Abstract
We obtained unique cell‐death‐inducing monoclonal antibodies (mAbs) named D18 and D19 against chicken DT40 cells. D18 and D19 caused several signs of apoptosis, such as exposed phosphatidyl serine on the cell surface, a sub G0/G1 peak, and DNA fragmentation, and inhibited the proliferation of DT40 cells. Flow cytometric and immunohistological analyses of various normal chicken tissues revealed the expression of the antigen recognized by these mAbs to be restricted to cells in lymphoid organs including bone marrow and bursa of fabricius, and to cells in some epithelial tissues. The cell death induced by the mAbs progressed through a mitochondrial pathway with loss of mitochondrial membrane potential. Apoptosis is generally characterized by cell shrinking; however, D18 and D19 elicited swelling, which preceded the cell death. We analyzed the antigen immunoprecipitated by the mAbs, and identified a 90‐ to 100‐kDa cell‐surface glycoprotein as the chicken transferrin receptor (TfR). Epitopes recognized by the two mAbs were confirmed to be different by the binding inhibition assay. The reactivity of the mAbs against DT40 cells was not inhibited by excess chicken serum, suggesting that the cell death induced by D18 and D19 was not caused by inhibition of the binding of transferrin (Tf) to chicken TfR. Since D18 and D19 have induced cell death in human embryonic kidney cells transfected with cDNA of the full‐length chicken TfR, we expect human TfR to be a promising target in antibody therapy for various human malignancies. (Cancer Sci 2008; 99: 894–900)
Abbreviations:
- BrdU
bromodeoxyuridine
- BSA
bovine serum albumin
- FBS
fetal bovine serum
- FITC
fluorescein‐isothiocyanate
- mAb
monoclonal antibody
- PBL
peripheral blood leukocytes
- PBS
phosphate‐buffered saline
- Tf
transferrin
- TfR
transferrin receptor.
The DT40 chicken B lymphoid tumor cell line shows an extremely high frequency of homologous recombination in higher eukaryotes, a property that can be used to generate cell lines in which a given target gene is completely inactivated.( 1 , 2 ) Preparing growth‐inhibitory or cell‐death‐inducing antibodies against DT40 cells seems attractive, since the mechanisms behind antibody‐provoking phenomena can be analyzed with gene‐knockout experiments( 3 ) leading to theoretical developments toward antibody‐based therapy for cancers. In this investigation, we report the production of antibodies to cell‐surface molecules on DT40 cells and the character of antigens recognized by cell‐death‐inducing mAbs.
Cell death can be classified into two categories: apoptosis and necrosis.( 4 ) Apoptotic cell death is defined by morphological and biochemical alterations. Apoptotic cells stimulated by a given death signal have several features( 5 ) such as exposed phosphatidyl serine (PS), activation of caspases,( 6 ) disruption of mitochondrial membrane potential (Δψm),( 7 , 8 ) cell shrinkage, and DNA fragmentation.( 4 ) Early in the apoptotic process, the plasma membrane and organelles can be intact. Finally, apoptotic bodies form and cells are rapidly engulfed by phagocytes such as macrophages. The sequence of events that results in the activation of caspases can be categorized into two pathways: types I and II.( 9 ) In either case, the ultimate cleavage and activation of the caspases lead to DNA fragmentation by DNase.
Cell swelling and destruction of the plasma membrane and organelles followed by inflammation occur during necrotic cell death.( 10 )
In this study, we have obtained two mAbs, which have cell‐death‐inducing activity against a cell‐surface receptor‐type antigen on chicken DT40 tumor cells. These mAbs induced several features of apoptosis, but instead of cell shrinkage, they caused swelling which is a characteristic of necrosis. Functional mAbs having unique cell death‐inducing activity will provide new insights into our understanding of cell‐death signaling pathways, and highlight the potential of mAbs for the treatment of malignancies.
Materials and Methods
Cells. PBL were isolated from heparinized chicken blood by density gradient centrifugation on Percoll (GE Healthcare, Bucks, UK). Blood was gently added on RPMI medium containing discontinuous Percoll gradients (57%, 52%, 47%, 42%, and 37%), and centrifuged at 600 × g for 30 min at room temperature. Chicken PBL were collected from the interface between 47% and 42%. DT40 chicken B lymphoid cells and freshly isolated chicken leukocytes were cultured at 39°C in RPMI‐1640 medium supplemented with heat‐inactivated 10% FBS (ICN Biomedicals, Aurora, OH, USA) and 1% chicken serum (Sigma‐Aldrich, Tokyo, Japan) in a humidified CO2 incubator. P3 × 63Ag8.653 mouse myeloma cells and hybridoma cells were cultured at 37°C in RPMI‐1640 medium (Sigma‐Aldrich) supplemented with heat‐inactivated 7% FBS in a humidified CO2 incubator. Human embryonic kidney (HEK) 293F cells (Invitrogen, Carlsbad, CA, USA) transfected with pcDNA4 plasmids (Invitrogen) expressing the full‐length chicken TfR were established and cultured in FreeStyle 293 Expression medium (Invitrogen) containing 10 µg/mL Zeocin (Invitrogen).
Immunization and cell fusion. DT40 cells (2.0 × 107) suspended in 900 µL of PBS were mixed with 10 µL of ImmunoEasy CpG DNA adjuvant (Qiagen, Tokyo, Japan), incubated for 15 min at room temperature, and injected into the quadriceps muscle of female BALB/c mice (Japan SLC, Kyoto, Japan). This procedure was repeated 3 times at 3‐week intervals in three mice.
Three days after the final injection, the immunized mice were sacrificed and spleen cells were fused with X63 mouse myeloma cells, as described previously.( 11 ) We established two hybridoma clones secreting cell death‐inducing mAbs, designated D18 and D19.
Cell growth inhibition assay. DT40 cells (1.5 × 104) in 96‐well plates (150 µL/well) were cultured in the presence or absence of mAbs (1:3 diluted culture fluids or 10 µg/mL purified mAb). We periodically counted the number of cells by the trypan blue dye exclusion method.
Morphological observation. DT40 cells untreated or treated with the mAbs were fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.2; postfixed with 2% OsO4; dehydrated in a graded series of ethanol; passed through propylene oxide; and then embedded in EPON 812 (Taab, Reading, UK). Semi‐thin sections (1 µm) were stained with 0.5% toluidine blue.
Electron microscopic analysis. For the transmission electron microscopy, specimens were fixed with a mixture of 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium cacodylate buffer, postfixed with 2% osmium tetraoxide, dehydrated with a graded series of ethanol concentrations, passed through propylene oxide, and then embedded in EPON 812. Ultra‐thin sections were stained with uranyl acetate and lead citrate. Samples were examined under a Hitachi H‐7100 electron microscope (Tokyo, Japan).
