Abstract
Mild periodic acid–Schiff (mPAS) staining can discriminate non‐O‐acetylated (mPAS‐positive) from O‐acetylated (mPAS‐negative) epithelial sialoglycoproteins in human colonic mucosa, allowing the three haplotypes expressed from a single polymorphic autosomal gene (oat) to be distinguished. In heterozygotes, we previously demonstrated wholly mPAS‐positive (stem cell mutated) crypts and clusters of two or more mPAS‐positive crypts to be significantly increased with duration of ulcerative colitis. To establish whether such an increase in the number of mutated crypts with age also occurs in normal individuals or in cases with diverticulosis, the O‐acetylation phenotype in the non‐cancerous colonic mucosa of 47 sporadic colorectal cancer patients who were heterozygotes for oat was tested with mild‐PAS staining. PAS‐positive crypts were assessed histologically in relation to age and compared between the left (sigmoid colon and rectum) and right (cecum and ascending colon) sides of the colorectum. Wholly mPAS‐positive (stem cell mutated) crypts and foci in heterozygotes were found to be increased significantly (P < 0.0001) in the left side with aging (r = 0.598 and 0.643, respectively). Such a positive correlation with aging was also confirmed in 19 diverticulosis cases without cancer (r = 0.797 and 0.793, respectively). The frequency of mutated crypts and foci on the right side was significantly lower than on the left side in both spontaneous colorectal cancer and diverticulosis cases. The results provide support for an intimate relationship between accumulation of mutated crypts with aging, possibly with significance for colorectal cancer development. Furthermore, the environment in the right side of the colon may be different from that in the left side in this regard. (Cancer Sci 2006; 97)
Abbreviation:
- mPAS
mild periodic acid–Schiff staining
It is widely accepted that the likelihood of developing sporadic colorectal carcinoma increases with age.( 1 , 2 , 3 ) Colorectal tumorigenesis is a multistage process,( 4 ) with increased colonic mucosal cell proliferation believed to be important for initiation and subsequent growth,( 5 , 6 ) acting together with the cumulative effects of protracted exposure to cancer‐causing agents. Recently, it was demonstrated that mPAS staining can discriminate non‐O‐acetylated (mPAS‐positive) from O‐acetylated (mPAS‐negative) epithelial sialoglycoproteins.( 7 ) Thus, three patterns of staining may result from expression of a single polymorphic autosomal gene (oat): (i) uniformly mPAS positive, reflecting the homozygous recessive genotype for low or absent O‐acetylation (oat b /oat b ); (ii) uniformly mPAS negative, reflecting the homozygous dominant genotype for normal or high O‐acetylation (oat a /oat a ); and (iii) mPAS‐negative mucosa containing scattered positive discordant crypts, representing heterozygotes (oat a /oat b ). In fact, the frequency distribution of these three phenotypes in different racial groups, including Japanese and British people, is consistent with that predicted by the Hardy–Weinberg law.( 8 ) This suggests that they result from expression of a single polymorphic autosomal gene (oat) encoding the O‐acetyl transferase active in generating colonic sialomucins. Further, the three phenotypes are similar to those seen in C57BL/6 J × SWR F1 mice, where the Dlb‐1 gene determines another intestinal mucus glycoprotein phenotype that can be visualized by virtue of Dolichos biflorus agglutinin binding.( 9 ) In this mouse model, discordant crypts are produced in heterozygotes by mutation. The altered crypts seen in humans using the mPAS technique are also similar in morphology to carcinogen‐induced crypts with changes in glucose‐6‐phosphate dehydrogenase expression in the mouse colon, indicating a role for somatic mutations.( 10 ) It is conceivable that somatic mutations of the high acetylator allele in colonic crypt stem cells in heterozygous subjects followed by crypt colonization by mutant progeny leads to conversion of the crypt phenotype from mPAS negative to mPAS positive. In fact, radiotherapy of heterozygotes induces a considerable increase in the mPAS‐positive crypt frequency, which subsequently remains significantly elevated for 2–34 years, indicating that crypts with a mutant phenotype are stable.( 11 , 12 )
Recently, we demonstrated that the number of wholly mPAS‐positive (stem cell mutated) crypts and clusters of two or more mPAS‐positive crypts in foci significantly increases with the duration of ulcerative colitis in heterozygote individuals, pointing to a possible role in the chronic inflammation–carcinoma sequence.( 13 ) Using the same staining approach in the present study, we assessed mutated crypts in non‐cancerous mucosa of patients with sporadic colorectal cancers and mucosa of diverticulosis patients without colorectal cancers to ascertain whether they might similarly accumulate with age without the background of ulcerative colitis. The frequency of mutated crypts was also compared between the right and left sides of the colorectum.
