Abstract
Standard treatment for elderly patients with relapsed or refractory DLBCL has not been established. CPT‐11 has a broad spectrum of anticancer activities including a cytotoxic effect in a variety of malignant tumors. The results of combined treatment with CPT‐11 and rituximab have not been reported. The R‐CMD regimen was given to elderly patients with relapsed or refractory DLBCL. The safety and efficacy of this regimen were studied. In addition, the serum nm23‐H1 level was determined to study whether or not it can serve as a prognostic factor. Thirty elderly patients with DLBCL were studied. The main non‐hematological toxicities were infusion‐related adverse events. Grade 3/4 hematological toxicity was seen in 19 patients. Following R‐CMD treatment, the BNP and troponin T levels did not increase. The CR rate was 57%, PR rate was 17%, 2‐year survival rate was 45.2%, and PFS rate was 37.2%. Patients with serum nm23‐H1 levels of higher than 80 ng/mL before the treatment showed significantly poorer prognosis. The serum nm23‐H1 level of the 30 subjects before the treatment was elevated at 39.4 ± 41.3 ng/mL, but it significantly decreased only in the subset of patients who achieved CR. The R‐CMD regimen was safe in elderly patients with DLBCL. No new signs of cardiotoxicity were observed with this regimen. It was also effective in patients with relapsed or refractory DLBCL who had previously used DXR. (Cancer Sci 2006; 97: 933–937)
Abbreviations:
- ANC
absolute neutrophil count
- CHOP
cyclophosphamide, doxorubicin, vincristine, prednisone
- CMD
CPT‐11, MIT, dexamethasone CR, complete remission
- DLBCL
diffuse large B‐cell lymphoma
- DXR
doxorubicin
- MIT
mitoxantrone
- ELISA
enzyme‐linked immunosorbent assay
- G‐CSF
granulocyte colony‐stimulating factor
- NHL
non‐Hodgkin's lymphoma
- PBS
phosphate‐buffered saline
- PFS
progression‐free survival
- PR
partial remission
- R‐CMD
rituximab, CPT‐11, mitoxantrone, dexamethasone
- T‐PBS
phosphate‐buffered saline containing 0.05% Tween 20
- ULN
upper limit of normal.
Rituximab combined with the CHOP regimen showed good results in patients older than 60 years with untreated DLBCL. The 5‐year overall survival rate was 58% and the 5‐year PFS rate was 54%.( 1 ) However, approximately half of the patients were either refractory to this treatment or relapsed after achieving remission. In young patients with relapsed DLBCL, autologous hematopoietic stem cell transplantation combined with high‐dose chemotherapy is given and the treatment results have improved. However, there are few reports of salvage therapy in elderly patients. Many elderly patients have complications such as myocardial damage, pulmonary dysfunction, liver dysfunction, and/or renal dysfunction prior to treatment. A consensus opinion has not been reached as to which drug or which combination of drugs is optimal for the treatment of elderly patients with DLBCL. Also, DXR is frequently used as pretreatment. However, the dosage or drugs chosen for salvage therapy are limited by DXR‐induced cardiomyopathy.
Irinotecan (CPT‐11) is a potent inhibitor of topoisomerase I, which is an enzyme that is normally active during DNA replication. The activity of CPT‐11 is not affected by P‐glycoprotein expression and there is minimum cross‐resistance with DXR or vincristine.( 2 ) Recently, CPT‐11 has been used in the treatment of cancer of the lung, ovary, colon, and stomach, and the efficacy of CPT‐11 as single therapy or in combination with other anticancer agents is being established. It has been reported that multiple small doses of CPT‐11 has a higher response rate than a single high dose in the treatment of NHL.( 3 ) However, a consensus opinion has not been reached.( 3 ) There are also many issues to evaluate, such as the kinds of drugs to combine with CPT‐11 and the order of administration of the drugs. In preclinical studies, the combination of topoisomerase I and topoisomerase II inhibitors showed an anticancer effect that was more than additive.( 4 , 5 ) In the treatment of NHL, a combination of CPT‐11 and etoposide failed to produce an anticancer effect that was stronger than CPT‐11 alone, and caused adverse reactions such as neutropenia and liver dysfunction.( 6 ) The therapeutic activity of MIT in NHL has been demonstrated in pretreated patients in phase I–II studies, with an objective response in 20–30% of patients with NHL.( 7 ) Therefore, we previously studied the combination of MIT, a topoisomerase II inhibitor that had not been used in previous treatments, and CPT‐11. The CMD regimen was previously given to 32 patients with relapsed or refractory NHL.( 8 ) CR was seen in 11 cases (34.4%) and PR in nine cases (28.1%). This regimen was found to be safe.
