Abstract
To study the mechanisms of the highly liver‐metastatic character of colon carcinoma cells, we studied the expression pattern of surface integrins on LS‐LM6 (a highly liver‐metastatic human colon cancer cell line) and the effects of hepatocyte‐derived soluble factors on cell migration. LS‐LM6 showed significantly higher expression of integrin αvβ5, a ligand for vitronectin (VN), as compared with its parental cell line (LS174T). A conditioned medium of cultured mouse hepatocytes enhanced VN‐mediated cell migration of LS‐LM6, which was blocked by neutralizing antibody against integrin αvβ5, while the medium did not affect cell adhesion to VN‐coated plastic surfaces. The conditioned medium induced phosphorylation of erbB3 and its heterodimeric partner, erbB2. Heregulin (HRG), a ligand for erbB3, exerted similar effects on VN‐mediated cell migration and phosphorylation of erbB3 and erbB2. The conditioned medium contained HRG, and depletion of HRG from the medium by pre‐absorption with HRG antibody abolished its effects on cell migration. Heregulin (HRG) was expressed in some hepatocytes in the liver with carcinoma cell metastasis. Furthermore, knockdown of integrin αv and erbB3 by small‐interfering RNAs significantly inhibited cell migration induced by HRG as well as liver metastasis in vivo. Finally, we found that HRG‐induced cell migration was associated with marked phosphorylation of Akt and that cell migration was suppressed by treatment with specific inhibitors of phosphatidylinositol 3‐kinase. Our study suggests that hepatocyte‐derived HRG might participate in a highly liver‐metastatic phenotype of LS‐LM6 through enhancement of integrin αvβ5‐mediated cell migration and erbB3/erbB2 signaling. (Cancer Sci 2010)
The liver is one of the major target organs for tumor metastasis, including colorectal carcinomas. Since liver metastasis is an important prognostic factor for patients suffering from colorectal carcinoma, understanding of the molecular mechanisms underlying the metastatic process is important. To elucidate the mechanisms of organ‐preferential metastasis, it should be important to consider complex interactions between tumor cells and the target organ’s microenvironment, which consists of the extracellular matrix (ECM), cytokines, and growth factors derived from the resident cells.( 1 , 2 )
It has been reported that cell adhesion and invasion (migration) play important roles in the development of liver metastasis of colorectal carcinoma cells in addition to angiogenesis and cell survival.( 3 ) At the initial stage of metastasis, tumor cells need to adhere to the luminal surface of hepatic sinusoidal endothelial cells via various cell adhesion molecules, such as E‐selectin, vascular cell adhesion molecule 1 (VCAM‐1), and intercellular adhesion molecule 1 (ICAM‐1) expressed on endothelial cell surfaces.( 4 , 5 , 6 , 7 , 8 ) At the next stage of metastasis, the interaction between ECM in the space of Disse and integrins, which are expressed on tumor cell surfaces, is required for invasion and migration of tumor cells into the liver parenchyma.( 9 )
The space of Disse in the normal liver has been shown to contain various ECM components, including fibronectin (FN) and several types of collagen.( 10 ) In addition, a large amount of vitronectin (VN) is synthesized by hepatocytes( 11 , 12 ) and secreted into the sinusoidal spaces, resulting in a considerable level of plasma VN.( 11 ) Vitronectin (VN) is known to interact with many types of integrins, including integrin αvβ5, which has been implicated in cell adhesion and migration in various cell types. Through the activation of receptor tyrosine kinases (RTK), such as epidermal growth factor receptor (EGFR)( 13 ) and insulin‐like growth factor (IGF)‐I receptor,( 14 ) integrin αvβ5 is implicated in migration on VN. Recently, the interaction of FN and integrin αvβ5 was reported to be crucial in the adhesion step of colon cancer cells to the liver parenchyma.( 15 ) However, the role of VN in the development of liver‐metastasis is unknown.
We previously established human colon carcinoma cell lines with a highly liver‐metastatic potential (a LS‐LM series) by repeated in vivo passages of a parental cell line (LS174T) in the liver of severe combined immunodeficient (SCID) mice.( 16 ) In the present study, we tried to examine the possible cooperation between integrins and hepatocyte‐derived factors in migration of LS‐LM6. We found that LS‐LM6 expressed higher levels of integrin αvβ5 as compared with LS174T, and that hepatocyte‐derived heregulin (HRG, also called neuregulin; a ligand for erbB3) strongly enhanced migration of LS‐LM6 on VN. Knockdown of integrin αv and erbB3 by small‐interfering RNAs significantly diminished cell migration induced by HRG in vitro, as well as liver metastasis in vivo. Our results indicate a novel integrin αvβ5‐mediated and erbB3‐dependent mechanism in a highly liver‐metastasis phenotype of colon carcinoma cells.