Cell‐death analysis. DT40 cells untreated or treated with D18 or D19 were assayed for apoptosis with an Annexin V‐FITC Apoptosis Detection Kit (Oncogene, San Diego, CA, USA). Briefly, cells (1.0 × 106) were washed, suspended in a binding buffer containing 280 ng/mL FITC‐Annexin V, and incubated for 15 min at room temperature in the dark. After being washed, the cells were resuspended in the binding buffer containing 600 ng/mL propidium iodide immediately before the flow cytometric analysis.
Mitochondrial membrane potential was assessed with 3, 3′‐dihexyloxacarbocyanine iodide (DiOC6(3), 10 µg/mL) and 4,6′‐diamidino‐2‐phenylindole (DAPI, 1 µg/mL) staining. Cells untreated or treated with the mAbs were suspended in PBS containing DiOC6 (3) and DAPI, and incubated for 30 min at 37°C. Cells were analyzed with a BD LSR flow cytometer (Becton Dickinson, Sunnyvale, CA, USA).
DNA fragmentation in apoptotic cells was analyzed as follows. Cells untreated or treated with the mAbs were harvested and resuspended in PBS containing 500 µg/mL proteinase K (Sigma‐Aldrich), 500 µg/mL RNase A (Sigma‐Aldrich), and 0.5% SDS (106 cells/200 µL buffer), and incubated for 30 min at 37°C. Then, 300 mL of NaI solution (6 M NaI, 13 mM EDTA, 0.5% sodium‐N‐lauroylsarcosinate, 10 mg/mL glycogen, and 26 mM Tris‐HCl [pH 8.0]) was added, and the cells were incubated for 15 min at 60°C. Fragmented DNA was precipitated in isopropanol, resuspended in TE, separated on 2% agarose gels, and visualized with ethidium bromide.
Cell cycle analysis. Cells were harvested periodically after the addition of mAb, washed with PBS, and fixed with 70% ethanol overnight at –20°C. After centrifugation and washing with PBS, cells were suspended in PBS containing DAPI (2 µg/mL). The DNA content of cells was analyzed using a BD LSR flow cytometer.
To evaluate cell‐cycle progression from G1 to S phase, a bromodeoxyuridine (BrdU) pulse‐chase experiment was performed. DT40 cells were incubated with BrdU (final concentration, 10 µM) for 20 min at 39°C, and were used for this experiment. After being washed with PBS, cells were cultured with D18 or D19 for 4‐h intervals. Washed cells were fixed, permeabilized, and refixed according to the manufacturer's protocol, using the BrdU Flow kit (Becton Dickinson). Following incubation with DNase for 1 h at 37°C, cells were stained with FITC‐conjugated anti‐BrdU mAb and DAPI, and BrdU‐labeled cells were analyzed with a BD LSR flow cytometer.
Immunostaining and flow cytometry. For cell‐surface immunostaining, cells (3.0 × 105) were incubated for 1 h at 4°C with primary antibodies. After three washes with PBS, cells were incubated for 30 min at 4°C with 1:200 diluted FITC‐conjugated antimouse immunoglobulins (DakoCytomation, Glostrup, Denmark). After three washes with PBS, cells were suspended in PBS containing 0.2% BSA, and analyzed with a BD LSR flow cytometer.
Chemical treatment. To characterize the antigenic determinant recognized by the mAbs, DT40 cells were treated with NaIO4 (5 mM, 5 min) or heated (95°C, 5 min), and incubated sequentially with primary mAbs and FITC‐conjugated secondary antibodies. Following three washes with PBS, the reactivity of the mAbs with DT40 cells was determined with a BD LSR flow cytometer.
Biotin‐labeling and immunoprecipitation. DT40 cells (5.0 × 106) were suspended in 50 µL of PBS (pH 8.0) containing 0.5 mg/mL sulfosuccinimidyl‐6′‐(biotinamide)‐6‐hexanamide hexanoate (EZ‐Link sulfo‐NHS‐LC‐LC‐Biotin; Pierce, Rockford, IL, US), and were incubated for 30 min at room temperature. After three washes, cells were treated with 250 µL of lysis buffer PBS containing 1% Nonidet P‐40, protease inhibitor cocktail (Nakalai, City, Japan) and benzonase (Merck, City, Country) for 60 min on ice. The lysates were spun at 10 000 × g for 20 min, and the supernatant was used for immunoprecipitation experiments. The supernatant was incubated with 5 µg of primary antibody and 5 µg of rabbit antimouse Ig (DakoCytomation) as the secondary antibody. This was followed by the addition of 20 µL of protein G Sepharose 4 fast flow beads (50% suspension, GE Healthcare). After the beads were intensively washed with lysis buffer, bound proteins were eluted by boiling the beads in SDS sample buffer (45 mM Tris‐HCl, pH 6.8; 1% SDS; 50 mM DTT; 10% glycerol; and 0.01% BPB) for 5 min at 95°C. After centrifugation, the supernatants were subject to SDS‐PAGE followed by Western blotting using Elite ABC reagent (Vector, Burlingame, CA, US).
In‐gel Tryptic Digestion and LC/MS/MS. Tryptic digestion was performed with an In‐Gel Tryptic Digestion Kit (Pierce). Briefly, gel slices containing proteins stained by Sypro Ruby were washed with destaining solution (2 mg/mL ammonium bicarbonate, 50% acetnitrile). Next, the gels were reduced with reducing buffer (2 mg/mL ammonium bicarbonate and 50 nM Tris [2‐carboxyethyl] phosphine) for 10 min at 60°C and alkylated with alkylation buffer (100 mM iodoacetamide and 2 mg/mL ammonium bicarbonate) for 1 h at room temperature in the dark. After washing with destaining solution, followed by the addition of 50 µL of acetonitrile for 15 min at room temperature, gel pieces were dried, 10 mg/mL activated trypsin was then added, and incubation continued for 15 min at room temperature. After the addition of digestion buffer, the gel pieces were incubated at 30°C overnight to digest the proteins.
Use of an ion trap mass spectrometer, LC/MS/MS LTQ Deca XP (Thermoelectrol), enabled the identification of proteins from their amino acid sequence (MS/MS sequence). The mass spectrometer is linked to a high performance liquid chromatography (HPLC) system. Tryptic peptides were injected onto a reversed‐phase column and separated based on hydrophobicity. Peptides desorbed from the column were eluted directly into the mass spectrometer. In the ion trap, the masses of the intact peptides were measured. Then each peptide in turn was isolated in the trap, and the collision energy was increased, fragmenting the peptide to result in an MS/MS spectrum that represents the sequence of the peptide. The MS/MS spectra were subjected to a search against a database (protein, DNA, or EST) to find a peptide with the corresponding intact mass and fragment masses. The protein identification software assigned a score for the match between the measured MS/MS mass spectrum, and the theoretical peptide mass spectrum was calculated from proteins in the database. If one or preferably more peptides had a well‐correlated match, the protein was identified with high confidence.
