Abstract
Peptide‐MHC tetramers have been engineered to allow accurate detection of antigen‐specific cytotoxic C lymphocytes (CTL) by flow cytometry. Here, we propose a novel use for peptide‐MHC tetramers in the specific and sensitive analysis of the cytotoxic function of antigen‐specific CTL by blocking MHC‐restricted antigen‐specific cytotoxicity. We found that pretreatment of ovalbumin (OVA)‐specific CD8+ CTL (OT‐1 CTL), derived from OT‐1 T‐cell receptor (TCR)‐transgenic mice, with OVA257−264 peptide‐H‐2Kb tetramer caused a marked inhibition of the cytotoxicity against OVA‐expressing EG‐7 tumor cells. OVA257−264 peptide‐H‐2Kb tetramer did not block the cytotoxicity mediated by 2C mouse (H‐2b)‐derived CD8+ CTL, which recognize allo (H‐2Ld) antigens. Moreover, OT‐I CTL activity was not inhibited by an irrelevant HBV208−216 peptide‐H‐2Kb tetramer. These results indicate that the blocking of CTL activity with peptide‐MHC tetramer was caused by interference with the interaction between the TCR and H‐2Kb‐OVA257−264 peptide complex, but not with the CD8‐MHC class I interaction. The blocking activity of OVA257−264 peptide‐H‐2Kb tetramer was reversible because OT‐I CTL pretreated with the tetramer recovered their cytotoxicity after culturing with interleukin‐2 for 24 h. The same results were also demonstrated in freshly isolated, in vivo‐primed OT‐1 CTL sorted by the tetramer. These results demonstrate that peptide‐MHC tetramer is a useful tool for defining MHC‐restricted antigen‐specific CTL function. Moreover, our finding implies that the measurement of CTL activity immediately after tetramer‐guided sorting is not a suitable method for evaluating the function of in vivo‐induced tetramer‐positive CTL. We believe that the tetramer‐blocking assay presented here will be useful for functionally monitor the induction of MHC‐restricted antigen‐specific CTL during vaccination therapy against tumor and infectious diseases. (Cancer Sci 2006; 97: 148 –154)
Abbreviations:
- CTL
cytotoxic T lymphocyte
- FCM
flow cytometry
- FITC
fluorescein‐isothiocyanate
- HBV
Hepatitis B virus
- HEPES
N‐2‐hydroxyethylpiperazine‐N′‐3‐propanesulfonic acid
- IFN
interferon
- IL
interleukin
- LLC
Lewis lung carcinoma
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- MMC
mitomycin C
- OVA
ovalbumin
- PE
phycoerythrin
- TCR
T cell receptor.
Tracking antigen‐specific immune responses in vivo is quite difficult because ligand‐induced physiological responses to TCR are very low.( 1 ) To overcome this problem, Altman et al. pioneered a biochemical approach to generate tetrameric complexes by combining purified, biotinylated, single peptide‐major histocompatibility complexes with PE‐conjugated avidin (MHC tetramers), which enable direct visualization and quantification of antigen‐specific effectors by FCM.( 2 ) The use of this new technology has been extended to evaluate not only quantitative but also qualitative aspects of T‐cell activation in combination with intracellular cytokine staining, as seen in both basic research and clinical settings,( 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 ) including the monitoring of tumor‐specific CTL induction during tumor‐vaccine therapy.( 11 , 12 , 13 , 14 )
Although the activation properties of peptide‐MHC tetramers with regard to T‐cell function have been reported,( 15 , 16 ) it remains unclear whether cell‐bound peptide‐MHC tetramers used in the isolation of CTL act as activators or as a blockers of CTL‐mediated cytotoxicity. Recently, during sorting of OVA‐specific CTL using a peptide‐loaded MHC tetramer, we found that the sorted tetramer‐bound CTL exhibited no significant cytotoxicity against OVA‐expressing tumors in a standard 4‐h 51Cr release assay. In contrast, similar OVA‐specific CTL isolated using magnetic beads exhibited a strong antigen‐specific cytotoxicity against OVA‐expressing tumor cells. This finding encouraged us to evaluate the precise role of cell‐bound peptide‐MHC tetramers in CTL‐mediated cytotoxicity. Here, we clearly demonstrate that cell‐bound peptide‐MHC tetramers potently block MHC‐restricted peptide‐specific cytotoxicity, and that this blocking activity is reversible. Thus, we propose a novel method for blocking specific cytotoxicity that we have termed the ‘tetramer‐blocking assay’. Our findings also indicate that it might be inappropriate to monitor tumor peptide‐specific CTL‐mediated cytotoxicity immediately after sorting CTL by labeling with peptide‐MHC tetramers.