Materials and Methods
Cell lines, antibodies, and other reagents. A human colon carcinoma cell line, LS174T, was purchased from the American Tissue Culture Collection (ATCC, Manussas, VA, USA). LS‐LM6 was established through six passages of LS174T in the liver of SCID mice as described previously.( 16 ) Cells were maintained in RPMI‐1640 medium with 10% fetal bovine serum. Mouse monoclonal antibody (mAb) against integrin αvβ3 and VN were from Chemicon (Temecula, CA, USA). Monoclonal antibodies (mAbs) against integrins α5, αvβ5, and β3 were from Pharmingen (San Jose, CA, USA); integrin β1 was from Immunotech (Marseille, France); integrins β6 and β8 were from R&D Systems (Minneapolis, MN, USA); Heregulin 1 (NRG1) was from Abnova (Taipei, Taiwan); phosphotyrosine (PY20) was from Takara (Tokyo, Japan); c‐neu was from Oncogene (San Diego, CA, USA); and phospho‐ERK was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rat mAb to integrin αv was from Immunotech. Rabbit Abs to Neu, erbB3, and erbB4 were from Santa Cruz Biotechnology; phospho‐erbB3, erbB3, phosho‐erbB2, erbB2, phospho‐Akt, Akt, ERK, and integrin αv were from Cell Signaling (Beverly, MA, USA); and HRG was from Neomarker (Fremont, CA, USA). Goat antibodies to HRG‐β1, biotinylated HRG‐β1, and normal goat IgG were from R&D Systems. LY294002 was from Cell Signaling. Wortmannin was from Calbiochem (San Diego, CA, USA). Recombinant HRG‐β1, IGF‐I, IGF‐II, hepatocyte growth factor (HGF), and epidermal growth factor (EGF) were from R&D Systems. Vitronectin (VN) was from BD (San Jose, CA, USA).
Flow cytometry. Cell surface expression of integrins was determined by using a flow cytometer (Coulter, Miami, FL, USA). We used the following six antihuman mouse monoclonal antibodies for analysis of expression of cell surface antigens: integrin αvβ3 from Chemicon, integrins α5 and αvβ5 from Pharmingen, integrin β1 from Immunotech, and integrins β6 and β8 from R&D Systems. Cells were incubated with 5 μg/mL of antibody for 30 min at 4°C. The cells were washed and further incubated with antimouse IgG antibody coupled with FITC (Dako, Tokyo, Japan) in the dark for 30 min on ice. They were again washed and sieved through 40‐μm pore mesh, then a total of 1 × 104 cells was analyzed. Cells were defined as positive when they had stronger fluorescence intensity than the cut‐off points (2% less than maximum points of control). Control cells were incubated with antimouse IgG antibody and FITC‐labeled secondary antibody.
Isolation and culture of hepatocytes. Hepatocytes were isolated from 10‐week‐old male SCID mice (Fox Chase C.B17/Icr‐Scid, Jcl; Clea, Tokyo, Japan) by the two‐step collagenase perfusion method, followed by repeated low‐speed (50g) centrifugations. Hepatocytes were plated at a density of 2 × 106 cells on 100‐mm culture dishes coated with type‐I collagen (BD) and cultured with Williams’ E medium. Hepatocyte‐conditioned media (HCM) were collected every 24 h and concentrated fourfold by ultrafiltration (Centricut; Kurabo, Osaka, Japan). The filtrated fractions containing substances of molecular weight <20 000 were also collected (FCM). In some experiments, to deplete HRG from the conditioned medium, HCM was incubated with streptavidin‐coated Dynabeads (Dynal, Invitrogen, Carlsbad, CA, USA) bound to biotinylated anti‐HRG‐β1 antibody for 12–16 h at 4°C, followed by removal of HRG‐conjugated magnetic beads.
Migration, adhesion, and proliferation assays. Migration assays were performed using modified Boyden chambers with 8‐μm pores (BD). The lower chambers were filled with RPMI‐1640 or Williams’ E medium containing 0.1% BSA and VN (10 μg/mL) at the final concentration. Williams’ E medium or conditioned media from hepatocytes (HCM or FCM) were added to the lower chambers at the indicated final concentration. In some experiments, various growth factors or conditioned media from LS174T and LS‐LM6 were applied. LS174T or LS‐LM6 (2 × 105 cells) was suspended in 100 μL of 0.1% BSA–RPMI and seeded into upper chambers. To examine the effects of neutralizing antibodies and kinase inhibitors, cells were treated with antibodies (10 μg/mL) and kinase inhibitors for 30 min before seeding. They were also added in the lower chambers at the same concentration. After incubation for 20 h, migrated cells on the lower surfaces of the filter were fixed and stained with hematoxylin–eosin or crystal violet, and counted after wiping off non‐migratory cells remaining on the upper surfaces.
For cell adhesion assay, 96‐well plates were coated with 100 μL of VN (10 μg/mL) and dried and blocked with 1% BSA for 1 h. Cells (1 × 105) were seeded with 100 μL of the conditioned medium. After incubation, unattached cells were removed, and attached cells were treated with bisbenzamide (20 μg/mL; Sigma, St. Louis, MO, USA) containing TNE buffer. Fluorescence was then measured using a fluorescent plate reader (PerSeptive Biosystems, Framingham, MA, USA). For cell proliferation assay, cells (1 × 105) were seeded to a 100 μL of VN (10 μg/mL)‐coated 96‐well plate and incubated with HCM without FBS, and cell numbers were measured the same way as for cell adhesion assay after culturing for 48 and 72 h. Hepatocyte‐conditioned media (HCM) concentrations: ×0.5, half of the time; ×1, one time; ×2, two times, and HRG concentration: 1, 1 ng/mL; 2, 2 ng/mL; 5, 5 ng/mL.
RNA interference. Small‐interfering RNAs (siRNAs) targeting human integrin αv (s7568 [αv‐1] and s7570 [αv‐2]) and erbB3 (s4778 [erbB3‐1] and s4780 [erbB3‐2]) were obtained from Ambion (Austin, TX, USA), and were transfected into LS‐LM6 cells using siPORT NeoFX agent (Ambion) according to the manufacturer’s protocol. The negative control siRNA was also purchased from Ambion. After transfection, silencing of integrin αv and erbB3 and effects on LS‐LM6 migration were examined.
Reverse transcriptase‐PCR (RT‐PCR), western blot analysis, immunohistochemistory staining, and experimental liver meta‐stasis. See the Supplementary Materials and Methods Data S1 for details.
