Abstract
Although S100A4 expression has reportedly been associated with metastasis of various malignancies, little is known about its biological significance in ovarian carcinomas. In this study, we investigated expression and secretion of S100A4 and its extracellular function in ovarian carcinoma cells. We first used immunohistochemistry to examine the expression and localization of S100A4 in 113 epithelial ovarian neoplasms (24 benign, 20 borderline, and 69 malignant tumors) and analyzed its prognostic significance in patients with ovarian carcinoma. Then we investigated the expression, subcellular localization, and secretion of S100A4 in four ovarian carcinoma cell lines. Finally, we examined the effect of S100A4 treatment on the cell proliferation and invasiveness of ovarian carcinoma cells, along with activation of small GTPase, RhoA. Both cytoplasmic and nuclear expressions of S100A4 were significantly stronger in carcinomas than those in benign and borderline tumors. Ovarian carcinoma patients with strong nuclear S100A4 expression showed a significantly shorter survival than those without (P = 0.0045). This was not the case for cytoplasmic S100A4 expression. Ovarian carcinoma cell lines were shown to express S100A4, and secrete S100A4 into the culture media. Treatment with recombinant S100A4 resulted in the upregulation of S100A4 expression, translocation of S100A4 into the nucleus, and enhancement of invasiveness, which was associated with the upregulation of small GTPase, RhoA. These findings suggest that the nuclear expression of S100A4 is involved in the aggressive behavior of ovarian carcinoma and S100A4 is an autocrine/paracrine factor that plays an important role in the aggressiveness of ovarian carcinoma cells. (Cancer Sci 2006; 97: 1061–1069)
Abbreviations:
- FIGO
International Federation of Gynecology and Obstetrics
- GAPDH
glyceraldehyde‐3‐phosphate dehydrogenase
- GTP
guanosine triphosphate
- OSE
ovarian surface epithelial
- RT‐PCR
reverse transcription–polymerase chain reaction.
Epithelial ovarian carcinoma is the leading cause of death in female genital malignancies, and more than half of the patients are diagnosed at the advanced stage of disease.( 1 ) The poor prognosis in patients with ovarian carcinoma is most likely related to the degree of peritoneal dissemination of cancer cells. The process of peritoneal dissemination is reportedly affected by a variety of gene products.( 2 , 3 , 4 , 5 , 6 , 7 , 8 ) However, little is known about the molecular aspects of the migration and invasion of ovarian carcinoma cells. Recent attention has focused on the intracellular molecules involved in the enhancement of motility and invasiveness of cancer cells, and we previously showed that upregulation and activation of a small GTPase, RhoA, plays an important role in the tumor progression of ovarian carcinoma in vivo and in vitro.( 9 )
The S100A4 (also known as mts‐l/metastasin/pEL98/p9ka) protein belongs to the S100 protein family, which consists of 21 small, acidic, calcium‐binding proteins with two common EF‐hand structure motifs.( 10 ) S100A4 has been identified so far as a cytoplasmic protein that cosediments with cytoskeletal proteins such as actin, non‐muscle myosin, and non‐muscle tropomyosin, implying a possible role in cell motility and invasion.( 11 ) Its relevance to the invasive or metastatic phenotype of cancer cells was shown by data from gene transfection,( 12 ) and from transgenic animals.( 13 ) S100A4 protein might also have functions in cell cycle progression.( 14 ) Increased expression of S100A4 protein has been reported in various human cancers.( 15 ) Interestingly, it has also been reported that some cells secrete S100 protein which might have extracellular functions, that is, extracellular S100A4 was reported to induce the outgrowth of hippocampal neurons,( 16 ) or stimulate the capillary formation of endothelial cells.( 17 ) Furthermore, a number of S100 proteins have been reported to translocate between the cytoplasm and the nucleus,( 18 ) although their nuclear function is largely unknown.
In human epithelial ovarian neoplasms, however, there have been no available data on the expression and subcellular localization of S100A4. Secretion of S100A4 and its extracellular function have not been analyzed in ovarian carcinoma cells. In the present study therefore we examined the expression and localization of S100A4 in various epithelial ovarian tumors using immunohistochemistry, its subcellular localization by Western blotting, and analyzed its prognostic significance in patients with ovarian carcinoma. Then, we investigated the expression, subcellular localization, and secretion of S100A4 in ovarian carcinoma cells in vitro. Finally, we examined the effect of S100A4 treatment on the cell proliferation and invasiveness of ovarian carcinoma cells, along with activation of small GTPase, RhoA.
Materials and Methods
Patients and tissue samples. A total of 113 primary epithelial ovarian tumors were examined for immunohistochemistry. Sixty‐nine patients with ovarian carcinoma visited Shinshu University Hospital (Nagano, Japan) between 1993 and 1999, and underwent surgery followed by cisplatin‐based chemotherapy. The follow‐up period ranged from 5 to 114 months (median: 47.3 months). According to the classification of FIGO, 33 carcinomas were classified as stage I, 10 were stage II, 21 were stage III, and 5 were stage IV. Histologically, 25 were serous, 5 mucinous, 22 clear cell and 17 endometrioid adenocarcinomas. With regard to histological grade,( 19 ) 31 were graded as G1, 27 were G2, and 11 were G3. In 24 of the 69 cases, the specimens of peritoneal dissemination were available and also examined for immunohistochemistry. In addition, 24 cases of benign cystadenomas (4 serous, 20 mucinous) and 20 cases of borderline malignant tumors (8 serous, 12 mucinous) were selected from the pathology file of Shinshu University Hospital. Specimens were reviewed to confirm the histopathological diagnosis using the standard criteria.( 20 ) These specimens were fixed in 10% phosphate‐buffered formalin and embedded in paraffin. Serial sections of 3 µm thickness were made for hematoxylin–eosin staining and immunohistochemistry. Some of the fresh tissue blocks from the excised ovarian carcinoma were stored at −80°C for Western blotting. Each tissue sample was used with the approval of the Ethics Committee of Shinshu University School of Medicine.
Immunohistochemical analysis. Immunohistochemical staining was carried out by the streptavidin–biotin–peroxidase complex method using a Histofine SAB‐PO kit (Nichirei, Tokyo, Japan). Three micrometer‐thick sections were deparaffinized and boiled in 0.01 M citrate buffer (pH 6.0) for 25 min in a microwave oven. They were then treated with 0.3% hydrogen peroxide and incubated with 10% normal mouse serum. The sections were incubated with an anti‐S100A4 rabbit polyclonal antibody (Dako, Glostrup, Denmark), which was used at a dilution of 1 : 50, at 4°C overnight. After washing in phosphate‐buffered saline, they were incubated with biotinylated goat antirabbit immunoglobulin G, followed by treatment with peroxidase‐conjugated streptavidin and stained with diaminobenzidine and 0.15% hydrogen peroxidase. Counterstaining was done with hematoxylin. Immunoreactive tumor cells were semiquantitatively estimated. Cytoplasmic staining was classified as: (–) negative, 0–10% positive cells; (+) weakly positive, 10–50% positive cells; and (+ +) strongly positive, more than 50% positive cells, by comparison with the positive internal control, that is, lymphocytes and vascular smooth muscle cells were positive for S100A4 in the cytoplasm.( 21 , 22 ) Nuclear staining was defined as: (–) negative, 0–5% positive cells; (+) weakly positive, 5–20% positive cells; and (+ +) strongly positive, more than 20% positive cells. The evaluation of immunostaining was carried out by two independent observers (N.K. and A.H.) unaware of the fate of the patient or the tissue site.
