Abstract
Diffuse‐type gastric carcinoma is characterized by rapid progression and poor prognosis. High expression of transforming growth factor (TGF)‐β and thick stromal fibrosis are observed in this type of gastric carcinoma. We have previously shown that disruption of TGF‐β signaling via introduction of a dominant negative form of the TGF‐β type II receptor (dnTβRII) into diffuse‐type gastric cancer cell lines, including OCUM‐2MLN, caused accelerated tumor growth through induction of tumor angiogenesis in vivo. In the present study, we show that TGF‐β induces upregulation of expression of tissue inhibitor of metalloproteinase 2 (TIMP2) in the OCUM‐2MLN cell line in vitro, and that expression of TIMP2 is repressed by dnTβRII expression in vivo. Transplantation of the OCUM‐2MLN cells to nude mice exhibited accelerated tumor growth in response to dnTβRII expression, which was completely abolished when TIMP2 was coexpressed with dnTβRII. Although the blood vessel density of TIMP2‐expressing tumors was only slightly decreased, the degree of hypoxia in tumor tissues was significantly increased and pericytes covering tumor vasculature were decreased by TIMP2 expression in OCUM‐2MLN cells, suggesting that the function of tumor vasculatures was repressed by TIMP2 and consequently tumor growth was reduced. These findings provide evidence that one of the mechanisms of the increase in angiogenesis in diffuse‐type gastric carcinoma is the downregulation of the anti‐angiogenic protein TIMP2. (Cancer Sci 2010; 101: 2398–2403)
Gastric cancer is the fourth most common cancer and the second most common cause of cancer death in the world, with an especially high incidence in East Asia, Eastern Europe and parts of Central and South America.( 1 ) There are two types of gastric cancer, that is, diffuse type and intestinal type, according to the Laurén classification.( 2 ) Of these two, the diffuse type is clinically characterized by rapid progression of disease and poor prognosis.( 3 ) Patients with this type of tumor often show thick stromal fibrosis with undifferentiated carcinoma cells scattered in the interstitium, which results in a stiff and thick gastric wall with reduced motility, but the tumors do not usually form ulcers or apparent mass lesions.
It has been suggested that transforming growth factor (TGF)‐β signaling is an important factor in this type of tumor. The thick stromal fibrosis observed in diffuse‐type gastric carcinoma has been reported to be induced by the TGF‐β produced by cancer cells and by cancer‐associated fibroblasts.( 4 ) Production of TGF‐β1 has been reported to be associated with the progression of diffuse‐type gastric carcinoma.( 5 ) Disruption of TGF‐β signaling by loss of Smad4 expression has also been observed in diffuse‐type gastric carcinoma.( 6 )
Therefore, we previously investigated the role of TGF‐β signaling in diffuse‐type gastric carcinoma( 7 ) using human diffuse‐type gastric carcinoma cell lines,( 8 , 9 ) including OCUM‐2MLN. In that study, we introduced to the cell lines a dominant negative form of the TGF‐β type II receptor (dnTβRII), which binds TGF‐β ligands but does not transduce intracellular signals. These cells, lacking the response to TGF‐β, did not show any significant difference in growth compared with control cells in vitro. However, growth of the tumors in vivo and tumor histology were significantly different depending on the functionality of TGF‐β signaling. Tumors expressing dnTβRII had less fibrosis, but had increased blood vessel formation and consequently exhibited accelerated tumor growth compared with tumors formed by the control cells.
To address the difference in gene expression due to repressed TGF‐β signaling in vivo, we performed oligonucleotide microarray analysis using RNA isolated from cancer cells from tumors with normal or dysfunctional TGF‐β signaling. We found that expression of an anti‐angiogenic factor, thrombospondin‐1 (TSP1), was regulated by TGF‐β signaling, and was involved in the increased tumor angiogenesis observed in dnTβRII‐expressing tumors.( 7 ) In addition, among the genes downregulated in dnTβRII‐expressing tumors in vivo to 50% of the expression level in control tumors, we identified a tissue inhibitor of metalloproteinase 2 (TIMP2), which had not been previously reported to be affected by TGF‐β signaling.
TIMP2 is an endogenous inhibitor of matrix metalloproteinases (MMP). In addition, TIMP2 is reported to function as an inhibitor of tumor progression and invasion in several cancer models,( 10 , 11 , 12 ) but the involvement of TIMP2 in gastric cancer models has not been fully elucidated. In the present study, we therefore investigated the function of TIMP2 in diffuse‐type gastric carcinoma cells. We showed that lentiviral introduction of the TIMP2 gene inhibited growth of the diffuse‐type gastric cancer using OCUM‐2MLN cells expressing dnTβRII in vivo.
