Abstract
Vaccine therapies are increasingly being used for the treatment of various diseases, and the antigen molecules themselves are being expanded from whole microorganisms to fine molecules such as peptides. Accordingly, there is a need for new adjuvants to support these new applications. In this paper, we used pharmaceutical grade mineral oil and sorbitan monooleate to develop a new oil adjuvant formula, NH2, and investigated its effects on peptide vaccination at both the pre‐clinical and clinical levels. The adjuvant effect of NH2 on peptide‐induced cellular immunity in mice was superior to that of Montanide ISA51VG, a commercially available incomplete Freund’s adjuvant for clinical use, although no significant difference was observed between the two adjuvants on peptide‐induced humoral immunity. The adjuvant effects of NH2 were also confirmed in a Phase‐I clinical trial of peptide vaccines for patients with advanced cancers. These results suggest that NH2 is a suitable adjuvant for peptide vaccination, particularly for cancer vaccines (Phase‐I clinical trial of pan‐HLA type personalized peptide vaccine for advanced cancer patients, UMIN clinical trial registry number: UMIN 000000619). (Cancer Sci 2010)
Vaccine therapies have long been used for the prevention of infectious diseases. However, recent advances in immunology and molecular pathology have led to the use of vaccines not only for the prevention but also for the treatment of allergies,( 1 , 2 ) autoimmune diseases,( 3 , 4 , 5 ) cancer,( 6 , 7 ) Alzheimer disease,( 8 ) and hypertension.( 9 ) At the same time, the antigens in the vaccine formulas have expanded from whole microorganisms to fine molecules such as epitope peptides. There is, thus, a need for new adjuvants to be used in these new applications.
Incomplete Freund’s adjuvant (IFA) is one of the most famous adjuvants and is widely used for protein and peptide antigens to induce both humoral and cellular immunity in animals and humans. There are currently only two IFA formulas commercially available for clinical use: Montanide ISA51(VG) and ISA720 (products of Seppic, Paris, France).( 10 , 11 ) Both Montanide ISA51(VG) and the original formula of IFA, consist of mineral oil and mannide monooleate.( 10 ) Although pharmaceutical‐grade (i.e. meeting the requirements of pharmacopeia such as the Japanese pharmacopeia and US pharmacopeia) mineral oil is commercially available, pharmaceutical‐grade formulas of mannide monooleate, such as Montanide 80 (Seppic), are difficult to obtain, particularly for academic researchers. A high‐purity oleic acid derivative, sorbitan monooleate (NOFABLE SO‐991), has recently been made available, and its regulatory status meets the requirements of the Japanese pharmaceutical excipients. Therefore, we attempted to develop a new oil adjuvant formula for clinical use using a pharmaceutical grade mineral oil and sorbitan monooleate. In this paper, we investigated the effects of the new adjuvant on peptide vaccination at both the pre‐clinical and clinical levels.
Materials and Methods
Adjuvants. The formula of the new adjuvant, NH2, is as follows: sorbitan monooleate (NOFABLE SO‐991; NOF Corporation, Tokyo, Japan) 11.4 w/w% and mineral oil (Hicall M‐72; Kaneda, Tokyo, Japan) 88.6 w/w%. Sorbitan monooleate and mineral oil were gently mixed at room temperature for 2 h and the precipitate was removed and sterilized by a tandem filtration using autoclaved Millex‐FA (1.0 μm) and Millex‐FG (0.2 μm) filters (Millipore, Billerica, MA, USA), and aseptically aliquoted into sterilized glass vials (5 mL per vial). Montanide ISA51VG was purchased from Seppic. The regulatory status of NOFABLE SO‐991 and Hicall M‐72 met the requirements of the Japanese pharmaceutical excipients and Japanese pharmacopeia, respectively. If NH2 is manufactured as an intra‐hospital product and only use in the hospital, Good Manufacturing Practice (GMP) is not applied, since intra‐hospital products are manufactured according to the physician’s prescriptions and not the drugs as provided for by the Pharmaceutical Affairs Law. The manufacturing of such intra‐hospital products is regulated by Medical Care Act, Medical Practitioners’ Act, and Pharmacists’ Act. Manufacturing and quality of our vaccine products including NH2 were controlled and assured by authorized pharmacists under manufacturing procedures and the facility had been authorized by the domestic government. Thus, the quality of the vaccine products including NH2 is sufficient for clinical use.