Survival analysis. The log‐rank test and Cox proportional hazards model were used to evaluate significant predictors of survival. The prognostic factors used in the survival analysis were as follows; FIGO stage, histological grade, results of S100A4 immunostaining. The log–rank test and Cox's univariate analysis were first carried out on each of the factors (PHREG procedure). Overall survival was then analyzed by the stepwise regression model using variables that showed significance by univariate analysis. Cumulative survival was also analyzed by the Kaplan–Meier method. Differences were considered to be significant at P < 0.05.
Preparation of nuclear and cytoplasmic protein fractions. Frozen ovarian carcinoma tissues were homogenized by a tissue homogenizer. Subfractioning of cell components was done using NE‐PER Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Rockford, IL) according to the manufacturer's protocol.
Cell culture. The ovarian cancer cell lines SKOV3 and OVCAR3 were purchased from the American Type Culture Collection (Rockville, MD). The ovarian cancer cell lines A2780 and A2780/CDDP (a cisplatin‐resistant cell line derived from A2780) were kind gifts from Dr Takashi Tsuruo (Cancer Chemotherapy Center, Tokyo, Japan),( 23 ) with the permission of Dr Thomas C. Hamilton (Fox Chase Cancer Institute, Philadelphia, PA). A2780, A2780/CDDP, and OVCAR3 were maintained in RPMI‐1640 (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (Biomeda, Foster City, CA). SKOV3 was cultured in Dulbecco's modified Eagle's medium (Sigma) with 10% fetal bovine serum. Incubation was carried out at 37°C under 5% CO2 in air.
OSE cells were obtained from three women who were treated surgically for benign gynecologic disease, after obtaining written consent from each patient. Cell culture was carried out as described previously.( 3 )
Treatment with recombinant S100A4. Human recombinant S100A4 protein was produced from human cDNA and kindly provided by Dr C.W. Heizmann (Division of Clinical Chemistry, Department of Pediatrics, University of Zurich, Switzerland). Recombinant A100A4 was added to the serum‐free culture media at a concentration of 10−14, 10−12 and 10−10 M.
Immunofluorescence staining. Cultured cells in a two‐well Laboratory‐Tek Chamber Slide were fixed in cold acetone for 15 min, then 0.3% hydrogen peroxide was applied to block endogenous peroxide activity, and the cells were incubated with normal mouse serum. The cells were incubated with an anti‐S100A4 antibody at room temperature for 1 h, and fluorescein‐isothiocyanate‐labeled goat antirabbit immunoglobulin G was used. Propidium iodide (Calbiochem, San Diego, CA) was used to stain DNA. All specimens were examined using a Laser Scanning Spectral Confocal Microscope (Leica TCS SP2; Leica Microsystems, Wetzlar, Germany).
Immunoprecipitation for secreted S100A4. The S100A4 secretion was examined using a Protein A Immunoprecipitation Kit (Kirkegaard & Perry Laboratories, Gaithersburg, MD). After cells were cultured for 24 h, 1 mL of culture medium was collected and incubated with 1 µg of anti‐S100A4 antibody overnight. Protein A agarose was added and the mixture was incubated at room temparature for 1 h. After heating the sample at 95°C for 2 min, it was centrifuged at 13 000 g for 20 min at 4°C and the supernatants were stored at −80°C.
Western blot analysis. Extracts equivalent to 30 µg of total protein were separated by sodium dodecylsulfate–polyacrylamide gel electrophoresis (12% acrylamide) and transferred onto nitrocellulose membranes (Hybond‐C super; Amersham Biosciences, Piscataway, NJ) as described previously.( 9 ) The first antibodies against S100A4, RhoA (Santa Cruz Biotechnology, Santa Cruz, CA), MT1‐MMP (Sigma) or β‐actin (BioMakor, Rehovot, Israel) were used.
RT‐PCR. Total RNA was extracted by the acid guanidinium–phenol–chloroform method.( 24 ) One microgram of total RNA was treated with 1 U/10 µL DNase I (Life Technologies, Gaithersburg, MD). RT‐PCR was carried out using an RNA PCR Kit (Takara Shuzo, Otsu, Japan) as described previously.( 3 , 9 ) Primers were synthesized to encompass a specific segment of the cDNA sequence of the S100A4 (sense, 5′‐agcttcttggggaaaaggac‐3′ and antisense, 5′‐ccccaaccacatcagagg‐3′), RhoA (sense, 5′‐ctggtgattgttggtgatgg‐3′ and antisense, 5′‐gcgatcataatcttcctgcc‐3′), or of GAPDH (sense, 5′‐acgaccactttgtcaagctc‐3′ and antisense, 5′‐ggtctacatggcaactgtga‐3′). The corresponding cDNA fragments were denatured at 94°C for 30 s, annealed at 58°C for 1 min, and extended at 72°C for 1 min. After 35 cycles of amplification, the PCR products were analyzed on a 2% agarose gel, and the bands were visualized using ethidium bromide.
Cell proliferation assay. Cell number and viability were further assessed by using the reagent WST‐1 (Roche, Indianapolis, IN). Cells under different treatments (n = 32 of each group) were plated at 1 × 104 cells/0.32 cm2 in a 96‐well plate and maintained for 48 h at 37°C with 5% CO2. This was followed by incubation with WST‐1 reagent for 4 h. After thorough shaking, each sample was measured at a wavelength of 450 nm with Multiscan JX (Thermo Labsystems, Vantee, Finland). Absorbance readings were normalized against control wells with medium alone.
In vitro invasion assay. Cell invasion through a reconstituted basement membrane (Matrigel; BD Biosciences, Bedford, MA), was assayed by a method reported previously.( 9 , 25 ) After 22 h of incubation under different treatments, serum‐free medium with 0.1% bovine serum albumin as control, recombinant S100A4, cultured medium of A2780/CDDP, C3 exoenzyme (Alexis Biochemicals, Lausen, Switzerland) and Y27632 (Calbiochem), the invaded cells were counted in 10 different fields under a light microscope at ×400 magnification. Each experiment was done in triplicate wells and repeated three times.