Materials and Methods
Mice and cell lines
Wild‐type BALB/c and C57BL/6 mice were obtained from Charles River Japan (Kanagawa, Japan). OT‐I transgenic mice were kindly provided by F. R. Carbone (The University of Melbourne, Victoria, Australia). 2C transgenic mice (C57BL/6 background) expressing class I‐restricted H‐2Ld‐specific TCR were kindly donated by K. Iwabuchi (Hokkaido, Japan). All mice were kept in a specific pathogen‐free chamber. EL‐4 cells, OVA‐transfected EL‐4 (E.G7), MBL‐2 cells and LLC cells were derived from C57BL/6 mice (H2Kb), and P815 cells were derived from a BDA/2 mouse (H2Kd).
Reagents and monoclonal antibodies
The PE‐conjugated tetramer of the H‐2Kb‐restricted OVA257−264 (SIINFEKL) peptide was purchased from MBL (Nagoya, Japan). The PE‐conjugated tetramer of the H‐2Kb‐restricted HBV antigenic peptide208−216 (ILSPFLPLL) was purchased from ProImmune (Littlemore, UK). Anti‐CD3 mAb and FITC‐labeled anti‐CD8 mAb were purchased from BD Pharmingen (San Diego, CA, USA). IL‐12 was kindly provided by Genetics Institute (Cambridge, MA, USA). Anti‐IL‐4 (11B11) mAb was purchased from American Type Culture Collection (Manassas, VA, USA). Recombinant IFN‐γ was purchased from Peprotech (London, UK). IL‐2 was supplied by T. Sawada (Shionogi Pharmaceutical Institute, Osaka, Japan). Anti‐CD8 mAb‐conjugated microbeads for the MACS system were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). OVA257−264 peptide was a kind gift of H. Tashiro (Fujiya, Hadano, Japan).
Cell preparation
The LLC cell line is a mouse lung carcinoma of C57BL/6 origin. The LLC‐OVA cell line was derived from LLC by transfection with chicken OVA cDNA. Cells were cultured in Iscove's Modified Dulbecco's Medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, l‐glutamine, 25 mM HEPES buffer, 0.05 mM 2‐mercaptoethanol, penicillin and streptomycin. To enhance the expression of MHC class I, LLC and LLC‐OVA were treated with 10 ng/mL IFN‐γ for 48 h at 37°C and were washed three times with medium before targeting in cytotoxicity assays. T‐cell leukemia EL‐4 and its OVA‐transfected counterpart E.G7, MBL‐2 lymphoma and P815 mastocytoma cells were cultured in RPMI‐1640 medium supplemented with 10% fetal bovine serum, 2 mM l‐glutamine, 0.05 mM 2‐mercaptoethanol, 25 mM HEPES, penicillin and streptomycin. OT‐I mouse derived‐CD8+ CTL (OT‐I CTL) were induced from isolated CD8+ CD44− naive T cells of OT‐I transgenic mice by stimulation with MMC‐treated splenocytes from wild‐type C57BL/6 mice in the presence of OVA257−264 peptide (5 µg/mL) and IL‐2 (100 U/mL), and were expanded for 8–9 days. To obtain 2C mouse‐derived CD8+ CTL (2C cells) specific to H‐2Ld targets, bulk splenocytes from 2C mice were cocultured with MMC‐treated spleen cells from BALB/c mice (2:1 ratio) in 12‐well flat culture plates and cultured for 3 days. The expanded cells were sorted by anti‐CD8 mAb‐conjugated microbeads, and then expanded with IL‐2 (100 U/mL) for another 4 days. We used these cells as effectors in the cytotoxicity assays. To prepare non‐specific CD8+ T cells, CD8+ T cells sorted using the MACS system were activated by 2 µg/mL of plate‐bound anti‐CD3 mAb with exposure to IL‐2 (100 U/mL) for 7 days.
