Abstract
Pemetrexed (MTA) is a multitargeted antifolate with promising clinical activity in lung cancer. We exposed the small cell lung cancer cell line PC6 to stepwise‐increasing pemetrexed concentrations of 0.4, 1.6, and 4.0 μm, and established three pemetrexed‐resistant lung cancer cell lines: PC6/MTA‐0.4, PC6/MTA‐1.6, and PC6/MTA‐4.0 cells. To investigate the mechanisms of acquired resistance to pemetrexed, we measured the expression levels of the thymidylate synthase (TS), reduced folate carrier (RFC), and folylpoly‐gamma‐glutamate synthetase (FPGS) genes. TS gene expression was significantly increased in PC6/MTA‐1.6 and PC6/MTA‐4.0 cells relative to parental cells in a pemetrexed dose‐dependent manner. In contrast, the levels of RFC gene expression in PC6/MTA‐0.4 cells and FPGS in PC6/MTA‐1.6 cells were significantly decreased, whereas the levels of both genes were restored in PC6/MTA‐4.0 cells. Knockdown of TS expression using siRNA enhanced pemetrexed cytotoxicity in PC6/MTA‐4.0 cells. The expression level of the TS gene was significantly correlated with the concentration of pemetrexed for 50% cell survival (IC50) in 11 non‐small cell lung cancer cell lines. These results suggest that the alteration of molecular pharmacological factors in relation with pemetrexed resistance is dose‐dependent, and that up‐regulation of the expression of the TS gene may have an important role in the acquired resistance to pemetrexed. In addition, TS may be a predictive marker for pemetrexed sensitivity in lung cancer. (Cancer Sci 2009)
Pemetrexed is an MTA that targets the folate‐dependent enzymes TS, DHFR, GARFT, and AICARFT, all of which are involved in the de novo biosynthesis of thymidine and purine nucleotides.( 1 ) Pemetrexed is transported intracellularly, predominantly via the RFC, where it is metabolized to polyglutamated forms. Pemetrexed was found to be one of the best substrates for mammalian FPGS, and it is believed that polyglutamation and the polyglutamated metabolites play important roles in determining both the selectivity and antitumor activity of this agent.( 2 , 3 ) The polyglutamated metabolites of pemetrexed are most active against TS, followed by DHFR, GARFT and AICARFT, and natural folate competes with this inhibition in all cases.( 4 ) Therefore, the primary mechanism of the action of pemetrexed is inhibition of TS, which results in a decrease in the available thymidine necessary for DNA synthesis.( 2 , 4 )
Pemetrexed is a single agent that is currently approved for second‐line treatment of advanced‐stage NSCLC.( 5 , 6 ) In chemotherapy‐naïve patients with advanced NSCLC, double combinations of platinum compounds with gemcitabine, vinorelbine, paclitaxel, and docetaxel are standard regimens. A recent phase III trial found that cisplatin/pemetrexed provides equivalent efficacy with significantly fewer side effects and more convenient administration than cisplatin/gemcitabine in advanced NSCLC.( 7 ) Preclinical examinations found that the combination of pemetrexed with gemcitabine or elrotinib synergistically interacted against NSCLC cells.( 8 , 9 , 10 ) These results were pivotal in the approval of pemetrexed for the treatment of NSCLC.
In previous studies, TS overexpression was reported to induce acquired resistance to pemetrexed in colon and breast cancer cell lines.( 11 , 12 ) Also, decreased expression of RFC and FPGS was also reported to be associated with induction of resistance to pemetrexed in an L1210 murine leukemia cell line.( 13 ) In gastric cancers on the other hand, the sensitivity of pemetrexed was not predicted by the expression levels of the TS, RFC, and FPGS genes.( 14 ) Thus, the mechanism of resistance to pemetrexed is not fully understood, but may involve impaired membrane transport, reduced polyglutamation, and/or increased levels of the target enzyme.( 15 ) These observations prompted us to determine whether TS, RFC, and/or FPGS may be a major determinant of sensitivity or resistance to pemetrexed and how they interact in lung cancer. We established three pemetrexed‐resistant lung cancer cell lines and used them to further understand the mechanism of resistance to pemetrexed in lung cancer.
