Abstract
Hepatitis C virus (HCV) infection has a high risk of liver cirrhosis and hepatocellular carcinoma at later stages. We recently identified a peptide derived from the HCV core protein capable of inducing both cellular and humoral responses to nearly all HCV‐positive patients in Japan with different human leukocyte antigen (HLA)‐class I‐A alleles. To assess the safety and immune responses to this novel peptide, we conducted a phase I dose‐escalation study of the vaccination for 26 HCV‐positive patients who were either non‐responders to the interferon‐based therapy (n = 23) or refused it (n = 3). The regimen was well tolerated, with no severe vaccine‐related toxicity. Twenty‐five and 22 patients completed the first and second cycle vaccination (6 and 12 vaccine injections), respectively. After a series of six vaccine injections, peptide‐specific CTL activity was augmented in peripheral blood mononuclear cells from 15 of 25 patient samples, with an expected optimal dose of 1 mg peptide. After 12 vaccine injections, peptide‐specific IgG production was augmented in plasma from the majority of patients (15 of 22 patients) tested, but not in a dose‐dependent fashion. There were two HCV RNA responders with >1 log declines. Among patients whose pre‐vaccination levels of alanine aminotransferase and alpha feto‐protein exceeded the normal ranges, a <30% decrease was found in 7 of 24 and three of six patients, respectively. Because of its tolerability and higher rate of immune boosting, this protocol is recommended for a phase II study to investigate its clinical efficacy. (Cancer Sci 2009; 100: 1935–1942)
Hepatitis C virus (HCV) is prevalent worldwide, with nearly 180 million individuals infected.( 1 , 2 ) Interferon (IFN)‐based therapies, although effective in 80% of patients infected with the HCV2 and HCV3 genotypes and also 50% of patients with the HCV1b genotype, have several limitations, including medical or physical contraindications, adverse events, and high cost.( 1 , 2 , 3 , 4 ) HCV1b is the most frequently observed genotype in Japan (70%) and is also frequently observed in the USA.( 3 , 4 ) HCV‐infected patients tend to develop liver cirrhosis (LC) and ultimately hepatocellular carcinoma (HCC). From this point of view, therapeutic HCV vaccines are also prophylactic cancer vaccines for HCV‐related HCC. Thus, there have been a variety of efforts to develop a HCV vaccine, including clinical trials of HCV vaccines with peptides capable of inducing human leukocyte antigen (HLA)‐A2‐ or HLA‐A24‐restricted CTL responses.( 5 , 6 , 7 ) However, no promising clinical outcome in those trials have been reported at the present time from the view of sustained viral responses. This might be in part due to insufficient activity to boost CTL activity. Alternatively, this might be in part due to an inability to induce humoral responses.
We recently identified a peptide derived from the HCV core protein capable of inducing both cellular and humoral responses to nearly all HCV‐positive patients in Japan with various HLA‐class I‐A alleles (Niu Y, Komatsu N, Komohara Y, Matsueda S, Yutani S, Ishihara Y, Itou M, Yamada A, Itoh K, Shichijo S unpubl. data). This peptide is well known as a HLA‐A2‐restricted CTL epitope,( 8 , 9 ) and its sequence is shared in the HCV1a, HCV1b, HCV2a, and HCV3a major genotypes found worldwide. In the present paper, we have reported the results of a phase I dose‐escalation study of this peptide vaccination.
Materials and Methods
Patient eligibility. This was a phase I dose‐escalation study. All laboratory tests required in order to assess eligibility had to be completed within 7 days prior to the start of treatment. The following inclusion criteria were mandatory:
-
1
Patients were required to have persistent HCV infection confirmed by HCV RNA test using serum.
-
2
All patients were diagnosed with chronic hepatitis (CH) or LC by laboratory tests (hepatic enzymes and platelet count) and ultrasonography.
-
3
Patients were either non‐responders to the previously conducted IFN‐based treatments (n = 23) or refused to receive them (n = 3).
-
4
Patients had no detectable levels of HCC at the time of entry.
-
5
Patients were required to be positive for one of the following alleles: HLA‐A2, HLA‐A11, HLA‐A24, HLA‐A26, HLA‐A31, or HLA‐A33.
-
6
Patients were required to have an Eastern Cooperative Oncology Group performance status of 0–1, age between 20 and 75 years, and adequate hematological (white blood cell count ≥2400/µL, hemoglobin level ≥8.0 g/dL, platelet counts ≥50 000/µL), renal (serum creatinine ≤1.4 g/dL), and hepatic (total bilirubin <2.5 mg/dL) functions.
-
7
Patients were required to be negative for hepatitis B antigens.
-
8
Patients were required to have had at least 4 weeks of recovery from the toxic effects of any previous therapy before trial entry.
Pregnant patients and patients with an autoimmune disease, an active infection, cancer, or hepatic encephalopathy were excluded. Patients with ascites were also excluded. The study protocol was approved by the Ethical Committee of Kurume University, and complete written informed consent was obtained from all patients at the time of enrollment. A total of 26 patients who were seen at our institution between October 2004 and June 2008 were included in this study.
Peptide and vaccination. The vaccinated peptide was originated from HCV core protein at positions 35–44 (C35‐44, YLLPRRGPRL), a well‐known HLA‐A2‐restricted CTL epitope( 8 , 9 ) that is conserved in various HCV genotypes (3, 4, 8, and 9). Our manuscript currently under submission elsewhere can be summarized as follows. This peptide demonstrated binding activity to HLA‐A*2402, HLA‐A*2601, HLA‐A*3101, and HLA‐A*3303 molecules, but showed no binding to HLA‐A*1101. With regard to HLA‐A2 subtypes, the peptide demonstrated binding activity to HLA‐A*0201 and HLA‐A*0206 molecules, but not to HLA‐A*0207 molecules. The peptide induced CTL activity in both patients and healthy donors with all the HLA‐class I‐A molecules mentioned above including HLA‐A*1101 and HLA‐A*0207, as far as tested by the IFN‐γ production assay.
