Abstract
Peritoneal dissemination is the most common cause of metastasis from malignancies in the abdominal cavity. There are no standard treatments for peritoneal dissemination and the results are poor. The reasons for this are as follows: (1) no effective chemotherapeutic agents have been identified or developed; (2) surgical cytoreduction has little effect on survival improvement; and (3) the molecular mechanisms of peritoneal dissemination have not been clarified and no therapy against the target molecules has been developed. However, studies on the molecular mechanisms of peritoneal dissemination have elucidated some of the target molecules and the development of new multimodal therapies has also improved survival. Early postoperative intraperitoneal chemotherapy, hyperthermic intraperitoneal perfusion chemotherapy and neoadjuvant intraperitoneal‐systemic chemotherapy have been newly developed, and a novel surgical therapy named peritonectomy has been proposed to perform complete cytoreduction of peritoneal dissemination. At present, these approaches appear to be effective therapeutic modalities for peritoneal dissemination. However, TS‐1 and capecitabine have shown worthwhile results in recent clinical trials for patients with advanced gastric cancer. We recently found that newly developed antitumor cytosine nucleoside analogs show a survival advantage in peritoneal dissemination models using human cancer cells. These non‐fluoropyrimidine nucleosides may potentially help to improve the poor prognosis observed in patients with advanced cancers involving peritoneal dissemination. (Cancer Sci 2007; 98: 11–18)
Peritoneal dissemination remains the most difficult type of metastasis to treat, and almost all surgeons believe that an operation is not indicated for such metastasis.( 1 , 2 , 3 ) Systemic chemotherapy tends to have little effect on the treatment of peritoneal dissemination, because the peritoneal–blood barrier existing between mesothelial cells and the submesothelial capillary hinders drug distribution throughout the peritoneal cavity. Recent advances in the studies of new drugs and multimodal therapies have proposed the therapeutic potency for advanced cancer with peritoneal dissemination. Neoadjuvant chemotherapy is also known to reduce the tumor burden, thus resulting in a stage reduction. As a result, the incidence of complete cytoreduction may increase after neoadjuvant chemotherapy. A new type of neoadjuvant intraperitoneal–systemic chemotherapy (NIPS) has been developed to increase the rate of complete cytoreduction.( 4 ) The complete removal of peritoneal dissemination is an independent prognostic factor. A peritonectomy is a novel surgical procedure by which complete cytoreduction for peritoneal dissemination is performed.( 5 , 6 , 7 , 8 ) These approaches are now being carried out as treatment modalities for peritoneal dissemination from appendiceal, colon and gastric cancer. Furthermore, several recent phase II studies that evaluated the efficacy of new anticancer drugs including taxanes, TS‐1 and capecitabine have shown promising results for patients with peritoneal dissemination of gastric cancer.( 9 , 10 ) In addition, potent therapeutic effects of non‐fluoropyrimidine antimetabolites have been demonstrated in clinical and preclinical studies. We recently found that newly developed antitumor cytosine nucleoside analogs such as 1‐(2‐deoxy‐2‐fluoro‐4‐thio‐β‐d‐arabino‐pentofuranosyl) cytosine (4′‐thio‐FAC), 1‐(2‐C‐cyano‐2‐deoxy‐1‐β‐d‐arabino‐pentofuranosyl) cytosine (CNDAC) and 1‐(3‐C‐ethynyl‐β‐d‐ribo‐pentofuranosyl) cytosine (ECyd) show a survival advantage in peritoneal dissemination models using human cancer cells.( 11 ) This review outlines the recent advances of the basic preclinical research and clinical trial results in the treatment of peritoneal dissemination from gastric cancer, including our recent findings. Furthermore, we also propose the possibility of non‐fluoropyrimidine nucleosides as an agent for patients with peritoneal dissemination of gastric cancer.
Molecular mechanisms of peritoneal dissemination
Peritoneal dissemination is established through a multistep process. Many metastasis‐related factors, including adhesion molecules, matrix proteases, motility factors and angiogenic factors, are involved in the formation of peritoneal dissemination. The first step is the detachment of cancer cells from the serosal surface of the primary tumor; such detached cancer cells are called peritoneal free cancer cells (Fig. 1, step 1). E‐cadherin is the key molecule for detachment.( 12 ) In addition, during an operation, blood or lymphatic fluid contaminated with cancer cells may spill into the peritoneal cavity from torn blood and lymphatic vessels.
Figure 1.
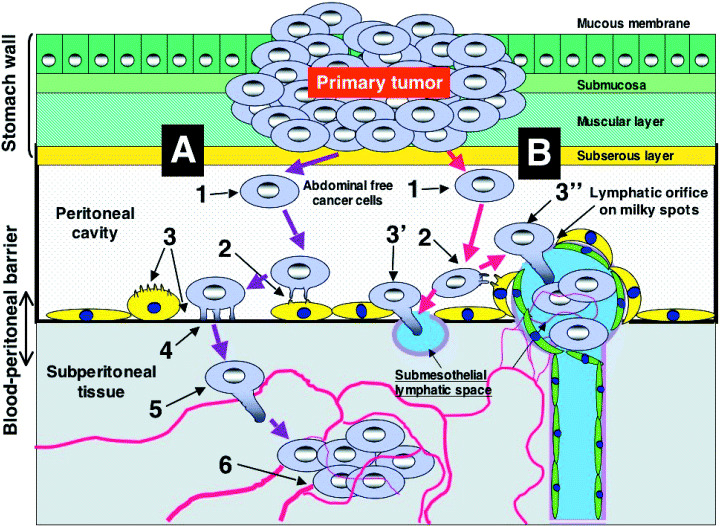
Peritoneal dissemination is established through a multistep process. There are two different processes for the attachment of peritoneal free cancer cells on the peritoneum. (A) Trans‐mesothelial process (steps 1–6): step 1, detachment from serosa; step 2, adhesion to mesothelial cells; step 3, contraction of mesothelial cells and submesothelial basement membrane; step 4, adhesion to submesothelial basement membrane; step 5, invasion of cancer cells into submesothelial tissue; step 6, vascular neogenesis, lymphangiogenesis and lymphatic dilatation. (B) Trans‐lymphatic metastasis process (steps 1–3′ or 3″): step 3′, exposure of lymphatic stomata or lymphatic orifices; step 3″, invasion through lymphatic stomata.
Two different processes have so far been proposed for the attachment of peritoneal free cancer cells on the peritoneum, designated as trans‐mesothelial (Fig. 1, process A, steps 1–6) and trans‐lymphatic metastasis (Fig. 1, process B, steps 1–3′ or 3″). The trans‐mesothelial process is the direct attachment of peritoneal free cancer cells on the peritoneal surface (step 2). CD44 is considered to be important for adhesion and interaction between gastric cancer cells and mesothelial cells.( 13 , 14 ) Using a high‐density cDNA microarray analysis, Sakakura et al. demonstrated that CD44 expression is upregulated in gastric cancer cell lines established through metastasis to the peritoneal cavity.( 15 ) The tissue that exists between mesothelial cells and the submesothelial capillary is named the peritoneal–blood barrier, which hinders the penetration of oxygen, nutrients and drugs from the submesothelial capillary to the peritoneal cavity.( 16 ) After the attachment of peritoneal free cancer cells on the peritoneum, most free cancer cells die off due to the existence of the peritoneal–blood barrier.( 17 ) However, some populations of free cancer cells can penetrate into the submesothelial space through gaps between the mesothelial cells (step 3).( 18 ) Cytokines such as tumor necrosis factor‐α and interleukin‐8 appear to be involved in the contraction of mesothelial cells and gap formation.( 19 ) Cancer cells attach themselves to the exposed submesothelial basement membrane through integrins expressed on the membrane (step 4)( 20 ) and thereafter invade the peritoneal–blood barrier (step 5).