Statistical analysis. Statistical significance among experimental groups was determined by the Student’s t‐test or two‐way anova test. P‐values lower than 0.05 were considered to be significant.
Results
Integrin αvβ5 is strongly expressed in a highly liver‐metastatic colon carcinoma cell line (LS‐LM6). At 6 weeks after intrasplenic transplantation, metastasized LS‐LM6 substituted most of the liver parenchyma, while LS174T formed many fewer and smaller metastatic nodules (Fig. 1a). Flow cytometric analyses of surface integrins revealed that the expression of integrin αvβ5 on LS‐LM6 was 6.4‐fold higher than that on LS174T, while there was no significant difference in the expression of integrin αvβ3, β1 (Fig. 1b), and β8 (Fig. S1), which were other β‐subunits dimerizing with αv‐subunit. Relative higher expression of β6 on LS‐LM6 was observed, but the expression (28.6%) was far lower as compared with that of integrin αvβ5 (75.4%).
Figure 1.
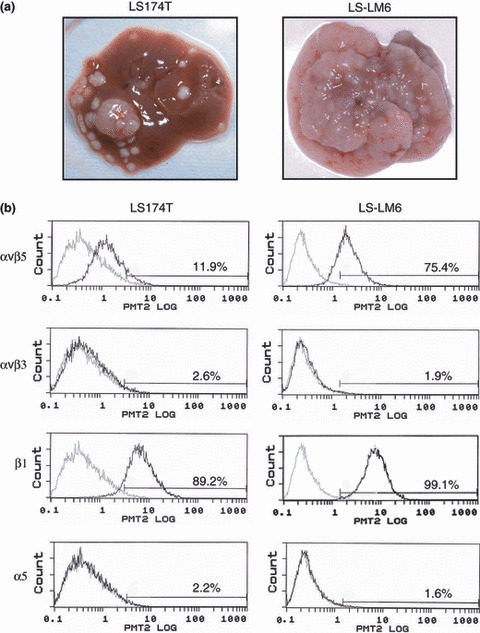
Liver metastasis of LS174T and LS‐LM6 and increased expression of integrin αvβ5 in LS‐LM6. (a) Livers at 6 weeks after intrasplenic injection of LS174T and LS‐LM6. (b) Flow cytometric analysis of surface integrins on LS174T and LS‐LM6. Three independent experiments were carried out and produced similar results.
Hepatocyte‐conditioned medium (HCM) enhances integrin αvβ5‐dependent migration of LS‐LM6 on VN. To identify host‐derived factors that affect LS‐LM6 migration and cooperate with integrin αvβ5, we examined the migration‐inducing activity of the HCM medium. Migration of LS‐LM6 was strongly enhanced by HCM in a concentration‐dependent manner, but not by FCM, suggesting the presence of a migration‐enhancing factor(s) larger than 20 kD (Fig. 2a). In contrast, neither HCM nor FCM enhanced migration of LS174T (Fig. 2a). Migration of LS‐LM6 on VN stimulated by HCM was markedly attenuated by neutralizing antibodies against integrin αvβ5, αv, and VN (Fig. 2b). Growth of LS174T and LS‐LM6 was significantly enhanced when treated with concentrated HCM for 48 h and LS174T for 72 h (Fig. 3a). Hepatocyte‐conditioned medium (HCM) did not show any discernible effect on cell adhesion of either cell line to VN‐coated plastic surfaces (Fig. 3b).
Figure 2.
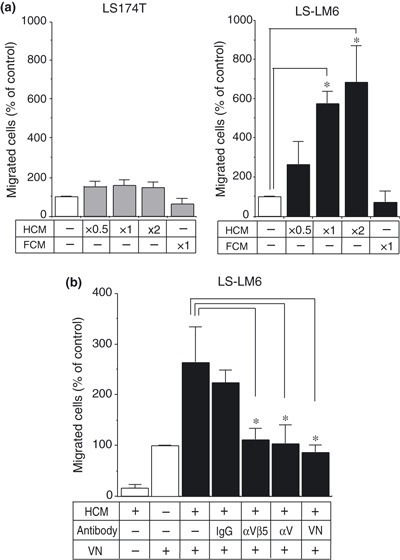
Enhancement of integrin αvβ5‐dependent migration of LS‐LM6 by hepatocyte‐conditioned medium (HCM). (a) Migration of LS‐LM6 on vitronectin (VN) by HCM. Both LS174T and LS‐LM6 can migrate 100–300 cells on VN without stimulation. The migration of LS‐LM6 is enhanced up to sixfold by HCM (×1) and 6.9‐fold by the twice‐concentrated HCM (×2) compared with the control medium (*P < 0.05, two‐way anova). LS174T shows no significant migration by HCM. The filtrated fraction containing the low‐molecular weight proteins (FCM) has no effect on cell migration. (b) The enhanced migration of LS‐LM6 on VN by HCM is significantly inhibited by the neutralizing antibodies against integrins αvβ5 and αv, and VN (*P < 0.05, two‐way anova).
Figure 3.