The peptide was prepared under the conditions of Good Manufacturing Practices by the American Peptide Company (San Diego, CA, USA), and was dissolved and stored at –80°C. Stock solutions were diluted with saline just before use. For injection of 0.3, 1, and 3 mg of peptide (levels 1, 2, and 3), 1 mL of the peptide, which was supplied in vials containing 1, 2, or 4 mg/mL sterile solution, was mixed with 1 mL of incomplete Freund's adjuvant (Montanide ISA51VG; Seppic, Paris, France) and emulsified in 5‐mL sterilized syringes followed by 0.6, 1, and 1.5 mL injection, respectively. The peptide emulsion was injected biweekly into the subcutaneous region of the side abdomen. All patients were treated in an outpatient clinic. Blood for immunological studies was obtained before vaccine injections, after the sixth and 12th injections. The first cycle consisted of six vaccinations, and second or later cycles of six vaccinations were conducted when the patients agreed and in the absence of severe toxicity. Twenty‐five and 22 patients completed the first and second cycle vaccination (six and 12 vaccine injections), respectively.
Cellular and humoral responses to peptides. Thirty milliliters of peripheral blood was obtained before and after vaccinations for the measurement of CTL precursors in peripheral blood mononuclear cells (PBMC) and IgG specific to C35‐44 peptide in plasma, according to previously reported methods.( 10 , 11 , 12 , 13 ) The positive control peptides used in this study were Epstein–Bar virus (EBV)‐ and influenza virus (Flu)‐derived peptides capable of inducing CTL activity restricted to HLA‐A2, HLA‐A3 supertype (A11, A31, A33), and HLA‐A24 alleles, as reported previously.( 10 , 11 , 14 ) The negative control peptides were human immunodeficiency virus (HIV)‐derived peptides capable of inducing CTL activity restricted to HLA‐A2, HLA‐A3 supertype, and HLA‐A24 alleles as reported previously,( 10 , 11 , 14 ) whereas the sequence of the peptide to the HLA‐A26 allele is EVIPMFSAL.( 15 ) In brief, for the CTL precursor assay, the peptide‐stimulated PBMC were harvested and tested for their ability to produce IFN‐γ in response to T2 cells for HLA‐A2+ (A0201, A0206, A0207) cases or CIR cells expressing HLA‐A1101, HLA‐A2401, HLA‐A2601, HLA‐A3101, or HLA‐A3303 molecules for the corresponding cases. These cells were preloaded with either a corresponding peptide or with a HIV peptide as a negative control. The level of IFN‐γ was determined by an ELISA carried out in quadruplicate. A two‐tailed Student's t‐test was used for the statistical analysis. A well was considered positive when the level of IFN‐γ production in response to a corresponding peptide was significantly higher (P < 0.05) than that in response to a HIV peptide, and when the amount of IFN‐γ produced in response to the peptide was more than 50 ng/mL greater than the amount produced in response to a HIV peptide.
The plasma levels of peptide‐specific IgG were measured by an ELISA system, and the results were shown as optical density as reported previously.( 12 ) An increment of peptide‐specific IgG was judged to have occurred if the IgG amount showed a more than 1.5‐fold increase.
The peptide‐specific antibody levels were also measured by microsuspension array technique as reported previously.( 7 ) Briefly, diluted plasma samples were incubated with peptide‐coated microspheres. After washing, the microspheres were incubated with antihuman Ig isotype‐specific antibodies. After washing, the microspheres bound with each antibody were reacted with the biotin‐labeled detection antibody and R‐phycoerythrin corresponding antibody, and the antibody levels were detected using a Luminex system, FLEXMAP3D, Luminex Corp., Austin, TX. As a control, IgG to HCV core protein was also measured by radioimmunoassay at the commercial level (SRL, Tokyo, Japan), and the levels were shown in international units (IU). All of the pre‐ and post‐vaccination samples were measured simultaneously to avoid any possible biases associated with in vitro assays.
Adverse events. Adverse events were monitored according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (http://ctep.cancer.gov).
Clinical laboratory data. Clinical laboratory values, such as serum levels of alanine aminotransferase (ALT), alpha fetoprotein (AFP), and blood platelet numbers, were measured by the clinical laboratory division of the Kurume University Hospital. Quantitation of HCV RNA was carried out by a clinical laboratory company (SRL).
Results
Patient demographics. Seven, seven, and 14 patients were initially planned for level 1 (0.3 mg per peptide), level 2 (1 mg per peptide), and level 3 (3 mg per peptide), respectively, in this phase I dose‐escalation study. As a result, a total of 26 patients infected with HCV (25 HCV1b and 1 HCV2a: patient 5) were enrolled (Table 1). Among them, three patients under level 1 setting (patients 2, 3, and 6) received at first 0.3 mg per peptide followed by 1 mg per peptide because of both no severe toxicity and stable disease with a new informed consent. Therefore, only five patients were invited for level 2 setting. One patient (patient 14) dropped out of the study after the second vaccination with vaccine‐unrelated death. Twenty‐five and 22 patients completed the first and second cycle vaccination (six and 12 vaccine injections), respectively, and thus were evaluable for toxicity and immunological evaluations. The patients’ characteristics are shown in Table 1. Twenty‐one patients were diagnosed with CH, and the remaining five were diagnosed with LC. Of the five LC patients, three had HCC that had been removed prior to their enrollment in the study. Twenty‐three patients were non‐responders to the IFN‐based treatments, whereas the remaining three had no history of IFN therapy.
Table 1.