Materials and Methods
Colorectal cancer cases
Large bowel resection specimens of 153 sporadic (non‐hereditary) primary sigmoid colon and rectum cancer patients (left side), and 29 cases of 70 years or older with cecum or ascending colon cancers (right side) were selected randomly. Tissue blocks sampled at a sufficient distance to guarantee non‐involvement with cancers were fixed with 10% buffered formalin (pH 7.0) and processed routinely for embedding in paraffin. The O‐acetylation phenotype was thus tested with mPAS staining in a total of 182 cases with sporadic colorectal carcinomas. Those for which tissue blocks were not sufficient for study were excluded from the final analysis.
Diverticulosis cases without colorectal cancer
Forty‐five large bowel resection specimens from patients with diverticulosis in the sigmoid colon and rectum on the left side and 28 specimens from patients with diverticulosis in the cecum and ascending colon on the right side were subjected to mPAS staining for comparison. A total of 19 heterozygotes for the left side (diverticula: median, 19; range, 7–40) and seven heterozygotes for the right side (diverticula: median, 11; range, 5–17) were available for the study.
mPAS‐positive crypts
The numbers of crypt profiles adjacent to the muscularis mucosa present in one central step section in each case were counted manually by a single observer (IO), according to the methods of Fuller et al. ( 7 ) and Campbell et al.( 8 ) The total number of crypt profiles present was then calculated by multiplying the number of step sections by the count for the central section. For mPAS staining, 4‐µm sections were cut from paraffin‐embedded blocks and step sections were taken at 80‐µm intervals to obtain the necessary size of sample while reducing the chance of counting the same crypt in adjacent sections. The sections were stained with the mPAS technique.( 14 ) Individual discordant crypts were considered to be wholly involved when all of the goblet cells showed mPAS positivity, and partially involved when both mPAS‐positive and mPAS‐negative cells were present in a crypt profile. Wholly involved, positive crypts, indicating stem cell mutation,( 11 ) were identified and counted in longitudinal sections showing uniform staining of goblet cells from the base of the crypt to the luminal surface (complete replacement by the mutant phenotype) in >10 000 crypt profiles and expressed as the number of positive crypts ×10−4. As goblet cells forming hyperplastic polyps and metaplastic Paneth cells show weak mPAS positivity, crypts consisting of these cells were strictly excluded. Identification of single crypts was carefully confirmed to avoid duplication. The total number of crypts examined with mPAS staining was 15 043 ± 5382 (mean ± SD; median, 12 996; range, 10 023–33 945) in the 88 mPAS‐heterozygote cases.
This work using surgical pathology samples was approved by the Kitasato University School of Medicine and the University Hospital Ethics Committee (B01‐20).
Statistics
Statistical comparisons between groups were carried out using the non‐parametric Mann–Whitney U‐test. For demonstration of associations, the Pearson's correlation coefficient test was applied. Significance was concluded at P < 0.05 and r > 0.5.
Results
Sigmoid colon and rectum cancer cases (left‐sided colon)
All 123 non‐cancerous colorectum specimens removed surgically for primary colorectal cancers in the left‐sided colon (sigmoid colon and rectum) clearly showed one of the three phenotypes of uniformly mPAS‐positive, uniformly mPAS‐negative or mPAS‐negative heterozygotes with scattered positive discordant crypts (44 : 25 : 54; Fig. 1A–C) by screening mPAS positivity (crypts tested for screening in latter, two phenotypes, median, 8340; range, 3630–17 760). Finally, 47 cases who were mPAS‐negative with scattered mPAS‐positive crypts (heterozygotes) were obtained (median age, 58; range, 30–89; men : women, 37 : 10). Seven cases (heterozygotes) were excluded from the analysis because their tissue blocks were not sufficient for further analysis.
Figure 1.