According to our findings, patients with matrix metalloproteinase (MMP)‐7‐positive gastric cancer have significantly poorer survival and die more frequently of peritoneal recurrence than those with MMP‐7‐negative tumors.( 21 ) Furthermore, treatment by antisense oligonucleotides specific for MMP‐7 effectively suppresses the peritoneal dissemination of MKN‐45‐P cells without influencing proliferation in a nude mouse model.( 22 ) The concerted expression of motility factors (hepatocyte growth factor receptor/c‐met, autocrine motility factor) and matrix‐digesting enzymes (urokinase‐type plasminogen activator and MMP‐7) plays an important role in invasion. We have reported that the simultaneous overexpression of c‐met, autocrine motility factor receptor and urokinase‐type plasminogen activator receptor are also closely associated with lymph node metastasis and peritoneal dissemination.( 23 ) Cancer cells invade near the subperitoneal blood vessels, proliferate by inducing vascular neogenesis and then evolve into the established peritoneal dissemination (step 6). Angiogenic factors such as vascular endothelial growth factor (VEGF)‐A and VEGF‐C secreted from peritoneal free cancer cells induce vascular neogenesis in the peritoneal–blood barrier.( 24 , 25 ) As a result, the distance of the peritoneal–blood barrier shortens and an environment with a rich vasculature is established. VEGF is a well‐known angiogenic factor that induces a mitogenic signal for endothelial cells by binding to endothelial cell receptors VEGFR‐1 (Flt‐1) and VEGFR‐2 (KDR). A soluble form of FLT‐1 binds VEGF specifically and inhibits its activity. Recently, it has been demonstrated that transduction of the soluble Flt‐1 gene to peritoneal mesothelial cells using adenoviral vector effectively suppresses the peritoneal metastasis of gastric cancer.( 26 ) From these findings, it is proposed that the development of antiangiogenic therapy targeted to peritoneal mesothelial cells may become a useful approach for regulating the peritoneal dissemination of gastric cancer.
The second metastatic process is trans‐lymphatic metastasis (Fig. 1, process B). Peritoneal free cancer cells migrate to the subperitoneal lymphatic sinus through the lymphatic orifices (stomata), and then progress to the peritoneal surface (step 3′). There are many lymphatic orifices on the greater omentum: appendices epiploicae of the colon, the inferior surface of diaphragm, the falciform ligament, Douglas’ pouch and the small bowel mesentery. These peritoneal parts have many milky spots, including the lymphatic apparatus, which is composed of peritoneal macrophages and lymphocytes in the lymphatic sinus. The lymphatic orifices are found on the milky spots. The peritoneal free cancer cells migrate to the lymphatic sinus in the milky spot, and undergo proliferation accompanied by neovascularization (step 3″). In the diaphragm, a large number of lymphatic orifices designated as stomata are found, and the stomata connect with the submesothelial lymphatic vessel. Peritoneal free cancer cells migrate into the submesothelial lymphatic space and then proliferate. In contrast, there are no lymphatic stomata and milky spots on the liver capsule, the peritoneum covering the abdominal wall, the serosal surface of the small bowel or the splenic capsule. These peritoneal parts are not affected until the late stages of peritoneal dissemination.
Recent advances in the multimodal therapy of peritoneal dissemination
The prognosis of patients with peritoneal dissemination from gastrointestinal cancer is very poor, with a median survival time (MST) of approximately 3 months.( 2 , 3 ) No standard treatments for peritoneal dissemination have been reported. Recent advances in the study of new drugs and multimodal therapies are summarized in Table 1. Wilke et al. reported that preoperative chemotherapy with etoposide, doxorubicin and cisplatin (EAP) could improve the prognosis of patients with locally advanced and non‐resectable gastric cancer, but a considerable number of patients could not be treated with EAP partly because of its severe toxicity (myelo‐suppression, nausea and vomiting).( 27 , 28 ) At present, intravenous 5‐fluorouracil (5‐FU) has been used either alone or in combination with other anticancer drugs such as FAM (5‐FU, doxorubicin, and mitomycin C) or FAMTX (5‐FU, doxorubicin, and methotrexate) as chemotherapy against advanced gastric cancer.( 29 , 30 ) However, systemic chemotherapy does not improve the survival of patients with peritoneal dissemination( 7 , 31 ) because the existence of the peritoneal–blood barrier hinders the distribution of drug throughout the peritoneal cavity. In contrast, intraperitoneal chemotherapy offers potential therapeutic advantages over systemic chemotherapy by generating high local concentrations of drugs.( 32 , 33 ) After intraperitoneal chemotherapy, drugs are absorbed from the lymphatic orifices, and cancer cells that are growing in the submesothelial lymphatic sinus are exposed to drugs at high concentrations. In intraperitoneal chemotherapy, the instillation time and kinds of drugs are the important factors. According to pharmacokinetic studies, 4 h intraperitoneal instillation of cisplatin shows a significantly higher area under the curve (AUC) than 2 h instillation.( 34 ) However, after intraperitoneal docetaxel administration in patients, higher drug concentration can be achieved for over 12 h, with very low plasma levels.( 35 , 36 ) In the experimental peritoneal dissemination model of gastric cancer cell line of MKN‐45‐P, the survival time of mice treated with intraperitoneal administration of docetaxel was markedly prolonged in comparison to that in the intravenous injection group.( 37 ) We monitored the intraperitoneal carcinoembryonic antigen (CEA) level of mice during docetaxel treatment. The CEA level was well correlated with the therapeutic efficacy of docetaxel. When docetaxel was injected into the mice either intravenously or intraperitoneally, the drug concentrations in the peritoneal cavity, peritoneal solid cancer tissue and ascites cancer cells were significantly higher for a longer time in the intraperitoneal injection group compared with the intravenous injection group.( 38 ) These results indicate that the intraperitoneal administration of docetaxel for peritoneal dissemination is likely to be an effective treatment method, without causing any increase in systemic toxicity. Currently, the therapeutic efficacy of docetaxel as a single agent and in combination with modalities using other agents in patients with advanced gastric cancer is being evaluated in clinical studies in many countries.
Table 1.