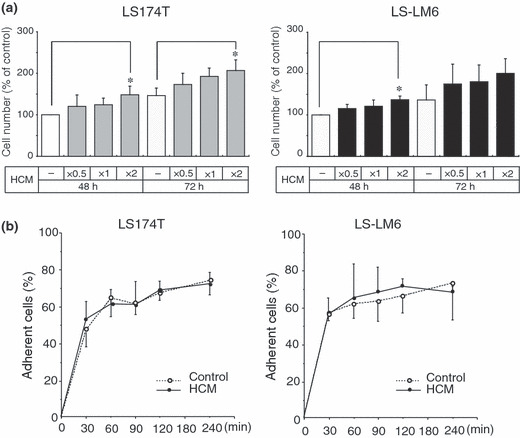
Effects of hepatocyte‐conditioned medium (HCM) on proliferation and adhesion of LS‐LM6 and LS174T. (a) Cell numbers at 48 and 72 h’ culture with HCM. Both cell lines show a slight increase in cell numbers by HCM. Hepatocyte‐conditioned medium (HCM) (×2) significantly increases cell numbers of both cell lines at 48 h as compared to the control medium and that of LS174T at 72 h (*P < 0.05, two‐way anova). (b) Effects of HCM on cell adhesion. Both cell lines show no significant difference in adhesion to vitronectin (VN)‐coated dishes by the treatment of either the control medium and HCM.
Hepatocyte‐conditioned medium (HCM) induces tyrosine phosphorylation of erbB3 and erbB2. Since integrin αvβ5 needs to be activated by receptor tyrosine kinases for its capacity to induce cell migration,( 13 , 14 ) we examined changes in the phosphorylation status of various receptor tyrosine kinases in LS174T and LS‐LM6 after HCM treatment. A band of 180 kD was found to be phosphorylated by HCM (Fig. 4a, asterisk). The EGF receptor family with similar molecular sizes, such as EGFR and erbB4, was not detectable in either cell line by western blot analysis (data not shown). Thus, we speculated that the band corresponded to erbB2 and erbB3, and performed western blotting using the specific antibodies. The 180 kD band did, in fact, coincide with both erbB2 and erbB3, which were tyrosine‐phosphorylated by HCM (Fig. 4a). Interestingly, HRG, a ligand for erbB3,( 17 ) alone induced phosphorylation of both erbB3 and erbB2 (Fig. 4a). Heregulin (HRG), also enhanced migration of LS‐LM6, and this was blocked by neutralizing antibodies against integrin αvβ5 and αv (Fig. 4b). The other ligands for RTK, such as IGF‐I, IGF‐II, HGF, and EGF, did not affect migration of LS174T and LS‐LM6 (Fig. 4c).
Figure 4.
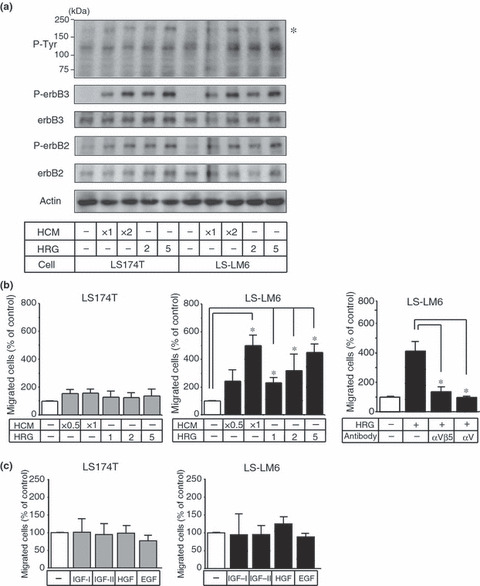
Tyrosine phosphorylation of erbB3/erbB2 and enhancement of the migration of LS‐LM6 by hepatocyte‐conditioned medium (HCM) and heregulin (HRG). (a) Western blot analysis for the tyrosin‐phosphorylated proteins stimulated by HCM and HRG in LS174T and LS‐LM6. A 180‐kD protein (asterisk) was phosphrylated by HCM and HRG. ErbB3 and erbB2, corresponding to the 180 kD band, were phosphorylated by HCM and HRG. (b) Both HCM and HRG significantly enhance the migration of LS‐LM6 cells (*P < 0.05, two‐way anova). However, they show no effect on the migration of LS174T. The migration induced by HRG is blocked by the neutralizing antibodies against integrins αvβ5 and αv. (c) Effect of the other growth factors on migration of LS174T and LS‐LM6. Insulin‐like growth factor (IGF)‐I, IGF‐II, HGF, as well as EGF, show no effect on the migration of both cell lines.
Heregulin (HRG) is a migration‐enhancing factor in hepatocyte‐conditioned medium. We then examined whether hepatocytes actually produced HRG and secreted into the culture medium. Although freshly isolated SCID mouse hepatocytes expressed low levels of HRG mRNA, HRG protein was not detected in HCM (Fig. 5a). However, HRG gene expression was increased in culture after 2 days and HRG protein (44 kD) was detectable in HCM (Fig. 5a). Heregulin (HRG) could be depleted from HCM by pre‐absorption with HRG antibody, and the HRG‐depleted HCM significantly lost its migration‐enhancing effect (Fig. 5b).
Figure 5.