Migration assay. Cell migration was examined using a monolayer wounding system as previously described.( 26 )
Rho‐GTP pull‐down assay. The Rho‐GTP pull‐down assay was carried out using a Rho Activation Assay Biochem Kit (Cytoskeleton, Denver, CO). In brief, all cells were washed with ice‐cold phosphate‐buffered saline and lyzed with lysis buffer. After being centrifuged at 8500 g for 5 min, Rhotekin (Rho effector)–Rho binding domain protein, which has high affinity for activated GTP‐Rho, bound to glutathione–agarose was added to the cell lysate and incubated for 1 h at 4°C on a rocker. After centrifugation, Rho‐GTP bound to beads was washed with lysis buffer and eluted in Laemmli sample buffer. Proteins were analyzed by Western blotting using antibodies against RhoA.
Statistical analyses. Statistical analysis was carried out with the Kruskal–Wallis rank test, Scheffe and Mann–Whitney's U‐test were used to compare the expression in immunohistochemistry.
Results
Expression of S100A4 in the cytoplasm and nuclei is increased in ovarian carcinomas compared with benign/borderline tumors. Representative profiles of immunostaining for S100A4 are shown in Figure 1. Specific staining for S100A4 was commonly observed in the cytoplasm. However, in some cases, both cytoplasmic and nuclear staining were observed. Correlations between S100A4 immunostaining and clinicopathological findings are summarized in Tables 1 and 2.
Figure 1.
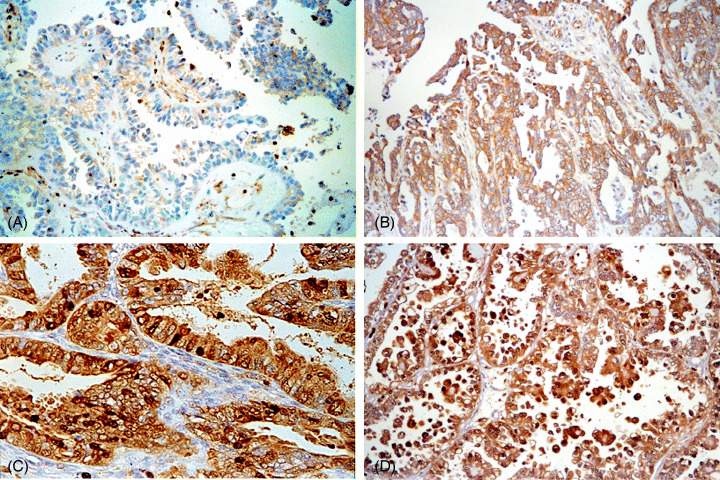
Immunohistochemical staining of S100A4 in various epithelial ovarian tumors. (A) Serous borderline tumor negative for S100A4 expression. (B) Serous carcinoma showing S100A4 positivity in the cytoplasm. (C) Endometrioid carcinoma showing S100A4 positivity both in the cytoplasm and in the nucleus. (D) Clear cell carcinoma showing S100A4 positivity both in the cytoplasm and in the nucleus (magnification: ×100).
Table 1.
Immunohistochemical cytoplasmic expression of S100A4 in epithelial ovarian neoplasms
| Total number of cases | Cytoplasmic staining | |||
|---|---|---|---|---|
| − | + | + + | ||
| Benign cystadenomas | 24 | 19(79%) | 1(4%) | 4(17%) |
| Serous | 4 | 0 | 1 | 3 |
| Mucinous | 20 | 19 | 0 | 1 |
| Borderline tumors | 20 | 8(40%) | 1(5%) | 11(55%) |
| Serous | 8 | 3 | 0 | 5 |
| Mucinous | 12 | 5 | 1 | 6 |
| Carcinomas | 69 | 5(7%) | 13(19%) | 51(74%) |
| FIGO stage | ||||
| I | 33 | 4 | 7 | 22 |
| II | 10 | 1 | 3 | 6 |
| III | 21 | 0 | 1 | 20 |
| IV | 5 | 0 | 2 | 3 |
| Histological type | ||||
| Serous | 25 | 0 | 3 | 22 |
| Mucinous | 5 | 0 | 1 | 4 |
| Endometrioid | 17 | 3 | 4 | 10 |
| Clear cell | 22 | 2 | 5 | 15 |
| Histological grade | ||||
| G1 | 31 | 2 | 3 | 26 |
| G2 | 27 | 2 | 9 | 16 |
| G3 | 11 | 1 | 1 | 9 |
–, 0–10% positive cells; +, 10–50% positive cells; + +, more than 50% positive cells.
Table 2.
Immunohistochemical nuclear expression of S100A4 in epithelial ovarian neoplasms
| Total number of cases | Nuclear staining | |||
|---|---|---|---|---|
| – | + | + + | ||
| Benign cystadenomas | 24 | 24(100%) | 0(0%) | 0(0%) |
| Serous | 4 | 4 | 0 | 0 |
| Mucinous | 20 | 20 | 0 | 0 |
| Borderline tumors | 20 | 17(85%) | 0(0%) | 3(15%) |
| Serous | 8 | 6 | 0 | 2 |
| Mucinous | 12 | 11 | 0 | 1 |
| Carcinomas | 69 | 34(49%) | 18(26%) | 17(25%) |
| FIGO stage | ||||
| I | 33 | 20 | 8 | 5 |
| II | 10 | 5 | 2 | 3 |
| III | 21 | 6 | 8 | 7 |
| IV | 5 | 3 | 0 | 2 |
| Histological type | ||||
| Serous | 25 | 11 | 6 | 8 |
| Mucinous | 5 | 1 | 3 | 1 |
| Endometrioid | 17 | 9 | 4 | 4 |
| Clear cell | 22 | 13 | 5 | 4 |
| Histological grade | ||||
| G1 | 31 | 17 | 7 | 7 |
| G2 | 27 | 13 | 8 | 6 |
| G3 | 11 | 4 | 3 | 4 |
–, 0–10% positive cells; +, 10–20% positive cells; + +, more than 20% positive cells.
The cytoplasmic S100A4 expression was significantly stronger in ovarian carcinomas than in benign (P < 0.0001) and borderline tumors (P = 0.0288). According to the FIGO stage classification, although the number of cases strongly positive for cytoplasmic expression was higher in stage III/IV (88%) than in stage I/II (65%) (P = 0.0672), there was no significant difference. The cytoplasmic expression of S100A4 in the metastatic lesion was compared to the respective primary lesion in 24 cases; it was increased or similar in 18 (75%) cases, and decreased in the remaining six cases.
Nuclear immunostaining strongly positive for S100A4 was observed in none of the 24 benign cystadenomas, 3 (15%) of the 20 borderline tumors, and 17 (25%) of the 69 ovarian carcinomas (Fig. 1c,d and Table 2). The rate of nuclear strong positivity was significantly higher in carcinoma than in benign (P < 0.0001) and borderline tumors (P = 0.0154). There was no significant difference according to the histological type, the FIGO stage or histological grade. The nuclear expression of S100A4 in the metastatic lesion was compared to the respective primary lesion in 24 cases; it was increased or similar in 14 (58%) cases, and decreased in the remaining 10 cases.