Materials and Methods
Cell lines. The OCUM‐2MLN cell line was established as described previously,( 8 ) and cultured in DMEM containing 10% fetal bovine serum (FBS) and 50 U/mL penicillin‐streptomycin (Invitrogen, Carlsbad, CA, USA). The 2MLN‐green fluorescent protein (GFP) and 2MLN‐dnTβRII cells were previously generated,( 7 ) whereas the GFP+GFP, dnTβRII+GFP and dnTβRII+TIMP2 cells were established using a lentiviral infection system as described.( 13 ) TIMP2 cDNA was amplified by PCR from OCUM‐2MLN cDNA using forward primer 5′‐AAAGAATTCATGGGCGCCGCGG ‐3′ and reverse primer 5′‐ATACTCGAGTTATGGGTCCTCGATGTC ‐3′, and inserted into the multicloning site of the lentiviral vector pCSII‐EF‐RfA using pENTR vector according to standard protocol (Invitrogen).
Antibodies and reagents. Antibodies to TIMP2, platelet/endothelial cell adhesion molecule‐1 (PECAM1), neuron‐glial antigen 2 (NG2) and Hypoxyprobe were from Abcam (Cambridge, UK), BD PharMingen (Flanklin Lakes, NJ, USA), Chemicon (Temecula, CA, USA) and HPI, Inc. (Burlington, MA, USA), respectively. Alexa 488‐, Alexa 594‐ and Alexa 633‐conjugated secondary antibodies were purchased from Invitrogen Molecular Probes (Eugene, OR, USA). TGF‐β1 (1 ng/mL; R& D Systems, Minneapolis, MN, USA) was used for the TGF‐β stimulation in vitro.
RNA isolation and quantitative RT‐PCR. Total RNA from cultured cells and excised subcutaneous tumors were extracted using the RNeasy Mini Kit (QIAGEN). First‐strand cDNA were synthesized using Quantitect Reverse Transcription kit (QIAGEN) with random hexamer primers. Quantitative real‐time RT‐PCR analysis was performed using 7500 Fast Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA). The primer sequences used were as follows:
| human GAPDH | forward 5′‐GAAGGTGAAGGTCGGAGTC‐3′ |
| reverse 5′‐GAAGATGGTGATGGGATTTC‐3′ | |
| human TIMP2 | forward 5′‐AGATGTAGTGATCAGGGCCAAAG‐3′ |
| reverse 5′‐GCTTGATCTCATACTGGATCCTCTTG‐3′ |
Cell growth assay. 2 × 104 cells were seeded in each well in 12‐well plates (day 0), and cultured in DMEM containing 1% FBS and 50 U/mL penicillin‐streptomycin (Invitrogen). The cell count was done in triplicate using a counter‐chamber at day 1, 2 and 3 after cell seeding.
Immunoblotting. Cultured cells were lysed in a buffer containing 50 mM Tris‐HCl, pH 8.0, 150 mM NaCl, 1% Nonidet P‐40 (Nacalai Tesque, Kyoto, Japan), 5 mM EDTA, 0.5% deoxycholic acid sodium salt‐monohydrate (Nacalai Tesque), 0.1% SDS (Nacalai Tesque), 1% aprotinin (Mitsubishi Pharma, Osaka, Japan) and 1 mM phenylmethylsulfonyl fluoride (Sigma). The cell lysates were boiled in SDS sample buffer (100 mM Tris‐HCl, pH 8.8, 0.01% bromophenol blue, 36% glycerol, 4% SDS and 10 mM dithiothreitol) and subjected to SDS‐PAGE. Proteins were electrotransferred to Pall Fluorotrans‐W membrane (Pall, East Hills, NY, USA), immunoblotted with antibodies and detected using an enhanced chemiluminescence (ECL) detection system (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
In vivo cancer models. BALB/c nude male mice aged 4–5 weeks were obtained from Oriental Yeast (Tokyo, Japan). All animal experimental protocols were performed in accordance with the policies of the Animal Ethics Committee of the University of Tokyo. In the subcutaneous xenograft model, a total of 5 × 106 cells in 100 μL of DMEM were injected into male nude mice. Tumor size was measured from 1 week after injection. In the orthotopic model, a total of 5 × 106 OCUM‐2MLN cells in 50 μL of DMEM were injected into the gastric walls of male nude mice under deep inhalation anesthesia with ether. Approximately 4 weeks later the mice were killed and tumors were measured and harvested.