Clinical studies in advanced gastric cancer
| Regimen | Reference | Patient | Case no. | Target metastasis | Effect | Survival | Side‐effect (grade 3 or 4) |
|---|---|---|---|---|---|---|---|
| FAM | MacDonald et al. ( 29 ) | Patients with measurable cancer | 62 | AM, H, N, bone | PR:42% | MST: 5.5 months responder: 12.5 months non‐responder: 3.5 months | Bone marrow: 7/62 |
| FAMTX | Wils et al. ( 30 ) | Advanced gastric cancer with or without measurable disease Randomized clinical trial: FAMTX versus FAM | 105 | PT, H, N, P | PR:41% (FAM 9%) | 1‐year survival: 41% 2 years survival 9% Survival was significantly better than FAM | Nausea: 63% Mucositis: 51% Diarrhea: 20% Alopecia: 24% |
| EAP | Wilke et al. ( 27 ) | Advanced gastric cancer with or without measurable disease | 34 | PT, H, N, P | PR + CR:64% | MST 18 months 2 years survival 26% | Bone marrow: 48% |
| XELOX | Park et al. ( 61 ) | Advanced gastric cancer with or without measurable disease | 20 | PT, H, N, P | PR(11) + CR(2): 65% | Median progression free survival: 7.5 months | No grade 3 and 4 |
| Gemcitabine + FOLFOX‐4 | Correale et al. ( 67 ) Chemother | Metastatic gastric cancer | 36 | H, N, P, ovary, lung | PR(15) + CR(4): 53% | Survival: 11 months | Neuropenia: 3/36 Mucositis: 4/36 Bone marrow: 6.7% |
| Docetaxel + capecitabine | Kim et al. ( 62 ) | Untreated metastatic or recurrent gastric cancer | 32 | PT, H, N, P | PR13 + CR(1): 44% | MST: 8.4 month 1 years survival 0% | |
| TS‐1 | Osugi et al.( 64 ) | Advanced gastric cancer with peritoneal dissemination | 18 | P | Non‐evaluable | MST: 211 days Control MST: 118 days | Bone marrow: 1/18 |
| TS‐1 | Yonemura et al. ( 58 ) | Advanced gastric cancer with positive cytology | 35 | Cy1/P0 | Non‐evaluable | 2 years survival 53% Control 2‐year survival 9% | Grade 3 and 4: 0% |
| Paclitaxel + TS‐1 | Kobayashi et al. ( 78 ) | Advanced gastric cancer Standard gastrectomy of more than a D2 dissection | 50 and CY0‐1 | T3‐4, N0‐2, P0, H0 M0, | Safe and feasible | Non‐evaluable | PTX: Neuropathy 1/50, leucopenia 1/50, neutropenia 4/50 S‐1: Diarrhea 1/50, neutropenia 3/50, anemia 1/50 |
| Docetaxel + TS‐1 | Yoshida et al. ( 57 ) | Advanced, recurrent gastric cancer | 48 | H, N, P, remnant, stomach | PR:56% | MST: 14.3 months Median time tumor progression: 7.3 months | Bone marrow: 58% Anorexia: 15% |
| HIPEC | Yonemura et al. ( 40 ) | Advanced, recurrent gastric cancer | 83 | P | PR:39% (17/43) | Complete resection1 and 5 years survival: 61%, 17% Incomplete resection: 1 and 5 years survival: 30%, 2% | Bowel perforation: 3/83 Bone marrow: 2/83 |
| EPIC | Jeung et al. ( 39 ) | Advanced gastric cancer with peritoneal dissemination Palliative gastrectomy | 49 | P | Better than control group | MST: 12 months Median progression free survival: 7 months | Bone marrow: 17/49 Nause vomiting: 10/49 |
| Peritonectomy + hyperthermic i.p. chemotherapy (cisplatin + mitomycin C) | Yonemura et al. ( 45 ) | Advanced gastric cancer with peritoneal dissemination Aggressive cytoreduction | 107 | P | Non‐evaluable | MST: 11.5 months 5 years survival: 6.7% | Not described |
| NIPS | Yonemura et al. ( 5 ) | Advanced gastric cancer Preoperative chemotherapy Clinical phase I | 51 | P | Cytology 75%, PR:45% | MST: 18 months, Peritonectomy: 18 months Complete cytoreduction: 20.4 months | Bone marrow: 16% Operative mortality: 4.5% |
5‐FU, 5‐fluorouracil; AM, abdominal mass; CR, complete response; EAP, etoposide + doxorubicin + cisplatin; EPIC, early postoperative intraperitoneal chemotherapy (5‐FU + cisplatin); FAM, 5‐FU + doxorubicin + mitomycin C; FAMTX, 5‐FU + doxorubicin + methotrexate; FOLFOX‐4, oxaliplatin + folinic acid + infusional 5‐FU; H, liver metastasis; HIPEC, hyperthermic intraperitoneal perfusion chemotherapy mitomycin C + etoposide + cisplatin; MST, median survival time; N, lymph node metastasis; NIPS, neoadjuvant intraperitoneal–systemic chemotherapy (methotrexate+ 5‐FU, i.v./docetaxel +carboplatin, i.p.); P, peritoneal dissemination; PR, partial response; PT, primary tumor; XELOX, oxaliplatin + capecitabine.
Early postoperative intraperitoneal chemotherapy (EPIC) is a new method designated as early postoperative intraperitoneal chemotherapy. In this treatment, the abdominal cavity is instilled with 500 mL saline with anticancer agents from postoperative day 1 for several postoperative days. The aim of EPIC is to kill the residual intraperitoneal cancer cells by intraperitoneal chemotherapy as early as possible before the cancer cells adhere to the peritoneal surface and are buried with fibrin materials. Jeung et al. reported the feasibility of EPIC in treating gastric cancer with peritoneal dissemination.( 39 ) When EPIC started on the day of operation with 5‐FU 500 mg/m2 and cisplatin 40 mg/m2 (days 1–3) over a 4‐week interval, the overall survival was 12 months. EPIC is thus considered to be a simple and useful method for the treatment of peritoneal dissemination.
Hyperthermic intraperitoneal perfusion chemotherapy (HIPEC) is a method used to treat the whole peritoneal cavity by circulating heated saline containing a high dose of anticancer drug.( 40 , 41 ) We have previously reported the efficacy of HIPEC in 83 patients with peritoneal dissemination of gastric cancer.( 40 ) Among the 43 evaluable patients, 17 (40%) exhibited a response. The overall 1‐ and 5‐year survival rates were 43 and 11%, respectively. However, HIPEC is indicated for tumors less than 2–3 mm in diameter because it is limited to a penetration depth of 1–2 mm from the peritoneal surface.( 42 ) Fujimoto et al. reported that the intraperitoneal hyperthermic perfusion (IPHP) treatment did not kill all of the gastric cancer cells that had penetrated deeply into subperitoneal layers, whereas gastric cancer cells in the abdominal effusion or lavage vanished.( 43 ) Verwaal et al. carried out a randomized controlled study in 105 patients with peritoneal dissemination from colorectal cancer. (41) Patients were assigned to receive either a standard treatment consisting of systemic chemotherapy (5‐FU–leucovorin) with or without palliative surgery, or experimental therapy consisting of aggressive cytoreduction with HIPEC, followed by the same systemic chemotherapy regime. The survival in the standard therapy arm was significantly poorer than that in the experimental therapy arm. If the cytoreduction was macroscopically complete, the median survival was significantly better than in patients with residual disease.( 41 ) Therefore, a cytoreduction followed by HIPEC is important to improve survival in patients with peritoneal dissemination of gastric and colorectal origin.