To prepare freshly isolated OT‐1 CTL, OT‐1 transgenic mice were primed in vivo with OVA protein 5 days before the experiment. OT‐1 CTL were then prepared by two different methods. We either used the isolation method using anti‐CD8 mAb and MACS beads, or the isolation method based on cell sorting by FACSVantage (Becton Dickinson, San Jose, CA, USA) after staining with the OVA tetramer. During the 24‐h incubation, the tetramer‐treated OT‐1 CTL were cultured in the presence of IL‐2 (10 µ/mL).
Tetramer staining
We used a slightly modified version of the original manufacturer's instructions to produce OVA‐MHC tetramers and produced HBV‐MHC tetramers according to the manufacturer's instructions. OT‐I CTL or 2C cells were washed twice with phosphate‐buffered saline and stained with 3.3 µL/mL of OVA‐MHC or HBV‐MHC tetramers per 1 × 106 cells at 4°C for 15 min (OVA‐MHC tetramer) or 30 min (HBV‐MHC tetramer), followed by FITC‐labeled anti‐CD8 mAb. The cells were washed again with phosphate‐buffered saline and analyzed by FCM. For use in cytotoxicity assays, the effectors were labeled with OVA‐MHC or HBV‐MHC tetramers in the same manner, with the exception that FITC‐labeled anti‐CD8 mAb was not used.
Flow cytometric analysis
Detailed procedures for staining and sorting were as described previously.( 17 ) Fluorescence data were collected on a FACSCalibur (Becton Dickinson, San Jose, CA, USA), and analyzed using CellQuest software (BD Biosciences, Mountain View, CA, USA).
Cytotoxicity assay
The cytotoxic activities of OT‐I CTL and 2C cells were assessed using standard 4‐h 51Cr release assays as described previously.( 17 ) Tumor‐specific cytotoxicity by OT‐I CTL was determined using LLC‐OVA and E.G7 as target cells. As controls, LLC, EL‐4 and MBL‐2 were used. In the case of 2C cells, the H‐2d‐specific cytolytic activity was determined using P815 (H‐2d) as a target, and MBL‐2 and E.G7 (both H‐2b) as control cells. The tests were run in triplicate at the indicated effector : target ratios. The percentage cytotoxicity was calculated as described previously.( 17 )
Results
Blockade of CTL‐mediated cytotoxicity by OVA‐MHC tetramers in a MHC‐restricted and antigenic peptide‐specific manner
The specificity of the OVA‐MHC tetramers was first examined by FCM using either non‐specific in vitro‐activated CD8+ T cells derived from the spleens of wild‐type C57BL/6 mice or by using OVA257−264 peptide‐specific CTL prepared from OT‐I transgenic mice. As shown in Fig. 1a,b, OVA‐MHC tetramers reacted with less than 1% of non‐specifically activated CD8+ T‐cell populations. In contrast, approximately 90% of OT‐I CTL were stained with OVA‐MHC tetramers. OT‐I CTL specifically killed OVA‐gene transduced EL‐4 (E.G7) cells, while they lysed neither the parental EL‐4 nor MBL‐2 cells (Fig. 1c). However, OVA‐specific cytotoxicity of OT‐I CTL was markedly blocked when the CTL were pretreated with the OVA‐MHC tetramer (Fig. 1c), and this blocking effect was enhanced in an OVA‐MHC tetramer dose‐dependent manner (Fig. 1d). Similar results were obtained using LLC and LLC‐OVA cells, instead of the EL‐4 and E.G7 cells, respectively (data not shown).
Figure 1.