In the present study, we gave rituximab combined with the CMD regimen to elderly patients with relapsed or refractory DLBCL, and its safety and efficacy were evaluated. The purpose of this clinical phase II study was to evaluate the safety and efficacy of the above therapies. Serum nm23‐H1 levels before and after the R‐CMD regimen were determined.( 9 ) We also studied whether the serum nm23‐H1 level is useful as a prognostic factor.
Patients and methods
Study design
This was an open‐label, single‐arm phase II study of the R‐CMD regimen for the treatment of relapsed or refractory DLBCL in elderly patients. We evaluated the response rate, survival, and PFS. The safety of this regimen was also investigated. This study was approved by the Ethics Committee of Kitasato University School of Medicine (Kanagawa, Japan) and was carried out in accordance with the guidelines of the Declaration of Helsinki.
Eligibility criteria
Patients with relapsed or refractory DLBCL who were being treated at Kitasato University School of Medicine were enrolled in the study. Eligible patients had histologically documented relapsed or refractory DLBCL as defined by the World Health Organization lymphoma classification.( 10 ) It was confirmed that CD20 antigen was expressed on the surface of lymphoma cells by either immunohistochemical analysis steins or flow cytometry using B1 or L26 anti‐CD20 monoclonal antibody. Patients who were between the ages of 65 and 79 years at the time of the study with expected survival of greater than 4 months and a performance status of 0–2 on the Eastern Cooperative Oncology Group scale, were included. Patients with stage II, III or IV disease, as assessed by the Ann Arbor classification, were included.( 11 ) None of the patients had received rituximab in previous treatments. We included patients who had previously received a total dose of DXR of less than 300 mg/m2. Pretreatment laboratory examination was completed within 2 weeks of study entry, and patients with the following range of laboratory results were included: ANC > 1.5 × 109/L; platelets > 75 × 109/L; creatinine < 1.5 × ULN; bilirubin < 2.0 × ULN; and aspartate transaminase < 5 × ULN. Patients with uncontrolled infection, concomitant malignancy, unstable angina pectoris, symptomatic cardiac arrhythmia, clinical heart failure or symptomatic pleural effusions were excluded. All patients gave informed consent for both treatment and sample collection in accordance with institutional policy.
Treatment
Patients were treated with the CMD regimen plus rituximab. The CMD regimen consisted of: CPT‐11, 25 mg/m2, i.v. on days 1 and 2; MIT, 8 mg/m2, i.v. on day 3; and dexamethasone, 40 mg/m2, i.v. on days 1–3. The patient was given six cycles of CMD, once every 3 weeks. Rituximab at a dose of 375 mg/m2 was given on day −2 of all cycles. Between 30 and 60 min prior to the start of rituximab infusion, the patient was given oral acetaminophen (650 mg) and diphenhydramine hydrochloride (50 mg). The schedule of giving G‐CSF was designed so as to maintain the dose intensity of chemotherapeutic agents. If the nadir ANC was less than 5 × 109/L on one or two measurements, the same doses of drugs were given in the present cycle as in the previous one. If the nadir ANC was less than 1.5 × 109/L on at least three measurements, the doses of CPT‐11 and MIT in the present cycle were reduced by 20% compared with the respective dose in the previous cycle. If the nadir platelet count was less than 25 × 109/L, the doses of CPT‐11 and MIT in the present cycle were reduced by 20% compared with the respective dose in the previous cycle.