According to the recent studies, complete removal of peritoneal dissemination is the independent prognostic factor.( 44 ) In 1995, peritonectomy was first described as a surgical procedure used to carry out complete cytoreduction for peritoneal dissemination.( 8 ) At present, this approach is being used as a treatment modality in combination with intraperitoneal chemotherapy including HIPEC.( 45 , 46 )
Neoadjuvant chemotherapy is known to reduce the tumor burden, thus resulting in a stage reduction. NIPS as a new type of neoadjuvant chemotherapy was developed to increase the rate of complete cytoreduction.( 4 , 5 ) The nutrition of peritoneal nodules can be derived from both the peritoneal surface and the blood supply.( 47 ) In NIPS, peritoneal dissemination is attacked both intraperitoneally and intravenously. Accordingly, two‐route chemotherapy may be the best method for preoperative chemotherapy. Carboplatin, docetaxel and 5‐FU are used for NIPS because these agents show high cytotoxicity against clinically obtained gastric cancer samples in in vitro chemosensitivity tests.( 48 ) Docetaxel and carboplatin are administered from a peritoneal port system. On the same day, methotrexate and 5‐FU are injected via the peripheral vein. This regimen is repeated weekly. After several cycles of NIPS, a complete cytoreduction is attempted by peritonectomy. A peritonectomy consists of two procedures, including a parietal and visceral peritonectomy. For the complete removal of visceral peritoneum bearing cancer, a total gastrectomy, combined with resection of the organs involved, is carried out. The final goal of a peritonectomy is complete removal of the primary tumor, lymph node metastasia and all of the peritoneal nodules. In our study, the MST of patients who received a peritonectomy after NIPS was 19.3 months, whereas that of patients who did not undergo an operation was 9.6 months (Fig. 2. P < 0.05).( 4 ) The patients who received a complete resection showed a significantly better prognosis than those who received an incomplete cytoreduction (Fig. 3). After NIPS, positive cytology changed to negative cytology in 24 (67%) of 35 patients. NIPS, therefore, can eradicate intraperitoneal free cancer cells prior to a peritonectomy.
Figure 2.
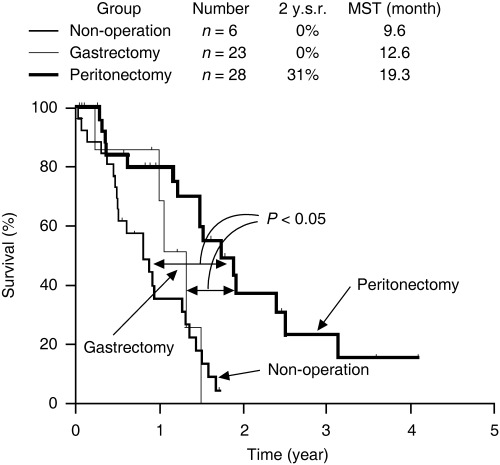
Survival curves of patients with clinically detectable peritoneal dissemination, treated by neoadjuvant intraperitoneal or systemic chemotherapy. All patients had P2 or P3 peritoneal dissemination. Fifty‐seven patients were treated with neoadjuvant intraperitoneal–systemic chemotherapy, using intraperitoneal administration of 30 mg/m2 docetaxel and 100 mg/m2 carboplatin with 1000 mL saline from a peritoneal port system, and intravenous infusion of 100 mg/m2 methotrexate and 600 mg/m2 5‐fluorouracil via a peripheral vein. Surgical exploration was done in 51 patients, and 28 patients received peritonectomy. Gastrectomy alone was done in 23 patients. Survival of patients who underwent peritonectomy showed the best prognosis. 2 y.s.r., 2‐year survival rate; MST, median survival time.
Figure 3.
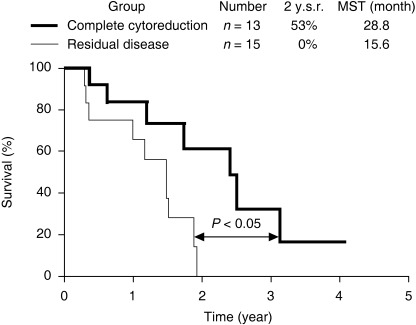
Survival curves of patients with clinically detectable peritoneal dissemination. Patients who received complete cytoreduction by peritonectomy after neoadjuvant intraperitoneal–systemic chemotherapy survived significantly better than those with residual disease (incomplete cytoreduction group). 2 y.s.r., 2‐year survival rate; MST, median survival time.
Although the aggressive combination treatment of a surgical cytoreduction and intraperitoneal chemotherapy to improve the prognosis of patients with peritoneal dissemination is an advance, the efficacy of this treatment is still limited. Major problems are not associated with the surgical techniques but rather in the regimens or efficacy of the anticancer agents themselves. To successfully control the peritoneal dissemination of gastric cancer, the monitoring of intraperitoneal free cancer cells is extremely important. In addition, both the intraperitoneal protein levels and the mRNA levels of tumor markers (e.g. CEA and CA19‐9) and metastasis‐ or angiogenesis‐related factors such as MMP‐7 and VEGF may be useful markers together with a cytological test.( 37 , 49 , 50 , 51 , 52 , 53 ) As the development of new drugs overcomes many intractable diseases, new regimens and drugs are needed to improve the poor prognosis of patients with peritoneal dissemination. We will introduce and propose agents that may be applicable for the treatment of peritoneal dissemination in the next section.
Future chemotherapy using novel antimetabolites
The administration of antimetabolic fluoropyrimidines such as doxifluridine (5′‐DFUR) and UFT has been used either alone or in combination with other antitumor drugs in chemotherapy for advanced gastrointestinal carcinomas. However, these regimens are not considered to be standard treatments because their therapeutic efficacy is inadequate. The development of new anticancer drugs has provided an opportunity for progress in cancer chemotherapy. Several recent clinical studies introducing new anticancer drugs and combination therapies have been reported to show promising results.( 9 , 10 )
TS‐1, which is composed of tegafur, gimestat (CDHP) and otastat potassium at a molar ratio of 1 : 0.4 : 1, is the standard first‐line anticancer drug used in the treatment of gastric cancer. Many recent reports have shown TS‐1 to be a promising drug for advanced and recurrent gastric cancer with metastasis, including peritoneal dissemination.( 9 , 54 , 55 , 56 , 57 , 58 ) We reported the effect of TS‐1 for potentially curable patients with peritoneal dissemination (P0/Cy1 status) as a type of postoperative chemotherapy.( 58 ) After a radical gastrectomy, 35 patients were treated with TS‐1 for 28 consecutive days with 14 days rest, and the schedule was repeated every 6 weeks (TS‐1 group). The patients in the TS‐1 group survived significantly longer than the control group without chemotherapy. The 2‐year survival rates of the control and TS‐1 groups were 9 and 53%, respectively (Fig. 4). The Cox proportional hazard model showed TS‐1 treatment as an independent prognostic factor, and the relative risk by TS‐1 treatment was 0.17‐fold lower than that of the control group. Major adverse reactions included myelosuppression and gastrointestinal toxicities, but they were generally mild and there were no treatment‐related deaths. From these results, we propose TS‐1 treatment to be safe and effective as a postoperative chemotherapy for patients with a P0/Cy1 status. At present, clinical studies of TS‐1 combined with taxanes or cisplatin in patients with advanced gastric cancer have been evaluated.( 9 , 59 , 60 ) It has been reported that docetaxel and S‐1 combination therapy are highly active and well tolerated in advanced or recurrent gastric cancer.( 57 )
Figure 4.