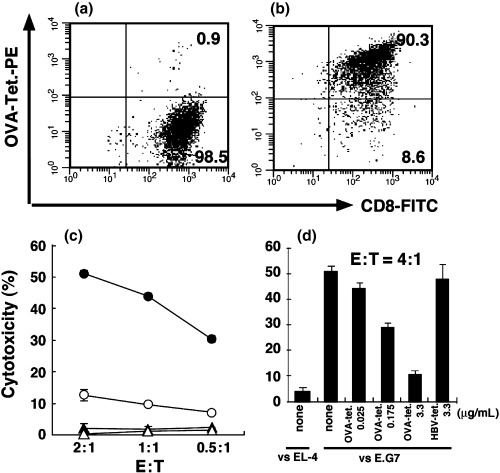
Cytotoxicity of OVA‐specific CTL is inhibited by OVA‐MHC tetramer. OT‐I CTL were generated in vitro with MMC‐treated C57BL/6 splenocytes and the necessary cytokines. (a) FCM analysis of in vitro‐generated non‐specific CD8+ T cells from wild‐type C57BL/6 mice was carried out using PE‐labeled SIINFEKL/H‐2Kb tetramer (OVA‐Tet‐PE) and FITC‐labeled anti‐CD8 mAb. (b) FCM analysis of the generated OT‐I CTL was carried out using PE‐labeled OVA‐MHC tetramer and FITC‐labeled anti‐CD8 mAb. The indicated number shows the percentage of the gated population within the total cell population. OT‐I CTL were added to produce the indicated effector : target (E:T) ratios, and then a standard 4‐h 51Cr release assay was carried out. (c) Cytotoxicity against E.G7 (•), EL‐4 (▴) and MBL‐2 (▵) cells mediated by OT‐I CTL without tetramer labeling. (○), Cytotoxicity against E.G7 cells mediated by OT‐I CTL prelabeled with OVA‐MHC tetramers. (d) 1 × 106 OT‐1 CTL were labeled with 0.025 µg/mL, 0.175 µg/mL and 3.3 µg/mL OVA‐MHC tetramer and 3.3 µg/mL HBV‐MHC tetramer. The cytotoxicity of OT‐I CTL with or without tetramer was examined against E.G7 or EL‐4 cells (E:T = 4:1). The bars represent the mean ± SE of duplicate samples.
Recently, it was reported that some peptide‐MHC tetramers bound to the CD8 coreceptor in addition to the TCR. Therefore, it was of great importance to determine whether the inhibition of CTL activity by OVA‐MHC tetramers was MHC‐restricted and antigen‐specific. As shown in Fig. 2a,b, the cytotoxicity of OVA‐specific OT‐I CTL was not inhibited by the antigen‐irrelevant MHC‐matched HBV peptide‐H‐2Kb tetramer. Moreover, the activity of allogeneic antigen (H‐2Ld)‐reactive CTL derived from 2C transgenic mice (H‐2b) was not blocked by OVA‐MHC tetramers (Fig. 2c). These results indicated that OVA‐MHC tetramers could block CTL‐mediated cytotoxicity in a MHC‐restricted and antigenic peptide‐specific manner.
Figure 2.
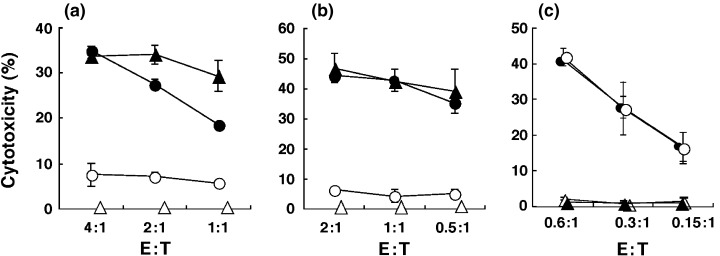
OVA‐specific cytotoxicity of OT‐1 CTL is not affected by irrelevant peptide‐loaded cognate MHC tetramers, and OVA‐MHC tetramer does not inhibit allo‐specific cytotoxicity. (a,b) OT‐1 CTL were labeled with (○) or without (•) OVA‐MHC tetramers, or with PE‐labeled HBV peptide (ILSPFLPLL)/H‐2Kb tetramers (HBV‐MHC tetramers) (▵), added to produce the indicated effector : target (E : T) ratios, and then a standard 4‐h 51Cr release assay was carried out against (a) LLC‐OVA and (b) E.G.7 cells. Cytotoxicity mediated by OT‐I CTL without tetramer binding against (a) LLC (▵) and (b) EL‐4 (▵) cells. (c) H‐2Ld‐specific 2C cells were prepared by culturing with MMC‐treated BALB/c splenocytes and the necessary cytokines. On day 7, the generated 2C cells were stained with or without OVA‐MHC tetramers, adjusted to give the indicated E:T ratios and then standard 4‐h 51Cr release assays were carried out. Cytotoxicity against P815 (H‐2d) cells mediated by 2C cells with (○) or without (•) tetramers is indicated. Cytotoxicity mediated by 2C cells in the absence of tetramer labeling against MBL‐2 (H‐2b) cells (▴) and E.G7 (H‐2b) cells (▵). The bars represent the mean ± SE of duplicate samples.