Response criteria
Tumor response was assessed after the six cycles of treatment or at the end of chemotherapy treatment. Tumor response was classified as CR, unconfirmed complete response, PR, stable disease, or progressive disease according to the International Workshop response criteria for NHL.( 12 ) These classifications were defined as follows: CR, the disappearance of all lesions and of radiologic or biologic abnormalities observed at diagnosis and the absence of new lesions; PR, regression of all measurable lesions by more than 50%, the disappearance of non‐measurable lesions, and the absence of new lesions; stable disease, regression of measurable lesions by 50% or less, or no change in the non‐measurable lesions, and no growth of existing lesions or no appearance of new lesions; progressive disease, the appearance of new lesions, growth of the initial lesions by more than 25%, or growth of measurable lesions that had regressed during treatment but that subsequently grew by more than 50% of their smallest dimensions.
ELISA for human nm23‐H1
We previously established an ELISA procedure to determine the nm23‐H1 protein level in the serum.( 13 ) Briefly, 96‐well plates (Corning, Corning, NY, USA) were coated with a monoclonal anti‐nm23‐H1 antibody (Seikagakukougyo, Tokyo, Japan), washed four times with PBS, and incubated with 25% Block Ace solution (Dainihon Seiyaku, Osaka, Japan). Serum samples were diluted two‐fold with PBS and 50‐µL aliquots were added to the wells. After incubation at room temperature for 1 h, the wells were washed four times with T‐PBS. The wells were then incubated at room temperature for 1 h with a polyclonal rabbit anti‐nm23‐H1 antibody (Santa Cruz Biotechnologies, Santa Cruz, CA, USA), washed four times with T‐PBS, and incubated with alkaline phosphatase‐conjugated antirabbit immunoglobulin G (Bio‐Rad Laboratory, Richmond, CA, USA). After four washes with T‐PBS, alkaline phosphatase activity was detected with diethanolamine as a substrate and an alkaline phosphatase‐detecting kit (Bio‐Rad Laboratory). The reaction was stopped with 50 µL of 0.4 N NaOH. Absorbance was measured at 405–415 nm with a correction wavelength of 620–630 nm using a microplate reader.
Statistical analyses
All statistical analyses were done with SAS software (version 9; SAS Institute, Cary, NC, USA). Data are expressed as mean ± standard deviation unless otherwise indicated. The duration of the response and survival were assessed using the method of Kaplan and Meier.( 14 ) A P‐value of <0.05 was considered to indicate statistical significance.
Results
Patient characteristics
The clinical characteristics of the 30 patients with relapsed or refractory DLBCL in this study are summarized in Table 1. There were 18 males and 12 females with a median age of 73 years (range, 65–79 years). Eight patients had stage II disease, 14 patients had stage III disease and eight patients had stage IV disease. There were 26 relapsed patients and four patients who were resistant to the initial treatment. Twenty‐two patients had previously undergone one chemotherapy (CHOP) regimen and eight patients had previously undergone two chemotherapy (CHOP and Ara C, carboplatine, etoposide and DEM) regimens. The total dose of DXR that had been given in the previous chemotherapy regimens was 250 mg/m2 (median; range, 200–300 mg/m2).
Table 1.
Patient characteristics (n = 30)
| Age; median (range) | 73 years (65–79) | |
| Gender (M/F) | 18/12 | |
| Stages (II/III/IV) | 8/14/8 | |
| LDH (normal/elevated) | 8/22 | |
| Disease status | ||
| Relapsed | 26 | |
| Primary refractory | 4 | |
| No. of prior chemotherapy regimens | ||
| 1 | 22 | |
| 2 | 8 | |
| Total dose of doxorubicin, median (range) | 250 mg/m2 (200–300) | |
Response to the R‐CMD regimen
Tumor response was assessed after six cycles of chemotherapy or at the end of treatment (Table 2). The overall response rate to the R‐CMD regimen was 74% (CR, 17 patients, 57%[confidence interval 46–67%] including unconfirmed complete response; PR, 5 patients, 17%). Sixteen (62%) of the 26 relapsed patients and one (25%) of the four primary refractory patients achieved CR. The 2‐year PFS rate was 37.2% and the 2‐year survival rate was 45.2% with a median follow‐up period of 23 months (Fig. 1).