Patient's characteristics
| Patient | HLA type † | Disease ‡ | Age/Sex | Previous treatment | Peptide dose (mg) | Total vaccination (times) |
|---|---|---|---|---|---|---|
| 1 | A*3303 | CH | 50/F | IFN + RBV | 0.3 | 15 |
| 2 | A*1101/3101 | CH | 61/F | IFN, IFN + RBV | 0.3, 1 | 33 |
| 3 | A*2602/A3101 | CH | 51/M | IFN | 0.3, 1 | 19 |
| 4 | A*0201/A3303 | LC | 50/M | IFN + RBV | 0.3 | 38 |
| 5 | A*0207/A2402 | CH | 71/M | IFN + RBV | 0.3 | 64 |
| 6 | A*0206/A2402 | CH | 55/M | IFN | 0.3, 1 | 57 |
| 7 | A*0206/A2402 | CH | 62/M | IFN + RBV | 0.3 | 8 |
| 8 | A*0206/A3303 | CH | 49/F | IFNβ, IFN + RBV | 1 | 26 |
| 9 | A*0206/A3303 | CH | 38/M | IFN (3 times), p‐IFN | 1 | 39 |
| 10 | A*0201 | LC | 61/M | IFN + RBV | 1 | 10 |
| 11 | A*3101 | CH | 58/M | – | 1 | 13 |
| 12 | A*0207/A2402 | CH | 60M | IFN + RBV, p‐IFN | 1 | 6 |
| 13 | A*0206/A2402 | LC | 61/F | IFN + RBV, IFN | 3 | 22 |
| 14 | A*0206 | LC | 69/F | IFN + RBV, p‐IFN | 3 | 2 |
| 15 | A*0201/A3303 | LC | 58/M | IFN, p‐IFN | 3 | 27 |
| 16 | A*1101/A3101 | CH | 70/M | IFN + RBV | 3 | 33 |
| 17 | A*0207/A2402 | CH | 68/M | IFNβ, IFN + RBV | 3 | 15 |
| 18 | A*2402 | CH | 64/M | IFNβ, IFN + RBV | 3 | 19 |
| 19 | A*2603/A3303 | CH | 70/M | IFNα, IFNβ | 3 | 29 |
| 20 | A*2402 | CH | 62/F | – | 3 | 25 |
| 21 | A*0201/A3303 | CH | 53/F | IFN | 3 | 22 |
| 22 | A*1101/A2402 | CH | 63/F | – | 3 | 26 |
| 23 | A*0201/A2603 | CH | 58/F | IFN + RBV | 3 | 25 |
| 24 | A*1101/A2402 | CH | 63/F | IFN + RBV (2 times) | 3 | 24 |
| 25 | A*2402 | CH | 57/F | IFN + RBV | 3 | 17 |
| 26 | A*0201/A2402 | CH | 58/F | IFN | 3 | 17 |
† HLA, human leukocyte antigen. ‡CH, chronic hepatitis; IFN, interferon; LC, liver cirrhosis; p‐IFN, peg interferon; RBV, ribavirin.
Toxicity. One LC patient (patient 14) had acute intestinal infection and pneumococcal infection with acidosis, disseminated intravascular coagulation, and renal insufficiency 11 days after the second vaccination, and died of sepsis 21 days after the vaccination. Any vaccination‐related symptomatic alterations were not observed after the last vaccination. The institutional safety evaluation committee concluded that the death of patient 14 was not a vaccine‐related event. It should be noted that patient 14 had a history of splenectomy 11 months earlier, which might have affected host immunity against the infection. Except for this case, no severe toxicity was observed during the study. Grade 1 or 2 local inflammation at the injection site was observed in 13 or 11 patients during the vaccinations, respectively. The local events disappeared within 1 week after the vaccination in most cases. Grade 1 fatigue and headache were observed in 11 and four patients, whereas grade 1 or 2 flu‐like symptoms were observed in three or one patients, respectively. No correlation was observed between the inoculated doses of vaccine peptides and the onset or duration of symptoms. These results indicated that this protocol was well tolerated.
Cellular immune responses. Peptide (C35‐44)‐specific cellular immune activities were measured using the PBMC before vaccination and after the sixth and 12th vaccinations (Table 2). Each of the two HLA allele‐restricted CTL activities was independently measured in the patients with heterozygous HLA‐class I‐A alleles. Augmentation of peptide‐specific CTL responses was judged to have occurred either if the number of positive wells increased or if the amounts of INF‐γ increased more than twofold in the case of equal numbers of positive wells in the quadruplicate assays. Under these circumstances, the peptide‐specific CTL responses at least by one of the HLA‐I‐A alleles in PBMC after the sixth vaccination were augmented in one of seven, five of five, and 9 of 13 of patients at levels 1, 2, and 3, respectively. The augmentation also occurred in PBMC after the 12th vaccination. CTL augmentation in more than two wells among four wells occurred mostly in the post‐vaccination samples from patients with levels 2 and 3. These results indicated that level 1 (0.3 mg peptide per injection) is not sufficient to induce peptide‐specific CTL activity, and level 2 (1 mg peptide) seemed to be sufficient to induce CTL activity under this biweekly protocol.
Table 2.
Immune responses during vaccination †
| Patient | CTL response (IFN‐γ, pg/mL) | IgG response (OD) § | |||||
|---|---|---|---|---|---|---|---|
| HLA‐restriction ‡ | Pre | Post‐sixth | Post‐12th | Pre | Post‐6th | Post‐12th | |
| 1 | A*3303 | 0 | 0 | 61/66 ¶ | 0.403 | 0.441 | 1.238 |
| 2 | A*1101 | 0 | 0 | 0 | 0.637 | 0.743 | 1.624 |
| A*3101 | 0 | 0 | 0 | ||||
| 3 | A*2602 | 0 | 179 | 50 | 0.526 | 1.786 | 2.335 |
| A*3101 | 0 | 56 | 0 | ||||
| 4 | A*0201 | 0 | 0 | 111 | 0.24 | 0.24 | 0.326 |
| A*3303 | 0 | 0 | 0 | ||||
| 5 | A*0207 | 61 | 0 | 130 | 0.8 | 2 | 2 |
| A*2402 | 0 | 0 | 0 | ||||
| 6 | A*0206 | 0 | 0 | 0 | 0.805 | 0.878 | 0.959 |
| A*2402 | 0 | 0 | 0 | ||||
| 7 | A*0206 | 0 | 0 | NA | 0.94 | 1 | 2.639 |
| A*2402 | 0 | 0 | 616 | ||||
| 8 | A*0206 | 182 | 60 | 94 | 0.512 | 0.65 | 2.735 |
| A*3303 | 0 | 55 | 0 | ||||
| 9 | A*0206 | 0 | 0 | 82 | 0.867 | 0.912 | 2.82 |
| A*3303 | 0 | 272 | 0 | ||||
| 10 | A*0201 | 0 | 52/107/116 | NA | 0.556 | 0.456 | NA |
| 11 | A*3101 | 82 | 168/213/571 | NA | 0.677 | 0.488 | NA |
| 12 | A*0207 | 0 | 94 | NA | 0.853 | 0.631 | NA |
| A*2402 | 0 | 467 | |||||
| 13 | A*0206 | 0 | 0 | 405/1094/1534 | 1.588 | 1.595 | 2.568 |
| A*2402 | 0 | 50 | 0 | ||||
| 15 | A*0201 | 340 | 277/278 | 0 | 0.764 | 0.66 | 1.164 |
| A*3303 | 0 | 0 | 0 | ||||
| 16 | A*1101 | 0 | 0 | 0 | 0.1 | 0.2 | 0.4 |
| A*3101 | 0 | 0 | 0 | ||||
| 17 | A*0207 | 0 | 0 | 0 | 1.296 | 1 | 1.631 |
| A*2402 | 0 | 153/2339 | 780 | ||||
| 18 | A*2402 | 0 | 0 | 0 | 0.5 | 0.5 | 0.8 |
| 19 | A*2603 | 0 | 0 | 152/1147 | 2 | 2 | 2.5 |
| A*3303 | 0 | 66 | 0 | ||||
| 20 | A*2402 | 0 | 69/343 | 276 | 2.538 | 2.33 | 2.761 |
| 21 | A*0201 | 169 | 1670 | 290/380/1520 | 0.514 | 0.922 | 2.703 |
| A*3303 | 161 | 92/643 | 163/650/678/5288 | ||||
| 22 | A*1101 | 86/332/395 | 0 | 0 | 0.824 | 0.685 | 0.985 |
| A*2402 | 151 | 513 | 88 | ||||
| 23 | A*0201 | 0 | 199 | 0 | 1.128 | 1.317 | 1.768 |
| A*2603 | 76 | 104 | 50 | ||||
| 24 | A*1101 | 134 | 0 | 0 | 1.087 | 2.084 | 2.757 |
| A*2402 | 90 | 0 | 111/250 | ||||
| 25 | A*2402 | 0 | 0 | 0 | 0.886 | 0.556 | 0.597 |
| 26 | A*0201 | 0 | 74/171 | 54/56/79 | 1.296 | 0.801 | 2.368 |
| A*2402 | 0 | 0 | 166 | ||||
CTL activity was measured by interferon (IFN)‐γ production assay, whereas IgG response was measured by ELISA;
‡ HLA alle‐restricted CTL responses were measured;
optical density at 450 nm;
IFN‐γ production levels in positive wells of quadruplicate culture are shown. Background levels of values (<50 pg/mL) are indicated as 0. NA, not available.