Cloning and expression of chicken TfR. Full‐length cDNA coding chicken TfR was cloned by reverse transcription–polymerase chain reaction (RT‐PCR) using forward 5′‐GGCAGGTATACTTTGGAGTCATTGC‐3′, reverse 5′‐ACAT TCGAGGCT CCAGTGAATTCG‐3′ primers, cDNA from DT40 cells as a template, and Ex Taq DNA polymerase (Takara Bio, Ohtsu, Japan). PCR product cloned into pGEM‐T Easy vector (Promega, Tokyo, Japan) was digested with Not I and subcloned into pcDNA4/HisMAX‐B (Invitrogen). Transfections of the expression vector into HEK293F cells were achieved with 293fectin (Invitrogen).
Results
Selection of cytotoxic mAbs against DT40 cells. BALB/c mice were immunized three times with DT40 cells and CpG DNA adjuvant, and the mouse with the highest antibody titer was further boosted intravenously with DT40 cells, and cell fusion with polyethylene glycol was done to generate hybridoma cultures. Based on observations of DT40 cells treated with culture media from 960 hybridoma cultures, we found morphological changes of DT40 cells followed by cell death in media from two hybridoma cultures. Hybridoma cells were cloned and mAbs, D18, and D19 (determined to mouse IgGγ2a, κ in both mAbs), were purified from ascite fluids of athymic (nu/nµ) mice by affinity chromatography with protein G. In this study, we extensively analyzed the biological effects of D18 and D19 on DT40 cells. First, we examined the effect of the mAbs on the growth of DT40 cells. Addition of D18 or D19 strongly inhibited the proliferation of DT40 cells (Fig. 1a). Next, morphological alterations of DT40 cells by mAbs were examined with phase‐contrast microscopy (Fig. 1b). At 18 h after treatment, DT40 cells were swelling and the contour of the cells had become round and smooth. At 48 h after the treatment, most of the cells underwent death with characteristic morphological features. Combination of both mAbs had a similar result to treatment with each individual mAb (data not shown). These morphological changes were also examined in semithin sections (Fig. 1c). To our surprise, typical signs of apoptosis, such as cell shrinkage, and necrosis, including cell swelling and ruptured plasma membrane, were observed simultaneously in semithin sections and in ultra‐thin sections by electron microscopic analysis (Fig. 1d). This complicated pattern of cell death was characteristically induced only by D18 or D19, and was not observed during the cell death caused by a typical apoptosis inducer, staurosporine (data not shown). Although it was reported that the Fas‐FasL system could induce a necrosis‐like cell death when the apoptosis signal was inhibited,( 12 ) systems simultaneously inducing both necrosis and apoptosis have not been reported. In this context, we have intensively investigated the mAb‐inducing cell death in detail.
Figure 1.
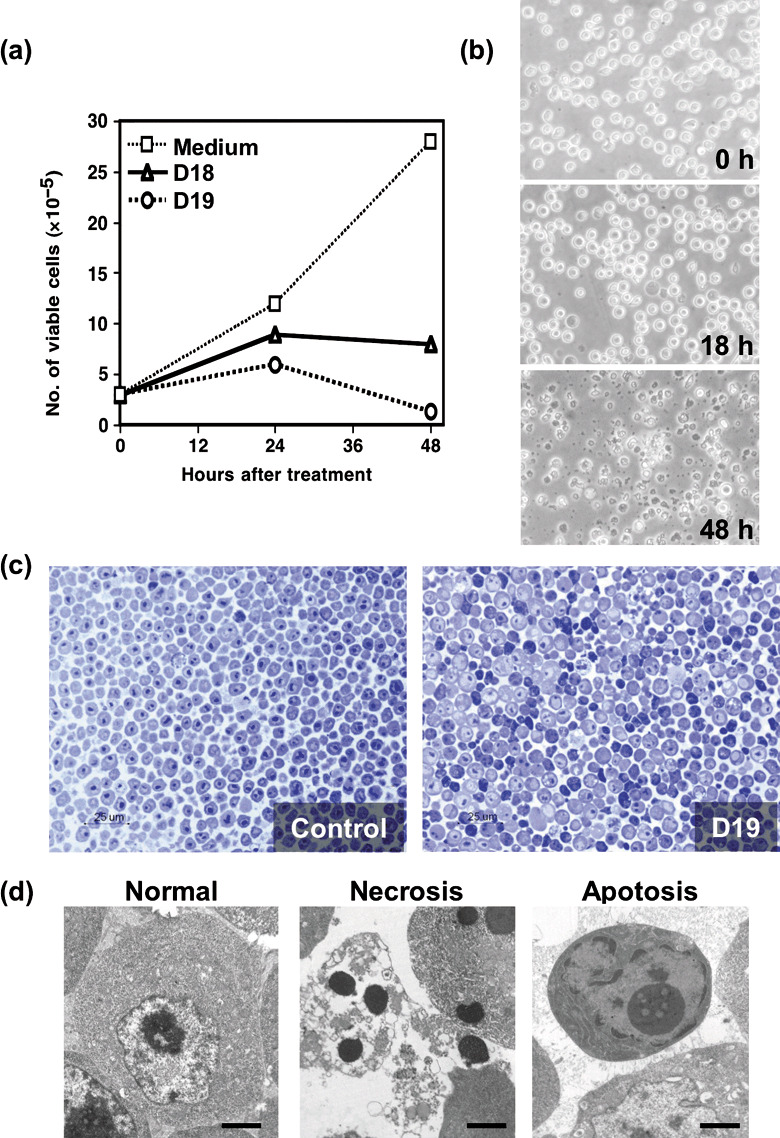
Effect of mAbs on the proliferation of DT40 cells. (a) DT40 cells were cultured in the presence or absence of monoclonal antibodies (mAbs) (D18 or D19), and cell growth of DT40 cells was evaluated by a trypan blue dye exclusion method. (b) Phase‐contrast micrographs of morphological alteration of DT40 cells cultured with D18 mAb are shown. (c) Semi‐thin sections of DT40 cells cultured for 18 h with or without D19 mAb were stained with toluidine blue solution. (d) Electron microscopic analysis of ultra‐thin sections of DT40 cells cultured for 18 h without (left) or with D19 (middle and right).