Elevated expression of S100A4 in the nuclei is correlated with poor prognosis of ovarian carcinoma patients. All 20 patients with borderline tumors were alive with no evidence of recurrence at the last follow‐up. Of the 69 patients with ovarian carcinoma, 25 had died of disease. Of the remaining 44 patients, 43 were alive and one had died of another disease. The prognosis was significantly poorer in patients with advanced FIGO stages (overall survival: stage I + II, 58.4 ± 27.1 months versus stage III + IV, 22.8 ± 22.6 months, P < 0.0001), in patients with high grade (G1, 52.3 ± 28.1 months versus G2 + G3, 43.1 ± 29.7 months, P = 0.0218). In patients of all stages, the prognosis was significantly poorer in patients with strong nuclear expression of S100A4 (strongly positive, 39.1 ± 30.1 months versus weakly positive/negative, 49.8 ± 28.7 months, P = 0.0045) (Fig. 2a). This was not the case for cytoplasmic S100A4 expression. Interestingly, among the patients with FIGO stage I + II, the prognosis was significantly poorer in those with strong nuclear positivity for S100A4 (strongly positive, 43.1 ± 31.2 months versus weakly positive/negative, 61.6 ± 25.5 months, P < 0.0001) (Fig. 2A), but was not statistically significant in those with stage III + IV (strongly positive, 35.6 ± 30.7 months versus weakly positive/negative, 25.4 ± 17.5 months, P = 0.7559). Multivariate analysis in stage I + II cases also showed that strong nuclear expression of S100A4 was an independent prognostic factor (P = 0.0009).
Figure 2.
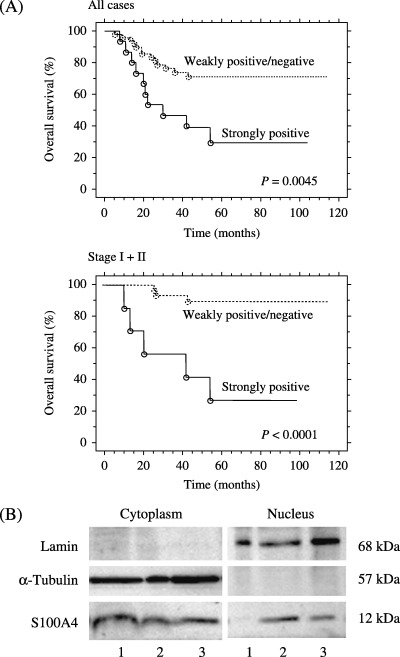
(A) Overall survival of ovarian carcinoma patients according to the nuclear S100A4 expression. Kaplan–Meier analysis shows significantly poorer survival in patients with strong nuclear S100A4 expression compared to those with weakly positive/negative nuclear S100A4 expression. (B) Western blot analysis of cytoplasmic and nuclear fractions obtained from three carcinoma tissues. Lane 1, serous carcinoma shown in Figure 1b; lane 2, endometrioid carcinoma shown in Figure 1C; lane 3, clear cell carcinoma shown in Figure 1D. The presence of S100A4 not only in the cytoplasmic but also in the nuclear fraction was observed in both cases (lanes 2 and 3) that showed nuclear staining of S100A4 (Fig. 1C,D). Lamin B and α‐tubulin were used as the markers for nuclear and cytoplasmic fractions, respectively.
To examine whether S100A4 protein is actually present in the nuclei of tumor cells, nuclear and cytoplasmic protein fractions were obtained from the three carcinoma cases, a serous carcinoma (Fig. 1B), an endometrioid carcinoma (Fig. 1C) and a clear cell carcinoma (Fig. 1D). In both cases that showed nuclear staining of S100A4 (Fig. 1C,D), the band for S100A4 was observed not only in the cytoplasmic compartment, but also in the nuclear compartment (Fig. 2B). These results confirmed that S100A4 protein existed in the nuclei of carcinoma cells.
S100A4 is expressed in ovarian carcinoma cells, localized either in the cytoplasm or in the nuclei, and secreted into culture media. Expression of S100A4 in the four ovarian carcinoma cell lines and normal OSE cells at the mRNA and protein levels was investigated with RT‐PCR and Western blot. RT‐PCR showed that an intense band observed at 200 bp for S100A4 was also observed in three (A2780, A2780/CDDP and SKOV3) of four ovarian carcinoma cells (Fig. 3A: a, lanes 2–4). In contrast, a band for S100A4 was not obtained in normal OSE cells (Fig. 3A: a, lane 1) or in OVCAR3 cells (Fig. 3A: a, lane 5). Western blot analysis revealed an intense band at 12 kDa for S100A4 was evident in three of four ovarian carcinoma cell lines (Fig. 3A: b, lanes 2–4). The specific band was not observed in normal OSE (Fig. 3A: b, lane 1) or OVCAR3 cells (Fig. 3A: b, lane 5), whereas the 42 kDa band for β‐actin was observed in all of the cell samples. Thus, the expression of S100A4 at mRNA and protein levels was shown in ovarian carcinoma cells such as A2780, A2780/CDDP and SKOV3, except for OVCAR3. In contrast, normal OSE cells did not express S100A4.
Figure 3.
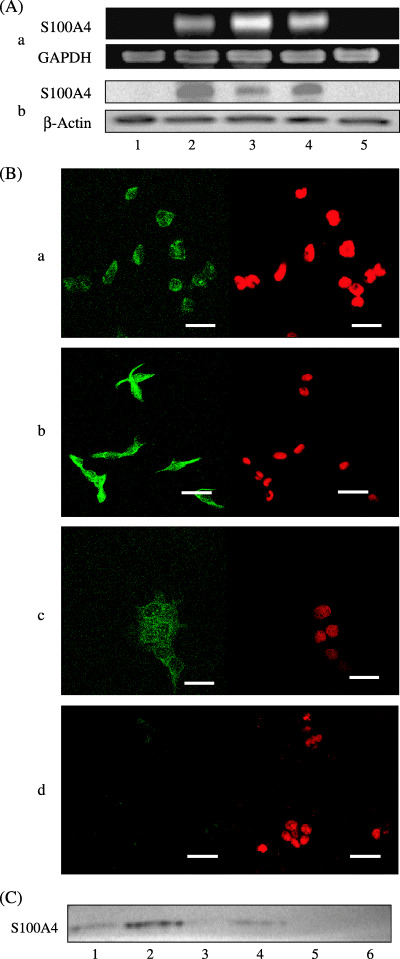
(A) RT‐PCR (a) and Western blot (b) analyses for the expression of S100A4 in normal OSE cells (1) and ovarian carcinoma cells (2, A2780; 3, A2780/CDDP; 4, SKOV3; 5, OVCAR3). S100A4 expressions at both mRNA and protein levels are observed in three ovarian carcinoma cells, but not in normal OSE or ovarian carcinoma OVCAR3 cells. (B) Immunofluorescent analysis for the subcellular localization of S100A4 in ovarian carcinoma cells. A2780 (a) and A2780/CDDP (b) cells show both cytoplasmic and nuclear expressions, whereas SKOV3 cells show only cytoplasmic expression (c) and OVCAR3 cells show faint cytoplasmic expression (d). Left panel, S100A4 staining; right panel, propidium iodide staining. Bar, 40 µm. (C) Western blot analysis for S100A4 in the culture media from ovarian carcinoma cells (lane 1, A2780; lane 2, A2780/CDDP; lane 3, OVCAR3; lane 4, SKOV3) and culture medium without cells (lane 5, RPMI‐1640; lane 6, Dulbecco's modified Eagle's medium). Secretion of S100A4 into the culture media is confirmed in A2780, A2780/CDDP, and SKOV3 cells, but not in OVCAR3 cells.