Quantification of tumors in vivo. Subcutaneous xenografts were measured externally every other day until the end of the evaluation periods, and the orthotopic xenografts were measured after harvest. Tumor volume was approximated using the equation vol = (a × b2)/2, where vol is the volume, a is the length of the major axis and b is the length of the minor axis. Relative tumor volume was calculated by dividing the tumor volume by that measured on the first day of the evaluation (6 days after implantation).
Histochemistry and immunohistochemistry. Tumors were excised, fixed for 1 h in 10% neutral‐buffered formalin at room temperature, washed for 1–4 h in PBS containing 10% sucrose, then washed for 1–4 h in PBS containing 15% sucrose, and finally washed overnight in PBS containing 20% sucrose. The tumors were embedded in optimal cutting temperature compound (Tissue‐Tek, Sakura Finetek, Tokyo, Japan), and snapped frozen in dry‐iced acetone for immunohistochemical examination. Frozen samples were further sectioned at 10‐μm thickness in a cryostat, and then incubated with primary and secondary antibodies. Samples were observed using a Zeiss LSM510 Meta confocal microscope (Tokyo, Japan) for immunohistochemistry. Images were imported into Adobe Photoshop and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
In vivo hypoxia detection. Hypoxyprobe (pimonidazole hydrochloride; HPI, Inc.; 60 mg/kg body weight), which binds to hypoxic cells, was injected through the tail vein of nude mice 30 min before tumor harvest. The hypoxic area was analyzed using immunohistochemistry with antibody against Hypoxyprobe.
Statistical analysis. For data storage and analysis, Excel software (Microsoft, Redmond, WA, USA) was used. Statistical differences were compared using two‐sided Student’s t‐test and considered significant when P < 0.05.
Results
Regulation of TIMP2 expression by TGF‐β signaling. From the oligonucleotide microarray analyses of mRNA extracted from the in vivo tumor model,( 7 ) we have identified genes affected by expression of dnTβRII in OCUM‐2MLN tumors (data not shown). Among them, here we investigated TIMP2, which has been reported to function as an inhibitor of tumor progression and invasion of certain types of tumors,( 10 , 11 , 12 ) but not been reported to be affected by TGF‐β signaling. The result of the microarray analysis, exhibiting reduction of TIMP2 expression in the OCUM‐2MLN tumors expressing dnTβRII, was confirmed by quantitative real‐time PCR of mRNA extracted from in vivo xenografts with primers specific to human TIMP2 (Fig. 1A). We next determined the expression of TIMP2 mRNA in OCUM‐2MLN cells in vitro by quantitative real‐time PCR. TIMP2 expression was upregulated by TGF‐β1 treatment (1 ng/mL) in the control cells (2MLN cells expressing GFP), but not in those expressing dnTβRII due to disrupted TGF‐β signaling (Fig. 1B).
Figure 1.
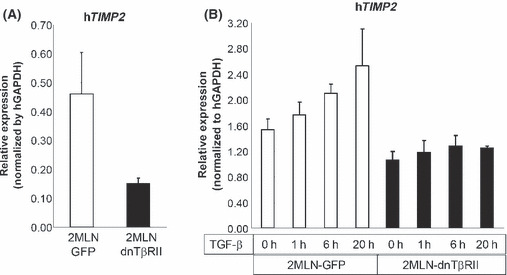
Regulation of tissue inhibitor of metallo‐proteinase 2 (TIMP2) expression by transforming growth factor (TGF)‐β signaling. (A) Expression levels of human TIMP2 mRNA in tissue from xenografted subcutaneous tumors expressing dominant negative form of the TGF‐β type II receptor (dnTβRII) or green fluorescent protein (GFP) were analyzed by quantitative real‐time PCR. (B) Levels of expression of TIMP2 mRNA in OCUM‐2MLN cells in vitro were determined by quantitative real‐time PCR. OCUM‐2MLN cells expressing GFP or dnTβRII were treated with TGF‐β1 (1 ng/mL) for 0, 1, 6 or 20 h before harvest and RNA isolation.
Establishment of cells overexpressing TIMP2. To investigate the effect of TIMP2 on the accelerated growth of dnTβRII tumors, we lentivirally re‐infected GFP or TIMP2 genes to the previously established OCUM‐2MLN cells expressing GFP or HA‐tagged dnTβRII. In this way, we established three new cell lines for OCUM‐2MLN: GFP+GFP, dnTβRII+GFP and dnTβRII+TIMP2. Overexpression of TIMP2 in OCUM‐2MLN in the cell lysates and conditioned medium was confirmed by immunoblot analysis and quantitative real‐time PCR (Fig. 2A,B). Next, we checked the growth of the TIMP2 expressing OCUM‐2MLN cells in vitro (Fig. 2C). No significant difference in the growth rate was observed between GFP+GFP, dnTβRII+GFP and dnTβRII+TIMP2 cells.