Stability of the NH2 adjuvant has not been examined, since the stability data of two components, NOFABLE SO‐991 and Hicall M‐72, suggest that each component is relatively stable in the mixture under anhydrous conditions in a sealed bottle, and we do not expect long term storage. On shelf stability test of the NH2 adjuvant might be needed for long term storage.
Antigens. The peptide antigens used in this study were as follows: SART3‐109 (VYDYNCHVDL) and SART3‐315 (AYIDFEMKI) derived from human SART3 at positions 109‐118 and 315‐323, respectively. Both the peptides were originally identified as epitope peptides recognized by human CTL in a human leukocyte antigen (HLA)‐A24 restricted manner.( 12 ) These two peptides are able to induce immune responses in H‐2d haplotype BALB/c mice, since the human HLA‐A2402 and mouse H‐2Kd molecules share similar antigen peptide‐binding motifs.( 13 ) The peptides were produced by NeoMPS (San Diego, CA, USA) under GMP conditions. The peptides were dissolved in DMSO at a concentration of 10 mg/mL and diluted with saline. After filter sterilization, the antigen solution was mixed with an equal volume of adjuvant (NH2 or ISA51VG) using two 5‐ or 10‐mL gasket‐free syringes (B. Braun, Melsungen, Germany) connected by a GP syringe‐connector (Green Peptide, Kurume, Japan) to produce a water‐in‐oil (W/O) type emulsion. The quality of the emulsion was confirmed by a droplet test. If a droplet of the antigen emulsion added to a beaker of water remained at the surface without dispersing, the emulsion was considered to have sufficiently high quality for use.
Mice. Female BALB/c and CD‐1 (ICR) mice aged 7 weeks were purchased from Charles River Japan (Yokohama, Japan) and housed in the animal facility at the Kurume University School of Medicine under specific‐pathogen‐free conditions. The experiments were conducted on mice aged 8–14 weeks. All animal experiments were approved by the Ethical Committee for Animal Experiments at Kurume University.
In vivo stability of emulsion. The emulsion was injected subcutaneously (s.c.) into both sides of the inguinal region of CD‐1 mice in a volume of 0.1 mL for each injection site (two sites per mouse) at day 0. At days 3, 7, 10, or 14, the mice were killed and the stability of the emulsion was evaluated based on the residual amount of the emulsion at the injection sites by the naked eye. Ten mice were used for each time point. The residual amount of emulsion was classified into five categories as follows: 4+, no significant reduction was observed; 3+, approximately three‐quarters of the injected amount remained; 2+, approximately half of the injected amount remained; 1+, approximately one‐quarter of the injected amount remained; 0, nothing remained.
Humoral immune response. BALB/c mice were immunized by s.c. injection of the antigens (100 μg/0.1 mL) once a week. After several immunizations, serum samples were obtained and anti‐peptide IgG antibody was measured by a Luminex fluorescence microarray system FLEXMAP3D (Luminex Corp., Austin, TX, USA) as previously reported.( 14 ) Anti‐peptide IgG antibody in human plasma was also measured by the Luminex system .