Restoration of antigen‐specific cytotoxicity of OT‐I CTL pretreated with OVA‐MHC tetramers
Next, we examined whether the blocking activities of OVA‐MHC tetramers against CTL activity is reversible or irreversible. Consistent with the above result (Fig. 1), pretreatment of OT‐I CTL with the OVA tetramer caused a marked inhibition of CTL activity against LLC‐OVA and EG‐7 (Fig. 3a,c). However, OT‐I CTL pretreated with the OVA tetramer recovered their cytotoxicity when the cells were cultured in the presence of IL‐2 for an additional 24 h (Fig. 3b,d). This is because of the disappearance of the OVA tetramer from OT‐I CTL during the final 24‐h incubation. Indeed, we found that over 88% of tetramer‐positive OT‐I CTL became negative for tetramer staining during the 24 h incubation (Fig. 3e,f). In contrast, control untreated OT‐I CTL cultured for 24 h maintained their antigen specificity and lysed OVA‐expressing LLC‐OVA and EG‐7 tumor cells but not their parental tumor cells (Fig. 3b,d).
Figure 3.
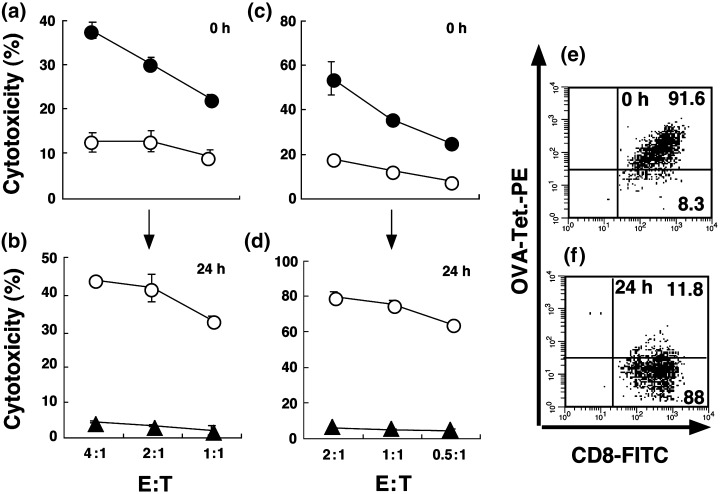
OVA‐specific cytotoxicity of OT‐I CTL prelabeled with OVA‐MHC tetramer is reversible if they are cultured for 24 h in the presence of IL‐2 before assay. (a,c) Cytotoxicity of OT‐I CTL was detected by a 4‐h 51Cr release assay against (a) LLC‐OVA and (c) E.G7 cells with (○) or without (•) OVA‐MHC tetramer. The OT‐I CTL pretreated with OVA‐MHC tetramer were cultured with IL‐2 for 24 h, and subjected to 4‐h 51Cr release assay. (b) Cytotoxicity against LLC‐OVA (○) or LLC (▵) cells mediated by the 24 h‐incubated OT‐I CTL pretreated with OVA‐MHC tetramer. (d) Cytotoxicity against E.G7 (○) or EL‐4 (▴) cells mediated by the 24 h‐incubated OT‐I CTL pretreated with OVA‐MHC tetramer. (e) FCM analysis of OT‐I CTL labeled with OVA‐MHC tetramer. (f) FCM analysis of 24 h‐incubated OT‐I CTL prelabeled with OVA‐MHC tetramer. The indicated number shows the percentage of the gated cell populations in the total cell population. The bars represent the mean ± SE of duplicate samples.
Blockade and subsequent restoration of cytotoxic activity of freshly isolated OT‐1 CTL with peptide‐MHC tetramer
Peptide‐MHC tetramer‐mediated blocking of cytotoxic activity was also demonstrated in freshly isolated OT‐1 CTL, which were prepared from OT‐1 transgenic mice primed in vivo with OVA protein 5 days before the experiment. Freshly isolated OT‐1 CTL were prepared by two different methods. One isolation method used anti‐CD8 mAb and MACS beads, whereas the other was based on isolation by cell‐sorting using FACSVantage after staining with the OVA tetramer. As shown in Fig. 4a, OT‐1 CTL freshly isolated with anti‐CD8 mAb and MACS beads revealed a specific cytotoxicity against EG‐7 but not against parental EL4 cells. However, FACS‐sorted tetramer‐bound OT‐1 CTL showed no significant cytotoxicity against EG‐7 cells. However, if tetramer‐isolated OT‐1 CTL were cultured with IL‐2 for 24 h before the cytotoxicity assay, the CTL recovered their specific cytotoxicity against EG‐7 cells in parallel with the disappearance of cell‐bound OVA tetramer (Fig. 4b–d), as observed in the tetramer blocking assay. We also confirmed that whole spleen cells, which were prepared from OT‐1 transgenic mice primed in vivo with OVA protein, were blocked by OVA tetramer (data not shown). During the 4‐h incubation of freshly isolated OT‐1 CTL, we detected neither apoptosis nor downregulation of TCR expression in tetramer‐bound OT‐1 CTL (data not shown).