Figure 2.
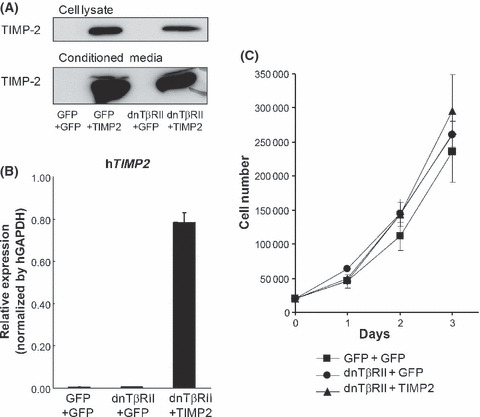
Establishment of cells overexpressing tissue inhibitor of metalloproteinase 2 (TIMP2). (A,B) Stable expression of TIMP2 in 2MLN‐dnTβRII cells. OCUM‐2MLN green fluorescent protein (GFP)+GFP, OCUM‐2MLN dnTβRII+GFP and OCUM‐2MLN dnTβRII+TIMP2 cell lines were established using a lentiviral system. Proteins and RNA were harvested from the cultured cell lines, and expression of TIMP2 was confirmed by immunoblotting using antibody against TIMP2 (A) and quantitative real‐time PCR using primers against human TIMP2 (B). (C) In vitro growth assay of OCUM‐2MLN cell lines (GFP+GFP, dnTβRII+GFP and dnTβRII+TIMP2 cell lines) cultured in growth medium containing 1% FBS. The cell count was done in triplicate at day 1, 2 and 3 after cell seeding.
Growth of gastric cancer cells overexpressing TIMP2 in vivo. Al‐though growth rates of GFP+GFP, dnTβRII+GFP and dnTβRII+TIMP2 cells in vitro were not different, we then performed subcutaneous transplantation into nude mice using these cells and monitored tumor growth. The growth of dnTβRII+GFP tumors was accelerated compared with GFP+GFP control tumors (P < 0.00005), which was completely abolished by overexpression of TIMP2 (P < 0.00005) in OCUM‐2MLN cells (Fig. 3A). We also orthotopically transplanted OCUM‐2MLN cells expressing GFP+GFP, dnTβRII+GFP or dnTβRII+TIMP2 into the gastric wall of nude mice, and determined the sizes of tumors (n = 6 for each group) on day 21 after implantation (Fig. 3B). The result in the orthotopic model confirmed that of the subcutaneous transplantation. The mean tumor volume of dnTβRII+GFP (0.79 cm3) was significantly larger than that of GFP+GFP (0.33 cm3) (P = 0.016), while the mean volume of dnTβRII+TIMP2 tumors (0.24 cm3) was significantly smaller than that of dnTβRII+GFP (P = 0.0025), and reduced to the same level as that of GFP+GFP tumors.
Figure 3.
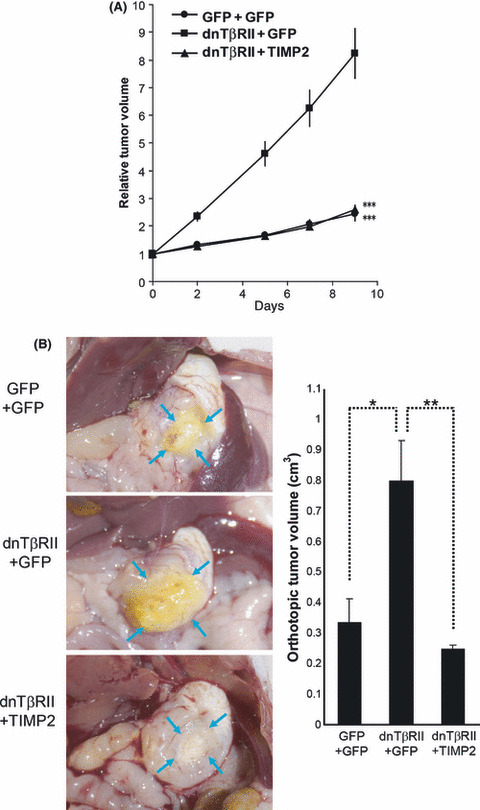
In vivo growth of OCUM‐2MLN tumors expressing the dominant negative form of the TGF‐β type II receptor (dnTβRII) and tissue inhibitor of metalloproteinase 2 (TIMP2). (A) green fluorescent protein (GFP)+GFP, dnTβRII+GFP or dnTβRII+TIMP2 expressing OCUM‐2MLN cells were transplanted subcutaneously into nude mice. Relative tumor volumes from the day of starting the evaluation (designated as day 0, 6 days after implantation) are shown. (B) The same cell lines as in (A) were orthotopically transplanted into the gastric walls of nude mice. Left panel: representative images of orthotopic tumors (marked by blue arrows) at day 21 after transplantation. Right panel: relative tumor volumes. Error bars represent standard errors (*P < 0.05, **P < 0.01, ***P < 0.001, n = 6–8).