Immunofluorescent analysis showed that specific staining for S100A4 was identified as a green signal in the nucleus and/or cytoplasm. In A2780 and A2780/CDDP cells, diffuse staining of S100A4 both in the cytoplasm and in the nucleus was observed (Fig. 3B: a,b). In SKOV3 cells, diffuse staining of S100A4 was observed only in the cytoplasm (Fig. 3B: c). In contrast, the expression of S100A4 was faint in OVCAR3 cells (Fig. 3B: d). These results were consistent with our data from Western blotting.
The existence of S100A4 in cultured media was investigated. Specific 12 kDa bands for S100A4 were detected in the cultured media from A2780, A2780/CDDP and SKOV3 (Fig. 3C, lanes 1, 2 and 4), all of which expressed S100A4. In contrast, S100A4 was not detected in culture medium from OVCAR3 (Fig. 3C, lane 3). These results confirmed that S100A4‐positive ovarian carcinoma cells secrete S100A4 protein into cultured medium.
S100A4 treatment induces upregulation of S100A4 along with its translocation into the nuclei. Next, we investigated the effect of extracellular S100A4 in ovarian carcinoma cells. In SKOV3 and OVCAR3, the intensity of the band for S100A4 mRNA was increased by treatment with recombinant S100A4 (Fig. 4A: a). The band for S100A4 protein was also increased by S100A4 treatment (Fig. 4A: b). Such an increase in the S100A4 expression at both the mRNA and protein levels occurred 2 h after addition of S100A4. The peak of the increase in the mRNA and protein expression was noted at 12 h after the S100A4 treatment (Fig. 4A: a,b).
Figure 4.
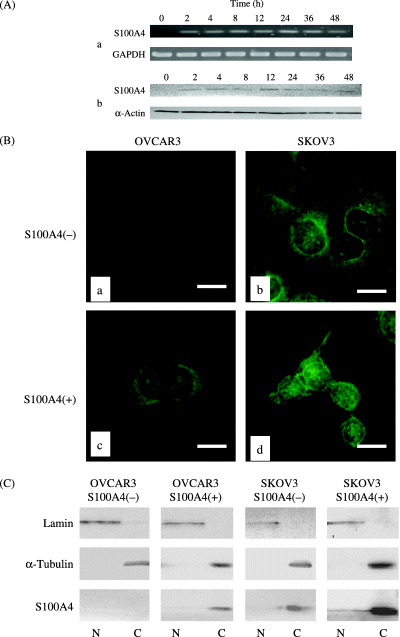
(A) RT‐PCR (a) and Western blot (b) analyses for the expression of S100A4 in ovarian carcinoma OVCAR3 cells after treatment with recombinant S100A4. Expression of S100A4 at both mRNA and protein levels occurred 2 h, and peaked at 12 h, after the treatment. (B) Immunofluorescent analysis for the subcellular localization of S100A4 showing SKOV3 cells (a) and OVCAR3 cells (b) without S100A4 treatment, and SKOV3 cells (c) and OVCAR3 cells (d) with S100A4 treatment. After the S100A4 treatment, nuclear expression of S100A4 appeared in SKOV3 cells (c), and cytoplasmic expression increased in OVCAR3 cells (d). Bar, 20 µm. (C) Western blot analysis of cytoplasmic and nuclear fractions showing the S100A4 treatment increased cytoplasmic expression in both SKOV3 and OVCAR3 cells. Moreover, the presence of S100A4 in the nuclear fraction was observed in SKOV3 cells after S100A4 treatment. C, cytoplasmic fractions; N, nuclear fractions.
We also analyzed the change in the subcellular localization of S100A4 in SKOV3 and OVCAR3 cells using immunofluorescence staining. Without addition of recombinant of S100A4, expression of S100A4 was observed only in cytoplasm in SKOV3 cells (Fig. 4B: b), however, nuclear expression of S100A4 appeared after the addition of recombinant S100A4 (Fig. 4B: d), indicating the translocation of S100A4 from the cytoplasm into the nucleus. In OVCAR3 cells, although S100A4 treatment increased the cytoplasmic expression of S100A4, its nuclear expression was not observed (Fig. 4B: c). To confirm the subcellular expression pattern of S100A4, the nuclear and cytoplasmic protein fractions were obtained from SKOV3 with or without addition of recombinant S100A4. Western blotting revealed that cytoplasmic S100A4 expression was increased in both cell lines and the nuclear compartments were induced in SKOV3 cells after the S100A4 treatment (Fig. 4C).
S100A4 treatment does not alter the cell proliferation, but enhances the invasiveness of ovarian cancer cells along with upregulation and activation of RhoA. We then analyzed the effect of extracellular S100A4 on the cell proliferation and invasiveness in two ovarian cancer cell lines, SKOV3 and OVCAR3. A WST‐1 assay revealed that treatment with recombinant S100A4 did not change the number of viable cells in either cell line (data not shown). A Matrigel invasion assay showed that treatment with recombinant S100A4 significantly stimulated the in vitro invasiveness in both cell lines in a dose‐dependent manner (Fig. 5A,B). As the results from Matrigel invasion assay present the sum of proteolysis and migration, we further investigated MT1‐MMP activity, according to a previous report,( 27 ) and scratch assay. Western blot analysis for MT1‐MMP revealed that the treatment with recombinant S100A4 increased the active form of MT1‐MMP (Fig. 5C) in OVCAR3 cells. In addition, migration of OVCAR3 cells was also stimulated by treatment with recombinant S100A4 (Fig. 5D). Treatment with conditioned media from A2780/CDDP, which actively secrete S100A4, also enhanced the invasiveness of SKOV3 and OVCAR3 cells (Fig. 6).
Figure 5.