We also measured HLA‐restricted CTL activity to EBV‐derived peptide in 15 patients and Flu‐derived peptide in nine patients, taken as positive peptides, to determine whether or not the vaccination of C35‐44 peptide influences cellular responses to the other viruses. Pre‐injection, post‐sixth injection, and post‐12th injection PBMC from 15 patients were incubated with each of C35‐44, EBV, or Flu peptide relevant to the patients’ HLA alleles, after which their CTL activity was measured in quadruplicate assays. CTL precursors were considered to be present when the level of IFN‐γ production in response to a corresponding peptide was significantly higher (P < 0.05) than that in response to a HIV peptide, and if the amount of IFN‐γ produced in response to the peptide was more than 50 ng/mL greater than the amount produced in response to a HIV peptide. Under these circumstances, the CTL precursors for C35‐44, EBV, and Flu were detectable in the pre‐vaccination PBMC from 6 of 15, 2 of 15, and one of nine patients tested; in the post‐sixth vaccination, PBMC from 9 of 15, 4 of 14, and six of nine patients; and in the post‐12th vaccination PBMC from 11 of 13, 4 of 13, and six of nine patients, respectively (data not shown). Representative results of four cases are shown in Figure 1. These results indicate that the C35‐44 peptide vaccination did not suppress, but rather had a trend to facilitate CTL activity to both EBV‐ and Flu‐derived peptides.
Figure 1.
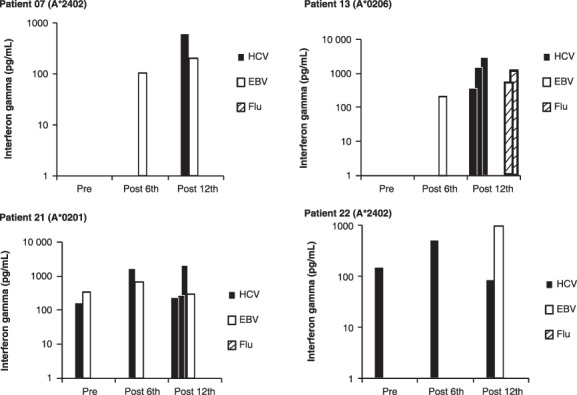
CTL activity to C35‐44, Epstein–Barr virus (EBV), and influenza virus (Flu) peptides. Peripheral blood mononuclear cells from pre‐, post‐6th, and post‐12th vaccination were incubated with each of C35‐44, EBV, or Flu peptide relevant to the patients’ human leukocyte antigen (HLA) alleles, after which their CTL activity was measured in quadruplicate assays. Representative results of four cases (patients 7, 13, 21, and 22) are shown. Each bar indicates the interferon‐γ value of positive wells of quadruplicate culture.
Humoral immune responses. Peptide (C35‐44)‐specific IgG responses were measured using plasma collected before vaccination and after the sixth and 12th vaccinations (Table 2). The increment was rarely observed in the samples collected after the sixth injections (5 of 26 patients, 19%), but was observed in the majority (15 of 22 patients, 68%) of the post‐12th vaccination samples without a dose‐dependent manner.
We further measured all the other Ig isotypes and all IgG subclasses reactive to the vaccinated peptide to examine a dominant type of vaccine‐induced immune reaction (Th1‐ or Th2‐type reactions), if any. IgG against HCV core protein was also measured as a control. As a result, augmentation in all the other Ig isotypes and all IgG subclasses (IgG1–IgG4) was observed in most patients whose plasma showed the increased IgG response shown in Table 2. Detailed results are given in 3, 4. The results suggest that both Th1 and Th2 cells are involved in the vaccination‐induced humoral responses. The vaccination, however, did not augment IgG reactive to a recombinant HCV core protein (Table 3).
Table 3.