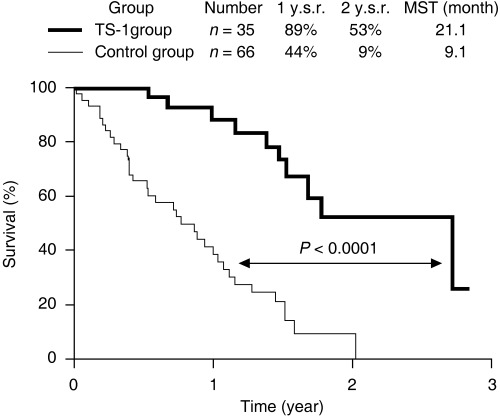
Survival improvement by TS‐1 in patients with peritoneal free cancer cells without macroscopic peritoneal dissemination (P0/Cy1 status). After radical gastrectomy, 35 patients were treated with oral TS‐1 (80 mg/m2) for 28 consecutive days with 14 days rest, and the schedule was repeated every 6 weeks (TS‐1 group). The other 66 patients did not receive any chemotherapy (control group). Patients in the TS‐1 group survived significantly longer than those in the control group (P < 0.0001). The Cox proportional hazard model showed TS‐1 treatment as an independent prognostic factor, and the relative risk for TS‐1 treatment was 0.17‐fold lower than that for the control group. Major adverse reactions included myelosuppression and gastrointestinal toxicities, but they were generally mild and there were no treatment‐related deaths. 1 y.s.r and 2 y.s.r., 1‐year and 2‐year survival rates; MST, median survival time.
Capecitabine is an orally administered fluoropyrimidine anticancer drug used in the treatment of metastatic breast and colorectal cancers. The therapeutic efficacy of capecitabine has been investigated in patients with advanced gastric cancer.( 61 , 62 , 63 , 64 ) Sakamoto et al. reported that the administration of capecitabine for the treatment of chemotherapy‐naive patients with unresectable advanced or metastatic gastric cancer showed promising activity and was well tolerated as a first‐line therapy for advanced and metastatic gastric cancer.( 65 ) In addition, the combination of capecitabine plus oxaliplatin every 3 weeks (XELOX regimen) in patients with previously untreated and non‐resectable advanced gastric adenocarcinoma has been carried out in Korea.( 61 )
Although the oral fluoropyrimidine antimetabolites are promising agents for advanced gastric cancer, the potent therapeutic effects of non‐fluoropyrimidine antimetabolites as typified by 2′‐deoxy‐2′,2′‐difuluorocytidine (gemcitabine) on various solid tumors have recently been demonstrated in preclinical and clinical studies. Gemcitabine, an antimetabolic fluorinated deoxycytidine analog, is used commonly to treat non‐small cell lung cancer, pancreatic cancer, bladder cancer and breast cancer. Among the antitumor 2′‐deoxycytidine analogs used clinically, such as Ara‐C and its prodrugs, only gemcitabine has been proven to be effective as a single agent against solid tumors. Although gemcitabine has no significant antitumor activity as a single agent in gastric cancer, De Lange et al. reported that cisplatin and gemcitabine had moderate efficacy in patients with advanced gastric cancer, with manageable toxicity, and that objective responses were observed in 30% of the patients, including two complete remissions and 10 partial remissions.( 66 ) In a multicenter phase II trial to evaluate gemcitabine plus FOLFOX‐4 (oxaliplatin, folinic acid and 5‐FU) in 36 patients with advanced gastric cancer, the gemcitabine plus FOLFOX‐4 regimen showed sufficient results to warrant a further evaluation of this multidrug combination in randomized phase III trials.( 67 )
Furthermore, newly developed cytosine nucleoside analogs such as 4′‐thio‐FAC, CNDAC and ECyd have been shown to have potent antitumor activity against a wide variety of solid tumors, including murine tumors and human adenocarcinomas.( 68 , 69 , 70 ) Miura et al. previously demonstrated that oral administration of the novel nucleoside analog 4′‐thio‐FAC, which is a strong inhibitor of DNA polymerase α, showed a marked inhibitory effect on the development of ascites and the survival of nude mice implanted with MKN‐45‐P cells into the peritoneal cavity.( 11 ) Moreover, Zajchowski et al. reported that daily oral administration of 4′‐thio‐FAC significantly inhibited the growth of gemcitabine‐resistant BxPC‐3 pancreatic tumors and also induced a regression of gemcitabine‐refractory Capan‐1 tumors.( 71 ) 4′‐thio‐FAC may thus be a potentially effective and therapeutic agent for advanced and metastatic cancers.
Clinical phase I studies of CNDAC and ECyd are now ongoing in the USA. A 2′‐deoxycytidine analog, CNDAC, is metabolically activated by cellular kinases including deoxycytidine kinase (DCK), Ara‐C and gemcitabine.( 72 ) CNDAC 5′‐triphosphate strongly inhibits DNA chain elongation by DNA polymerases. However, ECyd is a potent inhibitor of RNA polymerases.( 73 , 74 ) ECyd requires the activity of uridine/cytidine kinase 2 to produce the corresponding active metabolite (ECyd 5′‐triphosphate).( 75 ) Therefore, resistant cells harboring a mutation in the DCK gene show a crossresistance to 2′‐deoxycytidine analogs, whereas ECyd can effectively inhibit growth of 2′‐deoxycytidine analog‐resistant cells. Kazuno et al. reported that ECyd shows potent antitumor activity against an established gemcitabine‐resistant human pancreatic cancer cell line, which originated from MIAPaCa‐2.( 70 ) We recently investigated the therapeutic effects of CNDAC, Ara‐C, gemcitabine and ECyd on a peritoneal dissemination model using MKN‐45‐P. When either CNDAC or ECyd was injected intraperitoneally into the peritoneal cavity of MKN‐45‐P‐bearing mice every day from day 1 to day 21, both CNDAC and ECyd significantly prolonged survival time in MKN‐45‐P‐bearing mice (Fig. 5). In contrast, gemcitabine and Ara‐C were ineffective in this model. Furthermore, no survival benefit was obtained after the administration of TS‐1 (12 mg/kg) alone in this model.( 76 )
Figure 5.
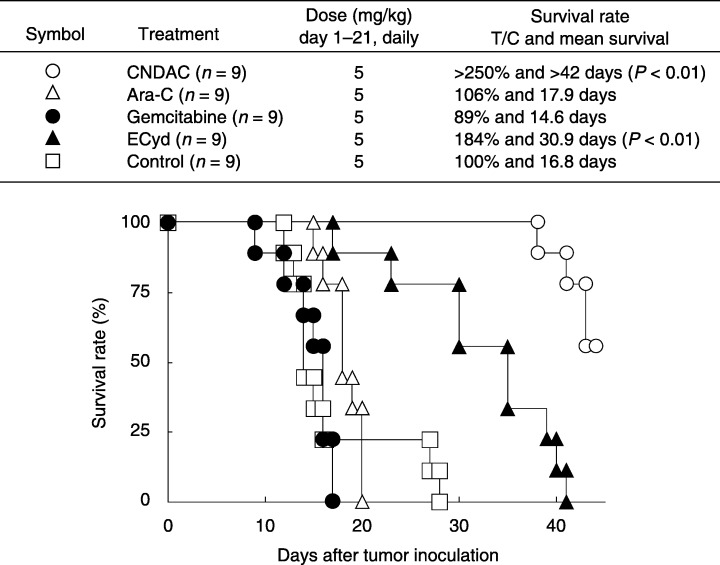
Antitumor effects of nucleoside analogs on peritoneal dissemination of MKN‐45‐P. MKN‐45‐P cells (1 × 107) were inoculated into the peritoneal cavity of nude mice on day 0. Survival curves were constructed using the Kaplan–Meier method. 2′‐Deoxycytidine analogs (CNDAC, Ara‐C and gemcitabine) at a dose of 5 mg/kg were injected intraperitoneally every day from day 1 to day 21. 1‐(3‐C‐ethynyl‐β‐d‐ribo‐pentofuranosyl) cytosine was administered at a dose of 0.1 mg/kg on the same schedule. T/C, treatment group/control group.