Materials and Methods
Cell lines and chemicals. The following human NSCLC cell lines were used in this study: eight adenocarcinomas (ACC‐LC‐94, ACC‐LC‐176, NCI‐H23, NCU‐LC‐201, RERF‐LC‐AI, RERF‐LC‐MT, RERF‐LC‐MS, and SK‐LC‐10), two squamous cell carcinomas (PC10 and QG56), and one large‐cell carcinoma (SK‐LC‐6). Cells were cultured in RPMI‐1640 medium supplemented with 10% heat‐inactivated FBS and 1% (v/w) penicillin/streptomycin in a humidified chamber (37°C, 5% CO2). The human small‐cell lung cancer cell line PC6 was continuously exposed to stepwise‐increasing pemetrexed concentrations up to 0.4 μm for 4 months, 1.6 μm for 6 months, and 4.0 μm for 8 months, which resulted in three pemetrexed‐resistant sublines; PC6/MTA‐0.4, PC6/MTA‐1.6, and PC6/MTA‐4.0. PC6/MTA‐0.4 cells were cultured in 0.4 μm pemetrexed, PC6/MTA‐1.6 cells in 1.6 μm pemetrexed, and PC6/MTA‐4.0 cells in 4.0 μm pemetrexed. Pemetrexed was provided by Eli Lilly Pharmaceuticals (Indianapolis, IN, USA), 5‐FU was obtained from Kyowa Hakko Kogyo (Tokyo, Japan), and methotrexate was purchased from Wako Chemicals (Osaka, Japan).
Total RNA extraction and quantitative real‐time RT‐PCR. Total RNA extraction and real‐time PCR with the LightCycler FastStart DNA SYBR Green kit (Roche Diagnostics, Indianapolis, IN, USA) were performed as described previously.( 16 ) The sequences of the TS, RFC, and FPGS primers have been described previously.( 14 ) We used a melting curve analysis to control for specificity of the amplification products. The number of transcripts was calculated from a standard curve obtained by plotting the known input of six different concentrations versus the PCR cycle number at which the detected fluorescence intensity reached a fixed value. The PCR program was 45 cycles at 94°C for 15 s and 60°C for 1 min. For each sample, the data were normalized to the housekeeping gene GAPDH.
Protein extraction and Western blotting. Equal amounts of total cell lysates were solubilized in the sample buffer (50 mm Tris‐HCL (pH6.8), 2% SDS, 1 mm EDTA, 10% glycerol) with Complete, Mini; Protease Inhibitor Cocktail Tablets; and PhosSTOP Phosphatase Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN, USA). Subsequently, these lysates were electrophoresed on 10% Ready Gel Tris‐HCl Gel (Bio‐Rad Laboratories, Harculies, CA, USA) and transferred to Immobilon‐P filters (Millipore, Billerica, MA, USA). The filters were first incubated with primary antibodies for 3 h (TS) and 1 h (vinculin) at room temperature and then with horseradish peroxidase–conjugated secondary antibodies (GE Healthcare Bioscience, Amersham Place, UK). Antibody against TS was purchased from Chemicon International (Temecula, CA, USA), and vinculin antibody was obtained from Sigma‐Aldrich (St. Louis, MO, USA). The relative band intensities were determined by densitometry using NIH Image.
TS copy number assays. The TS gene copy number was analyzed for three pemetrexed‐resistant cells compared with PC6 cells by quantitative real‐time PCR, performed on StepOnePlus (Applied Biosystems, Foster City, CA, USA) by Taqman Copy Number Assays (Applied Biosystems). The PCR program was 40 cycles at 95°C for 15 s and 60°C for 1 min. The primer for TS (predesigned copy number assays ID: Hs00560134) was purchased from Applied Biosystems. We used the Rnase P gene as an endogenous control.
In vitro MTS assay. Cells were cultured at 5000 per well in 96‐well tissue culture plates. To assess cell viability, stepwise 10‐fold dilutions of the anticancer drug were added 2 h after plating, and the cultures were incubated at 37°C for 96 h. At the end of the culture period, 20 μL of MTS solution (CellTiter 96 Aqueous One Solution Cell Proliferation Assay; Promega, Madison, WI, USA) was added, the cells were incubated for a further 4 h, and the absorbance was measured at 490 nm using an ELISA plate reader. Chemosensitivity is expressed as the drug concentration producing 50% growth inhibition, as described previously.( 16 )
Transfection and siRNA experiments. Cells (1 × 106) were transfected with siRNA oligonucleotides to result in a final RNA concentration of 100 nm. We used the X‐tremeGENE siRNA Transfection Reagent (Roche Applied Science) according to the manufacturer’s instructions. At 24 h after transfection, total RNA was extracted, or the cells were cultured at 5000 per well in 96‐well tissue culture plates for 2 h, and the cultures were incubated at 37°C for 48 h after adding stepwise dilutions of pemetrexed to assess cell viability. At the end of the culture period, 20 μL of MTS solution was added, the cells were incubated for an additional 4 h, and the absorbance was measured at 490 nm using an ELISA plate reader. The siRNA oligonucleotides for TS (predesigned siRNA, ID 116928) and the negative control siRNA (silencer negative control 1 siRNA) were purchased from Ambion (Austin, TX, USA).
Statistical analysis. Spearman’s test was used for correlation analysis to compare expression of the TS, RFC, and FPGS genes, and the IC50 values for pemetrexed. The differences in cell viability between samples were evaluated with the Student’s unpaired t‐test. The level of significance was set at 5% using two‐sided analysis.