Isotypes of antipeptide Ig during vaccination †
| Patient | Anti‐C35 peptide antibody | Anti‐HCV core IgG | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgG | IgA | IgM | IgE | |||||||
| Pre | Post‐12th | Pre | Post‐12th | Pre | Post‐12th | Pre | Post‐12th | Pre | Post‐12th | |
| 1 | 1721 | 2379 | 387 | 590 | 144 | 338 | 48 | 62 | 130 | 130 |
| 2 | 823 | 1071 | 174 | 227 | 88 | 103 | 16 | 17 | 210 | 180 |
| 5 | 13 803 | 12 571 | 6064 | 5479 | 393 | 276 | 651 | 596 | 350 | 330 |
| 6 | 432 | 507 | 85 | 123 | 94 | 75 | 10 | 15 | 47 | 49 |
| 8 | 4781 | 12 158 | 1668 | 5732 | 154 | 286 | 158 | 560 | 120 | 130 |
| 9 | 10 531 | 11 807 | 4541 | 4693 | 32 | 83 | 375 | 398 | 220 | 220 |
| 10 | 514 | 645 | 191 | 220 | 354 | 412 | 13 | 20 | 460 | 440 |
| 11 | 13 023 | NA † | 417 | NA | 70 | NA | 536 | NA | NA | NA |
| 12 | 2054 | NA | 417 | NA | 37 | NA | 58 | NA | NA | NA |
| 13 | 740 | 5070 | 378 | 1067 | <5 | <5 | 121 | 151 | 340 | 420 |
| 15 | 635 | 1052 | 33 | 426 | 127 | 916 | 19 | 39 | 86 | 100 |
| 16 | 407 | 1115 | 117 | 378 | <5 | 24 | 8 | 29 | 120 | 120 |
| 17 | 14 537 | 13 154 | 6502 | 6196 | 70 | 68 | 874 | 822 | 260 | 190 |
| 18 | 2357 | 2615 | 610 | 833 | 50 | 202 | 60 | 61 | 260 | 240 |
| 19 | 2449 | 6720 | 677 | 2244 | <5 | 626 | 43 | 175 | 230 | 250 |
| 20 | 8107 | 13 318 | 2990 | 6058 | 75 | 321 | 339 | 690 | 260 | 330 |
| 21 | 271 | 5743 | 68 | 2224 | <5 | 383 | 8 | 154 | 46 | 77 |
| 22 | 2987 | 2134 | 768 | 534 | 177 | 88 | 90 | 68 | 170 | 79 |
| 23 | 3445 | 2925 | 1148 | 966 | 82 | 72 | 99 | 88 | 170 | 130 |
| 24 | 477 | 5404 | 160 | 2489 | 415 | 441 | 15 | 185 | 49 | 32 |
| 25 | 13 490 | 11 111 | 5865 | 4168 | 96 | 242 | 474 | 369 | 430 | 330 |
| 26 | 1370 | 2943 | 417 | 1238 | 169 | 233 | 31 | 100 | 130 | 99 |
Isotypes of antipeptide (C35‐44) Ig in the pre‐ and post‐vaccination (12th) plasma were measured using a Luminex system, and the levels were shown by fluorescence intensity units (FIU). As a control, IgG to hepatitis C virus (HCV) core protein was also measured by radioimmunoassay, and the levels were shown by international units (IU). NA, not available.
Table 4.
Subclasses of antipeptide IgG during vaccination †
| Patient | Anti‐C35 IgG subclass | |||||||
|---|---|---|---|---|---|---|---|---|
| IgG1 | IgG2 | IgG3 | IgG4 | |||||
| Pre | Post‐12th | Pre | Post‐12th | Pre | Post‐12th | Pre | Post‐12th | |
| 1 | 1002 | 1735 | 462 | 641 | 309 | 487 | 596 | 783 |
| 2 | 301 | 410 | 241 | 294 | 265 | 209 | 335 | 404 |
| 5 | 17 950 | 17 095 | 9868 | 8498 | 6214 | 5432 | 8038 | 7302 |
| 6 | 291 | 372 | 117 | 124 | 57 | 73 | 119 | 166 |
| 8 | 4994 | 15 594 | 2681 | 9217 | 1341 | 5395 | 2228 | 7098 |
| 9 | 10 401 | 13 220 | 6351 | 7872 | 3821 | 4672 | 5303 | 5927 |
| 10 | 803 | 916 | 237 | 250 | 122 | 185 | 386 | 422 |
| 11 | 17 640 | NA | 6552 | NA | 5887 | NA | 5490 | NA |
| 12 | 2853 | NA | 495 | NA | 484 | NA | 363 | NA |
| 13 | 5541 | 6750 | 1927 | 2269 | 1550 | 1635 | 1881 | 2148 |
| 15 | 567 | 1123 | 148 | 373 | 57 | 227 | 184 | 604 |
| 16 | 557 | 1268 | 131 | 501 | 89 | 559 | 172 | 395 |
| 17 | 17 578 | 16 423 | 9124 | 8877 | 6977 | 6276 | 7796 | 7797 |
| 18 | 2151 | 3192 | 899 | 1104 | 600 | 760 | 928 | 1160 |
| 19 | 557 | 1268 | 131 | 501 | 611 | 2057 | 820 | 2761 |
| 20 | 9753 | 16 953 | 4718 | 9452 | 2915 | 6610 | 4423 | 8552 |
| 21 | 196 | 5939 | 122 | 3071 | 57 | 2240 | 66 | 2546 |
| 22 | 2249 | 1660 | 878 | 528 | 594 | 334 | 938 | 603 |
| 23 | 3795 | 3297 | 1368 | 1166 | 843 | 680 | 1436 | 1320 |
| 24 | 583 | 7089 | 131 | 3553 | 102 | 2323 | 98 | 2218 |
| 25 | 14 979 | 11 654 | 4428 | 2760 | 5166 | 3928 | 4647 | 3396 |
| 26 | 1979 | 3278 | 366 | 1182 | 403 | 2318 | 305 | 940 |
Subclasses of antipeptide (C35‐44) IgG in the pre‐ and post‐vaccination (12th) plasma were measured using a Luminex system, and the levels were shown by fluorescence intensity units (FIU). NA, not available.
Clinical evaluation. Clinical evaluation was not the objective of this phase 1 study. However, the available information, though very limited, might be important for developing further clinical studies of peptide‐based vaccination to HCV‐infected patients. During the vaccination period for up to the 12th vaccination, no patient received any treatment other than the vaccination; notably, none of them received injection of glycyrrhizin, a standard drug for patients unresponsive to IFN‐based therapies. Detailed results of the clinical evaluation are given in Table 5. A more than one log difference in HCV RNA was considered significant, whereas a more than 30% difference with a consistent trend throughout the vaccination period (1st to 12th) in ALT, platelet counts, and AFP was considered significant. Under these circumstances, no patient showed an increase in HCV RNA, whereas two patients showed a decrease. Two patients at level 1 showed an increase in ALT after the vaccination, whereas seven patients showed a significant decrease. No patient showed either a significant decrease or increase in platelet count. Three patients showed a significant decrease in AFP, a biomarker for liver cancer, among six patients whose pre‐vaccination AFP levels exceeded the normal range (>8.7 ng/mL). In addition, one patient at level 1 showed a sharp but transient decline in AFP after the sixth vaccination.