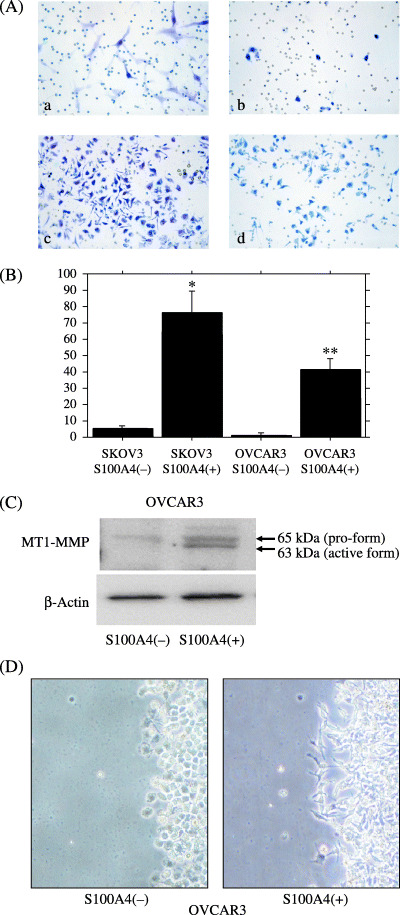
(A) Matrigel invasion assay showing that treatment with recombinant S100A4 resulted in an increase in invasiveness in both SKOV3 cells and OVCAR3 cells. Microphotographs showing SKOV3 cells (a) and OVCAR3 cells (b) without S100A4 treatment, and SKOV3 cells (c) and OVCAR3 cells (d) with S100A4 treatment. (B) Count of invaded cells showing the significant increase in invasiveness after the S100A4 treatment. *P < 0.0001; **P < 0.0001. (C) Western blot analysis showing S100A4 treatment increased the active form of MT1‐MMP in OVCAR3 cells. (D) Cells were incubated in serum‐free RPMI‐1640 with or without 10−10 M recombinant S100A4 for 22 h. Scratch wound assay showed that S100A4 treatment induced the migration of carcinoma cells along with the changes in cell shape.
Figure 6.
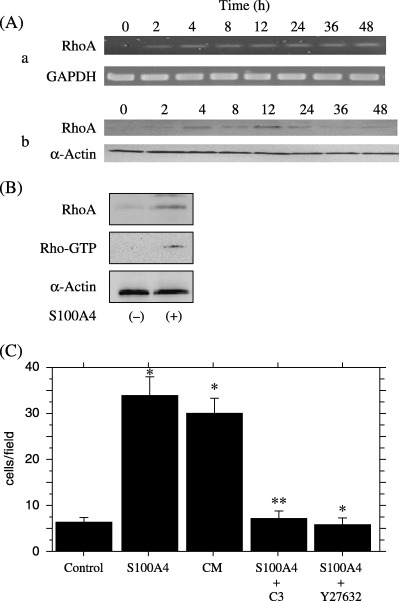
(A) RT‐PCR (a) and Western blot (b) analyses for the expression of RhoA in ovarian carcinoma OVCAR3 cells after the S100A4 treatment. Expression of RhoA is increased at both mRNA and protein levels after the S100A4 treatment. (B) Rho‐GTP pull‐down assay showing that the activated form of RhoA is increased after S100A4 treatment. (C) Matrigel invasion assay showing that invasiveness of OVCAR3 cells is also increased by treatment with conditioned media (CM) from A2780/CDDP containing S100A4. Increase in invasiveness by treatment with S100A4 or A2780/CDDP‐conditioned media is suppressed by simultaneous treatment with Rho inhibitors such as C3 exoenzyme and Y27632. *P < 0.0001; **P < 0.0001.
Finally, we investigated the role of small GTPase, RhoA, in the S100A4‐induced increase in invasiveness. The expression of RhoA at the mRNA and protein levels was increased by treatment with recombinant S100A4 (Fig. 6A: a,b). The peaks of their expressions were noted at 12 h after treatment, just after the peak of S100A4 expression. In addition, a Rho‐GTP pull‐down assay revealed that S100A4 treatment increased the amount of the GTP‐binding form of RhoA, that is, activation of RhoA occurred (Fig. 6B). To confirm the involvement of the Rho pathway in the stimulation of invasion under extracellular S100A4, we used C3 exoenzyme (a specific inhibitor of Rho) and Y27632 (Rho kinase inhibitor). When the SKOV3 cells were treated with C3 exoenzyme or A27632 for 22 h, the S100A4‐induced enhancement of cell invasion was completely suppressed (Fig. 6C).
Discussion
The present study showed that, among various epithelial ovarian neoplasms, the cytoplasmic and nuclear expressions of S100A4 obtained by immunohistochemistry were significantly stronger in invasive carcinomas than in benign cystadenomas. More interestingly, strong expression of S100A4 in the nucleus was an independent prognostic factor in patients with ovarian carcinoma. Kaplan–Meier analysis showed that patients with strong nuclear staining of S100A4 had a significantly shorter survival period compared to those without, especially in stage I/II disease. The preparation of cytoplasmic and nuclear fractions followed by Western blot analysis confirmed the presence of nuclear S100A4 protein in ovarian carcinoma tissues with nuclear immunostaining. Although the prognostic impact of cytoplasmic expression of S100A4 was reported in breast( 28 ) and gastric carcinoma,( 29 ) this is the first report showing the importance of nuclear S100A4 in the patient prognosis. Little is known about the prognostic indicators other than histological grade for early stage ovarian carcinomas, in which fertility‐sparing surgery is sometimes needed. Thus, development of a novel marker indicating aggressiveness in patients with early stage disease is needed in clinical practice. Further collection of such early stage ovarian carcinoma cases is needed to establish the prognostic significance of nuclear S100A4 expression.
Based on the in vivo data on S100A4 expression in ovarian carcinomas, we hypothesized that it plays an important role in the aggressive characteristics in ovarian carcinomas. To address this hypothesis, we tested in vitro S100A4 expression, secretion, and nuclear translocation, and its functional relevance to cell proliferation and invasiveness using four ovarian carcinoma cell lines, A2780, A2780/CDDP, SKOV3, and OVCAR3. RT‐PCR and Western blot analysis showed that ovarian carcinoma cells express S100A4 at the mRNA and protein levels, except for OVCAR3. Immunofluorescent analysis showed that nuclear localization of S100A4 was observed in A2780 and A2780/CDDP, but not in SKOV3. Interestingly, the difference in the expression and subcellular localization of S100A4 among the four cell lines paralleled the difference in aggressiveness in a nude mouse model of peritoneal dissemination; A2780 and A2780/CDDP were aggressive, SKOV3 was intermediate, and OVCAR3 was the least aggressive (A. Horiuchi et al, unpublished data, 2004). In addition, immunoprecipitation analysis showed that all of the cell lines except for OVCAR3 secreted S100A4 protein into the culture media. Furthermore, all four cell lines expressed the mRNA and protein of RAGE, the receptor for S100 family proteins (N. Kikuchi et al, unpublished data, 2004). These findings suggest that S100A4 can act as an autocrine/paracrine factor in ovarian carcinoma cells. Therefore, we used recombinant S100A4 in our experiments in order to analyze the effect of extracellular S100A4 on ovarian carcinoma cells.
