajima S, Shimer AL, Chadderdon RC et al. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real‐time polymerase chain reaction. Spine J 2005; 5: 14–23. [DOI] [PubMed] [Google Scholar]
- 21. Liu CH, Hwang SM. Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine 2005; 32: 270–9. [DOI] [PubMed] [Google Scholar]
- 22. Rougier F, Cornu E, Praloran V, Denizot Y. IL‐6 and IL‐8 production by human bone marrow stromal cells. Cytokine 1998; 10: 93–7. [DOI] [PubMed] [Google Scholar]
- 23. Manolagas SC. Role of cytokines in bone resorption. Bone 1995; 17: 63S–7S. [DOI] [PubMed] [Google Scholar]
- 24. Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med 1995; 332: 305–11. [DOI] [PubMed] [Google Scholar]
- 25. Horwitz EM, Prockop DJ, Fitzpatrick LA et al. Transplantability and therapeutic effects of bone marrow‐derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 1999; 5: 309–13. [DOI] [PubMed] [Google Scholar]
- 26. Wu GD, Tuan TL, Bowdish ME et al. Evidence for recipient derived fibroblast recruitment and activation during the development of chronic cardiac allograft rejection. Transplantation 2003; 76: 609–14. [DOI] [PubMed] [Google Scholar]
- 27. Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986; 315: 1650–9. [DOI] [PubMed] [Google Scholar]
- 28. Eustace D, Han X, Gooding R, Rowbottom A, Riches P, Heyderman E. Interleukin‐6 (IL‐6) functions as an autocrine growth factor in cervical carcinomas in vitro. Gynecol Oncol 1993; 50: 15–9. [DOI] [PubMed] [Google Scholar]
- 29. Kawano M, Hirano T, Matsuda T et al. Autocrine generation and requirement of BSF‐2/IL‐6 for human multiple myelomas. Nature 1988; 332: 83–5. [DOI] [PubMed] [Google Scholar]
- 30. Miki S, Iwano M, Miki Y et al. Interleukin‐6 (IL‐6) functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett 1989; 250: 607–10. [DOI] [PubMed] [Google Scholar]
- 31. Miles SA, Rezai AR, Salazar‐Gonzalez JF et al. AIDS Kaposi sarcoma‐derived cells produce and respond to interleukin 6. Proc Natl Acad Sci U S A 1990; 87: 4068–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shimizu S, Hirano T, Yoshioka R et al. Interleukin‐6 (B‐cell stimulatory factor 2)‐dependent growth of a Lennert’s lymphoma‐derived T‐cell line (KT‐3). Blood 1988; 72: 1826–8. [PubMed] [Google Scholar]
- 33. Yee C, Biondi A, Wang XH et al. A possible autocrine role for interleukin‐6 in two lymphoma cell lines. Blood 1989; 74: 798–804. [PubMed] [Google Scholar]
- 34. Honma S, Shimodaira K, Shimizu Y et al. The influence of inflammatory cytokines on estrogen production and cell proliferation in human breast cancer cells. Endocr J 2002; 49: 371–7. [DOI] [PubMed] [Google Scholar]