Table 5.
Data for clinical outcomes after vaccination
| Patient | HCV RNA (kIU/mL) | ALT (IU/L) | Plt. (× 104/mL) | AFP (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post‐6th | Post‐12th | Pre | Post‐6th | Post‐12th | Pre | Post‐6th | Post‐12th | Pre | Post‐6th | Post‐12th | |
| 1 | 1730 | 1830 | 2120 | 62 | 59 | 83 | 13.7 | 13.4 | 12.4 | 10.1 | 11.9 | 12.6 |
| 2 | 91 | 27 | NA | 77 | 51 | NA | 11.4 | 11.7 | NA | 32.6 | 51.1 | NA |
| 3 | 3420 | 4140 | 3710 | 41 | 51 | 64 | 22.4 | 25.4 | 24.0 | 3.2 | 3.5 | 3.7 |
| 4 | 500 | 96 | 6 | 206 | 157 | 105 | 11.5 | 8.7 | 10.6 | 74.3 | 32.2 | 67.5 |
| 5 | 114 | 102 | 328 | 114 | 154 | 186 | 9.3 | 10.2 | 8.7 | NA | 7.8 | 10.0 |
| 6 | 892 | 648 | 840 | 60 | 29 | 48 | 13.8 | 14.4 | 15.0 | 5.4 | 6.1 | 6.2 |
| 7 | 2100 | 1680 | NA | 95 | 100 | NA | 7.8 | 8.0 | NA | 7.0 | NA | NA |
| 8 | 2480 | 2660 | 2290 | 129 | 112 | 108 | 15.8 | 17.3 | 15.2 | 44.4 | 29.2 | 28.2 |
| 9 | 2870 | 2950 | 2330 | 358 | 233 | 245 | 5.8 | 7.4 | 6.8 | 28.7 | 19.5 | 19.7 |
| 10 | 32 000 | 16 000 | NA | 104 | 95 | NA | 11.2 | 10.3 | NA | 26.9 | 24.2 | NA |
| 11 | 20 | NA | NA | 83 | NA | NA | 13.9 | NA | NA | 5.6 | NA | NA |
| 12 | 25 000 | 25 000 | NA | 144 | 155 | NA | 14.9 | 14.6 | NA | 5.6 | 8.1 | NA |
| 13 | 1670 | 2130 | 2660 | 53 | 47 | 49 | 9.4 | 7.9 | 7.8 | 131 | 86.9 | 74.0 |
| 15 | 2130 | 2810 | 2600 | 45 | 34 | 25 | 8.0 | 8.4 | 9.2 | 6.8 | 7.6 | 7.9 |
| 16 | 2470 | 3510 | 2440 | 47 | 52 | 45 | 25.2 | 26.0 | 24.0 | 4.7 | NA | 5.0 |
| 17 | 2260 | 2140 | 1540 | 37 | 31 | 32 | 17.3 | 19.7 | 19.1 | 4 | 3.4 | NA |
| 18 | 3630 | 3300 | 2972 | 60 | 50 | 63 | 13.2 | 15.0 | 16.3 | 22.7 | 24.4 | 27.5 |
| 19 | 591 | 489 | 443 | 51 | 52 | 53 | 23.7 | 24.4 | 23.8 | 4.2 | NA | 3.3 |
| 20 | 583 | 591 | 346 | 24 | 22 | 22 | 19.2 | 17.3 | 20.4 | 4.9 | NA | NA |
| 21 | 4370 | 3575 | 3940 | 34 | 37 | 33 | 23.1 | 21.2 | 23.1 | NA | NA | 3.6 |
| 22 | 59 | <5 | 5 | 73 | 25 | 40 | 12.4 | 12.1 | 12.1 | 3.1 | NA | 1.5 |
| 23 | 3045 | 2380 | 2730 | 66 | 64 | 65 | 10.1 | 10.0 | 11.1 | 8.2 | 9.4 | 7.6 |
| 24 | 2230 | 2095 | 4510 | 52 | 36 | 27 | 11.7 | 12.2 | 11.4 | 2.9 | NA | NA |
| 25 | 2340 | 3160 | 5000 | 55 | 55 | 49 | 11.1 | 10.2 | 12.0 | 8.5 | NA | NA |
| 26 | 2150 | 3025 | NA | 80 | 58 | 56 | 12.4 | 14.4 | 14.9 | 6.8 | NA | NA |
AFP, α‐fetroprotein; ALT, alanine aminotransferase; NA, not assessed; Plt., platelet number.
Discussion
Liver damage may be induced by the boosted CTL‐directed destruction of HCV‐infected liver cells. However, there was no such liver damage throughout the vaccination period despite the fact that peptide vaccination induced both cellular and humoral responses in the majority (>60%) of patients. Rather, decreases in ALT and AFP were seen in a substantial numbers of patients. The other concern was the difficulty of inducing immune responses by the peptide vaccination for non‐responders to IFN‐based therapy, primarily because of the heterogeneity of HCV, and also because the immune system was suppressed in HCV‐positive patients.( 16 , 17 ) However, this concern also did not arise, and the vaccination successfully induced immune responses in the post‐vaccination samples of the majority (>60%) of patients in both the CTL and IgG assays. Our present results, along with the recent increased demand for development of a HCV vaccine,( 18 ) keep alive our hope of developing a clinically effective HCV vaccine.
Level 1 (0.3 mg per peptide) was considered too low to induce CTL activity. Level 2 (1 mg per peptide) was considered an optimal dose to induce CTL activity under this biweekly injection protocol, although level 3 (3 mg per peptide) was also recommended because of the higher rate of CTL induction. In contrast, the boosting of IgG production specific to this peptide seemed not to be dependent on the dose, and even level 1 was associated with an increase (>1.5 fold) in IgG in the post‐vaccination (12th) plasma from five of seven patients.