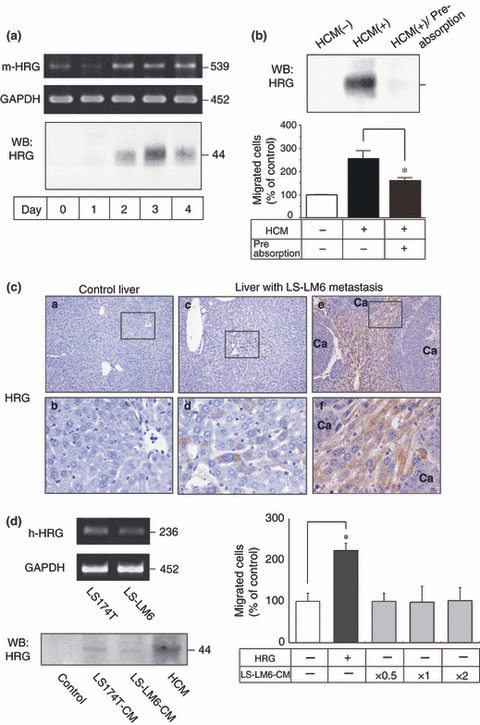
Detection of heregulin (HRG) in the cultured hepatocytes and liver with LS‐LM6 metastasis, and suppression of LS‐LM6 migration after removal of HRG from hepatocyte‐conditioned medium (HCM). (a) Reverse transcription (RT)‐PCR of the cultured hepatocytes demonstrates HRG (m‐HRG) at the expected 539‐bp band (GAPDH as a control). Western blot of concentrated HCM shows the presence of HRG with the maximum signal on day 3. (b) The enhanced LS‐LM6 migration is significantly attenuated by removal of HRG from the HCM (*P < 0.05, Student’s t‐test, lower panel). (c) In the liver with LS‐LM6 metastasis, some hepatocytes are positive for HRG immunohistochemistry whereas no positive staining is detected in the normal control liver nor metastatic nodules of LS‐LM6. Ca, carcinoma. (d) Reverse transcription (RT)‐PCR demonstrates HRG (h‐HRG) at the expected 236‐bp band in LS174T and LS‐LM6. Western blot shows a small amount of HRG in the concentrated conditioned medium of LS174T and LS‐LM6. The conditioned medium of LS‐LM6 does not induce the migration of LS‐LM6 (*P < 0.05, two‐way anova).
Hepatocytes express HRG in the liver with metastasis of carcinoma cells. We immunohistochemically (IHC) examined HRG expression in mouse liver tissues with or without LS‐LM6 metastasis. Although no HRG expression was detected in hepatocytes of normal control liver, hepatocytes of the LS‐LM6 metastasis liver focally exhibited a distinct positive staining in their cytoplasm. Interestingly, HRG expression seemed to be stronger in the hepatocytes surrounding metastatic nodules than in non‐metastatic area (Fig. 5c, left, middle, and right panels).
Production and secretion of HRG by LS174T and LS‐LM6. As previously reported,( 18 ) we confirmed that both LS174T and LS‐LM6 produced HRG mRNA (Fig. 5d, left and upper panel). However, HRG secreted into the medium by both cell lines was far less than that by hepatocytes (Fig. 5d, left and lower panel), and the conditioned medium of LS‐LM6 showed no effects on migration even after being concentrated (Fig. 5d, right panel). These results suggested that HRG secreted mainly by hepatocytes, but not by tumor cells, induced the integrin αvβ5‐mediated migration of LS‐LM6.
Inhibition of HRG‐induced migration and liver metastasis by knockdown of integrin αv and erbB3. The siRNAs of integrin αv and erbB3 effectively suppressed expression of the corresponding mRNAs in LS‐LM6 (Fig. 6a). Because of more effective suppression, si‐integrin αv‐2 and si‐erbB3‐2 cells were used for the following experiments. Western blot analysis confirmed that protein expression of integrin αv and erbB3 was decreased in these cells, while control siRNA did not exert any effect (Fig. 6b, left panel). In these si‐RNA transfected cells, HRG‐induced migration was strongly inhibited (Fig. 6b, right panel).
Figure 6.
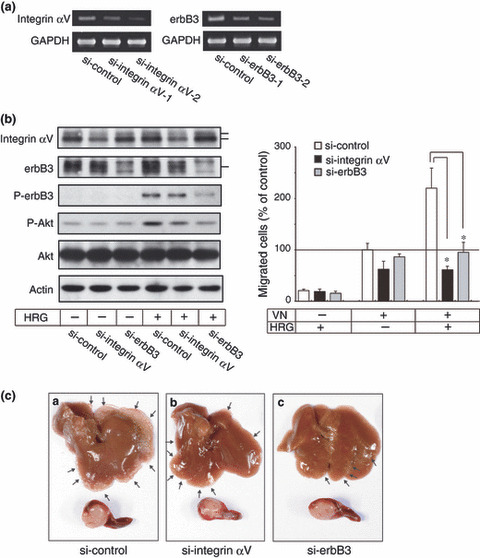
Abrogation of heregulin (HRG)‐induced migration by knock down of integrin αvβ5 and erbB3. (a) Reverse transcription (RT)‐PCR shows siRNAs of both integrin αv and erbB3 effectively suppressed the expression of their mRNAs in the transfected LS‐LM6 cells. (b) Western blot shows that synthesis of integrin αv and erbB3 are suppressed by siRNAs, but control siRNAs show no suppression (left panel). After HRG treatment, the phosphorylated erbB3 and Akt are suppressed in si‐erbB3 cells. Cells transfected with either integrin αv or erbB3 siRNAs show no response to HRG‐induced migration on vitronectin (VN) (right panel) (*P < 0.05, two‐way anova). (c) Liver metastasis lesions of both si‐integrin αv and si‐erbB3 cells are significantly suppressed compared with that of si‐control cells, but tumor sizes in the spleen are almost same. Arrows indicate metastatic nodules of LS‐LM6.
Liver metastasis of these si‐RNA transfected cells was significantly inhibited compared with that of control si‐RNA transfected cells, but growth of these cells at spleens (injection sites) was almost the same (Fig. 6c and Table 1). These indicate that integrin αv and erbB3, respectively, play an important role not only in the migration in vitro but also in the in vivo liver metastasis of LS‐LM6.
Table 1.