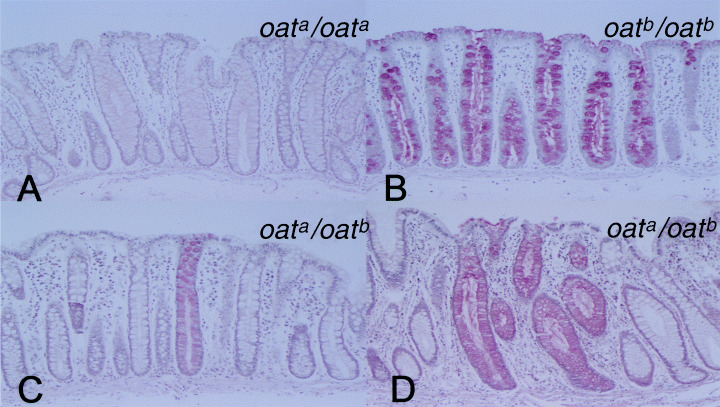
Mild periodic acid–Schiff (mPAS) staining (×128). (A) Uniformly mPAS‐negative mucosa (×128). (B) Uniformly mPAS‐positive mucosa (×128). (C) Uniformly mPAS‐negative mucosa with scattered positive discordant crypt (heterozygote). One mPAS‐positive crypt (positive from the base of the crypt in touch with the muscularis mucosa to the luminal surface) in a focus of mPAS‐positive crypts is evident in this figure. (D) Cluster of mPAS‐positive crypts in a heterozygote. Two mPAS‐positive crypts (positive at the base of crypts in touch with the muscularis mucosa) in a focus of mPAS‐positive crypts are shown.
The numbers of mPAS‐positive crypts, clusters of two or more positive crypts (Fig. 1D) and foci of mPAS‐positive crypts in the left‐sided colon in mPAS heterozygotes were median, 9.8 × 10−4 (range, 4.0–29.6 × 10−4), 0.9 × 10−4 (range, 0.0–2.8 × 10−4) and 10.7 × 10−4 (range, 2.4–25.1 × 10−4) crypts, respectively. Foci of mPAS‐positive crypts refer to both individual crypts and clusters of two or more positive crypts, each counted as one event. The numbers of mPAS‐positive crypts (range = 4.0–29.6 × 10−4, r = 0.598, P < 0.0001) and foci of mPAS‐positive crypts (range, 2.4–25.1 × 10−4, r = 0.643, P < 0.0001) were significantly increased with age (Fig. 2A,B). No significant increase with age could be shown for clusters of two or more positive crypts (range, 0.0–2.8 × 10−4, r = 0.050, P = 0.7369).
Figure 2.
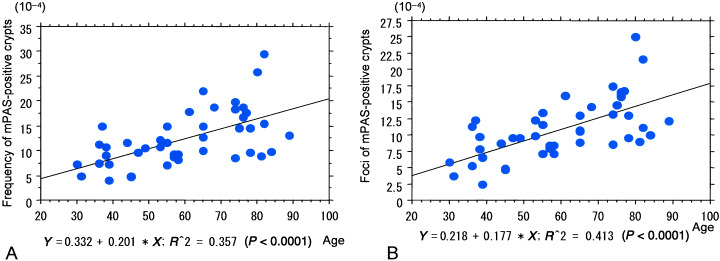
Colorectal cancer cases. (A) Note the positive correlation between the frequency of discordant mild periodic acid–Schiff (mPAS)‐positive crypts and age. (B) Positive correlation between values for foci of mPAS‐positive crypts and age.
Comparison between the left and right sides in colorectal cancer cases
Fifteen mPAS heterozygotes (median age, 73 years; range, 70–88 years; men : women, 9 : 6) were included in the 29 cecum or ascending colon cancer cases for the right‐sided colon. There were six uniformly mPAS‐negative cases and eight mPAS‐positive cases. The numbers of mPAS‐positive crypts, clusters of two or more positive crypts and foci of mPAS‐positive crypts were median, 3.8 × 10−4 (range, 2.9–7.9 × 10−4), 0.0 × 10−4 (range, 0.0–0.8 × 10−4) and 3.8 × 10−4 (range, 2.8–7.9 × 10−4), respectively. For comparison, 16 mPAS heterozygotes from the 47 sigmoid colon and rectum cancer cases (left‐sided) were selected as age‐matched counterparts (median age, 77 years; range, 74–89 years; men : women, 12 : 4). The numbers of mPAS‐positive crypts, clusters of two or more positive crypts and foci of mPAS‐positive crypts in the left‐sided colon were median, 16.1 × 10−4 (range, 8.5–29.6 × 10−4), 0.9 × 10−4 (range, 0.0–2.8 × 10−4) and 13.9 × 10−4 (range, 8.5–25.1 × 10−4), respectively, the difference being significant for those from the right‐sided colon (P < 0.0001, P = 0.0091 and P < 0.0001, respectively) (Table 1).