Results
Establishment of three pemetrexed‐resistant lung cancer sublines. To investigate the determinants of acquired resistance to pemetrexed in lung cancer, we established three pemetrexed‐resistant lung cancer cell lines PC6/MTA‐0.4, PC6/MTA‐1.6, and PC6/MTA‐4.0 cells (Fig. 1). The IC50 values for pemetrexed treatment of PC6/MTA‐0.4, PC6/MTA‐1.6, and PC6/MTA‐4.0 cells were approximately 0.5, 2.3, and 43.8 μm respectively, and they were more resistant by approximately 5‐, 23‐, and 438‐fold respectively, relative to PC6 cells (Table 1). The growth rates of PC6, PC6/MTA‐0.4, PC6/MTA‐1.6, and PC6/MTA‐4.0 cells were not changed (data not shown). We also observed cross‐resistance to MTX and 5‐FU in these three cell lines (Table 2).
Figure 1.
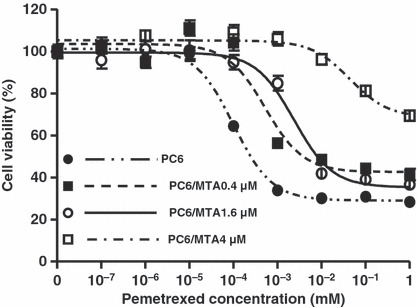
Establishment of three pemetrexed‐resistant lung cancer cell lines. Cytotoxicities of pemetrexed in PC6, PC6/MTA‐0.4, PC6/MTA, and PC6/MTA‐4.0 cells. PC6 cells were continuously exposed to stepwise‐increasing pemetrexed concentrations up to 0.4 μm for 4 months, 1.6 μm for 6 months, and 4.0 μm for 8 months, which resulted in three pemetrexed‐resistant sublines: PC6/MTA‐0.4, PC6/MTA‐1.6, and PC6/MTA‐4.0.
Table 1.
IC50values of pemetrexed in PC‐6, PC‐6/MTA‐0.4, PC‐6/MTA‐1.6, and PC‐6/MTA‐4.0 cells
| Cell lines | Pemetrexed IC50 (μm) | 95% CI (μm) | RR |
|---|---|---|---|
| PC6 | 0.1 | 0.07–0.13 | – |
| PC6/MTA‐0.4 | 0.53 | 0.32–0.88 | 5.3 |
| PC6/MTA‐1.6 | 2.38 | 1.51–3.76 | 23.8 |
| PC6/MTA‐4.0 | 43.87 | 22.08–87.20 | 438.7 |
RR, resistance rate: (IC50 in resistant subline)/(IC50 in the parental subline).
Table 2.
IC50values of Methotrexate and 5‐Fluorouracil in PC6, PC6/MTA‐0.4, PC‐6/MTA‐1.6, and PC6/MTA‐4.0 cells
| Cell lines | PC6 | PC6/MTA‐0.4 | PC6/MTA‐1.6 | PC6/MTA‐4.0 | |||
|---|---|---|---|---|---|---|---|
| Drug | IC50 (95% CI) | IC50 (95% CI) | RR | IC50 (95% CI) | RR | IC50 (95% CI) | RR |
| Methotrexate (nm) | 7.83 (5.04–12.16) | 29.32 (18.42–46.64) | 3.7 | 73.89 (24.31–224.60) | 9.4 | 126.1 (36.38–437.3) | 16.1 |
| 5‐Fluorouracil (nm) | 66.31 (49.45–88.92) | 353.4 (231.0–540.5) | 5.3 | 430.0 (341.1–552.6) | 6.5 | 1419 (1132–1778) | 21.4 |
RR, resistance rate: (IC50 in resistant subline)/(IC50 in the parental subline).
Expression levels of TS, RFC, and FPGS genes in three pemetrexed‐resistant lung cancer sublines. We used quantitative real‐time RT‐PCR to compare the expression levels of the TS, RFC, and FPGS genes in three pemetrexed‐resistant lung cancer cell lines with those in the parental PC6 cells. Compared to PC6 cells, the level of TS gene expression was significantly increased in PC6/MTA‐1.6 cells (2.5‐fold; P < 0.001) and PC6/MTA‐4.0 cells (20‐fold; P < 0.001), but was not changed in PC6/MTA‐0.4 cells (Fig. 2A). TS gene expression was higher with increasing stepwise concentrations of pemetrexed. RFC gene expression was decreased in PC6/MTA‐0.4 cells (45%; P <0.05) and PC6/MTA‐1.6 cells (62%; not significantly different) (Fig. 2B). FPGS gene expression was diminished in PC6/MTA‐0.4 cells (66%; P < 0.005) and PC6/MTA‐1.6 cells (33%; P < 0.005) (Fig. 2C). Interestingly, the levels of RFC and FPGS gene expression were restored in PC6/MTA‐4.0 cells to levels similar to those seen in the parental PC6 cells.