Currently, new nucleoside antimetabolites besides CNDAC and ECyd, such as tezacitabine and troxacitabine, are being investigated in clinical trials to evaluate their antitumor activity against solid tumors.( 77 ) These non‐fluoropyrimidine nucleosides may improve the poor prognosis of patients with advanced cancers involving peritoneal dissemination.
Conclusion
Peritoneal dissemination is established through at least two different processes: trans‐mesothelial metastasis and trans‐lymphatic metastasis. Such multimodality of peritoneal dissemination formation and isolated location in the peritoneal cavity make it difficult to treat advanced gastric cancer with peritoneal dissemination. At present, multimodal therapy with NIPS and peritonectomy is the best approach for curative treatment. However, the metabolism and pharmacodynamics of anticancer agents such as taxane and 5‐FU injected into the peritoneal cavity remain obscure. Further basic and pharmacological approaches are required to establish the standard regimen for treating peritoneal dissemination. The non‐fluoropyrimidine nucleoside antimetabolites may become good candidates for novel intraperitoneal combination chemotherapies. For example, combination therapy of the oral fluoropyrimidine and the non‐fluoropyrimidine nucleoside antimetabolites may be a worthwhile regimen for treatment of peritoneal dissemination in the future.
Acknowledgments
This work was supported in part by a Grant‐in‐Aid from the Ministry of Education, Culture, Sports, Japan.
References
- 1. Jacquet P, Vidal‐Jove J, Zhu B, Sugarbaker P. Peritoneal carcinomatosis from gastrointestinal malignancy: natural history and new prospects for management. Acta Chir Belg 1994; 94: 191–7. [PubMed] [Google Scholar]
- 2. Sadeghi B, Arvieux C, Glehen O et al. Peritoneal carcinomatosis from non‐gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000; 88: 358–63. [DOI] [PubMed] [Google Scholar]
- 3. Chu DZ, Lang NP, Thompson C, Osteen PK, Westbrook KC. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer 1989; 63: 364–7. [DOI] [PubMed] [Google Scholar]
- 4. Yonemura Y, Bandou E, Sawa T et al. Neoadjuvant treatment of gastric cancer with peritoneal dissemination. Eur J Surg Oncol 2006; 32: 661–5. [DOI] [PubMed] [Google Scholar]
- 5. Yonemura Y, Bandou E, Kinoshita K et al. Effective therapy for peritoneal dissemination in gastric cancer. Surg Oncol Clin N Am 2003; 12: 635–48. [DOI] [PubMed] [Google Scholar]
- 6. Yonemura Y, Fujimura T, Fushida S et al. A new surgical approach (peritonectomy) for the treatment of peritoneal dissemination. Hepatogastroenterology 1999; 46: 601–9. [PubMed] [Google Scholar]
- 7. Sugarbaker PH, Yonemura Y. Clinical pathway for the management of resectable gastric cancer with peritoneal seeding: best palliation with a ray of hope for cure. Oncology 2000; 58: 96–107. [DOI] [PubMed] [Google Scholar]
- 8. Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol 1999; 43 (Suppl): S15–25. [DOI] [PubMed] [Google Scholar]
- 9. Ajani JA. Evolving chemotherapy for advanced gastric cancer. Oncologist 2005; 10 (Suppl 3): 49–58. [DOI] [PubMed] [Google Scholar]
- 10. Sastre J, Garcia‐Saenz JA, Diaz‐Rubio E. Chemotherapy for gastric cancer. World J Gastroenterol 2006; 12: 204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miura S, Endo Y, Yoshimura Y, Endo M, Yonemura Y, Sasaki T. Potent antitumor effect of 1‐(2‐deoxy‐2‐fluoro‐4‐thio‐β‐d‐arabinofuranosyl) cytosine on peritoneal dissemination models of gastrointestinal cancers. Oncol Rep 2002; 9: 1319–22. [PubMed] [Google Scholar]
- 12. Yonemura Y, Nojima N, Kaji M et al. E‐cadherin and urokinase‐type plasminogen activator tissue status in gastric carcinoma. Cancer 1995; 76: 941–53. [DOI] [PubMed] [Google Scholar]
- 13. Nakashio T, Narita T, Sato M et al. The association of metastasis with the expression of adhesion molecules in cell lines derived from human gastric cancer. Anticancer Res 1997; 17: 293–9. [PubMed] [Google Scholar]
- 14. Koyama T, Yashiro M, Inoue T, Nishimura S, Hirakawa YSCK. TGF‐β1 secreted by gastric fibroblasts up‐regulates CD44H expression and stimulates the peritoneal metastatic ability of scirrhous gastric cancer cells. Int J Oncol 2000; 16: 355–62. [DOI] [PubMed] [Google Scholar]
- 15. Sakakura C, Hagiwara A, Nakanishi M et al. Differential gene expression profiles of gastric cancer cells established from primary tumour and malignant ascites. Br J Cancer 2002; 87: 1153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jacquet P, Sugarbaker PH. Peritoneal–plasma barrier. In: Sugarbaker PH, ed. Peritoneal Carcinomatosis: Principles and Management, 1st edn. Boston: Kluwer Academic Publishers, 1996: 53–63. [DOI] [PubMed] [Google Scholar]
- 17. Weiss L. Metastatic inefficiency. Adv Cancer Res 1990; 54: 159–211. [DOI] [PubMed] [Google Scholar]
- 18. Yonemura Y, Endou Y, Nojima N et al. A possible role of cytokines in the formation of peritoneal dissemination. Int J Oncol 1997; 111: 349–58. [DOI] [PubMed] [Google Scholar]
- 19. Mochizuki Y, Nakanishi H, Kodera Y et al. TNF‐α promotes progression of peritoneal metastasis as demonstrated using a green fluorescence protein (GFP)‐tagged human gastric cancer cell line. Clin Exp Metastasis 2004; 21: 39–47. [DOI] [PubMed] [Google Scholar]
- 20. Kawamura T, Endo Y, Yonemura Y et al. Significance of integrin α2/β1 in peritoneal dissemination of a human gastric cancer xenograft model. Int J Oncol 2001; 18: 809–15. [PubMed] [Google Scholar]
- 21. Yonemura Y, Endou Y, Fujita H et al. Role of MMP‐7 in the formation of peritoneal dissemination in gastric cancer. Gastric Cancer 2000; 3: 63–70. [DOI] [PubMed] [Google Scholar]
- 22. Yonemura Y, Endo Y, Fujita H et al. Inhibition of peritoneal dissemination in human gastric cancer by MMP‐7‐specific antisense oligonucleotide. J Exp Clin Cancer Res 2001; 20: 205–12. [PubMed] [Google Scholar]
- 23. Taniguchi K, Yonemura Y, Nojima N et al. The relation between the growth patterns of gastric carcinoma and the expression of hepatocyte growth factor receptor (c‐met), autocrine motility factor receptor, and urokinase‐type plasminogen activator receptor. Cancer 1998; 82: 2112–22. [PubMed] [Google Scholar]