Our study showed that treatment with recombinant S100A4 did not alter cell proliferation, but significantly enhanced the in vitro invasiveness of ovarian carcinoma cells, SKOV3 and OVCAR3, in a dose‐dependent manner. Interestingly, along with the increase in invasive capacity, S100A4 treatment induced evident expression of S100A4 in the cytoplasm in OVCAR3 cells, and also upregulated the S100A4 expression in SKOV3 cells, suggesting the presence of a positive feedback mechanism for the S100A4 expression in ovarian carcinoma cells. In addition, our immunofluorescent analysis showed that S100A4 treatment resulted in the translocation of S100A4 from the cytoplasm into the nucleus in SKOV3 cells. Although S100A4 was first identified as cytoplasmic protein, its translocation between the cytoplasm and the nucleus has been reported in human cells in vitro, such as endothelial cells,( 30 ) fibroblasts,( 31 ) glioblastoma cells,( 32 ) and colorectal carcinoma.( 33 ) S100A4 has been implicated in the regulation of gene transcription either through direct DNA binding, or through interaction with other DNA‐binding proteins.( 33 ) S100 family proteins are calcium‐binding proteins with two common EF‐hand structure motifs, and their change in the intracellular localization was shown to be dependent on the intracellular concentration of calcium.( 18 , 34 ) The intracellular translocation of an S100 protein has also been reported to occur under the extracellular presence of the same S100 protein.( 30 ) However, the question remains how S100A4 translocates between cytoplasm and nucleus in ovarian cancer cells. In ovarian carcinoma SKOV3 cells, nuclear translocation of S100A4 along with enhanced invasiveness was induced by extracellular S100A4. Nuclear expression of S100A4 was correlated with poor prognosis in ovarian carcinomas. All these data suggest that nuclear localization of S100A4 is important in the transformation of ovarian carcinoma cells into an aggressive phenotype.
Our study showed that treatment with S100A4 increased both MT1‐MMP activity and migration in ovarian cancer cells, although the signal transduction pathway of S100A4 remains unclear in the enhanced invasiveness. In this study, we found that the treatment with recombinant S100A4 resulted in upregulation and activation of RhoA, and that the increase in invasiveness due to extracellular S100A4 was suppressed by a specific inhibitor of Rho GTPase, C3 exoenzyme, and by a Rho kinase inhibitor, Y27632. We previously showed that a small GTPase, RhoA, plays an important role in the peritoneal dissemination of ovarian carcinoma cells both in vivo and in vitro.( 9 ) These findings suggest that the S100A4‐RhoA signal is essential in the invasion of ovarian carcinoma cells, although its signal effect is not yet clearly understood. As Rho GTPase is known to be involved in cell motility, an association between S100A4 and cell motility is reasonable.( 35 ) Noviskaya et al. reported that S100A4 stimulation was involved in the activation of both phospholipase C and protein kinase C.( 16 ) A possible relationship between S100A4, matrix metalloproteinases, and invasive capacity has also been reported.( 36 , 37 ) Extracellular function of S100A4 might also include angiogenesis.( 17 , 38 ) Thus, further research is needed to clarify the intercellular and extracellular mechanisms of S100A4 involvement in the progression of ovarian carcinomas.
In summary, the cytoplasmic and nuclear expression of S100A4 is stronger in ovarian carcinomas than in benign or borderline tumors, and nuclear expression of S100A4 could be an indicator for poor prognosis in ovarian carcinoma patients. In addition, ovarian carcinoma cells express S100A4 at the mRNA and protein levels and secrete S100A4, which might in turn work for its own upregulation as well as its nuclear translocation, along with an enhancement of invasiveness in vitro. Thus, S100A4 might be an important autocrine/paracrine factor involved in the aggressive characteristics of ovarian carcinoma cells, and could be a molecular target in the treatment of ovarian carcinoma. Development of a novel therapy targeted to the S100A4‐RhoA pathway is needed to improve the survival of ovarian carcinoma patients.
Acknowledgments
This work was supported in part by Grants‐in‐Aid for Scientific Research from the Ministry of Education, Science and Culture (No. 15390502, No. 16790947), Japan.
References
- 1. Murdoch WJ. Ovarian surface epithelium, ovulation and carcinogenesis. Biol Rev Camb Philos Soc 1996; 71: 529–43. [DOI] [PubMed] [Google Scholar]
- 2. Mandai M, Konishi I, Komatsu T et al. Mutation of the nm23 gene, loss of heterozygosity at the nm23 locus and K‐ras mutation in ovarian carcinoma: correlation with tumour progression and nm23 gene expression. Br J Cancer 1995; 72: 691–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Imai T, Horiuchi A, Wang C et al. Hypoxia attenuates the expression of E‐cadherin via up‐regulation of SNAIL in ovarian carcinoma cells. Am J Pathol 2003; 163: 1437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernstein LR, Liotta LA. Molecular mediators of interactions with extracellular matrix components in metastasis and angiogenesis. Curr Opin Oncol 1994; 6: 106–13. [DOI] [PubMed] [Google Scholar]
- 5. Schmalfeldt B, Prechtel D, Harting K et al. Increased expression of matrix metalloproteinases (MMP)‐2, MMP‐9, and the urokinase‐type plasminogen activator is associated with progression from benign to advanced ovarian cancer. Clin Cancer Res 2001; 7: 2396–404. [PubMed] [Google Scholar]
- 6. Baserga R. Oncogenes and the strategy of growth factors. Cell 1994; 79: 927–30. [DOI] [PubMed] [Google Scholar]
- 7. Yamamoto S, Konishi I, Mandai M et al. Expression of vascular endothelial growth factor (VEGF) in epithelial ovarian neoplasms: correlation with clinicopathology and patient survival, and analysis of serum VEGF levels. Br J Cancer 1997; 76: 1221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horiuchi A, Imai T, Shimizu M et al. Hypoxia‐induced changes in the expression of VEGF, HIF‐1 alpha and cell cycle‐related molecules in ovarian cancer cells. Anticancer Res 2002; 22: 2697–702. [PubMed] [Google Scholar]
- 9. Horiuchi A, Imai T, Wang C et al. Up‐regulation of small GTPases, RhoA and RhoC, is associated with tumor progression in ovarian carcinoma. Laboratory Invest 2003; 83: 861–70. [DOI] [PubMed] [Google Scholar]
- 10. Donato R. S100: a multigenic family of calcium‐modulated proteins of the EF‐hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol 2001; 33: 637–68. [DOI] [PubMed] [Google Scholar]