Cellular immune response. The spleens or lymph nodes were aseptically collected from the immunized mice 1 day after the last immunization. The tissues were teased gently to obtain a single cell suspension. Detection of cells secreting interferon gamma (IFN‐γ) was performed by an ELISPOT assay using a kit (BD ELISPOT mouse IFN‐γ ELISPOT set; BD Biosciences, San Diego, CA, USA) according to the manufacturer’s instructions. The cells were cultured overnight with 20 μg/mL of peptide in wells of an anti‐IFN‐γ antibody‐coated ELISPOT plate at 2 × 106 cells per well for the spleen cells or 5 × 105 cells per well for the lymph node cells. In the clinical study, IFN‐γ secretion of peptide‐induced CTL after stimulation with peptide was quantified by a conventional ELISA as previously reported.( 14 ) In brief, peripheral blood mononuclear cells of the patients were cultured in quadricate with 10 μg/mL of peptide for 2 weeks, and the peptide‐induced CTL were harvested and tested for their ability to produce IFN‐γ in response to CIR‐A2402 cells which were pre‐loaded with either a corresponding peptide, or an HIV peptide as a negative control. The level of IFN‐γ was determined by ELISA performed in quadruplicate. Student’s t‐test was employed for the statistical analysis. A well was considered positive when the level of IFN‐γ production in response to a corresponding peptide was significantly higher (P < 0.05) than that in response to an HIV peptide, and also the amount of IFN‐γ produced in response to the peptide was more than 10 ng/mL greater than the amount produced in response to an HIV peptide. The number of positive wells in the quadricate cultures was taken as the positive‐well frequency.
Clinical study. The clinical results presented in this paper are a part of the results of our previously performed “Phase‐I clinical trial of pan‐HLA type personalized peptide vaccine for advanced cancer patients.” The study protocol (UMIN clinical trial registry number: UMIN 000000619) was approved by the Ethical Committee of Kurume University, and complete written informed consent was obtained from all patients at the time of enrollment. In brief, a maximum of four peptides corresponding to the patients’ immunological memory were selected by measuring plasma IgG to the vaccine candidate peptide panel consisting of 24 peptides, including SART3‐109. The peptides were individually emulsified in a volume of 1.5 mL containing 3 mg of peptide and 0.75 mL of NH2 adjuvant, and s.c. injected into the lateral thigh at 2‐week intervals. In the clinical trial, eight patients were immunized with SART3‐109 peptide. The main results of the Phase‐I study, including the safety and immune responses to the vaccine peptides, will be reported elsewhere (Yamada A, Noguchi M, Komatsu N, Suekane S, Yutani S, Moriya F, Mine T, Momozono K, Kawano K, Itoh K, manuscript in preparation).
Results
Characteristics and stability of emulsion. Particle size and uniformity of the emulsion were analyzed by microscopic observation. NH2 or ISA51VG was mixed with an equivalent volume of saline to make the emulsion. The emulsion was then diluted 1:50 with Isoper L (Exon Mobile, Tokyo, Japan) and subjected to phase‐control microscopic observations. The majority of the particles formed by NH2 or ISA51VG were homogeneous and had a size of approximately 1 μm (Fig. 1). No separation of the emulsions made by NH2 or ISA51VG was observed at 3 months, and thus the emulsions were considered stable at room temperature for >3 months (data not shown). The in vivo stability of the emulsions was also analyzed. The residual amounts of the emulsion at the injection sites were classified into five categories (4+ to 0) as described above, and the number of sites in each category are shown in Figure 2. Both the emulsion made using NH2 and that made with ISA51VG were stable in vivo at least 3 days after the injection. A significant reduction (3+ or less) was observed in 9 (45%) and 12 (60%) of 20 injection sites in the NH2 and ISA51VG groups, respectively, at day 7, suggesting that in vivo degradation of the emulsions began 3–7 days after the injection in the majority of cases. There was no apparent difference in the in vivo stability between the NH2 and ISA51VG emulsions, although a marked reduction (1+ and 0) was more frequently observed in the NH2 emulsion.
Figure 1.
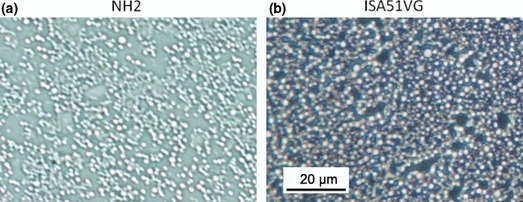
Microscopic observation of the emulsion. (a) NH2 or (b) ISA51VG was mixed 1:1 with saline to form an emulsion. The emulsion was then diluted 1:50 with Isoper L (Exon Mobile, Tokyo, Japan) and subjected to phase‐control microscopic observation.