We previously reported that a personalized peptide vaccination protocol is superior to a designated protocol from the standpoints of immune responses and clinical responses in patients with advanced stages of cancer.( 19 , 20 , 21 ) This superiority might be due in part to the fact that the pre‐designated peptide vaccination stimulates naive or resting T cells and suppresses memory or activated T cells in certain cases, whereas the personalized peptide vaccination stimulates the latter types of T cells.( 19 , 20 , 21 ) In the present study, significant levels of peptide‐specific IgG were detected in pre‐vaccination plasma of all 26 patients, indicating that memory B cells at least exist in all 26 patients. The vaccination did not suppress CTL activity against Flu or EBV, but rather had a trend to facilitate CTL activity to EVB‐ and Flu‐derived peptides. Based on these results, we considered that this peptide stimulated memory or activated T cells in those HCV‐infected patients, which in turn resulted in the higher rates of immune boosting without suppression of the CTL activity to other viruses in HCV‐infected patients who failed the IFN‐based therapies.
This peptide boosted the CTL activity restricted to each of the HLA‐A2 (A0201, A0206, A0207), HLA‐A24 (A2402), HLA‐A26 (A2602, A2603), HLA‐A31 (A3101), and HLA‐A33 (A3303) molecules in the post‐vaccination samples as far as tested. This is consistent with the results of an in vitro analysis (Niu Y, Komatsu N, Komohara Y, Matsueda S, Yutani S, Ishihara Y, Itou M, Yamada A, Itoh K, Shichijo S unpubl. data). Although the CTL boosting was observed in none of the four HLA‐A1101+ patients, further studies with additional cases should be conducted to determine whether or not this peptide can boost CTL activity by in vivo vaccination for HLA‐A1101+ patients.
The sequence of this peptide is well conserved in the different genotypes of HCV, and thus could be applicable not only to HCV1b patients but also to HCV2a patients. Indeed, this peptide boosted both CTL and IgG responses in one HCV2a patient (patient 05) who received 0.3 mg vaccination, although further studies with additional HCV2a cases should be conducted to confirm this possibility.
Although the clinical benefit of this peptide vaccination will be addressed in a future phase II clinical study, it is of note that a decrease in ALT was observed in 7 of 24 patients (29%) during the vaccination. Th1‐type immune responses are suggested to be involved in liver damage at chronic phase.( 22 , 23 ) Therefore, the vaccination‐induced CTL boosting is expected to be associated with increased ALT. Indeed, Klade et al. reported two such cases who had a transient decrease in HCV RNA concomitant with an ALT increase during the HCV peptide vaccination.( 6 ) In contrast, our two cases showed declines of both HCV RNA and ALT. Although a reason for this discrepancy is unclear at the present time, the different protocols would be at least responsible for this discrepancy. We used only one CTL epitope (C35‐44) emulsified with ISA51 adjuvant, whereas they used five peptides containing four CTL epitopes and three helper epitopes with poly‐l‐arginine as an adjuvant. Augmentation of CTL or IgG responses was observed in six of seven patients showing a decline in ALT in this trial. Nelson et al. reported that interleukin‐10 treatment results in normalization of ALT in the majority of CH patients who are non‐responders to IFN‐based treatments.( 24 ) Interleukin‐10 promotes the production of IgA, IgG1, and IgG2.( 25 , 26 ) We showed that all three of these Ig were increased in the post‐vaccination samples, suggesting that Th2‐type immune responses are at least boosted by the vaccination. Therefore, an increment in Th2‐type reactions might be in part responsible for the decline of ALT in these seven patients, although the biological function of these antibodies specific to C35‐44 peptide is unknown at the present time.
In addition to ALT, a decrease in AFP level was also observed in three of six evaluable patients. It is well known that HCV core protein plays a pivotal role in the development of HCC.( 27 , 28 ) Therefore, the vaccination‐induced CTL boosting might eliminate precancerous liver cells expressing the HLA‐class I‐A–C35‐44 peptide complex, which may in turn result in decreased AFP. If this is the case, this vaccine may be effective as a cancer prophylactic in CH or LC patients who are resistant to IFN‐based therapies and have a high risk of HCC. One LC patient (patient 4) who had a history of HCC showed a decrease in HCV RNA by the vaccination alone. This patient had received IFN‐based therapy combined with the vaccination with the result of sustained viral responses. These results suggest the possible benefit of vaccination and IFN therapy for certain HCV patients who fail the standard IFN‐based treatment.
HCV is known as a highly variable virus, but the amino acid sequence of this peptide is well conserved in the entire HCV genotype. HLA‐A2, HLA‐A11, HLA‐A24, HLA‐A26, HLA‐A31, and HLA‐A33 types constitute >99% of Japanese, 98% of Asian, 74% of Caucasian, and 50–67% of Black people.( 29 ) This peptide can induce both cellular and humoral responses in patients with these HLA‐class I‐A alleles. Therefore, this peptide might be useful as a therapeutic HCV vaccine, as well as a prophylactic cancer vaccine for HCV‐related HCC, for the majority of people in the world.
Disclosure Statement
Yamada, Itoh, and Shichijo received a research grant from the Green Peptide Co.; Yamada, Itoh, and Shichijo have stock ownership of the Green Peptide; Yamada is an executive of the Green Peptide.
Acknowledgments
This study was supported in part by Grants‐in‐Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, by the 21st Century COE Program for Medical Science, and by Toshi‐area Program from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- 1. Initiative for Vaccine Research: Hepatitis C . (Website on the internet). 2006. Available from URL: http://www.who.int/vaccine_research/diseases/viral_cancers/en/index2.html#disease%20burden. World Health Organization, Geneve, Switzerland, cited at July 6, 2009.