Figure 2.
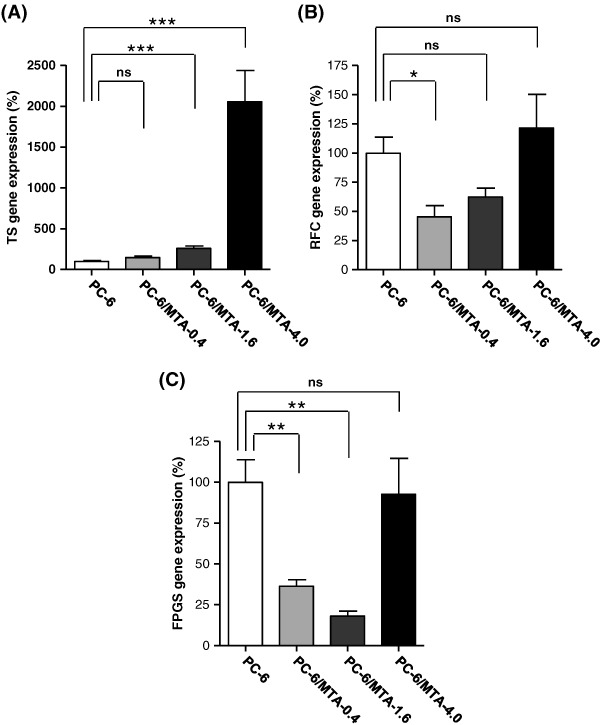
Expression levels of thymidylate synthase (TS), reduced folate carrier (RFC), and folylpoly‐gamma‐glutamate synthetase (FPGS) genes in PC‐6, PC‐6/MTA‐0.4, and PC6/MTA‐4.0 cells. Expression levels of TS, RFC, and FPGS genes were determined by real‐time PCR. ***P < 0.001. **P < 0.005. *P < 0.05. ns, not significantly different.
Expression levels of TS protein in three pemetrexed‐resistant lung cancer sublines. We examined the TS protein levels by Western blotting. The relative band intensities were determined by densitometry using Scion Image. Compared to PC6 cells, the level of TS protein was significantly up‐regulated in PC6/MTA‐0.4 cells (2.5‐fold; P < 0.05), PC6/MTA‐1.6 cells (3.5‐fold; P < 0.05), and PC6/MTA‐4.0 cells (6.0‐fold; P < 0.001) (Fig. 3A,B). TS protein expression was higher with increasing stepwise concentrations of pemetrexed. In addition, we examined the TS protein level of PC6/MTA‐4.0 cells with a pemetrexed‐free medium for 1 month. It was not changed relative to PC6/MTA‐4.0 cells with 4 μm pemetrexed concentration medium (data not shown). Therefore, we think that PC6/MTA‐4.0 cells are not revertant.
Figure 3.

Expression levels of thymidylate synthase (TS) protein and TS gene copy number in PC‐6, PC‐6/MTA‐0.4, and PC6/MTA‐4.0 cells. (A) TS protein expression in PC6, PC6/MTA‐0.4, PC6/MTA1.6, and PC‐6/MTA‐4.0 cells by Western blotting. (B) The relative band intensities by densitometry using NIH Image. (C) The relative TS gene copy numbers in PC6, PC6/MTA‐0.4, PC6/MTA1.6, and PC‐6/MTA‐4.0 cells compared with PC6 cells.
TS gene amplification in three pemetrexed‐resistant lung cancer sublines. We examined the TS gene copy number in three pemetrexed‐resistant cells and PC6 cells. The relative DNA copy numbers of PC6/MTA‐0.4, PC6/MTA‐1.6, and PC6/MTA‐4.0 cells was increased by about 2.1‐, 2.3‐, and 23.5‐fold compared with the parental cells (Fig. 3C). These data suggested that the TS gene amplification might be one of mechanisms of acquired resistance to pemetrexed.
Enhanced cytotoxicity of pemetrexed in PC‐6/MTA‐4.0 cells by TS siRNA. Given the importance of TS overexpression for acquired resistance to pemetrexed, we transfected PC6/MTA‐4.0 cells with TS siRNA and examined whether modification of TS gene expression altered pemetrexed cytotoxicity. At 24 h after transfection, total RNA was extracted and TS gene expression was measured using real‐time PCR. Relative to PC6/MTA‐4.0 cells transfected with negative‐control siRNA, the level of TS gene expression was significantly diminished by approximately 35% in cells treated with TS siRNA (P < 0.005) and was not changed in non‐transfected cells (Fig. 4A). At 72 h after transfection, we assessed cell viability using the MTS assay. The cytotoxicity induced by pemetrexed was greater in PC6/MTA‐4.0 cells transfected with TS siRNA than in cells transfected with negative‐control siRNA (Fig. 4B). Thus, decreased TS gene expression resulted in alteration of pemetrexed sensitivity.