Unique process of cell death after treatment with the mAbs. Since the inhibition of cell growth by the mAbs seems at least partly due to cell death, we investigated the effects of mAbs on the emergence of cells in the sub G0/G1 phase. After each treatment with the mAbs, cells were harvested and fixed. Subsequently, total DNA content was evaluated by the staining of cells with DAPI and was analyzed by flow cytometry. We found a significant proportion of cells in the sub G0/G1 phase (<2 N) suggesting DNA‐fragmentation of apoptotic cells (Fig. 2a). Figure 2a also shows that cells in the sub G0/G1 phase emerged 18 h after the addition of D18 or D19. In fact, nucleosomal‐level DNA degradation was observed as a DNA‐ladder in DT40 cells cultured with the mAbs for 18 h (Fig. 2b). Because DNA fragmentation is a typical feature of apoptotic cells, the cell death induced by the mAbs could be due to apoptosis. However, in much of the sub G0/G1 population of DT40 cells treated with D18 or D19, swelling was observed by the flow cytometric analysis of dual parameters, forward scatter, and DAPI‐staining (Fig. 2c). Thus, we next investigated exposed PS and mitochondrial membrane potential on the cell surface. PS is exposed on the outer membrane in early apoptotic cells, although PS exists in inner membrane in viable cells. Exposed PS in cells treated with mAbs was analyzed by flow cytometry using FITC‐conjugated Annexin V and propidium iodide. DT40 cells were cultured in the absence or presence of mAbs for appropriate lengths of time in individual experiments. Exposed PS was observed little at 6 h after treatment with mAbs, and it apparently increased at 12–18 h after treatment with mAbs (Fig. 2d). Since it has been recently reported that mitochondria play a central role in cell death signaling, mitochondrial membrane potential was assessed using DiOC6(3) and DAPI. Disruption of mitochondrial membrane potential was observed at 12 h after treatment with mAbs (Fig. 2e). From these results, D18 and D19 mAbs seemed to induce cell death by way of the mitochondrial pathway in DT40 cells.
Figure 2.
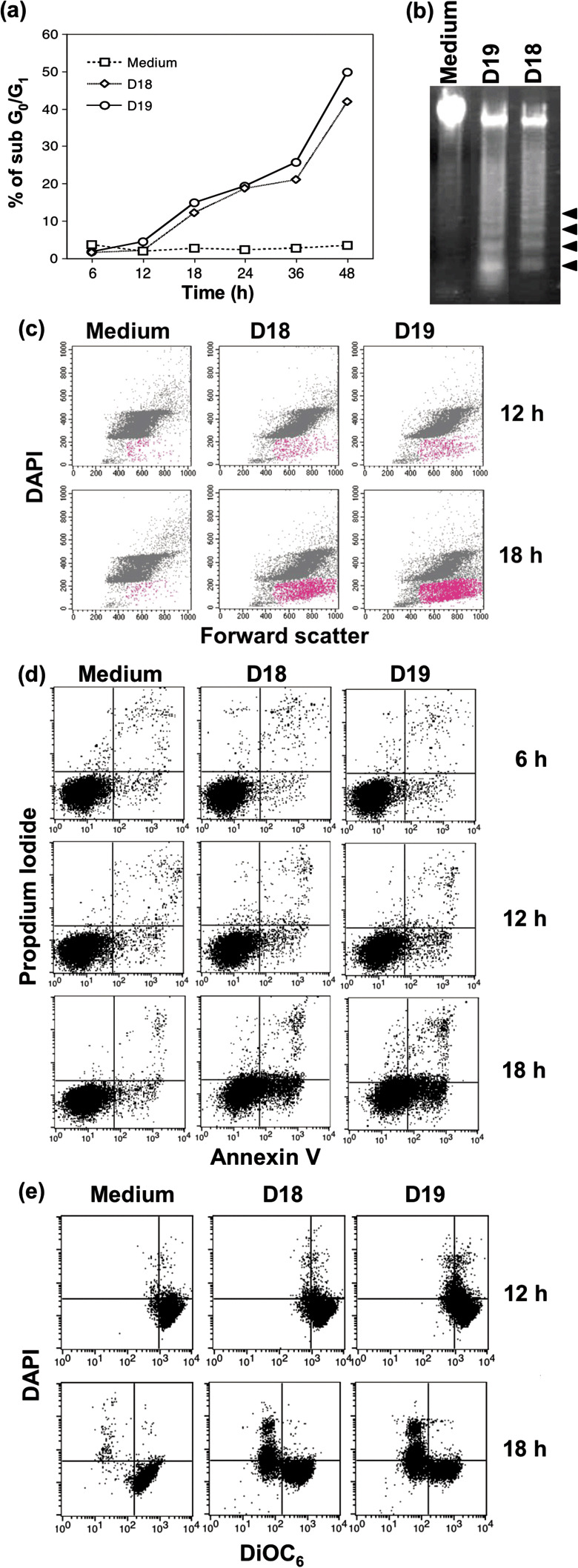
Apoptosis‐like cell death observed in DT40 cells treated with monoclonal antibodies (mAbs). (a) Cells were cultured with D18 or D19 mAb for the time indicated, stained with DAPI, and analyzed for sub G0/G1 cells by flow cytometry. (b) Agarose gel electrophoresis to detect DNA laddering in DT40 cells. The arrowheads show nucleosomal fragmentation of DNA. (c) DT40 cells were cultured with or without D18 or D19 mAb for 12 or 18 h and stained with DAPI, and 10 000 cells were analyzed by dual‐parameter flow cytometry with DAPI and forward scatter. Apoptosis‐like cell population is indicated in red. (d) Exposed PS was analyzed by staining with Annexin V fluorescein‐isothiocyanate (FITC) and propidium iodide. The percentage of cells located in right histogram quadrants is indicated. (e) Disruption of mitochondrial membrane potential (Δψ) was determined by measuring decreased DiOC6.
Effect of D18 and D19 mAbs on the cell cycle progression. Next, in order to analyze the effects of mAbs on progression or arrest of the cell cycle in DT40, particularly on G1 arrest, we performed two‐color flow cytometric analysis. We labeled cells in the S‐phase using BrdU which is a thymidine analog, and chased the cell cycle progression. BrdU‐labeled cells were cultured in the presence of mAbs or TN‐16, which is known to reversibly induce M‐phase arrest,( 13 ) and analyzed the effect on the cell cycle. After each treatment with mAbs or TN‐16, DT40 cells were harvested and stained with FITC‐conjugated anti‐BrdU mAb and DAPI. As shown in Figure 3, populations of S‐phase (62%), G1‐phase (29%), and G2/M‐phase (9%) were observed in BrdU‐labeled control DT40 cells. Although BrdU‐labeled cells were arrested in M‐phase by the culture with TN‐16 for 4 h, D19 mAb‐treated BrdU‐labeled cells were not arrested in specified point of the cell cycle. However, obvious delay of the cell cycle progression was induced in D19 mAb‐treated cells as compared with the control medium.