Table 1.
Comparison of frequencies of mild periodic acid–Schiff (mPAS)‐positive crypts in colonic mucosa between the right and left sides of the colorectum (colorectal cancer cases)
| Side | No. cases | Age (years) median (range) | Sex | Frequency of mPAS‐ positive crypts (×10−4) median (range) | Clusters of mPAS‐ positive crypts (×10−4) median (range) † | Foci of mPAS‐ positive crypts (×10−4) median (range) ‡ |
|---|---|---|---|---|---|---|
| Right | 15 | 73 (70–88) | M9 : F6 | 3.8 (2.9–7.9) | 0.0 (0.0–0.8) | 3.8 (7.9–2.8) |
| Left | 16 | 77 (74–89) | M12 : F4 | 16.1 (8.5–29.6) | 0.9 (0.0–2.8) | 13.9 (8.5–25.1) |
| P‐value | <0.0001 § | <0.0091 § | <0.0001 § |
Clusters of two or more mPAS‐positive crypts.
‡ Both individual crypts and clusters of two or more positive crypts, each counting as one event.
§ Compared with the right‐sided colon.
Diverticulosis cases without colorectal cancers
Twenty‐two mPAS heterozygotes were included in the 45 diverticulosis patients who received rectosigmoidectomy for diverticulosis of the sigmoid colon or rectum. Three heterozygotes were excluded because their tissue blocks were not sufficient for further analysis, so that 19 mPAS heterozygotes were examined (median age, 66 years; range, 42–91 years; men : women, 11 : 8). There were eight uniformly mPAS‐negative cases and 15 mPAS‐positive cases. The numbers of mPAS‐positive crypts, clusters of two or more positive crypts, and foci of mPAS‐positive crypts in the left‐sided colon in mPAS heterozygotes were median, 8.8 × 10−4 (range, 5.3–17.9 × 10−4), 1.1 × 10−4 (range, 0.0–5.7 × 10−4) and 7.9 × 10−4 (range, 1.0–16.6 × 10−4), respectively. The numbers of mPAS‐positive crypts (r = 0.797, P < 0.0001) and foci (r = 0.793, P < 0.0001) increased significantly with age (Fig. 3A,B). No significant increase with age could be shown for clusters of two or more positive crypts (r = 0.031, P = 0.9008).
Figure 3.
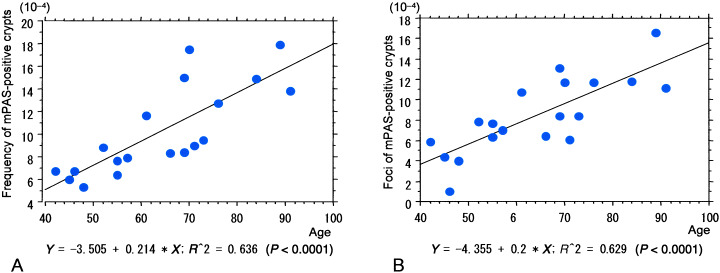
Diverticulosis cases without colorectal cancers. (A) Note the positive correlation between the frequency of discordant mild periodic acid–Schiff (mPAS)‐positive crypts and age. (B) Values for foci of mPAS‐positive crypts also correlate with age.
Comparison between the left and right sides in diverticulosis cases without colorectal cancers
Fifteen mPAS heterozygotes were included in the 28 cases with diverticulosis of the cecum or ascending colon (right‐sided). There were five uniformly mPAS‐negative cases and eight mPAS‐positive cases. Finally, seven mPAS heterozygotes (median age, 49 years; range, 42–53 years; men : women, 7 : 0) were examined. Eight heterozygotes were excluded because their tissue blocks were not sufficient for further analysis. The median numbers of mPAS‐positive crypts, clusters of two or more positive crypts and foci of mPAS‐positive crypts were 3.8 × 10−4 (range, 2.0–7.0 × 10−4), 0.0 × 10−4 (range, 0.0–1.6 × 10−4) and 3.8 × 10−4 (range, 1.0–5.4 × 10−4), respectively. For comparison, eight mPAS heterozygotes from the 19 cases with diverticulosis of the sigmoid colon and rectum (left‐sided) were selected as age‐matched counterparts (median age, 50 years; range, 42–57 years; men : women, 7 : 1). The median numbers of mPAS‐positive crypts, clusters of two or more positive crypts and foci of mPAS‐positive crypts were 6.7 × 10−4 (range, 5.3–8.8 × 10−4), 0.9 × 10−4 (range, 0.0–5.7 × 10−4) and 6.1 × 10−4 (range, 1.0–7.9 × 10−4), respectively, the differences being significant from the right‐sided values (P = 0.0078 and P < 0.0372, respectively) except for the clusters of two or more positive crypts (Table 2).