Figure 4.
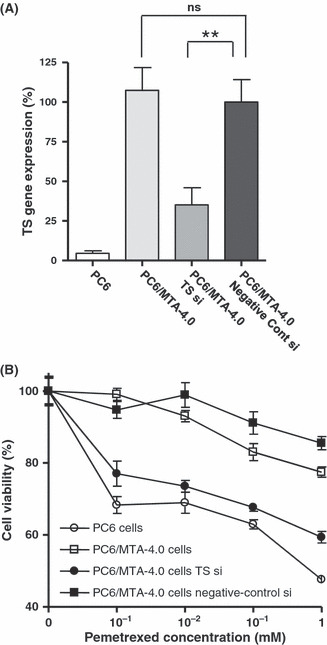
Modification of pemetrexed cytotoxicity by thymidylate synthase (TS) siRNA. (A) Expression level of the TS gene in PC‐6/MTA‐4.0 cells transfected with TS siRNAs, in control siRNA‐transfected and non‐transfected PC‐6/MTA‐4.0 cells. **P < 0.005. ns, not significantly different. (B) Cytotoxicity of 1 μm, 10 μm, 100 μm, and 1 mM pemetrexed in PC‐6/MTA‐4.0 cells transfected with TS siRNA, control siRNA, non‐transfected PC‐6/MTA‐4.0 cells, and non‐transfected PC‐6 cells. Open circle (○), PC6 cells; open square (□), PC6/MTA‐4.0 cells; closed circle (•), PC6/MTA‐4.0 cells transfected with TS siRNA; closed square (), PC6/MTA‐4.0 cells transfected with negative‐control siRNA.
Relationship between pemetrexed cytotoxicity and TS gene expression. We determined the expression levels of the TS, RFC, and FPGS genes in 11 NSCLC cell lines using real‐time PCR and compared the IC50 values for pemetrexed in these cell lines with the relative expression levels of TS, RFC, and FPGS. We found a significant correlation between the TS gene expression and pemetrexed sensitivity in all 11 NSCLC cell lines (r = 0.624, P < 0.005) (Fig. 5A), but the expression levels of the RFC and FPGS genes were not significantly correlated with pemetrexed sensitivity (Fig. 5B,C).
Figure 5.
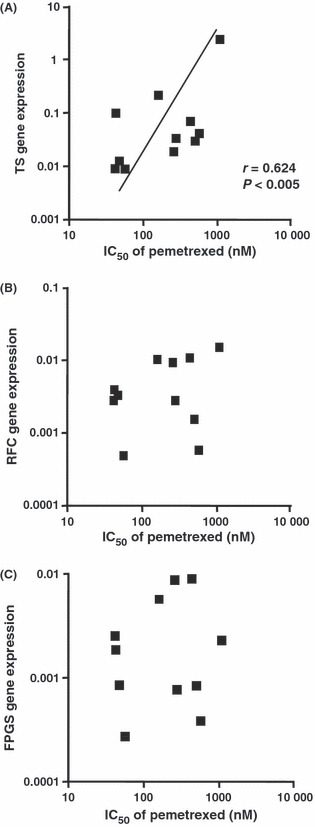
Relationship between pemetrexed sensitivity and the expression levels of thymidylate synthase (TS), reduced folate carrier (RFC)‐1, and folylpoly‐gamma‐glutamate synthetase (FPGS) genes in non‐small‐cell lung cancer (NSCLC) cell lines. TS expression and pemetrexed sensitivity are strongly correlated in all NSCLC cell lines investigated (n = 11, r = 0.624, P < 0.005), but no significant correlation was observed between the expression levels of the other genes investigated and pemetrexed sensitivity. Each IC50 value is the mean of three independent sensitivity tests performed in quadruplicate. Expression levels were normalized to GAPDH expression. The statistical significance of each correlation was determined by Spearman’s correlation test. Closed square (), NSCLC cells.
Discussion
In this study, we have established for the first time three pemetrexed resistant lung cancer cell lines with three different pemetrexed concentrations to elucidate whether the TS, RFC, and/or FPGS genes were a major determinant of acquired resistance to pemetrexed and to examine any interactions among the expression of these genes.