Figure 3.
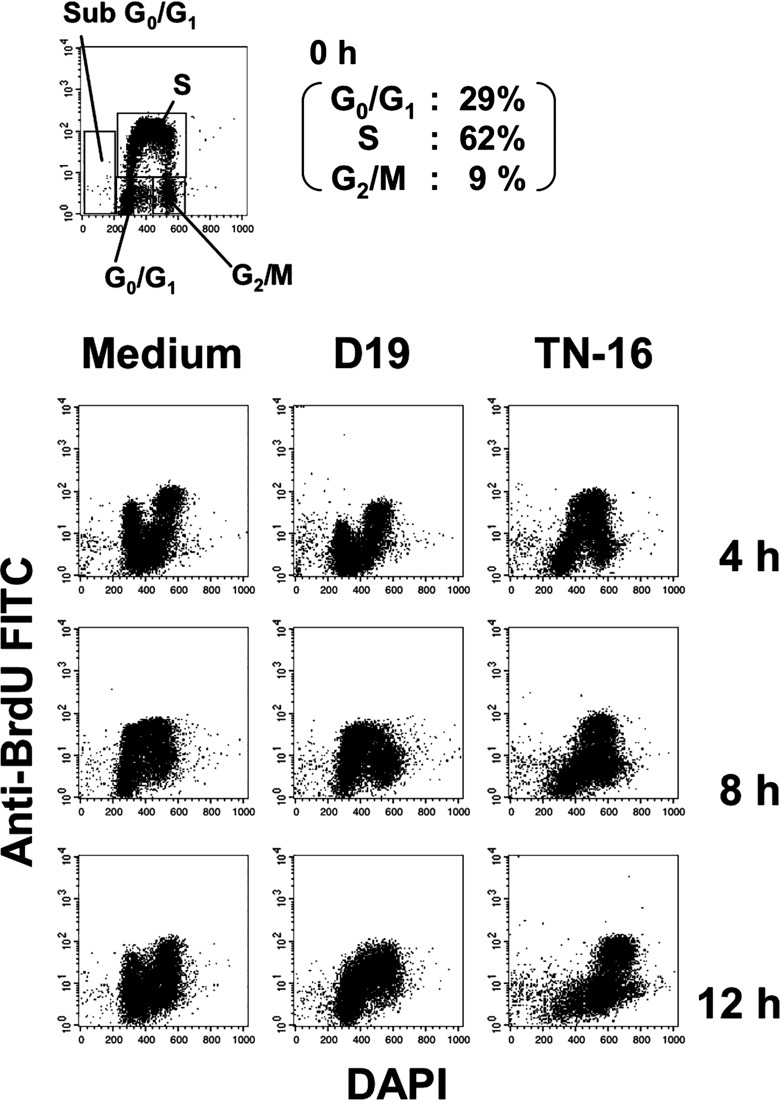
Effect of D18 or D19 mAb on the cell‐cycle progression in the DT40 cell. Following DT40 cells in the S‐phase were pulsed with BrdU for 20 min at 39°C; the labeled cells were cultured in the presence or absence of D18 or D19 mAb. Bromodeoxyuridine (BrdU)‐labeled DT40 cells untreated or treated with mAbs were stained with DAPI and fluorescein‐isothiocyanate (FITC)‐conjugated anti BrdU antibodies, and analyzed by flow cytometry. The gate for the sub G0/G1, G0/G1, S, G2/M phase is shown in the untreated panels at 0 h. Cells were treated or untreated with D19 or TN‐16, and collected at 4 h, 8 h, and 12 h.
Tissue distribution of mAb‐defined antigen in the chicken. We investigated the tissue distribution of antigens recognized by D18 and D19 mAbs. Reactivity of mAbs with freshly isolated leukocytes from bursa of fabricius (BF), bone marrow, spleen, thymus, and peripheral blood were investigated by flow cytometry.
As shown in Figure 4, D18 or D19‐defined antigen was strongly expressed in DT40 tumor cells and cells of BF, moderately in cells of spleen and bone marrow, and very weakly in thymocytes and peripheral blood cells. It is noteworthy that reactivity of D19 with DT40 cells was approximately 20‐fold stronger than that with BF cells expressing the highest TfR in the normal chicken cells, from the values of mean fluorescence intensity in flow cytometric analysis (Fig. 4). In addition, D18 and D19 mAbs reacted with cells in basal layer of squamous (tongue) and digestive (stomach and intestine) epithlia, but reacted little with normal chicken tissue such as the brain, lung, liver, pancreas, and kidney, in immunohistological staining (data not shown). Consequently, it was suggested that D18 and D19 mAbs recognized a cell‐surface antigen selectively expressed on a part of chicken lymphoid and epithelial cells.
Figure 4.
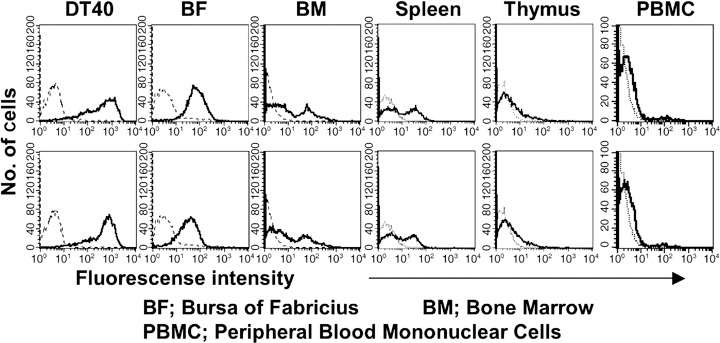
Expression of the antigen defined with D18 or D19 monoclonal antibody (mAb) on DT40 tumor cells and chicken normal leukocytes by flow cytometry. Expression of the antigen on freshly isolated leukocytes from bursa of fabricius (BF) and bone marrow (BM) (spleen and thymus) was analyzed with D18 (upper panel) or D19 (lower panel) mAb, followed by fluorescein‐isothiocyanate (FITC)‐conjugated secondary antibody by flow cytometry (solid lines, stained with mAbs; dotted lines, control). For peripheral blood leukocytes (PBL), two‐color flow cytometric analysis was performed with CyChrome‐labeled antichicken IgY antibody.