- 24. Aoyagi K, Kouhuji K, Yano S et al. VEGF significance in peritoneal recurrence from gastric cancer. Gastric Cancer 2005; 8: 155–63. [DOI] [PubMed] [Google Scholar]
- 25. Yonemura Y, Endo Y, Tabata K et al. Role of VEGF‐C and VEGF‐D in lymphangiogenesis in gastric cancer. Int J Clin Oncol 2005; 10: 318–27. [DOI] [PubMed] [Google Scholar]
- 26. Sako A, Kitayama J, Koyama H et al. Transduction of soluble Flt‐1 gene to peritoneal mesothelial cells can effectively suppress peritoneal metastasis of gastric cancer. Cancer Res 2004; 64: 3624–8. [DOI] [PubMed] [Google Scholar]
- 27. Wilke H, Preusser P, Fink U et al. Preoperative chemotherapy in locally advanced and nonresectable gastric cancer: a phase II study with etoposide, doxorubicin, and cisplatin. J Clin Oncol 1989; 7: 1318–26. [DOI] [PubMed] [Google Scholar]
- 28. Wilke H, Preusser P, Fink U et al. New developments in the treatment of gastric carcinoma. Cancer Treat Res 1991; 55: 363–73. [DOI] [PubMed] [Google Scholar]
- 29. MacDonald JS, Schein PS, Woolley PV et al. 5‐Fluorouracil, doxorubicin, and mitomycin (FAM) combination chemotherapy for advanced gastric cancer. Ann Intern Med 1980; 93: 533–6. [DOI] [PubMed] [Google Scholar]
- 30. Wils JA, Klein HO, Wagener DJ et al. Sequential high‐dose methotrexate and fluorouracil combined with doxorubicin – a step ahead in the treatment of advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cooperative Group. J Clin Oncol 1991; 9: 827–31. [DOI] [PubMed] [Google Scholar]
- 31. Ajani JA, Ota DM, Jessup JM et al. Resectable gastric carcinoma. An evaluation of preoperative and postoperative chemotherapy. Cancer 1991; 68: 1501–6. [DOI] [PubMed] [Google Scholar]
- 32. Markman M. Intraperitoneal chemotherapy. Semin Oncol 1991; 18: 248–54. [PubMed] [Google Scholar]
- 33. Armstrong DK, Bundy B, Wenzel L et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006; 354: 34–43. [DOI] [PubMed] [Google Scholar]
- 34. Goel R, Cleary SM, Horton C et al. Effect of sodium thiosulfate on the pharmacokinetics and toxicity of cisplatin. J Natl Cancer Inst 1989; 81: 1552–60. [DOI] [PubMed] [Google Scholar]
- 35. O'Dwyer PJ, LaCreta F, Hogan M, Rosenblum N, O'Dwyer JL, Comis RL. Pharmacologic study of etoposide and cisplatin by the intraperitoneal route. J Clin Pharmacol 1991; 31: 253–8. [DOI] [PubMed] [Google Scholar]
- 36. Fushida S, Furui N, Kinami S et al. Pharmacologic study of intraperitoneal paclitaxel in gastric cancer patients with peritoneal dissemination. Gan Kagaku Ryoho 2002; 29: 2164–7. (In Japanese.) [PubMed] [Google Scholar]
- 37. Yonemura Y, Endou Y, Bando E et al. Effect of intraperitoneal administration of docetaxel on peritoneal dissemination of gastric cancer. Cancer Lett 2004; 210: 189–96. [DOI] [PubMed] [Google Scholar]
- 38. Shimada T, Nomura M, Yokogawa K et al. Pharmacokinetic advantage of intraperitoneal injection of docetaxel in the treatment for peritoneal dissemination of cancer in mice. J Pharm Pharmacol 2005; 57: 177–81. [DOI] [PubMed] [Google Scholar]
- 39. Jeung HC, Rha SY, Jang WI, Noh SH, Chung HC. Treatment of advanced gastric cancer by palliative gastrectomy, cytoreductive therapy and postoperative intraperitoneal chemotherapy. Br J Surg 2002; 89: 460–6. [DOI] [PubMed] [Google Scholar]
- 40. Yonemura Y, Fujimura T, Nishimura G et al. Effects of intraoperative chemohyperthermia in patients with gastric cancer with peritoneal dissemination. Surgery 1996; 119: 437–44. [DOI] [PubMed] [Google Scholar]
- 41. Verwaal VJ, Van Ruth S, De Bree E et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003; 21: 3737–43. [DOI] [PubMed] [Google Scholar]
- 42. Los G, Van Vugt MJ, Pinedo HM. Response of peritoneal solid tumours after intraperitoneal chemohyperthermia treatment with cisplatin or carboplatin. Br J Cancer 1994; 69: 235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fujimoto S, Takahashi M, Kobayashi K et al. Relation between clinical and histologic outcome of intraperitoneal hyperthermic perfusion for patients with gastric cancer and peritoneal metastasis. Oncology 1993; 50: 338–43. [DOI] [PubMed] [Google Scholar]
- 44. Yonemura Y, Bandou E, Kawamura T, Endou Y, Sasaki T. Quantitative prognostic indicators of peritoneal dissemination of gastric cancer. Eur J Surg Oncol 2006; 32: 602–6. [DOI] [PubMed] [Google Scholar]
- 45. Yonemura Y, Kawamura T, Bandou E, Takahashi S, Sawa T, Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg 2005; 92: 370–5. [DOI] [PubMed] [Google Scholar]
- 46. Culliford A, Brooks AD, Sharma S et al. Surgical debulking and intraperitoneal chemotherapy for established peritoneal metastases from colon and appendix cancer. Ann Surg Oncol 2001; 8: 787–95. [DOI] [PubMed] [Google Scholar]
- 47. Cunliffe WJ. The rationale for early postoperative intraperitoneal chemotherapy for gastric cancer. Cancer Treat Res 1991; 55: 143–59. [DOI] [PubMed] [Google Scholar]
- 48. Tanaka M, Sasaki T. Cell culture (collagen gel matrix) and its application for chemosensitivity test. Gan Kagaku Ryoho 1992; 19: 743–8. (In Japanese.) [PubMed] [Google Scholar]
- 49. Vogel I, Kalthoff H. Disseminated tumour cells: Their detection and significance for prognosis of gastrointestinal and pancreatic carcinomas. Virchows Arch 2001; 439: 109–17. [DOI] [PubMed] [Google Scholar]
- 50. Kim JC, Hong HK, Lee KH et al. Experimental radioimmunoguided surgery for peritoneal metastases of gastric cancer using anticarcinoembryonic antigen‐specific T84.66 F(ab′) 2. J Cancer Res Clin Oncol 2005; 131: 495–503. [DOI] [PubMed] [Google Scholar]
- 51. Kodera Y, Nakanishi H, Ito S et al. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: detection of cytokeratin 20 mRNA in peritoneal washes, in addition to detection of carcinoembryonic antigen. Gastric Cancer 2005; 8: 142–8. [DOI] [PubMed] [Google Scholar]
- 52. Yamamoto K, Shimada S, Hirota M, Yagi Y, Matsuda M, Baba H. EIPL (extensive intraoperative peritoneal lavage) therapy significantly reduces peritoneal recurrence after pancreatectomy in patients with pancreatic cancer. Int J Oncol 2005; 27: 1321–8. [PubMed] [Google Scholar]