Figure 4.
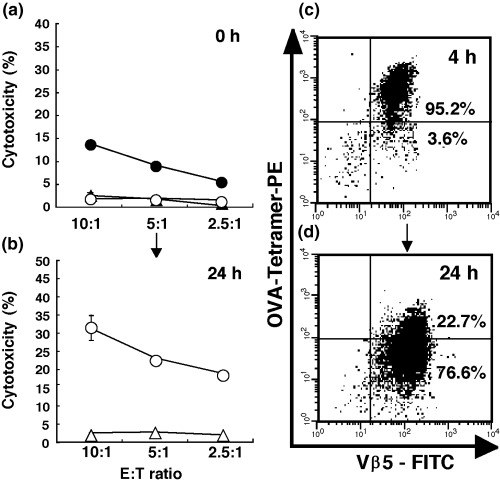
The cytotoxicity of freshly isolated OT‐1 CTL is inhibited by OVA‐MHC tetramer. (a) The cytotoxiciy of OT‐1 CTL isolated by OVA‐MHC tetramer (○) or by anti‐CD8 monoclonal antibody (•, ▴) was determined by 4‐h 51Cr release assay against E.G7 (•, ○) or against EL‐4 (▴) cells. (b) Upon 24 h incubation with IL‐2 after tetramer treatment, the cytotoxicity of the OT‐1 CTL was determined against E.G7 (○) and EL‐4 (▵) cells. (c) FCM analysis of freshly isolated‐OT‐I CTL labeled with OVA‐MHC tetramer. (d) FCM analysis of 24 h‐incubated OT‐I CTL prelabeled with OVA‐MHC tetramer. The bars represent the mean ± SE of duplicate samples. Indicated numbers stand for the percentages of the gated populations within the total cell population.
Discussion
In the present study, we demonstrated that OVA peptide‐loaded MHC tetramer is a useful tool for defining MHC‐restricted antigen‐specific CTL‐mediated cytotoxicity. Pretreatment of OVA‐specific CTL with OVA‐MHC tetramers caused a strong inhibition of their cytotoxicity in a MHC‐restricted and antigenic peptide‐specific manner. We called this cytotoxic blocking assay the ‘tetramer‐blocking assay’.
Peptide‐MHC tetramers have been developed for the direct visualization and quantification of antigen‐specific CTL by FCM. Indeed, peptide‐MHC tetramers enabled us to detect the frequency of tumor peptide‐specific CTL in tumor patients after tumor vaccine therapy. Recently, peptide‐MHC tetramers were reported to act as strong T‐cell activating reagents in addition to diagnostic reagents.( 15 , 16 ) However, we initially demonstrated that peptide‐MHC tetramers act to block CTL‐mediated cytotoxicity. Generally, we carried out cold target inhibition assays or blocking assays using anti‐MHC mAb in 51Cr release assays to define antigen specificity and MHC restriction of the CTL. However, these assays have some limitations for analyzing the killing mechanism of CTL. We were unable to exclude the effects of another shared antigen recognized by tumor‐specific T cells in our cold inhibition assay. Anti‐MHC molecule mAb possess cross reactivity toward different antigens loaded on the cognate MHC molecules. Compared with those assays, our novel tetramer‐blocking assay is a very critical and reliable assay for defining MHC‐restricted antigen‐specific cytotoxicity because peptide‐MHC tetramers bind specifically to the TCR on CTL. As it was possible that the cytotoxicity of the CTL was blocked by the MHC tetramers non‐specifically binding to the CD8 coreceptor, we avoided it by using a MHC‐tetramer complex with an α3 domain mutant of the MHC heavy chain, which Bodinier et al. engineered to abrogate its non‐specific binding to the TCR.( 18 ) Xu et al. reproted that this structure diminished its engagement with the CD8 coreceptor molecule on T cells.( 19 ) Consistent with their results, we also deduced that tetramers did not block CTL activity via binding to CD8 coreceptors from the following results: (i) antigen‐irrelevant HBV peptide‐MHC tetramers did not inhibit OVA‐specific CTL activity; and (ii) BALB/c‐derived alloantigen (H‐2Ld)‐reactive CTL activity was not blocked by OVA257−264 peptide‐H‐2Kb tetramers.