Table 2.
Comparison of frequencies of mild periodic acid–Schiff (mPAS)‐positive crypts in colonic mucosa between the right and left sides of the colorectum (diverticulosis cases)
| Side | No. cases | Age (years) median (range) | Sex | Diverticula n (range) | Frequency of mPAS‐ positive crypts (×10−4) median (range) | Clusters of mPAS‐ positive crypts (×10−4) median (range) † | Foci of mPAS‐ positive crypts (×10−4) median (range) ‡ |
|---|---|---|---|---|---|---|---|
| Right | 7 | 49 (42–53) | M7 : F0 | 11 (5–17) | 3.8 (2.0–7.0) | 0.0 (0.0–1.6) | 3.8 (1.0–5.4) |
| Left | 8 | 50 (42–57) | M7 : F1 | 16.5 (7–34) | 6.7 (5.3–8.8) | 0.9 (0.0–5.7) | 6.1 (1.0–7.9) |
| P‐value | 0.0078 § | 0.0372 § |
Clusters of two or more mPAS‐positive crypts.
‡ Both individual crypts and clusters of two or more positive crypts, each counting as one event.
§ Compared with the right‐sided colon.
Discussion
Concerning mPAS positivity in the present study, the ratios of uniformly mPAS‐positive mucosa : uniformly mPAS‐negative mucosa : uniformly mPAS‐negative with scattered positive discordant crypts (heterozygotes) were 44 : 25 : 54 in colorectal cancer cases and 23 : 13 : 37 in diverticulosis cases, in line with those predicted for Japanese cases in a previous report (34 : 17 : 49),( 8 ) indicating consistency of the staining method. The median frequency of mPAS‐positive crypts in 104 crypts in sigmoid colon or rectum cancer cases was 9.8 × 10−4 (range, 4.0–29.6 × 10−4) (median age, 58 years; age range, 30–89 years), similar to the values in another earlier report (median 10.0 × 10−4; range, 1.5–44.0× 10−4; median age, 70 years; age range, 51–83 years),( 15 ) suggesting that our assessment method was accurate. All of the quantitative data were obtained by one pathologist (IO) in the present study.
The results clearly showed the number of both mPAS‐positive crypts and foci in heterozygotes to increase significantly with age in the left‐sided colon, indicating accumulation of mutations in line with expectations. Although materials from individuals younger than 30 years were not available in the present study, coefficient curves showed almost zero values of mPAS‐positive crypts and foci of mPAS‐positive crypts at age 0 years (Fig. 2), suggesting that mutated crypts appear after birth and increase with age. This is in line with an earlier report by Fuller et al.( 7 ) in which the colonic mucosa of 18 infants and children revealed only two patterns of staining reaction, either uniformly negative or uniformly positive mPAS staining, indicating no demonstration of mutated crypts. This tendency would explain the increase in colorectal cancer development with age,( 16 ) supporting multistep carcinogenesis.( 4 )
According to the previous report,( 7 ) no difference was found in the mutation frequencies of colonic crypts between age‐matched individuals with sporadic colorectal cancer and benign colonic conditions. Therefore, it is worthwhile to see the age influence on mutation frequencies in the present study, although non‐cancerous mucosa is not completely normal in cases with sporadic colorectal cancer. No significant correlations were described between any categories of mPAS‐positive crypts and patients’ ages in previous reports,( 13 ) and the frequency of mutated crypts was clearly lower in our elderly patients than in patients with long‐standing ulcerative colitis in our previous study (mPAS‐positive crypts, 37.7 ± 16.5 × 10−4 (mean ± SD); clusters of two or more mPAS‐positive crypts, 7.9 ± 3.6 × 10−4; and foci of mPAS‐positive crypts, 12.5 ± 4.0 × 10−4).( 13 ) This suggests differences in the genesis of ulcerative colitis‐associated cancers from sporadic colorectal cancers, although mild, non‐specific latent colitis may be able to accelerate sporadic colorectal cancer development, according to recent reports that anti‐inflammation treatment suppresses colorectal cancer development and progression.( 17 , 18 , 19 ) Thus, mPAS staining might be a useful biomarker to predict mutation status in colonic crypts, although histological analysis of many colonic crypts with mPAS staining is rather difficult in practice.