Character of the antigen recognized by D18 or D19 mAb. The cross‐linking of death receptors on the cell surface by death ligands induces many apoptotic cell deaths. Well‐known death ligands or regulators of apoptosis are tumor necrosis factor alpha (TNF‐α)( 14 ) and Fas‐ligand (FasL)( 15 , 16 ) which induce apoptosis by activation of their corresponding receptors, TNFR‐1( 17 ) and Fas.( 18 ) Here, we investigated the character of antigens recognized by D18 or D19. First, we investigated the molecular weight of mAb‐defined antigens by the immunoprecipitation of biotin‐labeled cell‐surface proteins. An approximately 100‐kDa glycoprotein was detected by both D18 and D19 mAbs in the reducing condition. In the non‐reducing condition, an approximately 200‐kDa glycoprotein in addition to a 100‐kDa protein was detected, suggesting that the antigen recognized by each mAb is a 100‐kDa protein, part of which forms homodimeric glycoproteins by the disulfide bond between cysteine residues (Fig. 5a). We purified the antigen(s) defined with D18 or D19 from total cell lysates of DT40 by the immunoprecipitation with D18 or D19 mAb. Ninety‐five kDa proteins detected with Sypro Ruby staining were digested with modified trypsin in polyacrylamide gel, and identification of the protein was performed with LC/MS/MS analysis. As a result of database research, the protein immunoprecipitated with D18 or D19 was identified to chicken TfR with credible cover ratio (32% and 31% in D18 and D19, respectively). We showed the MS/MS spectrum of the digested peptide sequence NPAAVSESLYNR, corresponding to the region 528–539 in chicken TfR by MS/MS ion search (Fig. 5b).
Figure 5.
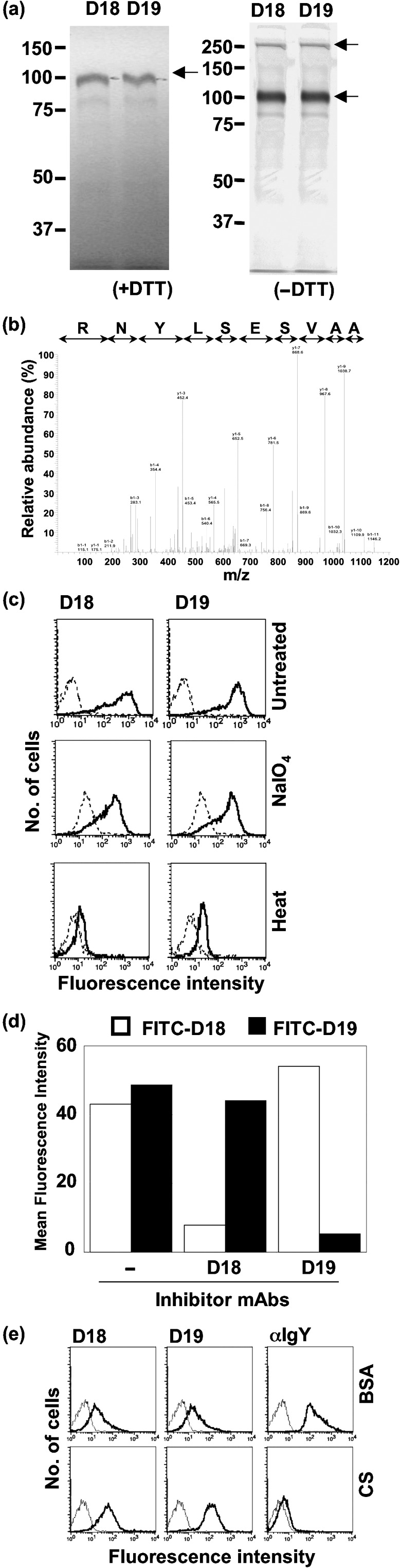
Characterization of the antigen defined with monoclonal antibodies (mAbs). (a) Immunoprecipitated proteins by D18 and D19 mAbs were analyzed with SDS‐PAGE in the reducing (left) or non‐reducing (right) condition. (b) MS/MS spectrum of D19‐immunoprecipitated protein ([M + H +] = 1320.65). Peptide sequence was indicated by matching b ion (red) and y ion (blue) fragments. (c) DT40 cells were untreated or subjected to periodate or heat treatment, stained with D18 or D19 mAb, and analyzed by flow cytometry. (d) DT40 cells were stained with fluorescein‐isothiocynate (FITC)‐conjugated D18 or D19 mAb in the absence or presence of excess (100‐folds) inhibitor mAbs, and analyzed by flow cytometry. (e) DT40 cells were stained with D18, D19, or antichicken IgY in the absence or presence of 1% BSA or 30% chicken serum, and analyzed by flow cytometry. Data are shown by mean fluorescence intensity (MFI).
Next, we investigated the antigenic epitopes recognized by D18 or D19. We assessed the chemical nature of the epitope recognized with mAb by flow cytometry (Fig. 5c). Reactivity of both D18 and D19 against DT40 cells decreased a little after the treatment of cells with NaIO4, and was remarkably affected by heat‐treatment, suggesting that the mAb‐defined epitope is peptide, but not carbohydrate included in a chicken TfR glycoprotein. From mAb‐binding inhibition experiment, these two mAbs seem to recognize different epitopes on the chicken TfR (Fig. 5d). Although the reactivity of anti‐IgY antibody was inhibited by chicken serum, the reactivity of D18 or D19 mAb with DT40 cells was not inhibited by the chicken serum (30%) or bovine Tf (1 mg/mL), indicating that cell death induced by mAbs is not caused by the binding inhibition of Tf to chicken TfR (Fig. 5e).
MAb‐induced cell death in human cells transfected with chicken TfR. In order to assess the possibility that antibody binding to TfR in human cells could induce cell death, we have established a stable human cell clone transfected with full‐length chicken TfR cDNA cells (cTfR‐293) (Fig. 6a). D18 and D19 mAbs were definitely reactive with cTfR‐293, substantiating that D18‐ or D19‐defined antigen is certainly chicken TfR. Furthermore, cell death was induced in cTfR‐293 with D18 or D19 mAb (10 µg/mL), but not in HEK293 cells transfected with control plasmids (Fig. 6b).
Figure 6.