Figure 2.
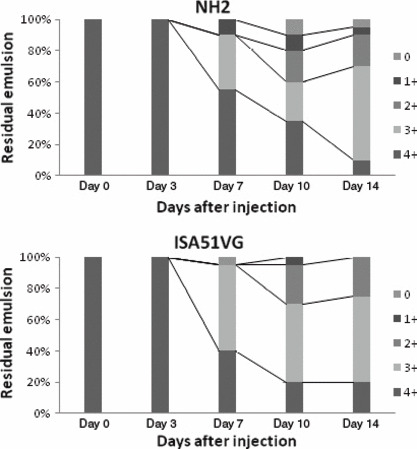
In vivo stability of the emulsion. The emulsion was injected subcutaneously into both sides of the inguinal region of CD‐1 mice in a volume of 0.1 mL for each injection site (two sites per mouse) at day 0. At days 3, 7, 10, or 14, the mice were killed and the stability of the emulsion was evaluated based on the residual amount of the emulsion at the injection sites by the naked eye. Ten mice were used for each time point. The residual amount of emulsion was classified into five categories as follows: 4+, no significant reduction was observed; 3+, approximately three‐quarters of the injected amount remained; 2+, approximately half of the injected amount remained; 1+, approximately one‐quarter of the injected amount remained; 0, nothing remained.
Effects of NH2 and ISA51VG on humoral immunity in mice. SART3‐109 peptide was mixed with NH2 or ISA51VG and injected weekly into BALB/c mice. Serum samples were collected 7 days after the 4th or 6th immunization, and their SART3‐109‐specific IgG1 or IgG2 antibody levels were measured. SART3‐109 peptide alone (without any adjuvant) was also used for immunization (Fig. 3). Augmentation of peptide‐specific IgG1 responses was detected in 5/8 (63%) and 4/8 (50%) of mice in the NH2 and ISA51VG groups, respectively, after the 4th immunization, and 5/8 (63%) and 6/8 (75%) after the 6th immunization. Similarly, augmentation of peptide‐specific IgG2a responses was detected in 5/9 (56%) and 4/8 (50%) of mice in the NH2 and ISA51VG groups, respectively, after the 4th immunization, and 4/8 (50%) and 6/8 (75%) after the 6th immunization.
Figure 3.
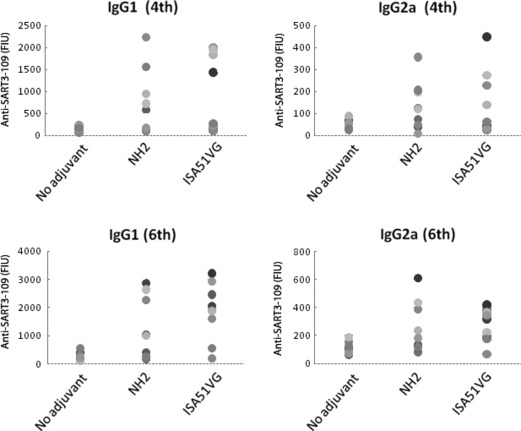
Effects of NH2 and ISA51VG on humoral immunity in mice. BALB/c mice were immunized by subcutaneously injection of 100 μg of SART3‐109 peptide with/without adjuvant (total volume 0.1 mL) once a week. After the 4th and 6th immunizations, serum samples were obtained and anti‐SART3 IgG1 and IgG2a were measured by a Luminex fluorescence microarray system.