In the present study, the frequencies of mPAS‐positive (mutated) crypts and foci were both significantly higher (approximately three times more) on the left side (sigmoid colon and rectum) than on the right side (cecum and ascending colon). Furthermore, clusters of two or more mPAS‐positive crypts were also significantly higher (approximately 10 times more) in the left‐sided colon. Whereas Campbell et al. described no significant difference between the frequencies of wholly involved mPAS‐positive crypts in background mucosa of left‐sided and right‐sided colon cancers (median frequency, 10.0 × 10−4 vs 6.8 × 10−4, P = 0.4569),( 15 ) partially involved mPAS‐positive crypts were significantly more frequent in mucosa from the left‐sided cases (P < 0.05). Our results differ from those obtained in a study carried out in British patients but the assessment of mPAS‐positive crypts were carried out consistently by one pathologist, with the same method used by Campbell et al.( 15 ) As all of our samples were obtained from Japanese patients, the differences might have been caused by racial or environmental factors, including food intake and lifestyle.
Our results may help to explain why the prevalence of colorectal cancers in Japanese people is higher (approximately 3.3 times more) in the left‐sided colon (sigmoid colon and rectum) than in the right‐sided colon (cecum and ascending colon).( 20 ) It is in line with the fact that right‐sided colon cancers have a tendency for distinct clinicopathological features, including relatively more variable differentiation types, serrated tumor structures, high microsatellite instability and low incidence of p53 mutations, suggesting differences in carcinogenesis from left‐sided colon cancers.( 21 , 22 ) It has been suggested that chronic exposure to carcinogenic substance might be more important for left‐sided colorectal carcinogenesis.( 23 )
The frequencies of mPAS‐positive crypts and foci of mPAS‐positive crypts in diverticulosis patients without colorectal cancers showed basically the same tendencies as for colorectal cancer cases. It was confirmed that mPAS‐positive crypts and foci increase significantly with age in the left‐sided colon with diverticulosis, although the available sample numbers were relatively small. Furthermore, no significant differences were revealed between colorectal cancer cases and diverticulosis cases by comparison of two regression slopes with age. The frequencies of mPAS‐positive crypts and foci in patients aged 40–60 years were significantly higher in the left‐sided than in the right‐sided colon with diverticulosis, similar to the colorectal cancer cases, although with comparatively lower values.
In conclusion, the present study demonstrated a significant increase in the number of wholly mPAS‐positive (somatic‐mutated) crypts in heterozygotes with age, indicative of accumulation of genetic mutations. This is in line with an increase in colorectal carcinoma development in the elderly.
Acknowledgments
This work was supported in part by Grants‐in‐Aid for Scientific Research from Kitasato University Graduate School of Medical Sciences, from the Ministry of Education, Culture, Sports, Science and Technology, Japan (No. 13214093) and from the Uehara Memorial Foundation.