- 53. Yonemura Y, Fujimura T, Ninomiya I et al. Prediction of peritoneal micrometastasis by peritoneal lavaged cytology and reverse transcriptase–polymerase chain reaction for matrix metalloproteinase‐7 mRNA. Clin Cancer Res 2001; 7: 1647–53. [PubMed] [Google Scholar]
- 54. Osugi H, Takada N, Takemura M et al. Oral fluoropyrimidine anticancer drug TS‐1 for gastric cancer patients with peritoneal dissemination. Oncol Rep 2002; 9: 811–15. [PubMed] [Google Scholar]
- 55. Iwahashi M, Nakamori M, Tani M et al. Complete response of highly advanced gastric cancer with peritoneal dissemination after new combined chemotherapy of S‐1 and low‐dose cisplatin: report of a case. Oncology 2001; 61: 16–22. [DOI] [PubMed] [Google Scholar]
- 56. Maehara Y. S‐1 in gastric cancer: a comprehensive review. Gastric Cancer 2003; 6 (Suppl 1): 2–8. [DOI] [PubMed] [Google Scholar]
- 57. Yoshida K, Ninomiya M, Takakura N et al. Phase II study of docetaxel and S‐1 combination therapy for advanced or recurrent gastric cancer. Clin Cancer Res 2006; 12: 3402–7. [DOI] [PubMed] [Google Scholar]
- 58. Yonemura Y, Endou Y, Bandou E et al. The usefulness of oral TS‐1 treatment for potentially curable gastric cancer patients with intraperitoneal free cancer cells. Cancer Ther 2006; 4: 135–42. [Google Scholar]
- 59. Ueda Y, Yamagishi H, Ichikawa D et al. Phase I study of a combination of s‐1 and weekly paclitaxel in patients with advanced or recurrent gastric cancer. Oncology 2005; 69: 261–8. [DOI] [PubMed] [Google Scholar]
- 60. Sakaguchi Y, Kabashima A, Okita K et al. Long‐term outcome of S‐1 and cisplatin combination therapy in patients with advanced or recurrent gastric cancer. Gastric Cancer 2005; 8: 111–16. [DOI] [PubMed] [Google Scholar]
- 61. Park YH, Kim BS, Ryoo BY, Yang SH. A phase II study of capecitabine plus 3‐weekly oxaliplatin as first‐line therapy for patients with advanced gastric cancer. Br J Cancer 2006; 94: 959–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim JG, Sohn SK, Kim DH et al. Phase II study of docetaxel and capecitabine in patients with metastatic or recurrent gastric cancer. Oncology 2005; 68: 190–5. [DOI] [PubMed] [Google Scholar]
- 63. Cho EK, Lee WK, Im SA et al. A phase II study of epirubicin, cisplatin and capecitabine combination chemotherapy in patients with metastatic or advanced gastric cancer. Oncology 2005; 68: 333–40. [DOI] [PubMed] [Google Scholar]
- 64. Park YH, Ryoo BY, Choi SJ, Kim HT. A phase II study of capecitabine and docetaxel combination chemotherapy in patients with advanced gastric cancer. Br J Cancer 2004; 90: 1329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sakamoto J, Chin K, Kondo K et al. Phase II study of a 4‐week capecitabine regimen in advanced or recurrent gastric cancer. Anticancer Drugs 2006; 17: 231–6. [DOI] [PubMed] [Google Scholar]
- 66. De Lange SM, Van Groeningen CJ, Kroep JR et al. Phase II trial of cisplatin and gemcitabine in patients with advanced gastric cancer. Ann Oncol 2004; 15: 484–8. [DOI] [PubMed] [Google Scholar]
- 67. Correale P, Fulfaro F, Marsili S et al. Gemcitabine (GEM) plus oxaliplatin, folinic acid, and 5‐fluorouracil (FOLFOX‐4) in patients with advanced gastric cancer. Cancer Chemother Pharmacol 2005; 56: 563–8. [DOI] [PubMed] [Google Scholar]
- 68. Miura S, Yoshimura Y, Endo M, Satoh H, Machida H, Sasaki T. Comparison of 1‐(2‐deoxy‐2‐fluoro‐4‐thio‐β‐d‐arabinofuranosyl) cytosine with gemcitabine in its antitumor activity. Cancer Lett 1999; 144: 177–82. [DOI] [PubMed] [Google Scholar]
- 69. Matsuda A, Sasaki T. Antitumor activity of sugar‐modified cytosine nucleosides. Cancer Sci 2004; 95: 105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kazuno H, Sakamoto K, Fujioka A, Fukushima M, Matsuda A, Sasaki T. Possible antitumor activity of 1‐(3‐C‐ethynyl‐β‐d‐ribo‐pentofuranosyl) cytosine (ECyd, TAS‐106) against an established gemcitabine (dFdCyd)‐resistant human pancreatic cancer cell line. Cancer Sci 2005; 96: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zajchowski DA, Biroc SL, Liu HL et al. Anti‐tumor efficacy of the nucleoside analog 1‐(2‐deoxy‐2‐fluoro‐4‐thio‐β‐d‐arabinofuranosyl) cytosine (4′‐thio‐FAC) in human pancreatic and ovarian tumor xenograft models. Int J Cancer 2005; 114: 1002–9. [DOI] [PubMed] [Google Scholar]
- 72. Obata T, Endo Y, Murata D, Sakamoto K, Sasaki T. The molecular targets of antitumor 2′‐deoxycytidine analogues. Curr Drug Targets 2003; 4: 305–13. [DOI] [PubMed] [Google Scholar]
- 73. Hattori H, Tanaka M, Fukushima M, Sasaki T, Matsuda A. Nucleosides and nucleotides. 158. 1‐(3‐C‐ethynyl‐β‐d‐ribo‐pentofuranosyl)‐cytosine, 1‐(3‐C‐ethynyl‐β‐d‐ribo‐pentofuranosyl) uracil, and their nucleobase analogues as new potential multifunctional antitumor nucleosides with a broad spectrum of activity. J Medical Chem 1996; 39: 5005–11. [DOI] [PubMed] [Google Scholar]
- 74. Tabata S, Tanaka M, Endo Y, Obata T, Matsuda A, Sasaki T. Anti‐tumor mechanisms of 3′‐ethynyluridine and 3′‐ethynylcytidine as RNA synthesis inhibitors: development and characterization of 3′‐ethynyluridine‐resistant cells. Cancer Lett 1997; 116: 225–31. [DOI] [PubMed] [Google Scholar]
- 75. Murata D, Endo Y, Obata T et al. A crucial role of uridine/cytidine kinase 2 in antitumor activity of 3′‐ethynyl nucleosides. Drug Metab Dispos 2004; 32: 1178–82. [DOI] [PubMed] [Google Scholar]
- 76. Yonemura Y, Endou Y, Tochiori S et al. Effect of intraperitoneal chemotherapy on experimental peritoneal dissemination of gastric cancer. Gan Kagaku Ryoho 2005; 32: 1635–9. (In Japanese.) [PubMed] [Google Scholar]
- 77. Szafraniec SI, Stachnik KJ, Skierski JS. New nucleoside analogs in the treatment of solid tumors. Acta Pol Pharm 2004; 61: 297–305. [PubMed] [Google Scholar]
- 78. Kobayashi M, Tsuburaya A, Nagata N, Miyashita Y, Oba K, Sakamoto J. A feasibility study of sequential paclitaxel and S‐1(PTX/S‐1) chemotherapy as post‐operative adjuvant chemotherapy for advanced gastric cancer. Gastric Cancer 2006; 9: 114–19. [DOI] [PubMed] [Google Scholar]