Effects of NH2 and ISA51VG on cellular immunity in mice. SART3‐109 peptide was mixed with/without NH2 or ISA51VG and injected weekly into BALB/c mice. Spleens or lymph nodes were collected 1 day after the last immunization, the cells were stimulated with/without SART3‐109 or an irrelevant peptide, SART3‐315, and the numbers of IFN‐γ producing cells were counted by ELISPOT analyses (Fig. 4). “No Ag” means no in vitro stimulation with antigen; however, all the groups including “no Ag” and “SART3‐315” groups had been stimulated in vivo with SART3‐109 1 day before the assay. Therefore, values of “no Ag” and “SART3‐315” groups show sum of SART3‐109‐specific immunity stimulated by in vivo and background response. Levels of background responses may be similar to those of post 2nd to 4th vaccination. A SART3‐109‐specific IFN‐γ response was not detected in the spleens of the mice immunized with SART3‐109 alone at any time point. After the 5th immunization, weak augmentation of the IFN‐γ response was observed in the spleens of the ISA51VG group, and a stronger augmentation effect was detected in spleens of the NH2 group. In the lymph nodes, such apparent augmentation of IFN‐γ response was not observed in both the ISA51VG and NH2 groups.
Figure 4.
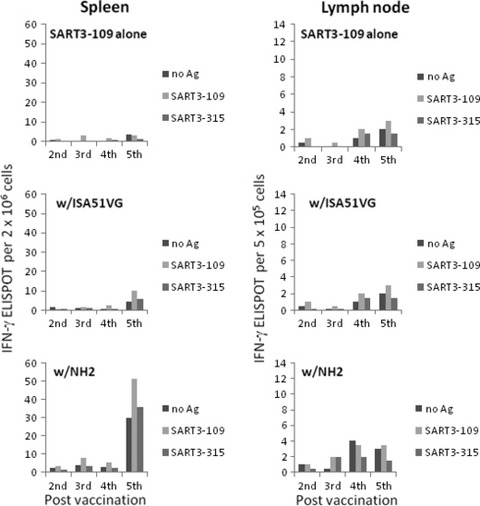
Effects of NH2 and ISA51VG on cellular immunity in mice. BALB/c mice were immunized with SART3‐109 peptide with/without adjuvant. The numbers of IFN‐γ producing cells in the spleen and lymph nodes of the immunized mice were determined by ELISPOT assay.
Effects of NH2 on humoral immunity in humans. In the human Phase‐I trial, peptide vaccinations with NH2 adjuvant were well tolerated in all patients with no vaccine‐related severe adverse events. The main results of the Phase‐I study, including the safety and immune responses to the vaccine peptides, will be reported elsewhere (Yamada A, et al., manuscript in preparation). SART3‐109‐specific IgG antibody levels in the plasma samples gathered in the pre‐vaccination period and at 1 week after the 6th or 12th vaccination from the patients who received SART3‐109 vaccination with NH2 adjuvant were analyzed (Fig. 5). SART3‐109‐specific IgG levels were increased in three of eight cases after the 6th vaccination. Although no augmentation of the IgG response was observed in the remaining five cases after the 6th vaccination, augmentation of the IgG was observed after the 12th vaccination in all three of the patients who continued the vaccination (vaccination was ceased in the remaining two patients after the 6th vaccination).
Figure 5.
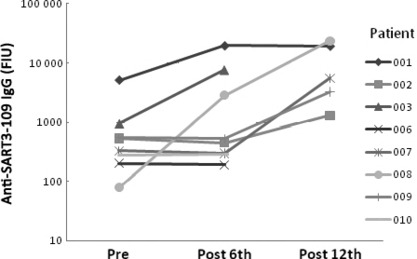
Effects of NH2 on humoral immunity in humans. Anti‐SART3‐109 IgG levels were measured in the plasma of patients who were immunized with a cancer vaccine containing SART3‐109 peptide with NH2 adjuvant.
Effects of NH2 on cellular immunity in humans. SART3‐109‐specific CTL induction in peripheral blood mononuclear cells during the pre‐vaccination period and 1 week after the 6th vaccination of the patients whose IgG response had been augmented after the 6th vaccination was measured. As shown in Table 1, the SART3‐109‐specific CTL response was increased in two of three cases.
Table 1.