Inhibition of hepatic metastasis of LS‐LM6 cells by si‐RNA
| si‐RNA | Hepatic metastasis | Weight of spleen‡ (mg) | ||
|---|---|---|---|---|
| Incidence† | Number of nodules‡ | Tumor volume§ | ||
| si‐control | 8/8 | 100.7 ± 54.7** | 125.6 ± 106* | 283 ± 66 |
| si‐integrin αv | 8/8 | 14.9 ± 11.4** | 15.6 ± 12* | 233 ± 44.7 |
| si‐erbB3 | 8/8 | 11.3 ± 11.7** | 11.7 ± 12.2* | 214 ± 65.9 |
*P < 0.05; **P < 0.01. †Number of mice with hepatic metastasis/number of mice which received splenic injection. ‡Average ± SD. §Tumor volume was calculated by (diameter of nodules/2)3 × 4π/3 and summed for each nodule, average ± SD.
It has been demonstrated that the MAPK and PI3K pathways are involved in the downstream signaling of erbB2/erbB3.( 19 , 20 ) The level of phosphorylated Akt was decreased when erbB3 siRNA was transfected (Fig. 6b). Phosphorylation levels of ERK did not change in cells transfected with erbB3 siRNA (data not shown). The specific PI3K inhibitors (LY294002 and wortmannin) suppressed Akt phosphorylation without affecting the levels of erbB3 phosphorylation (Fig. S2). Both inhibitors significantly suppressed HRG‐induced migration of LS‐LM6 (Fig. S2).
Discussion
There is increasing evidence suggesting that integrin‐mediated interaction between tumor cells and ECM is one of the key steps in liver metastasis of colon carcinoma cells.( 21 ) Involvement of α6, β1, and β4 integrins in tumor cell–endothelial cell adhesion within the hepatic microcirculation and involvement of α2, α6, β1, and β4 integrins in extravasation into the parenchyma in liver metastasis of colon carcinoma cells has been reported.( 9 ) In the present study, we found that there was an increase in integrin αvβ5 expression in LS‐LM6, and that αvβ5‐mediated migration of LS‐LM6 was enhanced by hepatocyte‐derived HRG, suggesting the possible importance of VN‐dependent cell migration in the enhanced liver metastasis of colon carcinomas.
Integrin αvβ5 has been reported to be associated with adhesion of HT‐29 colon carcinoma cells to the surfaces of hepatic sinusoids in the early stage of liver metastasis, possibly through interaction with FN.( 15 ) Our study highlighted the importance of integrin αvβ5 expressed on LS‐LM6 in cell migration, rather than cell adhesion or proliferation. Recently, Schluter et al. ( 22 ) demonstrated that migration activity of tumor cells was closely related to their metastatic potential. Activation of several RTK has been demonstrated to be a prerequisite for integrin αvβ5‐mediated induction of cell spreading and migration on VN‐coated surfaces.( 13 , 14 ) Interestingly, RTK signaling is not required for integrin αvβ5‐dependent cell adhesion to VN and proliferation of tumor cells.( 13 ) It has been reported that activation of IGF‐I receptor plays an important role in promoting metastasis to the lung in vivo by enhancing αvβ5‐mediated cell migration.( 14 ) In our study, although the growth factors, including IGF‐I, IGF‐II, HGF, and EGF, did not have a significant effect on migration of LS174T and LS‐LM6, these growth factors might affect the other processes, such as proliferation and survival of the tumor cells and angiogenesis.( 23 , 24 , 25 , 26 )
The HCM strongly phosphorylated erbB3 and erbB2 in both LS174T and LS‐LM6. The erbB family consists of four members, erbB1, erbB2, erbB3, and erbB4. The expression levels of erbB2, an orphan receptor, in colorectal carcinomas have been shown to correlate well with the tendency to metastasize to the liver.( 27 ) In addition, an association between erbB2 and integrin β4 has been shown to promote invasion and metastasis of breast carcinoma.( 28 , 29 ) ErbB3 is a receptor for HRG and phosphorylates its heterodimeric partner, erbB2, upon stimulation.( 30 ) While the erbB3 monomer exhibits low‐affinity binding to HRG, the erbB3/erbB2 heterodimer binds to HRG with high affinity.( 31 ) Activation of the erbB3 signaling pathway has been shown to increase cell migration and enhance metastasis of breast carcinoma( 19 , 32 ) and melanoma.( 33 ) Interestingly, Maurer et al. ( 34 ) demonstrated that erbB3 and erbB2 might act together with heterodimeric formation in human colorectal carcinomas by immunohistochemical and northern blot analyses. Our results suggest an important role of erbB3/erbB2 heterodimer formation in inducing migration of colon carcinoma cells on VN.
We showed that cultured hepatocytes produced and secreted HRG in the medium, and that depletion of HRG from the conditioned medium significantly reduced its activity to enhance VN‐mediated cell migration, suggesting that HRG was the major migration‐enhancing factor in the medium. Although LS174T and LS‐LM6 themselves produced HRG as reported previously,( 18 ) the production levels were low and were insufficient to promote their migration, suggesting that contribution of an autocrine mechanism is at most minor. Heregulin (HRG) mRNA was detected at a low level in freshly isolated hepatocytes, but it increased in cultured hepatocytes. This suggests that some alterations of hepatocytes in vivo are necessary to produce sufficient HRG to promote tumor cell migration and metastasis. In fact, IHC staining for HRG showed the HRG positive hepatocytes only in the liver with carcinoma cell metastasis. Regarding the possibility of induction of HRG in hepatocytes by factors secreated from tumor cells, our preliminary data indicated that conditioned media of LS‐LM6 and LS174T did not induce HRG mRNA in cultured hepatocytes (data not shown). Further study on changes of the hepatic microenvironment by metastasized tumor cells to induce HRG expression in resident hepatocytes is required.