Effect of TIMP2 overexpression on tumor angiogenesis. Since we previously showed that expression of dnTβRII induced tumor angiogenesis in vivo and accelerated tumor growth, we next examined whether the effect of TIMP2 on tumor growth in this model was due to inhibition of angiogenesis. We studied the vascular density of OCUM‐2MLN subcutaneous tumors by immunohistochemistry using PECAM1, a specific marker of vascular endothelium (Fig. 4). The PECAM1‐positive area was significantly increased in dnTβRII+GFP tumors (5.3% per microscopic field) compared with GFP+GFP tumors (2.1%) (P < 0.000001). Coexpression of TIMP2 with dnTβRII only weakly reduced the PECAM1‐positive area (4.3% per microscopic field) compared with dnTβRII+GFP tumors (P = 0.035), although the length of the vessels appeared to be reduced in the tumors expressing TIMP2.
Figure 4.
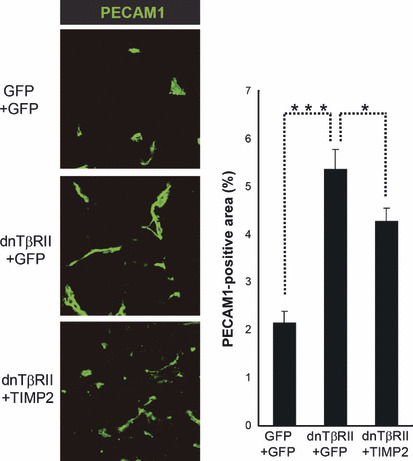
Effect of tissue inhibitor of metalloproteinase 2 (TIMP2) overexpression on tumor angiogenesis. The vascular density of tumor vasculature in OCUM‐2MLN subcutaneous tumors (n = 3–4 tumors for each condition). The vascular density was determined by immunostaining with an antibody against platelet/endothelial cell adhesion molecule‐1 (PECAM1). Left panel: representative micrographs of immunostained tumor sections photographed at ×40 magnification, with PECAM1‐positive areas shown in green. Right panel: percentage of the PECAM1‐positive area per microscopic field (at least 18 microscopic fields were analyzed for each condition). dnTβRII, dominant negative form of the TGF‐β type II receptor; GFP, green fluorescent protein. Error bars represent standard errors (*P < 0.05, ***P < 0.001).
TIMP2 overexpression reduces the function of tumor vessels. Although the decrease of the PECAM1‐positive area by TIMP2 was small, the altered vessel architecture suggests that the function of blood vessels may be reduced significantly in dnTβRII+TIMP2 tumors. As a way of evaluating the function of tumor vessels, we analyzed the degree of oxygen delivery using Hypoxyprobe (pimonidazole hydrochloride), which is a molecule that binds to hypoxic cells. Subcutaneous transplantation of GFP+GFP, dnTβRII+GFP or dnTβRII+TIMP2 cells to nude mice was performed. At day 15 after transplantation in OCUM‐2MLN models, Hypoxyprobe (60 mg/kg bodyweight) was injected through the tail vein of the mice 30 min before the harvest of tumors. The hypoxic area was then analyzed by immunohistochemistry with an antibody against Hypoxyprobe. The intratumor hypoxic area was significantly reduced in tumors expressing dnTβRII+GFP (12.3% per microscopic field) compared with GFP+GFP controls (34.8%) (P < 0.00005), correlating with increased PECAM1‐positive blood vessel density (Fig. 5A). On the other hand, the hypoxic area was significantly increased in dnTβRII+TIMP2 tumors (35.7% per microscopic field) compared with dnTβRII+GFP (P < 0.00005), and Hypoxyprobe‐positive staining was observed even in areas with high vessel density. These observations suggest that the shorter vessels in dnTβRII+TIMP2 tumors were less functional and unable to deliver oxygen efficiently to the surrounding tumor tissue. To further elucidate the mechanism of this reduction of vessel function in dnTβRII+TIMP2 tumors, we investigated the degree of pericyte coverage around the tumor vessels. Pericytes have been reported to be important for maturation of vasculature, and reported to be identified as NG2‐positive cells adjacent to the vessels, where NG2 is a marker of stromal cells including pericytes. Here we performed double staining of PECAM1 and NG2, and found that dnTβRII+TIMP2 tumors had less pericyte‐covered vasculature than dnTβRII+GFP tumors (Fig. 5B).