The central role of TS in DNA biosynthesis and tumor biology has been established. Therefore, significant efforts have focused on designing novel inhibitors of TS that exhibit higher potency. 5‐FU represents the first class of TS inhibitors. It is converted intracellularly to FdUMP, which can form a stable covalent bond with TS, resulting in its inhibition. Acute induction of TS expression is one of several mechanisms of acquired resistance to 5‐FU, because TS that is stably bound to FdUMP is no longer able to bind its mRNA and suppress its own translation, which results in increased TS protein expression.( 17 , 18 ) We have also reported that up‐regulation of TS gene expression contributes to acquired resistance to 5‐FU in lung cancer.( 16 ) Interestingly, incubation of TS with pemetrexed also significantly impairs its ability to interact with TS mRNA.( 18 ) Therefore, acute induction of TS may play an important role in acquired resistance to pemetrexed in the same manner as 5‐FU. In this study, the level of TS mRNA expression was significantly increased in PC6/MTA‐1.6 (2.5‐fold) and PC6/MTA‐4.0 cells (20‐fold) compared with PC6 cells. In addition, modification of TS gene expression by TS siRNA in PC6/MTA‐4.0 cells altered the cytotoxicity of pemetrexed. Previous studies have also reported that induction of TS is associated with resistance to pemetrexed in colon and breast cancer cells.( 11 , 12 ) Our findings here are consistent with these previous studies, and are the first to report the importance of TS in acquired resistance to pemetrexed in lung cancer.
RFC and FPGS activities may also be determinants of pemetrexed cytotoxicity,( 19 , 20 ) since pemetrexed utilizes RFC for entry into cells and requires polyglutamation by FPGS to exert maximal inhibitory effects on various target enzymes. Decreased expression of RFC and FPGS were also reported to be associated with induction of resistance to pemetrexed in an L1210 murine leukemia cell line.( 13 ) In this study, RFC and FPGS gene expression in PC6/MTA‐0.4 and PC6/MTA‐1.6 cells was potently decreased. Therefore, acquired resistance to pemetrexed may result from reduction in the intracellular concentration of pemetrexed due to decreased levels of RFC gene expression and/or inhibition of polyglutamation due to decreased levels of FPGS gene expression in low (0.4 μm) or middle (1.6 μm) concentrations. However, at a high (4 μm) concentration of pemetrexed, expression of both genes was restored to levels similar to those seen in the parental PC6 cells, and TS gene expression was significantly increased by 20‐fold. These results suggest that although the determinants of acquired resistance to pemetrexed may be altered by the pemetrexed concentration, TS overexpression may be one of major determinants of the acquired resistance to pemetrexed.
Decreased intracellular concentration of drug is often indicative of drug resistance, and many multidrug‐resistant proteins mediate cellular exclusion and resistance to antifolate drugs.( 21 ) Reduced accumulation of pemetrexed has been reported to be important for pemetrexed resistance.( 15 ) We think that the reduced cellular accumulation of pemetrexed was associated with not only RFC and FPGS but also ABC efflux transporters.( 22 ) Therefore, in future studies, we will explore other resistance genes including ABC transporters.
Given the importance of TS, RFC, and FPGS expression in pemetrexed cytotoxicity in NSCLC cells, we analyzed the relationship between the expression of these genes and the sensitivity to pemetrexed in lung cancer cell lines. TS, RFC, and FPGS have been reported to be predictive biomarkers for 5‐FU but not pemetrexed sensitivity in gastric and colon cancer cell lines.( 14 , 23 ) In the present study, however, the level of TS gene expression but not RFC and FPGS was significantly correlated with pemetrexed sensitivity in NSCLC cells. We previously reported that the expression level of DPD, the first rate‐limiting enzyme for conversion of 5‐FU into an inactive metabolite,( 17 ) was significantly correlated with 5‐FU sensitivity in NSCLC, whereas that of TS was not.( 16 ) This discrepancy may reflect differences in the expression levels of TS and DPD in different cancer types. NSCLC is characterized by high DPD and low TS activity,( 24 ) such that DPD levels and not TS levels predominantly affect the sensitivity to 5‐FU in NSCLC cells. However, TS‐targeting drugs may still be useful in NSCLC treatment since DPD does not affect pemetrexed metabolism. Thus, the alteration of TS activity may directly mediate pemetrexed cytotoxicity in NSCLC cells. Furthermore, our findings are consistent with two recent clinical trials. A phase II clinical trial looking at the effects of pemetrexed in advanced breast cancer revealed a potential association between low pemetrexed TS expression levels and response to pemetrexed chemotherapy.( 25 ) A phase III clinical trial of patients with lung cancer demonstrated that the overall survival of patients with adenocarcinoma but not squamous cell carcinoma who were randomly assigned to receive cisplatin/pemetrexed was significantly better than those receiving cisplatin/gemcitabine.( 7 ) TS expression was significantly higher in chemotherapy‐naive patients with lung squamous cell carcinoma than adenocarcinoma.( 26 ) Our in vitro study confirmed that the level of TS gene expression was significantly associated with sensitivity to pemetrexed in lung cancer cell lines.