Knockdown experiments clearly indicate that both integrin αv and erbB3 are important molecules conferring the enhanced liver metastatic ability to LS‐LM6. In further signaling analysis, we showed that HRG‐induced migration of LS‐LM6 was associated with strong phosphorylation of Akt, and knockdown of erbB3 expression suppressed both migration and Akt phosphorylation. The importance of the PI3K pathway in erbB3/erbB2 signaling has been reported by several investigators.( 19 , 20 ) Our study also demonstrated that inhibition of Akt phosphorylation by the inhibitors of PI3K effectively suppressed migration. Our results provide a rationale for the use of PI3K inhibitors for prevention of liver metastasis in colorectal carcinoma patients.
In conclusion, the present study demonstrated that HRG secreted from hepatocytes plays a crucial role in the VN‐dependent motility of an integrin αvβ5‐expressing colon carcinoma cell line, LS‐LM6, by a mechanism in which HRG and its receptors (erbB3/erbB2) as well as activation of the PI3K pathway were involved. Our study suggests the intriguing possibility that hepatocytes might contribute to the enhanced liver metastasis by providing factors required for tumor cell migration into the liver parenchyma.
Supporting information
Fig. S1. (a)Flow cytometric analysis shows the low level expression of β6 and β8 integrins in LS174T and LS‐LM6.
Fig. S2. Western blot shows that the phosphorylation of Akt is clearly suppressed by LY294002 (25 mM) and wortmannin (0.5 mM) without influence of erbB3 phosphorylation (left panel). In migration assay, LY294002 (25 mM) and wartmannin (0.5 mM) significantly suppress the heregulin (HRG)‐induced migration of LS‐LM6 (*P < 0.05, two‐way anova).
Data S1. Materials and Methods.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Acknowledgments
We thank the late Dr K. Koyama for his advice, Ms Yoko Mitobe for technical assistance, and the members of the Department of Molecular Pathology and Tumor Pathology for their helpful discussion.
References
- 1. Nicolson GL. Cancer progression and growth: relationship of paracrine and autocrine growth mechanisms to organ preference of metastasis. Exp Cell Res 1993; 204: 171–80. [DOI] [PubMed] [Google Scholar]
- 2. Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 2003; 3: 453–8. [DOI] [PubMed] [Google Scholar]
- 3. Rudmik LR, Magliocco AM. Molecular mechanisms of hepatic metastasis in colorectal cancer. J Surg Oncol 2005; 92: 347–59. [DOI] [PubMed] [Google Scholar]
- 4. Brodt P, Fallavollita L, Bresalier RS, Meterissian S, Norton CR, Wolitzky BA. Liver endothelial E‐selectin mediates carcinoma cell adhesion and promotes liver metastasis. Int J Cancer 1997; 71: 612–9. [DOI] [PubMed] [Google Scholar]
- 5. Bresalier RS, Byrd JC, Brodt P, Ogata S, Itzkowitz SH, Yunker CK. Liver metastasis and adhesion to the sinusoidal endothelium by human colon cancer cells is related to mucin carbohydrate chain length. Int J Cancer 1998; 76: 556–62. [DOI] [PubMed] [Google Scholar]
- 6. Kitakata H, Nemoto‐Sasaki Y, Takahashi Y, Kondo T, Mai M, Mukaida N. Essential roles of tumor necrosis factor receptor p55 in liver metastasis of intrasplenic administration of colon 26 cells. Cancer Res 2002; 62: 6682–7. [PubMed] [Google Scholar]
- 7. Matsushita Y, Kitajima S, Goto M et al. Selectins induced by interleukin‐1beta on the human liver endothelial cells act as ligands for sialyl Lewis X‐expressing human colon cancer cell metastasis. Cancer Lett 1998; 133: 151–60. [DOI] [PubMed] [Google Scholar]
- 8. Sturm JW, Magdeburg R, Berger K et al. Influence of TNFA on the formation of liver metastases in a syngenic mouse model. Int J Cancer 2003; 107: 11–21. [DOI] [PubMed] [Google Scholar]
- 9. Enns A, Gassmann P, Schluter K et al. Integrins can directly mediate metastatic tumor cell adhesion within the liver sinusoids. J Gastrointest Surg 2004; 8: 1049–59; discussion 60. [DOI] [PubMed] [Google Scholar]
- 10. Martinez‐Hernandez A, Amenta PS. The hepatic extracellular matrix. I. Components and distribution in normal liver. Virchows Arch 1993; 423: 1–11. [DOI] [PubMed] [Google Scholar]
- 11. Seiffert D, Keeton M, Eguchi Y, Sawdey M, Loskutoff DJ. Detection of vitronectin mRNA in tissues and cells of the mouse. Proc Natl Acad Sci USA 1991; 88: 9402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Otter M, Kuiper J, Rijken D, Van Zonneveld AJ. Hepatocellular localisation of biosynthesis of vitronectin. Characterisation of the primary structure of rat vitronectin. Biochem Mol Biol Int 1995; 37: 563–72. [PubMed] [Google Scholar]
- 13. Klemke RL, Yebra M, Bayna EM, Cheresh DA. Receptor tyrosine kinase signaling required for integrin alpha v beta 5‐directed cell motility but not adhesion on vitronectin. J Cell Biol 1994; 127: 859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brooks PC, Klemke RL, Schon S, Lewis JM, Schwartz MA, Cheresh DA. Insulin‐like growth factor receptor cooperates with integrin alpha v beta 5 to promote tumor cell dissemination in vivo . J Clin Invest 1997; 99: 1390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Enns A, Korb T, Schluter K et al. Alphavbeta5‐integrins mediate early steps of metastasis formation. Eur J Cancer 2005; 41: 1065–72. [DOI] [PubMed] [Google Scholar]