Effect of NH2 adjuvant on human CTL induction
| Patient | SART3‐109‐induced IFN‐γ production | |||
|---|---|---|---|---|
| Pre‐vaccination | Post 6th vaccination | |||
| Positive well frequency | IFN‐γ of positive wells (pg/mL) | Positive well frequency | IFN‐ γ of positive wells (pg/mL) | |
| 001 | 1/4 | 63 | 2/4 | 182, 273 |
| 003 | 0/4 | – | 0/4 | – |
| 008 | 1/4 | 12 | 3/4 | 27, 62, 281 |
Discussion
The importance of translational research is now widely accepted in the various fields of medical science, and particularly for the development of cancer vaccines. Nonetheless, the development of peptide vaccines against cancers presents several difficulties that are not encountered when developing vaccines for infectious diseases: (i) the major purpose of the cancer vaccines is therapeutic rather than prophylactic; (ii) the characteristics of cancer cells, as well as the origin, tissue type, and differentiation stages, vary among individual patients; (iii) major histocompatibility complex restriction is a factor when developing cancer vaccines; and (iv) the target population of the clinical trials is patients rather than healthy donors. Together with the above issues, several hundreds of CTL‐epitope peptides derived from various types of cancers have been identified by academia, and thus the majority of the clinical trials are currently initiated by academic researchers, but not pharmaceutical industries. For academic researchers, peptides of GMP grade are now readily available from several companies, but with the exception of Montanide ISA51VG and ISA720, clinical‐grade adjuvants for peptide vaccination are difficult to obtain. These facts hamper the promotion of translational research on peptide cancer vaccines, and thus the development of a new clinical‐grade adjuvant available for academic researchers is needed.
Different types of adjuvants have been reported previously.( 10 , 11 ) However, suitable adjuvants for peptide vaccination are limited, since most of the vaccine peptides are usually 9‐10‐mer in size (MW is around 1100 Da) and sensitive to various peptidases and proteases; moreover, the majority of cancer vaccines are designed to induce CTL rather than antibody to the vaccinated peptides.( 6 ) W/O emulsion‐type adjuvants, such as ISA51VG and NH2, are suitable for peptide vaccination, since peptides solubilized in water phase are sequestered from peptidase‐containing body fluid, and slow release of the peptides from the emulsion provides sustained antigenic stimulation.( 10 ) However, the molecular mechanisms of the immune‐enhancing effects of oil adjuvants are still unclear.( 15 ) Induction of toll‐like receptor (TLR) 2 by IFA has been reported.( 16 ) A signaling pathway through TLR is thought to be important for the expression of adjuvant activity, since TLR9 agonists such as oligonucleotides containing unmethylated CpG motifs or non‐toxic variants of TLR4‐activating lipids such as monophosphoryl lipid A express adjuvant activity.( 17 , 18 ) However, a recent study using MyD88−/−; Trif Lps2/Lps2 mice demonstrated that TLR signaling did not account for the action of adjuvants, including IFA, in this model.( 15 , 19 ) Therefore, further study regarding molecular mechanisms of oil adjuvants is needed to develop safer and more effective adjuvants for vaccination.
In this study, we demonstrated that a new formula of W/O emulsion adjuvant, NH2, could be used for translational studies performed by academic researchers, and that the adjuvant activity of NH2 on the peptide‐induced CTL response was higher than that of ISA51VG in a pre‐clinical study using mice. The effectiveness of NH2 adjuvant was further confirmed by a clinical study of peptide‐based cancer vaccines. These results suggest the usefulness of NH2 adjuvant for further translational research on peptide vaccines, particularly peptide‐based cancer vaccines, by academic researchers. It should be noted that after several months of shelf storage of NH2 at room temperature, a very small amount of precipitates attached to the glass surface were observed in some vials. Therefore, further improvement might be needed for industrial use.
Disclosure Statement
Yamada and Itoh received a research funding from the Green Peptide Co. Ltd.; Yamada and Itoh own stock in the Green Peptide Co., and Yamada is a part‐time executive of the Green Peptide Co.