- 2. Chander G, Sulkowski MS, Jenckes MW et al . Treatment of chronic hepatitis C: a systematic review. Hepatol 2002; 36: S135–4. [DOI] [PubMed] [Google Scholar]
- 3. Kato N, Hijikata M, Ootsuyama Y et al . Molecular cloning of the human hepatitis C virus genome from Japanese patients with non‐A, non‐B hepatitis. Proc Natl Acad Sci USA 1990; 87: 9524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blatt LM, Mutchnick MG, Tong MJ et al . Assessment of hepatitis C virus RNA and genotype from 6807 patients with chronic hepatitis C in the United States. J Viral Hepat 2000; 7: 196–202. [DOI] [PubMed] [Google Scholar]
- 5. Schlaphoff V, Klade CS, Jilma B et al . Functional and phenotypic characterization of peptide‐vaccine‐induced HCV‐specific CD8+ T cells in healthy individuals and chronic hepatitis C patients. Vaccine 2007; 25: 6793–806. [DOI] [PubMed] [Google Scholar]
- 6. Klade CS, Wedemeyer H, Berg T et al . Therapeutic vaccination of chronic hepatitis C nonresponder patients with the peptide vaccine IC41. Gastroenterology 2008; 134: 1385–95. [DOI] [PubMed] [Google Scholar]
- 7. Yutani S, Yamada A, Yoshida K et al . Phase I clinical study of a personalized peptide vaccination for patients infected with hepatitis C virus (HCV) 1b who failed to respond to interferon‐based therapy. Vaccine 2007; 25: 7429–35. [DOI] [PubMed] [Google Scholar]
- 8. Battegay M, Fikes J, Di Bisceglie AM et al . Patients with chronic hepatitis C have circulating cytotoxic T cells which recognize hepatitis C virus‐encoded peptides binding to HLA‐A2.1 molecules. J Virol 1995; 69: 2462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cerny A, McHutchison JG, Pasquinelli C et al . Cytotoxic T lymphocyte response to hepatitis C virus‐derived peptides containing the HLA A2.1 binding motif. J Clin Invest 1995; 95: 521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsueda S, Takedatsu H, Sasada T et al . New peptide vaccine candidates for epithelial cancer patients with HLA‐A3 supertype alleles. J Immunother 2007; 30: 274–81. [DOI] [PubMed] [Google Scholar]
- 11. Wang Y, Takao Y, Harada M et al . New epitope peptides derived from hepatitis C virus (HCV) 2a which have the capacity to induce cytotoxic t lymphocytes in HLA‐A2+ HCV‐infected patients. Microbiol Immunol 2006; 50: 857–65. [DOI] [PubMed] [Google Scholar]
- 12. Takao Y, Yamada A, Yutani S, Sata M, Itoh K. Antibody reactive a hepatitis C virus (HCV)‐derived peptide capable of inducing HLA‐A2 restricted cytotoxic T lymphocytes is detectable in a majority of HCV‐infected individuals without HLA‐A2 restriction. Microbiol Immunol 2004; 48: 507–17. [DOI] [PubMed] [Google Scholar]
- 13. Matsueda S, Yamada A, Takao Y et al . A new epitope peptide derived from hepatitis C virus 1b possessing the capacity to induce cytotoxic T‐lymphocytes in HCV1b‐infected patients with HLA‐A11‐A31 and ‐A33. Cancer Immunol Immunother 2007; 56: 1359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ogata R, Matsueda S, Yao A, Noguchi M, Itoh K, Harada M. Identification of polycomb group protein enhancer of zeste homolog 2 (EZH2)‐derived peptides immunogenic in HLA‐A24+ prostate cancer patients. Prostate 2004; 60: 273–81. [DOI] [PubMed] [Google Scholar]
- 15. Yamada N, Ishikawa Y, Dumrese T et al . Role of anchor residues in peptide binding to three HLA‐A26 molecules. Tissue Antigens 1999; 54: 325–32. [DOI] [PubMed] [Google Scholar]
- 16. Bowen DG, Walker CM. Mutational escape from CD8+ T cell immunity: HCV evolution, from chimpanzees to man. J Exp Med 2005; 201: 1709–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rushbrook SM, Ward SM, Unitt E et al . Regulatory T cells suppress in vitro proliferation of virus‐specific CD8+ T cells during persistent hepatitis C virus infection. J Virol 2005; 79: 7852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Strickland GT, EI‐Kamary SS, Klenerman P, Nicosia A. Hepatitis C vaccine: supply and demand. Lancet Infect Dis 2008; 8: 379–86. [DOI] [PubMed] [Google Scholar]
- 19. Mine T, Sato Y, Noguchi M et al . Humoral responses to peptides correlate with overall survival in advanced cancer patients vaccinated with peptides based on pre‐existing, peptide‐specific cellular responses. Clin Cancer Res 2004; 10: 929–37. [DOI] [PubMed] [Google Scholar]
- 20. Noguchi M, Yao A, Harada M et al . Immunological evaluation of neoadjuvant peptide vaccination before radical prostatectomy for patients with localized prostate cancer. Prostate 2007; 67: 933–42. [DOI] [PubMed] [Google Scholar]
- 21. Yajima N, Yamanaka R, Mine T et al . Immunologic evaluation of personalized peptide vaccination for patients with advanced malignant glioma. Clin Cancer Res 2005; 11: 5900–11. [DOI] [PubMed] [Google Scholar]
- 22. Bertoketti A, Bertoletti A, D’Elios MM et al . Different cytokine profiles of intraphepatic T cells in chronic hepatitis B and hepatitis C virus infections. Gastroenterology 1997; 112: 193–9. [DOI] [PubMed] [Google Scholar]
- 23. McGuinness PH, McGuinness PH, Painter D, Davies S, McCaughan GW. Increases in intrahepatic CD68 positive cells, MAC387 positive cells, and proinflammatory cytokines (particularly interleukin 18) in chronic hepatitis C infection. Gut 2000; 46: 260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nelson DR, Lauwers GY, Lau JY, Davis GL. Interleukin 10 treatment reduces fibrosis in patients with chronic hepatitis C: a pilot trial of interferon nonresponders. Gastroenterology 2000; 118: 655–60. [DOI] [PubMed] [Google Scholar]
- 25. Brière F, Servet‐Delprat C, Bridon JM, Saint‐Remy JM, Banchereau J. Human interleukin 10 induces naive surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3. J Exp Med 1994; 179: 757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Defrance T, Vanbervliet B, Brière F, Durand I, Rousset F, Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti‐CD40‐activated naive human B cells to secrete immunoglobulin A. J Exp Med 1992; 175: 671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moriya K, Fujie H, Shintani Y et al . The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med 1998; 4: 1065–7. [DOI] [PubMed] [Google Scholar]
- 28. Tanaka N, Moriya K, Kiyosawa K, Koike K, Aoyama T. Hepatitis C virus core protein induces spontaneous and persistent activation of peroxisome proliferator‐activated receptor alpha in transgenic mice: implications for HCV‐associated hepatocarcinogenesis. Int J Cancer 2008; 122: 124–31. [DOI] [PubMed] [Google Scholar]
- 29. Imanishi T, Akaza T, Kimura A, Tokunaga K, Gojobori T. Allele and haplotype frequencies for HLA and complement loci in various ethnic groups. In: Proceedings of the Eleventh International Histocompatibility Workshop and Conference. Oxford, UK: Oxford University Press, 1992; 1065–220. [Google Scholar]