- 16. Yoshioka T, Masuko T, Kotanagi H et al. Homotypic adhesion through carcinoembryonic antigen plays a role in hepatic metastasis development. Jpn J Cancer Res 1998; 89: 177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001; 2: 127–37. [DOI] [PubMed] [Google Scholar]
- 18. Zvibel I, Brill S, Papa M, Halpern Z. Hepatocyte‐derived soluble factors regulate proliferation and autocrine growth factor expression in colon cancer cell lines of varying liver‐colonizing capability. Tumour Biol 2000; 21: 187–96. [DOI] [PubMed] [Google Scholar]
- 19. Adam L, Vadlamudi R, Kondapaka SB, Chernoff J, Mendelsohn J, Kumar R. Heregulin regulates cytoskeletal reorganization and cell migration through the p21‐activated kinase‐1 via phosphatidylinositol‐3 kinase. J Biol Chem 1998; 273: 28238–46. [DOI] [PubMed] [Google Scholar]
- 20. Vijapurkar U, Kim MS, Koland JG. Roles of mitogen‐activated protein kinase and phosphoinositide 3′‐kinase in ErbB2/ErbB3 coreceptor‐mediated heregulin signaling. Exp Cell Res 2003; 284: 291–302. [DOI] [PubMed] [Google Scholar]
- 21. Felding‐Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis 2003; 20: 203–13. [DOI] [PubMed] [Google Scholar]
- 22. Schluter K, Gassmann P, Enns A et al. Organ‐specific metastatic tumor cell adhesion and extravasation of colon carcinoma cells with different metastatic potential. Am J Pathol 2006; 169: 1064–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Long L, Nip J, Brodt P. Paracrine growth stimulation by hepatocyte‐derived insulin‐like growth factor‐1: a regulatory mechanism for carcinoma cells metastatic to the liver. Cancer Res 1994; 54: 3732–7. [PubMed] [Google Scholar]
- 24. Kawamoto K, Onodera H, Kan S, Kondo S, Imamura M. Possible paracrine mechanism of insulin‐like growth factor‐2 in the development of liver metastases from colorectal carcinoma. Cancer 1999; 85: 18–25. [DOI] [PubMed] [Google Scholar]
- 25. Miyamoto S, Nakamura M, Shitara K et al. Blockade of paracrine supply of insulin‐like growth factors using neutralizing antibodies suppresses the liver metastasis of human colorectal cancers. Clin Cancer Res 2005; 11: 3494–502. [DOI] [PubMed] [Google Scholar]
- 26. Wen J, Matsumoto K, Taniura N, Tomioka D, Nakamura T. Hepatic gene expression of NK4, an HGF‐antagonist/angiogenesis inhibitor, suppresses liver metastasis and invasive growth of colon cancer in mice. Cancer Gene Ther 2004; 11: 419–30. [DOI] [PubMed] [Google Scholar]
- 27. Ishida H, Sadahiro S, Suzuki T, Ishikawa K, Tajima T, Makuuchi H. c‐erbB‐2 protein expression and clinicopathologic features in colorectal cancer. Oncol Rep 2000; 7: 1229–33. [DOI] [PubMed] [Google Scholar]
- 28. Gambaletta D, Marchetti A, Benedetti L, Mercurio AM, Sacchi A, Falcioni R. Cooperative signaling between alpha(6)beta(4) integrin and ErbB‐2 receptor is required to promote phosphatidylinositol 3‐kinase‐dependent invasion. J Biol Chem 2000; 275: 10604–10. [DOI] [PubMed] [Google Scholar]
- 29. Guo W, Pylayeva Y, Pepe A et al. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 2006; 126: 489–502. [DOI] [PubMed] [Google Scholar]
- 30. Bublil EM, Yarden Y. The EGF receptor family: spearheading a merger of signaling and therapeutics. Curr Opin Cell Biol 2007; 19: 124–34. [DOI] [PubMed] [Google Scholar]
- 31. Sliwkowski MX, Schaefer G, Akita RW et al. Coexpression of erbB2 and erbB3 proteins reconstitutes a high affinity receptor for heregulin. J Biol Chem 1994; 269: 14661–5. [PubMed] [Google Scholar]
- 32. Xue C, Liang F, Mahmood R et al. ErbB3‐dependent motility and intravasation in breast cancer metastasis. Cancer Res 2006; 66: 1418–26. [DOI] [PubMed] [Google Scholar]
- 33. Ueno Y, Sakurai H, Tsunoda S et al. Heregulin‐induced activation of ErbB3 by EGFR tyrosine kinase activity promotes tumor growth and metastasis in melanoma cells. Int J Cancer 2008; 123: 340–7. [DOI] [PubMed] [Google Scholar]
- 34. Maurer CA, Friess H, Kretschmann B et al. Increased expression of erbB3 in colorectal cancer is associated with concomitant increase in the level of erbB2. Hum Pathol 1998; 29: 771–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. (a)Flow cytometric analysis shows the low level expression of β6 and β8 integrins in LS174T and LS‐LM6.
Fig. S2. Western blot shows that the phosphorylation of Akt is clearly suppressed by LY294002 (25 mM) and wortmannin (0.5 mM) without influence of erbB3 phosphorylation (left panel). In migration assay, LY294002 (25 mM) and wartmannin (0.5 mM) significantly suppress the heregulin (HRG)‐induced migration of LS‐LM6 (*P < 0.05, two‐way anova).
Data S1. Materials and Methods.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item