- 11. Selinfreund RH, Barger SW, Welsh MJ, Van Eldik LJ. Antisense inhibition of glial S100 beta production results in alterations in cell morphology, cytoskeletal organization, and cell proliferation. J Cell Biol 1990; 111: 2021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davies BR, Davies MP, Gibbs FE, Barraclough R, Rudland PS. Induction of the metastatic phenotype by transfection of a benign rat mammary epithelial cell line with the gene for p9Ka, a rat calcium‐binding protein, but not with the oncogene EJ‐ras‐1. Oncogene 1993; 8: 999–1008. [PubMed] [Google Scholar]
- 13. Davies MP, Rudland PS, Robertson L, Parry EW, Jolicoeur P, Barraclough R. Expression of the calcium‐binding protein S100A4 (p9Ka) in MMTV‐neu transgenic mice induces metastasis of mammary tumors. Oncogene 1996; 13: 1631–7. [PubMed] [Google Scholar]
- 14. Parker C, Whittaker PA, Usmani BA, Lakshmi MS, Sherbet GV. Induction of 18A2/mts1 gene expression and its effects on metastasis and cell cycle control. DNA Cell Biol 1994; 13: 1021–8. [DOI] [PubMed] [Google Scholar]
- 15. Ilg EC, Schafer BW, Heizmann CW. Expression pattern of S100 calcium‐binding proteins in human tumors. Int J Cancer 1996; 68: 325–2. [DOI] [PubMed] [Google Scholar]
- 16. Novitskaya V, Grigorian M, Kriajevska M et al. Oligomeric forms of the metastasis‐related Mts1 (S100A4): protein stimulate neuronal differentiation in cultures of rat hippocampal neurons. J Biol Chem 2000; 275: 41278–86. [DOI] [PubMed] [Google Scholar]
- 17. Semov A, Moreno MJ, Onichtchenko A et al. Metastasis‐associated protein S100A4 induces angiogenesis through interaction with annexin II and accelerated plasmin formation. J Biol Chem 2005; 280: 20833–41. [DOI] [PubMed] [Google Scholar]
- 18. Mueller A, Bachi T, Hochli M, Schafer BW, Heizmann CW. Subcellular distribution of S100 proteins in tumor cells and their relocation in response to calcium activation. Histochem Cell Biol 1999; 111: 453–9. [DOI] [PubMed] [Google Scholar]
- 19. Silverberg SG. Histopathologic grading of ovarian carcinoma: a review and proposal. Int J Gynecol Pathol 2000; 19: 7–15. [DOI] [PubMed] [Google Scholar]
- 20. Scully RE, Young RH, Clement PB. Surface epithelial‐stromal tumors and serous tumors. In: Rosai J, Sobin LH, eds. Atlas of Tumor Pathology. Tumors of the Ovary, Maldeveloped Gonads, Fallopian Tube, and Broad Ligamant. Third series, Fascicle 23. Washington DC: Armed Forces Institute of Pathology, 1998. [Google Scholar]
- 21. Davies BR, O'Donnell M, Durkan GC et al. Expression of S100A4 protein is associated with metastasis and reduced survival in human bladder cancer. J Pathol 2002; 196: 292–9. [DOI] [PubMed] [Google Scholar]
- 22. Pedersen KB, Nesland JM, Fodstad O, Maelandsmo GM. Expression of S100A4, E‐cadherin, alpha‐ and beta‐catenin in breast cancer biopsies. Br J Cancer 2002; 87: 1281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsuruo T, Hamilton TC, Louie KG, Behrens BC, Young RC, Ozols RF. Collateral susceptibility of adriamycin‐, melphalan‐ and cisplatin‐resistant human ovarian tumor cells to bleomycin. Jpn J Cancer Res 1986; 77: 941–5. [PubMed] [Google Scholar]
- 24. Chomczynski P, Sacchi N. Single‐step method of RNA isolation by acid guanidinium thiocyanate‐phenol‐chloroform extraction. Anal Biochem 1987; 162: 156–9. [DOI] [PubMed] [Google Scholar]
- 25. Albini A, Iwamoto Y, Kleinman HK et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 1987; 47: 3239–45. [PubMed] [Google Scholar]
- 26. Ito T, Williams JD, Fraser D, Phillips AO. Hyaluronan attenuates transforming growth factor‐beta1‐mediated signaling in renal proximal tubular epithelial cells. Am J Pathol 2004; 164: 1979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bjornland K, Winberg JO, Odegaard OT et al. S100A4 involvement in metastasis: deregulation of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in osteosarcoma cells transfected with an anti‐S100A4 ribozyme. Cancer Res 1999; 59: 4702–8. [PubMed] [Google Scholar]
- 28. Sato N, Fukushima N, Maitra A et al. Gene expression profiling identifies genes associated with invasive intraductal papillary mucinous neoplasms of the pancreas. Am J Pathol 2004; 164: 903–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rudland PS, Platt‐Higgins A, Renshaw C et al. Prognostic significance of the metastasis‐inducing protein S100A4 (p9Ka) in human breast cancer. Cancer Res 2000; 60: 1595–603. [PubMed] [Google Scholar]
- 30. Hsieh HL, Schafer BW, Weigle B, Heizmann CW. S100 protein translocation in response to extracellular S100 is mediated by receptor for advanced glycation endproducts in human endothelial cells. Biochem Biophys Res Commun 2004; 316: 949–59. [DOI] [PubMed] [Google Scholar]
- 31. Sakaguchi M, Miyazaki M, Inoue Y et al. Relationship between contact inhibition and intranuclear S100C of normal human fibroblasts. J Cell Biol 2000; 149: 1193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Davey GE, Murmann P, Heizmann CW. Intracellular Ca2+ and Zn2+ levels regulate the alternative cell density‐dependent secretion of S100B in human glioblastoma cells. Biol Chem 2001; 276: 30819–26. [DOI] [PubMed] [Google Scholar]
- 33. Flatmark K, Pedersen KB, Nesland JM et al. Nuclear localization of the metastasis‐related protein S100A4 correlates with tumour stage in colorectal cancer. J Pathol 2003; 200: 589–95. [DOI] [PubMed] [Google Scholar]
- 34. Mandinova A, Atar D, Schafer BW, Spiess M, Aebi U, Heizmann CW. Distinct subcellular localization of calcium binding S100 proteins in human smooth muscle cells and their relocation in response to rises in intracellular calcium. J Cell Sci 1998; 111: 2043–54. [DOI] [PubMed] [Google Scholar]
- 35. Jenkinson SR, Barraclough R, West CR, Rudland PS. S100A4 regulates cell motility and invasion in an in vitro model for breast cancer metastasis. Br J Cancer 2004; 90: 253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schmidt‐Hansen B, Ornas D, Grigorian M et al. Extracellular S100A4 (mts1) stimulates invasive growth of mouse endothelial cells and modulates MMP‐13 matrix metalloproteinase activity. Oncogene 2004; 23: 5487–95. [DOI] [PubMed] [Google Scholar]
- 37. Mathisen B, Lindstad RI, Hansen J et al. S100A4 regulates membrane induced activation of matrix metalloproteinase‐2 in osteosarcoma cells. Clin Exp Metastasis 2003; 20: 701–11. [DOI] [PubMed] [Google Scholar]
- 38. Ambartsumian N, Klingelhofer J, Grigorian M et al. The metastasis‐associated Mts1 (S100A4): protein could act as an angiogenic factor. Oncogene 2001; 20: 4685–95. [DOI] [PubMed] [Google Scholar]