Figure 5.
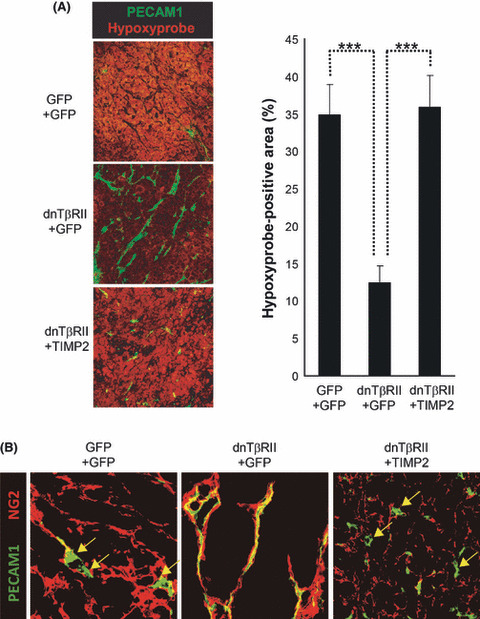
Effect of tissue inhibitor of metallo‐proteinase 2 (TIMP2) overexpression on the function of tumor vessels. (A) The hypoxic area in OCUM‐2MLN subcutaneous tumors (n = 3–4 tumors for each condition). The hypoxic area of tumors was determined by immunostaining with an antibody against Hypoxyprobe. Left panel: representative micrographs of immunostained tumor sections photographed at ×20 magnification, with areas positive for Hypoxyprobe shown in red. Platelet/endothelial cell adhesion molecule‐1 (PECAM1), green. Right panel: percentage of Hypoxyprobe‐positive area per microscopic field (18–20 microscopic fields were analyzed for each condition). Error bars represent standard errors (***P < 0.001). (B) Vessel structure in OCUM‐2MLN subcutaneous tumors. Representative micrographs of tumor sections photographed at ×40 magnification with immunostaining of endothelial cells and pericytes using an antibody against PECAM1 (green) and neuron‐glial antigen 2 (NG2) (red), respectively. Yellow arrows indicate PECAM1‐positive vessels lacking co‐staining of NG2. dnTβRII, dominant negative form of the TGF‐β type II receptor; GFP, green fluorescent protein.
Discussion
Tissue inhibitors of metalloproteinases are endogenous inhibitors of MMP, endopeptidases that regulate tissue remodeling through the proteolytic degradation or activation of cell surface and extracellular matrix proteins.( 14 ) Increased MMP activity can lead to tissue damage and progression of various diseases, playing an important role in angiogenesis and tumor progression.( 15 , 16 )
Tissue inhibitor of metalloproteinase 2 binds to the catalytic site of activated MMP, thus inhibiting protease activity,( 17 , 18 ) but apart from its inhibitory effect TIMP2 is also a cofactor for MMP2 activation mediated by membrane‐type 1 MMP (MT1‐MMP),( 19 ) suggesting a complex role for TIMP2 in MMP regulation. In addition, TIMP2 can function in an MMP‐independent manner, as TIMP2 has been shown to inhibit fibroblast growth factor 2 (FGF2)‐ or vascular endothelial growth factor (VEGF)‐A‐induced proliferation of endothelial cells in vitro and angiogenesis in vivo. ( 20 ) Interaction of TIMP2 with integrin α3β1 on the endothelial cell surface results in dissociation of the protein tyrosine phosphatase Shp‐1 from the integrin complex and increased Shp‐1 association with and inactivation of the receptor tyrosine kinases FGF receptor (FGFR)‐1 and KDR (receptors for FGF2 and VEGF‐A, respectively), leading to reduced cell proliferation.( 20 ) Furthermore, TIMP2 was shown to induce Shp‐1 mediated induction of p27Kip1 expression and subsequent G1 growth arrest of endothelial cells in vitro. ( 21 )
This is the first report showing the TGF‐β‐induced upregulation of TIMP2 expression in gastric carcinoma cells in vitro and in vivo. Tumors formed by subcutaneous transplantation of the diffuse‐type gastric carcinoma OCUM‐2MLN cells, lacking intracellular TGF‐β signaling due to stable expression of dnTβRII, are more angiogenic and show more accelerated growth compared with control tumors with functional TGF‐β signaling.( 7 ) In addition to reduced expression of anti‐angiogenic factor TSP1, we have found that they expressed less TIMP2 compared with control tumors. Furthermore, we have shown that in both orthotopic and subcutaneous transplantation models of OCUM‐2MLN cells applied to nude mice, accelerated tumor growth due to expression of dnTβRII was completely abolished when TIMP2 was coexpressed with dnTβRII.