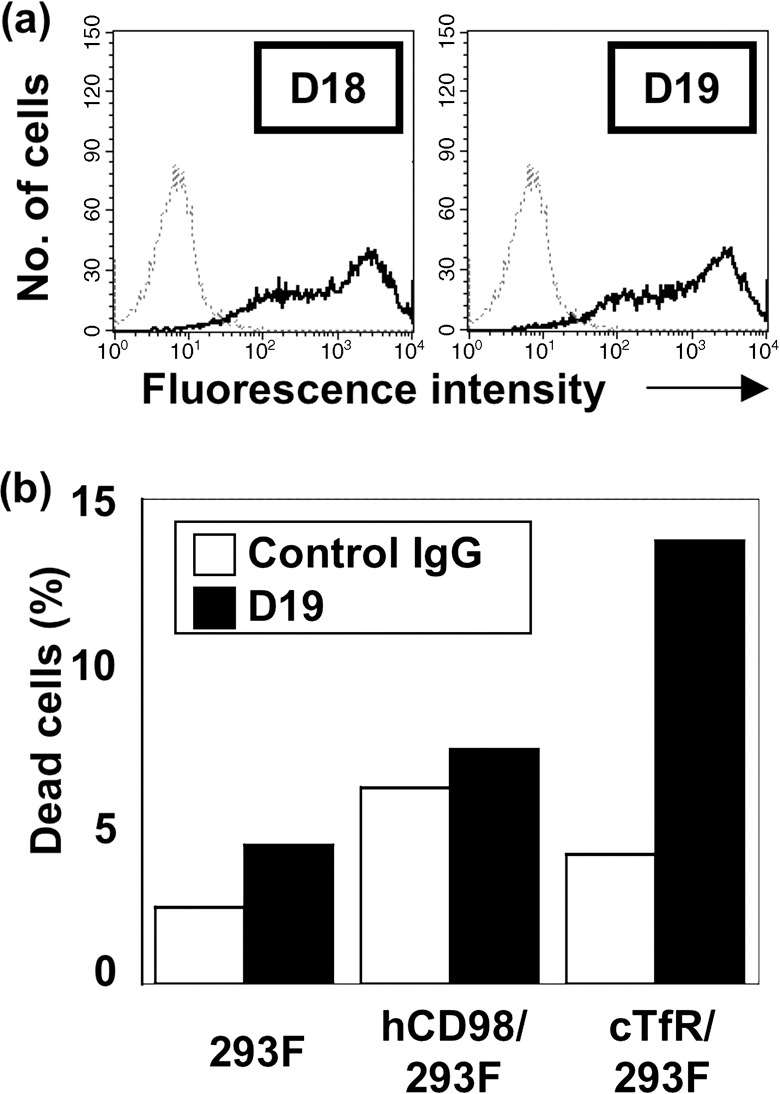
Cell death induced by monoclonal antibodies (mAbs) in human cells transfected with full‐length chicken transferrin receptor (TfR) cDNA. (a) HEK293F human embryonic kidney cells were transfected with chicken TfR cDNA, selected by Zeocin and clone‐sorted, as described in ‘Materials and Methods’, and stained with D18 or D19 mAb. (b) HEK293F cells transfected with chicken TfR cDNA or control (human CD98hc) cDNA were treated with mAb, and examined for cell death.
Discussion
Treatment of chicken DT40 tumor cells with D18 or D19 mAb resulted in the exposed PS, loss of mitochondrial membrane potential, and DNA fragmentation; however, cell shrinkage was not detected and instead cell swelling was observed. Additionally, in the electron microphotograph (Fig. 1d), apoptotic and necrotic cell death were simultaneously induced by these mAbs. However, a typical apoptosis was induced by staurosporin treatment, and necrosis‐like cell death accompanying the elevated cell size was not observed (data not shown). It has been reported that necrosis‐like cell death was induced by the inhibition of the caspase activities that were central factors inducing apoptosis.( 12 , 19 , 20 , 21 ) However, simultaneous induction of both apoptotic and necrotic cell death through cell‐surface receptors has not been reported until now. In this study, we identified a chicken TfR as the antigen defined with D18 and D19 cell‐death‐inducing mAbs. As for small molecular weight compounds, gambogic acid (GA) was reported to bind TfR and induce apoptosis by using caspase‐8 and the mitochondrial pathway.( 22 ) Cell death induced by D18 or D19 mAb seems different from apoptosis induced by GA in morphological characteristics including cell swelling.
In contrast to apoptotic cell death, the molecular mechanisms of necrotic cell death are not well understood, although several features including the release of lysosomal enzymes, generation of toxic oxygen radicals, and the activation of calcium‐dependent phospholipases, have been proposed.( 19 ) In this context, the possibility that difference in the mode of dysfunction in mitochondria, namely difference in the pattern of release of cytochrome c, might be greatly involved in cellular signals leading to or dividing necrosis or apoptosis.( 12 ) Necrosis has been regarded as non‐physiological cell death, and has been paid little attention, as compared with apoptosis. In this study, however, we have shown necrosis‐like cell death can be induced by mAbs, suggesting that necrosis, as in apoptosis, can also be caused by physiological events and regulated by physiological cellular mechanisms. The epitope in the chicken TfR defined with D18 or D19 mAb is restricted to a small cell population in normal lymphoid and epithelial tissues. In contrast to the induction of cell death in DT40 tumor cells, D18 or D19 did not induce cell death in chicken BF cells reactive with D18 or D19 (data mot shown). This might be explained by the difference of expression level of TfR between DT40 tumor and BF cells, as shown in Figure 4. TfR is expressed at low levels on various normal cells containing pluripotent hematopoietic stem cells from the mouse, rat, or human species, but is expressed at greater level on cells from various human malignancies irrespective of their tissue origin.( 23 ) Fas/CD95 and DR5/TRAIL receptor have been reported to be typical cell death‐inducing molecules.( 24 ) Fas/CD95 is expressed abundantly on almost all normal cells;( 25 ) in particular, hepatocytes are sensitive to Fas‐ligand( 26 ) and anti‐Fas/CD95 mAb (Jo2) induces the death of hepatocytes in the murine system. (27) DR5/TRAIL receptor is infrequently expressed on normal cells( 28 ) and could selectively induce apoptosis in tumor cells; however, tumor cells could frequently acquire resistance to apoptosis stimuli.( 29 ) Various studies have shown low or no TfR expression in normal cells, and elevated levels of expression of the TfR on cancer cells when compared to their counterparts. Internalization of TfR by mAb could induce secondary cell death by the deprivation of iron‐bound Tf, even if tumor cells acquire resistance to apoptosis stimuli through a given cell death receptor. In this context, we have confirmed rapid internalization of TfR by D18 or D19 mAbs (data not shown). Therefore, TfR could be an excellent molecular target for antibody therapy against various malignancies, possibly even superior to typical cell‐death‐inducing molecules such as Fas/CD95 and DR5.
Preparation of mAbs recognizing equivalent epitope in human TfR will be promising for antibody therapy for various human malignancies, because D18 and D19 mAbs have tumor specificity and the ability to internalize TfR and induce cell death in the chicken system. Although some mAbs recognizing TfR mAbs have been reported to inhibit cell growth,( 30 ) precise mechanisms remain solved, especially in relation to cell death. Anti‐TfR mAb‐mediated cell death has been mainly regarded as apoptotic cell death caused by the binding inhibition of TfR to the iron‐bound Tf. In this context, partial necrosis‐induction by D18 or D19 might cause migration of leukocytes and immune response, which might contribute to the augmentation of an antitumor effect, compared with anti‐TfR mAbs reported up to now. Furthermore, D18‐ and D19‐mediated internalization of TfR suggests the therapeutic benefit of these mAbs. The unique pathway of the cell death in the chicken, shown in this study, seems to be conserved in the human system, since D18 or D19 mAb could induce cell death in human embryonic kidney cells transfected with chicken full‐length cDNA of TfR. However, cell swelling was not observed in these cells, suggesting that deprivation of iron‐bound Tf by the mAb‐induced internalization of TfR was insufficient possibly because of the existence of endogenous human TfR in HEK293 cells.