It is conceivable that the mechanism of ‘inhibited’ cytotoxicity of effectors by the OVA‐MHC tetramer can be explained by its constitutive binding to the T cells, allowing competitive blocking of binding to receptor targets. It has been reported that the presence of peptide‐MHC complexes on the T‐cell surface could make these T cells susceptible to lysis by neighboring T cells with peptide‐specific CTL activity.( 20 ) However, in our experiment, OVA‐MHC‐pretreated CTL did not show significant apoptotic cell death during 4‐h incubation (data not shown). This may be because we used a MHC‐tetramer complex with an α3 domain mutant of MHC, which diminished the TCR signal and perforin/granzyme release essential for inducing apoptosis of T cells. Therefore, tetramer blocking of cytotoxicity appeared not to derive from its apoptosis‐inducing mechanisms. We also confirmed that cytotoxicity blocking by the tetramer was not derived from TCR downregulation due to shedding( 21 ) or internalization( 22 , 23 ) of this receptor after engagement with peptide‐MHC complexes as reported previously.
Recently, tetramer‐positive tumor peptide‐specific CTL were reportedly detected in tumor patients after tumor peptide vaccine therapy.( 4 , 24 ) Nevertheless, while clinical studies have indicated that tumor‐rejection antigen (TRA) peptides can elicit antigen‐specific tetramer‐positive CTL in vivo, the increased frequency of CTL is not associated with clinical outcome in terms of tumor rejection in these patients.( 4 , 24 ) In such cases, the generation of CTL alone is not a good indicator of clinical response and it therefore becomes necessary to investigate the functional capacity of these tetramers with or without CTL. However, our present findings reveal that measuring the CTL activity immediately after tetramer‐guided sorting is not a reliable method to evaluate the function of in vivo‐induced tetramer‐positive CTL. Although we need to examine whether our novel tetramer blocking assay is suitable for other target specificities, it is important to note that the effectors obtained by tetramer‐guided sorting should be cultured in vitro for 24 h in the presence of IL‐2 in order to obtain an accurate evaluation of their specific killing activities in the cytotoxicity assay.
Acknowledgments
We would like to thank Dr Mark J. Micallef (TORAY, Kamakura, Japan) for helpful proofreading during the preparation of this manuscript. We would like to thank Dr Michiko Kobayashi (Genetics Institute, Cambridge, MA, USA) and Ms Takuko Sawada (Shionogi Pharmaceutical Institute, Osaka, Japan) for their kind donations of IL‐12 and IL‐2, respectively. This work was supported in part by a Grant‐in‐Aid for Science Research on Priority Areas and Millennium Project from the Ministry of Education, Culture, Sports, Science and Technology.