We confirmed that the levels of TS protein expression in three pemetrexed resistant lung cancer cells were increased in a dose‐dependent manner. TS protein expression is affected not only by functional polymorphism of the TS gene, but also by TS gene amplification.( 27 , 28 ) The functional polymorphisms of the TS gene were reported to be a VNTR containing 2 (2R) or 3 (3R) repeats of 28 bp, an SNP of a G to C substitution in the 5′UTRs and a 6‐bp deletion at nucleotide 1494 in the 3′UTR (1494del6). However a previous study showed that the predictive marker of 5‐FU chemotherapy was not the polymorphism but the DNA copy numbers of the TS gene.( 27 ) Therefore, we comfirmed the DNA copy numbers of the TS gene in three pemetrexed‐resistant cells. We found that the TS copy numbers in three pemetrexed‐resistant cells were increased relative to the parental PC6 cells. Taken together with our results, TS gene amplification may be one of predictive markers of the resistance to pemetrexed.
We have established three pemetrexed‐resistant lung cancer cell lines and propose that TS overexpression may be one of major determinants of acquired resistance to pemetrexed, although its interaction with other genes, including RFC and FPGS, may also be important. In addition, we propose that the level of TS gene expression may predict drug sensitivity to pemetrexed. As pemetrexed has been approved for use in Japan, we plan to examine the association between TS gene amplification and sensitivity to pemetrexed in patients of lung cancer.
Abbreviations
- ABC
ATP‐binding cassette
- AICARFT
Aminoimidazole carboxamide formyl transferase
- DHFR
Dihydrofolate reductase
- DPD
Dihydropyrimidine dehydrogenase
- FdUMP
Fluorodeoxyuridine monophosphate
- FPGS
Folylpoly‐gamma‐glutamate synthetase
- GAPDH
Glyceraldehyde‐3‐phosphatedehydrogenase
- GARFT
Glycinamide ribonucleotide formyltransferase
- MTA
Multitargeted antifolate (pemetrexed)
- MTS
3‐(4,5‐dimethylthiazol‐2‐yl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐tetrazolium, inner salt
- MTX
Methotrexate
- NSCLC
Non‐small‐cell lung cancer
- RFC
Reduced folate carrier
- Rnase
P Ribonuclease P
- SNP
Single nucleotide polymorphism
- TS
Thymidylate synthase
- 5′UTRs
5′‐untranslated regions
- VNTR
Variable number of tandem repeats
Acknowledgement
We thank Shino Nakamura for her skillful technical assistance.
References
- 1. Adjei AA. Pemetrexed: a multitargeted antifolate agent with promising activity in solid tumors. Ann Oncol 2000; 11: 1335–41. [DOI] [PubMed] [Google Scholar]
- 2. Shih C, Chen VJ, Gossett LS et al. LY231514, a pyrrolo[2,3‐d]pyrimidine‐based antifolate that inhibits multiple folate‐requiring enzymes. Cancer Res 1997; 57: 1116–23. [PubMed] [Google Scholar]
- 3. Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther 2007; 6: 404–17. [DOI] [PubMed] [Google Scholar]
- 4. Shih C, Habeck LL, Mendelsohn LG, Chen VJ, Schultz RM. Multiple folate enzyme inhibition: mechanism of a novel pyrrolopyrimidine‐based antifolate LY231514 (MTA). Adv Enzyme Regul 1998; 38: 135–52. [DOI] [PubMed] [Google Scholar]
- 5. Hanna N, Shepherd FA, Fossella FV et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non‐small‐cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004; 22: 1589–97. [DOI] [PubMed] [Google Scholar]
- 6. Weiss GJ, Langer C, Rosell R et al. Elderly patients benefit from second‐line cytotoxic chemotherapy: a subset analysis of a randomized phase III trial of pemetrexed compared with docetaxel in patients with previously treated advanced non‐small‐cell lung cancer. J Clin Oncol 2006; 24: 4405–11. [DOI] [PubMed] [Google Scholar]
- 7. Scagliotti GV, Parikh P, Von Pawel J et al. Phase III Study Comparing Cisplatin Plus Gemcitabine With Cisplatin Plus Pemetrexed in Chemotherapy‐Naive Patients With Advanced‐Stage Non‐Small‐Cell Lung Cancer. J Clin Oncol 2008; 26: 1–11. [DOI] [PubMed] [Google Scholar]
- 8. Giovannetti E, Mey V, Nannizzi S et al. Cellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human non‐small‐cell lung cancer cells. Mol Pharmacol 2005; 68: 110–8. [DOI] [PubMed] [Google Scholar]
- 9. Oguri T, Achiwa H, Sato S et al. The determinants of sensitivity and acquired resistance to gemcitabine differ in non‐small cell lung cancer: a role of ABCC5 in gemcitabine sensitivity. Mol Cancer Ther 2006; 5: 1800–6. [DOI] [PubMed] [Google Scholar]