Table 2.
Results of treatment
| Response | ||
| CR (incl. CRu) | 17/30 (57%) | |
| PR | 5/30 (17%) | |
| Disease status | ||
| Relapsed CR | 16/26 (62%) | |
| Primary refractory | 1/4 (25%) | |
| Survival (median follow up; 19 months) | ||
| Overall survival (2 years) | 45.2% | |
| Progression‐free survival (2 years) | 37.2% | |
Figure 1.
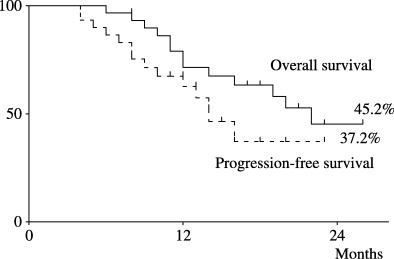
Overall survival and PFS curves of the 30 elderly patients with relapsed or refractory DLBCL who were treated with the R‐CMD regimen.
Correlation of serum nm23‐H1 to survival and PFS
The 30 elderly patients with relapsed or refractory DLBCL were divided into two groups according to the nm23‐H1 level before starting the R‐CMD regimen (cut‐off value of 80 ng/mL). The 2‐year survival rates of the high nm23‐H1 (80, n = 12) and low nm23‐H1 (<80, n = 18) groups were 0% and 72.1%, respectively (P = 0.002 on the log‐rank test and P = 0.005 on the generalized Wilcoxon test), and the 2‐year PFS rates were 0% and 67.7%, respectively (P = 0.002 for the log‐rank test and P = 0.007 for the generalized Wilcoxon test) (Fig. 2). The serum nm23‐H1 level was measured in blood samples obtained before starting the R‐CMD regimen and after completion of six courses of chemotherapy. The mean serum nm23‐H1 level prior to the treatment was 39.4 ± 41.3 ng/mL, which was elevated. Among patients who achieved CR, the mean serum nm23‐H1 level prior to the therapy was 31.2 ± 22.3 ng/mL, and it decreased significantly to 2.2 ± 1.3 ng/mL after the therapy (P = 0.0001). Among the non‐CR patients, the mean serum nm23‐H1 level prior to the therapy was 78.6 ± 39.8 ng/mL and it decreased to 67.3 ± 36.1 ng/mL after the therapy, but this decrease was not statistically significant (P = 0.086). It was found that the nm23‐H1 level already decreased after two of the six courses of chemotherapy in both the CR and non‐CR patients, although the degree of the decrease was smaller in the latter patients.
Figure 2.
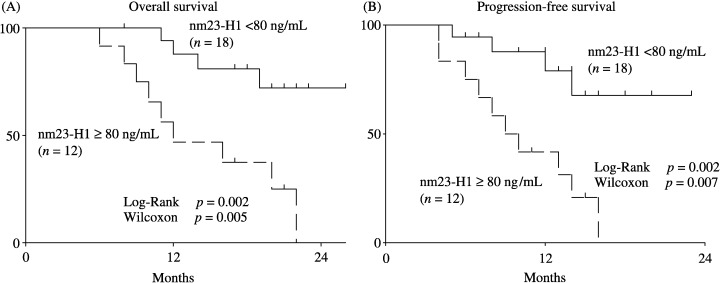
Overall survival (A) and PFS (B) curves of elderly patients with relapsed or refractory DLBCL with a high or low level of serum nm23‐H1. The patients with a high level of nm23‐H1(≥80 ng/mL; n = 12) had a worse prognosis than patients with a low level of nm23‐H1 (<80 ng/mL; n = 18).