Acknowledgments
This study was supported in part by Grants‐in‐Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and by the 21st Century COE Program for Medical Science.
References
- 1. Erb KJ, Wohlleben G. Novel vaccines protecting against the development of allergic disorders: a double‐edged sword? Curr Opin Immunol 2002; 14: 633–43. [DOI] [PubMed] [Google Scholar]
- 2. Weiss R, Hammerl P, Hartl A et al. Design of protective and therapeutic DNA vaccines for the treatment of allergic diseases. Curr Drug Targets Inflamm Allergy 2005; 4: 585–97. [DOI] [PubMed] [Google Scholar]
- 3. Cohen‐Kaminsky S, Jambou F. Prospects for a T‐cell receptor vaccination against myasthenia gravis. Expert Rev Vaccines 2005; 4: 473–92. [DOI] [PubMed] [Google Scholar]
- 4. Sela M. Immunomodulatory vaccines against autoimmune diseases. Rejuvenation Res 2006; 9: 126–33. [DOI] [PubMed] [Google Scholar]
- 5. Correale J, Farez M, Gilmore W. Vaccines for multiple sclerosis: progress to date. CNS Drugs 2008; 22: 175–98. [DOI] [PubMed] [Google Scholar]
- 6. Itoh K, Yamada A. Personalized peptide vaccines: a new therapeutic modality for cancer. Cancer Sci 2006; 97: 970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Itoh K, Yamada A, Mine T, Noguchi M. Recent advances in cancer vaccines: an overview. Jpn J Clin Oncol 2009; 39: 73–80. [DOI] [PubMed] [Google Scholar]
- 8. Wisniewski T, Konietzko U. Amyloid‐beta immunisation for Alzheimer’s disease. Lancet Neurol 2008; 7: 805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown MJ. Therapeutic potential of vaccines in the management of hypertension. Drugs 2008; 68: 2557–60. [DOI] [PubMed] [Google Scholar]
- 10. Aucouturier J, Dupuis L, Ganne V. Adjuvants designed for veterinary and human vaccines. Vaccine 2001; 19: 2666–72. [DOI] [PubMed] [Google Scholar]
- 11. Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev 2008; 60: 915–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niiya F, Nishizaka S, Matsunaga K et al. Expression of SART3 tumor‐rejection antigen in gastric cancers. Jpn J Cancer Res 2000; 91: 337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamada A, Yano H, Takao Y, Ono T, Matsumoto T, Itoh K. Nonmutated self‐antigen‐derived cancer vaccine peptides elicit an IgE‐independent but mast cell‐dependent immediate‐type skin reaction without systemic anaphylaxis. J Immunol 2006; 176: 857–63. [DOI] [PubMed] [Google Scholar]
- 14. Yutani S, Yamada A, Yoshida K et al. Phase I clinical study of a personalized peptide vaccination for patients infected with hepatitis C virus (HCV) 1b who failed to respond to interferon‐based therapy. Vaccine 2007; 25: 7429–35. [DOI] [PubMed] [Google Scholar]
- 15. Moreira LO, Smith AM, DeFreitas AA, Qualls JE, El Kasmi KC, Murray PJ. Modulation of adaptive immunity by different adjuvant‐antigen combinations in mice lacking Nod2. Vaccine 2008; 26: 5808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lim SK. Freund adjuvant induces TLR2 but not TLR4 expression in the liver of mice. Int Immunopharmacol 2003; 3: 115–8. [DOI] [PubMed] [Google Scholar]
- 17. Krieg AM. Toll‐free vaccines? Nat Biotechnol 2007; 25: 303–5. [DOI] [PubMed] [Google Scholar]
- 18. Mata‐Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF‐biased agonist of TLR4. Science 2007; 316: 1574–6. [DOI] [PubMed] [Google Scholar]
- 19. Gavin AL, Hoebe K, Duong B et al. Adjuvant‐enhanced antibody responses in the absence of toll‐like receptor signaling. Science 2006; 314: 1859–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