- 10. Giovannetti E, Lemos C, Tekle C et al. Molecular mechanisms underlying the synergistic interaction of erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor, with the multitargeted antifolate pemetrexed in non‐small‐cell lung cancer cells. Mol Pharmacol 2008; 73: 1290–300. [DOI] [PubMed] [Google Scholar]
- 11. Sigmond J, Backus HH, Wouters D, Temmink OH, Jansen G, Peters GJ. Induction of resistance to the multitargeted antifolate Pemetrexed (ALIMTA) in WiDr human colon cancer cells is associated with thymidylate synthase overexpression. Biochem Pharmacol 2003; 66: 431–8. [DOI] [PubMed] [Google Scholar]
- 12. Longley DB, Ferguson PR, Boyer J et al. Characterization of a thymidylate synthase (TS)‐inducible cell line: a model system for studying sensitivity to TS‐ and non‐TS‐targeted chemotherapies. Clin Cancer Res 2001; 11: 3533–9. [PubMed] [Google Scholar]
- 13. Wang Y, Zhao R, Goldman ID. Decreased expression of the reduced folate carrier and folypolyglutamate synthetase is the basis for acquired resistance to the pemetrexed antifolate (LY231514) in an L1210 murine leukemia cell line. Biochem Pharmacol 2003; 65: 1163–70. [DOI] [PubMed] [Google Scholar]
- 14. Kim JH, Lee KW, Jung Y et al. Cytotoxic effects of pemetrexed in gastric cancer cells. Cancer Sci 2005; 96: 365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schultz RM, Chen VJ, Bewley JR, Roberts EF, Shih C, Dempsey JA. Biological activity of the multitargeted antifolate, MTA (LY231514), in human cell lines with different resistance mechanisms to antifolate drugs. Semin Oncol 1999; 2: 68–73. [PubMed] [Google Scholar]
- 16. Oguri T, Achiwa H, Bessho Y et al. The role of thymidylate synthase and dihydropyrimidine dehydrogenase in resistance to 5‐fluorouracil in human lung cancer cells. Lung cancer 2005; 49: 345–51. [DOI] [PubMed] [Google Scholar]
- 17. Longley DB, Harkin DP, Johnston PG. 5‐fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 2003; 3: 330–8. [DOI] [PubMed] [Google Scholar]
- 18. Chu E, Callender MA, Farrell MP, Schmitz JC. Thymidylate synthase inhibitors as anticancer agents: from bench to bedside. Cancer Chemother Pharmacol 2003; 52: S80–9. [DOI] [PubMed] [Google Scholar]
- 19. Chattopadhyay S, Zhao R, Krupenko SA, Krupenko N, Goldman ID. The inverse relationship between reduced folate carrier function and pemetrexed activity in a human colon cancer cell line. Mol Cancer Ther 2006; 5: 438–49. [DOI] [PubMed] [Google Scholar]
- 20. Leil TA, Endo C, Adjei AA et al. Identification and characterization of genetic variation in the folylpolyglutamate synthase gene. Cancer Res 2007; 67: 8772–82. [DOI] [PubMed] [Google Scholar]
- 21. Hooijberg JH, De Vries NA, Kaspers GJ, Pieters R, Jansen G, Peters GJ. Multidrug resistance proteins and folate supplementation: therapeutic implications for antifolates and other classes of drugs in cancer treatment. Cancer Chemother Pharmacol 2006; 58: 1–12. [DOI] [PubMed] [Google Scholar]
- 22. Assaraf YG. Molecular basis of antifolate resistance. Cancer Metastasis Rev 2007; 26(1): 153–81. [DOI] [PubMed] [Google Scholar]
- 23. Van Triest B, Pinedo HM, Van Hensbergen Y et al. Thymidylate synthase level as the main predictive parameter for sensitivity to 5‐fluorouracil, but not for folate‐based thymidylate synthase inhibitors, in 13 nonselected colon cancer cell lines. Clin Cancer Res 1999; 5: 643–54. [PubMed] [Google Scholar]
- 24. Fukushima M, Morita M, Ikeda K, Nagayama S. Population study of expression of thymidylate synthase and dihydropyrimidine dehydrogenase in patients with solid tumors. Int J Mol Med 2003; 12: 839–44. [PubMed] [Google Scholar]
- 25. Gomez HL, Santillana SL, Vallejos CS et al. A phase II trial of pemetrexed in advanced breast cancer: clinical response and association with molecular target expression. Clin Cancer Res 2006; 12: 832–8. [DOI] [PubMed] [Google Scholar]
- 26. Ceppi P, Volante M, Saviozzi S et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer 2006; 107: 1589–96. [DOI] [PubMed] [Google Scholar]
- 27. Ooyama A, Okayama Y, Takechi T, Sugimoto Y, Oka T, Fukushima M. Genome‐wide screening of loci associated with drug resistance to 5‐fluorouracil‐based drugs. Cancer Sci 2007; 98 (4): 577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gosens MJ, Moerland E, Lemmens VP, Rutten HT, Tan‐Go I, Van den Brule AJ. Thymidylate synthase genotyping is more predictive for therapy response than immunohistochemistry in patients with colon cancer. Int J Cancer 2008; 123 (8): 1941–9. [DOI] [PubMed] [Google Scholar]