Adverse drug reactions
Grade 3–4 hematological toxicities were observed in 19 (63%) of the 30 patients (Table 3). Eight patients (35%) developed grade 4 neutropenia. Four of the eight patients developed uncomplicated fever following treatment with G‐CSF; they were treated with antibiotics and recovered. The other four patients with grade 4 neutropenia did not develop fever after G‐CSF treatment only. Five patients developed grade 3 thrombocytopenia and they required platelet transfusion. Four patients developed grade 3 hemoglobin decrease, and two of the four patients received red blood cell transfusion. Toxicity during rituximab treatment was observed in 10 patients and consisted mainly of infusion‐related adverse events. The four most frequently observed adverse events following rituximab treatment were fever, chills/rigor, rash, and headache. Infusion‐related adverse events typically occurred 1–2 h after the start of infusion. The majority of the infusion‐related adverse events were classified as mild to moderate (toxicity grade 1 or 2) and occurred during the first infusion. They were effectively managed with prophylactic or supportive antihistamines and antipyretics, and resolved within 24 h. Two patients developed nausea/vomiting of grade 3. One patient developed alanine aminotransferase/aspartate transaminase elevation of grade 3. There were no deaths associated with the treatment.
Table 3.
Adverse drug reactions hematological
| Grade | 0/1 | 2 | 3 | 4 |
| WBC | 1 | 10 | 11 | 8 |
| Neutrophil | 1 | 10 | 10 | 9 |
| Hb | 12 | 14 | 4 | 0 |
| Plt | 15 | 10 | 5 | 0 |
| Non‐hematological | ||||
| Fever | 26 | 0 | 4 | 0 |
| Chills/rigor | 19 | 11 | 0 | 0 |
Discussion
The treatment results and adverse drug reactions of CPT‐11 differ depending on the method of administration (multiple small dose or single high dose), which drugs CPT‐11 is combined with in combination treatment, and the order in which the drugs are given. Therefore, a consensus opinion has not been reached. An early phase II study of CPT‐11 for relapsed or refractory lymphoma( 3 ) showed CR in two (23.2%) of nine cases and PR in one case (11.1%) by CPT‐11 40 mg/m2 for 3 consecutive days and repeated every week, and CR in two (12.5%) of 16 cases and PR in three cases (18.8%) by CPT‐11 40 mg/m2 for 5 consecutive days and repeated once every 3–4 weeks. However, all cases showed resistance to treatment with CPT‐11 200 mg/m2 for 1 day and repeated once every 3–4 weeks. Therefore, the usefulness of multiple small doses of CPT‐11 was suggested. Sarris et al.( 15 ) reported that eight (44%) of 18 cases of relapsed aggressive lymphoma and one (20%) of five cases of refractory aggressive lymphoma showed remission (CR + PR) with CPT‐11 300 mg/m2 given every 21 days. As for adverse drug reactions, they reported diarrhea of grade 3 or higher in 15% of cases and nausea/vomiting in 14%. However, it was reported that when CPT‐11 350–500 mg/m2 was given on only 1 day and repeated every 3–4 weeks, CR was seen in one (3%) of 28 cases and PR in only one case. The duration of remission was short, and digestive and hematological toxicities were frequently seen. Therefore, the usefulness of single high‐dose CPT‐11 was reported to be low.( 16 ) Sugiyama et al.( 17 ) reported that CPT‐11 40 mg/m2 was given for 3 consecutive days and repeated 2–3 times every week. They observed CR in two of 48 cases and PR in 15 cases. The main adverse drug reactions were diarrhea and hematological toxicities. Therefore, the possibility was suggested that multiple small doses have higher treatment efficacy than a single high dose of CPT‐11 for lymphoma.