Although vessel density determined by PECAM1 immunostaining of tumor samples was only slightly reduced in tumors overexpressing TIMP2, the vessels in TIMP2 tumors were shorter, at least in cross‐sections. These vessels in TIMP2 tumors also had less pericyte coverage, suggesting a reduced function of the tumor vessels, since pericytes are needed for stabilization of vascular structure.( 22 , 23 ) Consequently, the degree of hypoxia in dnTβRII+TIMP2 tumors was significantly higher than that in dnTβRII+GFP tumors, even in areas with high vessel density, suggesting that the blood vessels in dnTβRII+ TIMP2 tumors are unable to efficiently deliver oxygen to the surrounding tumor tissue. This is similar to the results seen by blocking Delta‐like ligand 4 (Dll4)/Notch signaling in C6 glioma tumors, which, despite increased blood vessel density, resulted in increased hypoxia and reduced tumor growth.( 24 ) We investigated whether TIMP2 expression repressed Notch signaling, but found no significant effect of TIMP2 overexpression on expression of the known Notch‐regulated genes that we analyzed (E. Johansson et al., unpublished data, 2008).
While we showed that TIMP2 expression inhibited angiogenesis and tumor growth induced by dnTβRII expression, we have not fully elucidated the mechanisms behind this. Tissue inhibitor of metalloproteinase 2 has been reported to directly inhibit endothelial cell migration, as well as growth in vitro. ( 20 , 25 ) The anti‐angiogenic effect of TIMP2 in this model could also be indirect, due to inhibition of MMP. Since the metalloproteinase MMP1 is highly expressed in OCUM‐2MLN cells and induced in tumors expressing dnTβRII in vivo (E. Johansson et al., unpublished data, 2007), it would be interesting to further study the MMP/TIMP balance in these cells and its effect on tumor growth.
In conclusion, the present study provides further evidence that induction of tumor angiogenesis is an important factor behind the increased tumor growth in diffuse‐type gastric carcinoma with disrupted TGF‐β signaling, and that one of the mechanisms of this increased angiogenesis may be the downregulation of the anti‐angiogenic protein TIMP2. Induction of TIMP2 expression might thus be a potential treatment strategy for diffuse‐type gastric cancer with disrupted TGF‐β signaling.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This work was supported by Grant‐in‐Aid for Scientific Research on Priority Areas (17016011) and Grant‐in‐Aid for Young Scientists (B) (19790282) from the Ministry of Education, Culture, Sports, Science and Technology, and by Health and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare. E.J. was a scholarship holder from the Swedish Foundation for International Cooperation and Higher Education (STINT) and from the Takeda Science Foundation.