In conclusion, our present report describes the unique cell death in which apoptotic and necrotic cell deaths occur simultaneously. We believe that human TfR is an attractive target molecule, and that the development of functional mAbs inducing the death of tumor cells will be promising for the antibody therapy for human cancers. Studies are currently underway to analyze the cell death induced in human transfectant cells expressing chicken TfR treated with D18 or D19 mAb, and to produce cell‐death‐inducing mAbs recognizing human TfR.
Acknowledgment
This work was supported in part by the ‘Academic Frontier’ Project for Private Universities, matching fund subsidies from MEXT (Ministry of Education, Culture, Sports, Science, and Technology of Japan), 2005–7.
References
- 1. Buerstedde JM, Takeda S. Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell 1991; 67: 179–88. [DOI] [PubMed] [Google Scholar]
- 2. Buerstedde JM, Reynaud CA, Humphries EH, Olson W, Ewert DL, Weill JC. Light chain gene conversion continues at high rate in an ALV‐induced cell line. EMBO J 1990; 9: 921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lahti JM. Use of gene knockouts in cultured cells to study apoptosis. Methods 1999; 17: 305–12. [DOI] [PubMed] [Google Scholar]
- 4. Wyllie AH, Kerr JF, Currie AR. Cell death. the significance of apoptosis. Int Rev Cytol 1980; 68: 251–306. [DOI] [PubMed] [Google Scholar]
- 5. Rathmell JC, Thompson CB. The central effectors of cell death in the immune system. Annu Rev Immunol 1999; 17: 781–828. [DOI] [PubMed] [Google Scholar]
- 6. Thornberry NA, Lazebnik Y. Caspases: enemies within. Science 1998; 281: 1312–6. [DOI] [PubMed] [Google Scholar]
- 7. Shimizu S, Eguchi Y, Kamiike W et al . Bcl‐2 blocks loss of mitochondrial membrane potential while ICE inhibitors act at a different step during inhibition of death induced by respiratory chain inhibitors. Oncogene 1996; 13: 21–9. [PubMed] [Google Scholar]
- 8. Zamzami N, Susin SA, Marchetti P et al . Mitochondrial control of nuclear apoptosis. J Exp Med 1996; 183: 1533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scaffidi C, Fulda S, Srinivasan A et al . Two CD95 (APO‐1/Fas) signaling pathways. EMBO J 1998; 17: 1675–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol 1995; 146: 3–15. [PMC free article] [PubMed] [Google Scholar]
- 11. Masuko T, Sugahara K, Kozono M et al . A murine monoclonal antibody that recognizes an extracellular domain of the human c‐erbB‐2 protooncogene product. Jpn J Cancer Res 1989; 80: 10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsumura H, Shimizu Y, Ohsawa Y, Kawahara A, Uchiyama Y, Nagata S. Necrotic death pathway in Fas receptor signaling. J Cell Biol 2000; 151 (6): 1247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miwa M, Sugimura T, Inui N, Takayama S. Poly (adenosine diphosphate ribose) synthesis during the cell cycle of transformed hamster lung cells. Cancer Res 1973; 33: 1306–9. [PubMed] [Google Scholar]
- 14. Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 2001; 104: 487–501. [DOI] [PubMed] [Google Scholar]
- 15. Nagata S. Apoptosis by death factor. Cell 1997; 88: 355–65. [DOI] [PubMed] [Google Scholar]
- 16. Nagata S, Golstein P. The Fas death factor. Science 1995; 267: 1449–56. [DOI] [PubMed] [Google Scholar]
- 17. Vercammen D, Beyaert R, Denecker G et al . Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med 1998; 187: 1477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 1993; 75: 1169–78. [DOI] [PubMed] [Google Scholar]
- 19. Fiers W, Beyaert R, Declercq W, Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene 1999; 18: 7719–30. [DOI] [PubMed] [Google Scholar]
- 20. Vercammen D, Brouckaert G, Denecker G et al . Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med 1998; 188: 919–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lemaire C, Andreau K, Souvannavong V, Adam A. Inhibition of caspase activity induces a switch from apoptosis to necrosis. FEBS Lett 1998; 425: 266–70. [DOI] [PubMed] [Google Scholar]
- 22. Kasibhatla S, Jessen KA, Maliartchouk S et al . A role for transferrin receptor in triggering apoptosis when targeted with gambogic acid. Proc Natl Acad Sci USA 2005; 102: 12095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The transferrin receptor part I. Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol 2006; 121: 144–58. [DOI] [PubMed] [Google Scholar]
- 24. Yagita H, Takeda K, Hayakawa Y, Smyth MJ, Okumura K. TRAIL and its receptors as targets for cancer therapy. Cancer Sci 2004; 95: 777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leithauser F, Dhein J, Mechtersheimer G et al . Constitutive and induced expression of APO‐1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest 1993; 69: 415–29. [PubMed] [Google Scholar]
- 26. Galle PR, Hofmann WJ, Walczak H et al . Involvement of the CD95 (APO‐1/Fas) receptor and ligand in liver damage. J Exp Med 1995; 182: 1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ogasawara J, Watanabe‐Fukunaga R, Adachi M et al . Lethal effect of the anti‐Fas antibody in mice. Nature 1993; 364: 806–9. [DOI] [PubMed] [Google Scholar]
- 28. Spierings DC, De Vries EG, Vellenga E et al . Tissue distribution of the death ligand TRAIL and its receptors. J Histochem Cytochem 2004; 52: 821–31. [DOI] [PubMed] [Google Scholar]
- 29. Gratas C, Tohma Y, Barnas C, Taniere P, Hainaut P, Ohgaki H. Up‐regulation of Fas (APO‐1/CD95) ligand and down‐regulation of Fas expression in human esophageal cancer. Cancer Res 1998; 58: 2057–62. [PubMed] [Google Scholar]
- 30. Moura IC, Lepelletier Y, Arnulf B et al . A neutralizing monoclonal antibody (mAb A24) directed against the transferrin receptor induces apoptosis of tumor T lymphocytes from ATL patients. Blood 2004; 103: 1838–45. [DOI] [PubMed] [Google Scholar]