Studies regarding drug combinations reported that a combination of CPT‐11 and carboplatin could not improve the treatment efficacy of CPT‐11 alone. Therefore, the usefulness of carboplatin combination was low.( 18 , 19 ) A study on combined treatment of CPT‐11 and etoposide( 6 ) reported that it caused unexpected and frequent hepatotoxicities. Therefore, it was recommended that etoposide not be combined with CPT‐11. A preclinical study showed that the anticancer effect of the combination of topoisomerase I inhibitor and topoisomerase II inhibitor was more than additive.( 3 , 4 ) We reported the safety and efficacy of the CMD regimen which was composed of MIT (which is less cardiotoxic than DXR) combined with CPT‐11 and dexamethasone, in patients with relapsed NHL.( 7 ) As there has been no report on the safety and efficacy of the combination of rituximab and CPT‐11, we evaluated the treatment efficacy and safety (especially cardiotoxicities) of the combination of rituximab and the CMD regimen in the present study. The response rate was 74% (22 of 30 cases) (CR 57%, PR 17%), the 2‐year PFS rate was 37.2%, and the 2‐year survival rate was 45.2%. In our previous study, the CMD regimen( 7 ) was given to patients with relapsed or refractory NHL aged 18–79 years. The response rate was 62.5% (CR 34.4%, PR 28.1%), the 2‐year event‐free survival rate was 16.1%, and the 2‐year survival rate was 31.8%. Among the nine patients with relapsed or refractory DLBCL aged 65–79 years in our previous study( 7 ) three cases achieved CR and two cases achieved PR (response rate of 55.6%). Therefore, it appears that by combining rituximab with the CMD regimen, the response rate can be increased by approximately 10–20% without significantly increasing definite adverse drug reactions.
We previously established an ELISA technique to determine the serum level of nm23‐H1 protein.( 13 ) nm23‐H1 was originally identified as a protein that was expressed at a lower level in metastatic cancer cells.( 20 ) The nm23 genes play critical roles in cellular proliferation, differentiation, oncogenesis, and tumor metastasis. The mechanisms responsible for these pleiomorphic effects are not well understood. We previously reported that serum nm23‐H1 levels in patients with aggressive NHL were significantly higher than that in controls, and that a high nm23‐H1 level was associated with poor prognosis in patients with aggressive NHL.( 9 , 13 ) In a multi‐institutional study involving a large number of patients, we found that serum nm23‐H1 was an important and independent prognostic factor in patients with DLBCL.( 9 ) In this study, the serum nm23‐H1 level was measured before and after treatment with the R‐CMD regimen and we evaluated whether it is useful for determining the prognosis and treatment efficacy of this regimen. The results showed that patients with a serum nm23‐H1 level of higher than 80 ng/mL had significantly poorer prognosis. In the patients who achieved CR, the serum nm23‐H1 level decreased to within the normal range quickly after the treatment, but in patients who did not achieve CR, it remained at a high level after the treatment. These results suggest that the serum nm23‐H1 level is a prognostic factor for not only the initial onset of lymphoma but also relapsed or refractory lymphoma, and that it is a prognostic factor for not only chemotherapy but also rituximab‐combined chemotherapy. The serum nm23‐H1 level quickly decreased in the CR cases; therefore, there is a possibility that it can be used to determine the treatment efficacy.
The R‐CMD regimen could be used safely in elderly patients and no new signs of cardiotoxicity were found. It was effective for patients with relapsed or refractory DLBCL who were previously treated with DXR. However, further long‐term follow‐up is needed to evaluate the survival rate.