References
- 1. Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol 2009; 472: 467–77. [DOI] [PubMed] [Google Scholar]
- 2. Lauren P. The two histological main types of gastric carcinoma: diffuse and so‐called intestinal‐type carcinoma. An attempt at a histo‐clinical classification. Acta Pathol Microbiol Scand 1965; 64: 31–49. [DOI] [PubMed] [Google Scholar]
- 3. Yashiro M, Chung YS, Nishimura S, Inoue T, Sowa M. Establishment of two new scirrhous gastric cancer cell lines: analysis of factors associated with disseminated metastasis. Br J Cancer 1995; 72: 1200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mizoi T, Ohtani H, Miyazono K, Miyazawa M, Matsuno S, Nagura H. Immunoelectron microscopic localization of transforming growth factor beta 1 and latent transforming growth factor beta 1 binding protein in human gastrointestinal carcinomas: qualitative difference between cancer cells and stromal cells. Cancer Res 1993; 53: 183–90. [PubMed] [Google Scholar]
- 5. Kinugasa S, Abe S, Tachibana M et al. Overexpression of transforming growth factor‐beta1 in scirrhous carcinoma of the stomach correlates with decreased survival. Oncology 1998; 55: 582–7. [DOI] [PubMed] [Google Scholar]
- 6. Kim JY, Park DY, Kim GH et al. Smad4 expression in gastric adenoma and adenocarcinoma: frequent loss of expression in diffuse type of gastric adenocarcinoma. Histol Histopathol 2005; 20: 543–9. [DOI] [PubMed] [Google Scholar]
- 7. Komuro A, Yashiro M, Iwata C et al. Diffuse‐type gastric carcinoma: progression, angiogenesis, and transforming growth factor beta signaling. J Natl Cancer Inst 2009; 101: 592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fujihara T, Sawada T, Hirakawa K et al. Establishment of lymph node metastatic model for human gastric cancer in nude mice and analysis of factors associated with metastasis. Clin Exp Metastasis 1998; 16: 389–98. [DOI] [PubMed] [Google Scholar]
- 9. Qiu H, Yashiro M, Shinto O, Matsuzaki T, Hirakawa K. DNA methyltransferase inhibitor 5‐aza‐CdR enhances the radiosensitivity of gastric cancer cells. Cancer Sci 2009; 100: 181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mendes O, Kim HT, Lungu G, Stoica G. MMP2 role in breast cancer brain metastasis development and its regulation by TIMP2 and ERK1/2. Clin Exp Metastasis 2007; 24: 341–51. [DOI] [PubMed] [Google Scholar]
- 11. Lee YK, So IS, Lee SC et al. Suppression of distant pulmonary metastasis of MDA‐MB 435 human breast carcinoma established in mammary fat pads of nude mice by retroviral‐mediated TIMP‐2 gene transfer. J Gene Med 2005; 7: 145–57. [DOI] [PubMed] [Google Scholar]
- 12. Brand K, Baker AH, Perez‐Canto A et al. Treatment of colorectal liver metastases by adenoviral transfer of tissue inhibitor of metalloproteinases‐2 into the liver tissue. Cancer Res 2000; 60: 5723–30. [PubMed] [Google Scholar]
- 13. Shibuya K, Shirakawa J, Kameyama T et al. CD226 (DNAM‐1) is involved in lymphocyte function‐associated antigen 1 costimulatory signal for naive T cell differentiation and proliferation. J Exp Med 2003; 198: 1829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol 2001; 11: S37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heissig B, Hattori K, Friedrich M, Rafii S, Werb Z. Angiogenesis: vascular remodeling of the extracellular matrix involves metalloproteinases. Curr Opin Hematol 2003; 10: 136–41. [DOI] [PubMed] [Google Scholar]
- 16. Parks WC, Mecham RP. Matrix Metalloproteinases. San Diego: Academic Press, 1998. [Google Scholar]
- 17. Umenishi F, Umeda M, Miyazaki K. Efficient purification of TIMP‐2 from culture medium conditioned by human hepatoma cell line, and its inhibitory effects on metalloproteinases and in vitro tumor invasion. J Biochem 1991; 110: 189–95. [DOI] [PubMed] [Google Scholar]
- 18. Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci 2002; 115: 3719–27. [DOI] [PubMed] [Google Scholar]
- 19. Kudo T, Takino T, Miyamori H, Thompson EW, Sato H. Substrate choice of membrane‐type 1 matrix metalloproteinase is dictated by tissue inhibitor of metalloproteinase‐2 levels. Cancer Sci 2007; 98: 563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seo D‐W, Li H, Guedez L et al. TIMP‐2 mediated inhibition of angiogenesis: an MMP‐independent mechanism. Cell 2003; 114: 171–80. [DOI] [PubMed] [Google Scholar]
- 21. Seo D‐W, Li H, Qu C‐K et al. Shp‐1 mediates the antiproliferative activity of tissue inhibitor of metalloproteinase‐2 in human microvascular endothelial cells. J Biol Chem 2006; 281: 3711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res 2006; 312: 623–9. [DOI] [PubMed] [Google Scholar]
- 23. Kano MR, Komuta Y, Iwata C et al. Comparison of the effects of the kinase inhibitors imatinib, sorafenib, and transforming growth factor‐beta receptor inhibitor on extravasation of nanoparticles from neovasculature. Cancer Sci 2009; 100: 173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noguera‐Troise I, Daly C, Papadopoulos NJ et al. Blockade of Dll4 inhibits tumour growth by promoting non‐productive angiogenesis. Nature 2006; 444: 1032–7. [DOI] [PubMed] [Google Scholar]
- 25. Oh J, Seo D‐W, Diaz T et al. Tissue inhibitors of metalloproteinase 2 inhibits endothelial cell migration through increased expression of RECK. Cancer Res 2004; 64: 9062–9. [DOI] [PubMed] [Google Scholar]