References
- 1. Benson DD, Mitchell N, Dix D. On the role of aging in carcinogenesis. Mutat Res 1996; 356: 209–16. [DOI] [PubMed] [Google Scholar]
- 2. Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with ageing. Nature 1990; 346: 866–8. [DOI] [PubMed] [Google Scholar]
- 3. Issa JP, Ottaviano L, Celano P, Hamilton SR, Davidson NE, Baylin SB. Methylation of the oestrogen receptor CpG island links ageing and neoplasia in human colon. Nature Genet 1994; 7: 536–40. [DOI] [PubMed] [Google Scholar]
- 4. Vogelstein B, Fearon ER, Hamilton SR et al. Genetic alterations during colorectal‐tumor development. N Engl J Med 1988; 319: 525–32. [DOI] [PubMed] [Google Scholar]
- 5. Lipkin M. Biomarkers of increased susceptibility to gastrointestinal cancer. Their development and application to studies of cancer prevention. Gastroenterology 1987; 92: 1083–6. [DOI] [PubMed] [Google Scholar]
- 6. Holt P, Yeh KY. Colonic proliferation is increased in senescent rats. Gastroenterology 1988; 95: 1556–63. [DOI] [PubMed] [Google Scholar]
- 7. Fuller CE, Davies RP, Williams GT, Williams ED. Crypt restricted heterogeneity of goblet cell mucus glycoprotein in histologically normal human colonic mucosa: a potential marker of somatic mutation. Br J Cancer 1990; 61: 382–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campbell F, Appleton MAC, Fuller CE et al. Racial variation in the O‐acetylation phenotype of human colonic mucosa. J Pathol 1994; 174: 169–74. [DOI] [PubMed] [Google Scholar]
- 9. Winton DJ, Gooderham NJ, Boobis AR, Davies DS, Ponder BA. Mutagenesis of mouse intestine in vivo using the Dlb‐1 specific locus test: studies with 1,2‐dimethylhydrazine, dimethylnitrosamine, and the dietary mutagen 2‐amino‐3,8‐dimethylimidazo[4,5‐f]1uinoxaline. Cancer Res 1990; 50: 7992–6. [PubMed] [Google Scholar]
- 10. Griffiths DF, Davies SJ, Williams D, Williams GT, Williams ED. Demonstration of somatic mutation and colonic crypt clonality by X‐linked enzyme histochemistry. Nature 1988; 333: 461–3. [DOI] [PubMed] [Google Scholar]
- 11. Campbell F, Fuller CE, Williams GT, Williams ED. Human colonic stem cell mutation frequency with and without irradiation. J Pathol 1994; 174: 175–82. [DOI] [PubMed] [Google Scholar]
- 12. Campbell F, Williams GT, Appleton MAC, Dixon MF, Harris M, Williams ED. Post‐irradiation somatic mutation and clonal stabilization time in the human colon. Gut 1996; 39: 569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Okayasu I, Hana K, Yoshida T, Mikami T, Kanno J, Fujiwara M. Significant increase of colonic mutated crypts in ulcerative colitis correlatively with duration of illness. Cancer Res 2002; 62: 2236–8. [PubMed] [Google Scholar]
- 14. Veh RW, Meessen D, Kuntz D, May B. A new method for histochemical demonstration of side chain substituted sialic acids. In: Malt RA, Willamson RCN, eds. Colonic Carcinogenesis. Lancaster: MTP, 1982; 355–65. [Google Scholar]
- 15. Campbell F, Appleton MA, Shields CJ, Williams GT. No difference in stem cell somatic mutation between the background mucosa of right‐ and left‐sided sporadic colorectal carcinomas. J Pathol 1998; 186: 31–5. [DOI] [PubMed] [Google Scholar]
- 16. Japanese Ministry of Health and Welfare. Fifth Report of Statistics of Malignant Neoplasm in Japan (Supervized ed. Masuzoe K). Tokyo: Sougouigakusha, 1990; 19–43. [Google Scholar]
- 17. Thun MJ, Namboodiri MM, Heath CW Jr. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med 1991; 325: 1593–6. [DOI] [PubMed] [Google Scholar]
- 18. Giardiello FM, Yang VW, Hylind LM et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N Engl J Med 2002; 346: 1054–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oshima M, Dinchuk JE, Kargman SL et al. Suppression of intestinal polyposis in Apcdelta716 knockout mice by inhibition of cyclooxygenase 2 (COX‐2). Cell 1996; 87: 803–9. [DOI] [PubMed] [Google Scholar]
- 20. Japanese Society for Cancer of the Colon and Rectum . Multi‐Institutional Registry of Large Bowel Cancer in Japan, Vol. 24. Utsunomiya, Japan: Matsui PTO, 2003; 9–21. [Google Scholar]
- 21. Miyakura Y, Sugano K, Konishi F et al. Extensive methylation of hMLH1 promoter region predominates in proximal colon cancer with microsatellite instability. Gastroenterology 2001; 121: 1300–9. [DOI] [PubMed] [Google Scholar]
- 22. Tou SIH, Drye ER, Boulos PB, Hollingsworth SJ. Activity (transcription) of the genes for MLH1, MSH2 and p53 in sporadic colorectal tumours with micro‐satellite instability. Br J Cancer 2004; 90: 2006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nagengast FM, Van Der Werf SD, Lamers HL, Hectors MP, Buys WC, Van Tongeren JM. Influence of age, intestinal transit time, and dietary composition on fecal bile acid profiles in healthy subjects. Dig Dis Sci 1988; 33: 673–8. [DOI] [PubMed] [Google Scholar]