References
- 1. Feugier P, Van Hoof A, Sebban C et al. Long‐term results of the R‐CHOP study in the treatment of elderly patients with diffuse large B‐cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 2005; 23: 4117–26. [DOI] [PubMed] [Google Scholar]
- 2. Pommier Y. DNA topoisomerase I and II in cancer chemotherapy: update and perspectives. Cancer Chemother Pharmacol 1993; 32: 103–8. [DOI] [PubMed] [Google Scholar]
- 3. Ohno R, Yoshida Y, Oguro M et al. An early phase II study of CPT‐11 (Irinotecan hydrochloride) in patients with hematological malignancies. Jpn J Cancer Chemother 1994; 21: 75–82. [PubMed] [Google Scholar]
- 4. Kano Y, Suzuki K, Akutsu M, Suda K, Inoue Y, Yoshida M. Effects of CPT‐11 in combination with other anti‐cancer agents in culture. Int J Cancer 1992; 50: 604–10. [DOI] [PubMed] [Google Scholar]
- 5. Kim R, Hirabayashi N, Nishiyama M, Jinushi K, Toge T, Okada K. Experiment studies on biochemical modulation targeting topoisomerase I and II in human tumor xenografts in nude mice. Int J Cancer 1992; 50: 760–6. [DOI] [PubMed] [Google Scholar]
- 6. Ohtsu T, Sasaki Y, Igarashi T, Murayama T, Kobayashi Y, Tobinai K. Unexpected hepatotoxicities in patients with non‐Hodgkin's lymphoma treated with irinotecan (CPT‐11) and etoposide. Jpn J Clin Oncol 1998; 28: 502–6. [DOI] [PubMed] [Google Scholar]
- 7. Coltman CA Jr, Coltman TM, Balcerzak SP, Morrison FS, Von Hoff DD. Mitoxantrone in refractory nonHodgkin's lymphoma. A Southwest Oncology Group study. Semin Oncol 1984; 11: 50–3. [PubMed] [Google Scholar]
- 8. Niitsu N, Iijima K, Chizuka A. Combination therapy with irinotecan (CPT‐11), mitoxantrone, and dexamethasone in relapsed or refractory non‐Hodgkin's lymphoma: a pilot study. Ann Hematol 2001; 7: 411–6. [DOI] [PubMed] [Google Scholar]
- 9. Niitsu N, Okabe‐Kado J, Okamoto M et al. Serum nm23–H1 protein as a prognostic factor in aggressive non‐Hodgkin's lymphoma. Blood 2001; 97: 1202–10. [DOI] [PubMed] [Google Scholar]
- 10. Jaffe ES, Harris NL, Stein H, Vardiman JW (eds). WHO Health Organazation Classification of Tumours. Pathology and Genetics. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press, 2001. [Google Scholar]
- 11. Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res 1971; 31: 1860–1. [PubMed] [Google Scholar]
- 12. Cheson BD, Horning SJ, Coiffier B et al. Report of an international workshop to standardize response criteria for non‐Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999; 17: 1244–53. [DOI] [PubMed] [Google Scholar]
- 13. Niitsu N, Okabe‐Kado J, Kasukabe T, Yamamoto‐Yamaguchi Y, Umeda M, Honma Y. Prognostic implications of the differentiation inhibitory factor nm23–H1 protein in the plasma of aggressive non‐Hodgkin's lymphoma. Blood 1999; 94: 3541–50. [PubMed] [Google Scholar]
- 14. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81. [Google Scholar]
- 15. Sarris AH, Phan A, Goy A et al. Irinotecan in relapsed or refractory non‐Hodgkin's lymphomas. Indications of activity in a phase II trial. Oncology 2002; 16: 27–31. [PubMed] [Google Scholar]
- 16. Ribrag V, Koscielny S, Vantelon JM et al. Phase II trial of irinotecan (CPT‐11) in relapsed or refractory non‐Hodgkin's lymphomas. Leuk Lymphoma 2003; 44: 1529–33. [DOI] [PubMed] [Google Scholar]
- 17. Sugiyama K, Omachi K, Fujiwara K et al. Irinotecan hydrochloride for the treatment of recurrent and refractory non‐Hodgkin lymphoma: a single institution experience. Cancer 2002; 94: 594–600. [DOI] [PubMed] [Google Scholar]
- 18. Tobinai K, Hotta T, Saito H et al. Combination phase I/II study of irinotecan hydrochloride (CPT‐11) and carboplatin in relapsed or refractory non‐Hodgkin's lymphoma. CPT‐11/Lymphoma Study Group. Jpn J Clin Oncol 1996; 26: 455–60. [DOI] [PubMed] [Google Scholar]
- 19. Suzumiya J, Suzushima H, Maeda K et al. Kyushu Hematology Organization for Treatment Study Group . Phase I study of the combination of irinotecan hydrochloride, carboplatin, and dexamethasone for the treatment of relapsed or refractory malignant lymphoma. Int J Hematol 2004; 79: 266–70. [DOI] [PubMed] [Google Scholar]
- 20. Steeg PS, Bevilacqua G, Kopper L et al. Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst 1988; 80: 200–4. [DOI] [PubMed] [Google Scholar]