References
- 1. Howard MC, Spack EG, Choudhury K, Greten TF, Schneck JP. MHC‐based diagnostics and therapeutics − clinical applications for disease genes. Immunol Today 1999; 20: 161–5. [DOI] [PubMed] [Google Scholar]
- 2. Altman JD, Moss PAH, Goulder PJR et al. Phenotypic analysis of antigen‐specific T lymphocytes. Science 1996; 274: 94–6. [PubMed] [Google Scholar]
- 3. Bousso P, Casrouge A, Altman JD et al. Individual variations in the murine T‐cell response to a specific peptide reflect variability in naïve repertoires. Immunity 1998; 9: 169–78. [DOI] [PubMed] [Google Scholar]
- 4. Lee PP, Savage PA, Fong L et al. Characterization of circulating T cells specific for tumor‐associated antigens in melanoma patients. Nature Med 1999; 5: 677–85. [DOI] [PubMed] [Google Scholar]
- 5. Yee C, Riddell SR, Greenberg PD. In vivo tracking of tumor‐specific T cells. Cur Opin Immmunol 2001; 13: 141–6. [DOI] [PubMed] [Google Scholar]
- 6. Hoffman TK, Donnenberg AD, Finkeistein SD et al. Frequencies of tetramer+ T cells specific for the wild‐type sequence p53264−272 peptide in the circulation of patients with head and neck cancer. Cancer Res 2002; 62: 3521–9. [PubMed] [Google Scholar]
- 7. Palmowski M, Salio M, Dunbar RP, Cerundolo V. The use of HLA class I tetramers to design a vaccination strategy for melanoma patients. Immunol Rev 2002; 188: 155–63. [DOI] [PubMed] [Google Scholar]
- 8. Cohen CJ, Denkberg G, Schiffenbauer YS et al. Simultaneous monitoring of binding to and activation of tumor‐specific T lymphocytes by peptide‐MHC. J Immunol Meth 2003; 277: 39–52. [DOI] [PubMed] [Google Scholar]
- 9. Rosato A, Santa SD, Zoso A et al. The cytotoxic T‐lymphocyte response against a poorly immunogenic mammary adenocarcinoma is focused on a single immunodominant class I epitope derived from the gp70 env product of an endogenous retrovirus. Cancer Res 2003; 63: 2158–63. [PubMed] [Google Scholar]
- 10. Whiteside TL, Zhao Y, Tsukishiro T, Elder EM, Gooding W, Baar J. Enzyme‐linked immunospot, cytokine flow cytometry, and tetramers in the detection of T‐cell response to a dendritic cell‐based multipeptide vaccine in patients with melanoma. Clin Cancer Res 2003; 9: 641–9. [PubMed] [Google Scholar]
- 11. Rivoltini L, Castelli C, Carrabba M et al. Human tumor‐derived heat shock protein 96 mediates in vitro activation and in vivo expansion of melanoma‐ and colon carcinoma‐specific T cells. J Immunol 2003; 171: 3467–74. [DOI] [PubMed] [Google Scholar]
- 12. Zajac P, Oertli D, Marti W et al. Phase I/II clinical trial of a nonreplicative vaccinia virus expressing multiple HLA‐A0201‐restricted tumor‐associated epitopes and costimulatory molecules in metastatic melanoma patients. Hum Gene Ther 2003; 14: 1497–510. [DOI] [PubMed] [Google Scholar]
- 13. Walker EB, Haley D, Miller W et al. gp100209‐2M peptide immunization of human lymphocyte antigen A2+ stage I‐III melanoma patients induces significant increase in antigen‐specific effector and long‐term memory CD8+ T cells. Clin Cancer Res 2004; 10: 668–80. [DOI] [PubMed] [Google Scholar]
- 14. Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate‐pulsed dendritic cells elicits antigen‐specific, cytotoxic T‐cells in patients with malignant glioma. Cancer Res 2004; 64: 4973–9. [DOI] [PubMed] [Google Scholar]
- 15. Cochran JR, Cameron TO, Stern LJ. The relationship of MHC‐peptide binding and T cell activation probed using chemically defined MHC class II oligomers. Immunity 2000; 12: 241–50. [DOI] [PubMed] [Google Scholar]
- 16. Cohen CJ, Denkberg G, Schiffenbauer YS et al. Simultaneous monitoring of binding to and activation of tumor‐specific T lymphocytes by peptide‐MHC. J Immunol Meth 2003; 277: 39–52. [DOI] [PubMed] [Google Scholar]
- 17. Nishimura T, Iwakabe K, Sekimoto M et al. Distinct role of specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo . J Exp Med 1999; 190: 617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bodinier M, Peyrat M, Tournay C et al. Efficient detection and immunomagnetic sorting of specific T cells using multimers of MHC class I and peptide with reduced CD8 binding. Nature Med 2000; 6: 707–10. [DOI] [PubMed] [Google Scholar]
- 19. Xu XN, Purbhoo MA, Chen N et al. A novel approach to antigen‐specific deletion of CTL with minimal cellular activation using α3 domain mutants of MHC class I/peptide complex. Immunity 2001; 14: 591–602. [DOI] [PubMed] [Google Scholar]
- 20. Huang JF, Yand Y, Spulveda H et al. TCR‐mediated internalization of peptide‐MHC complexes acquired by T cells. Science 1999; 286: 952–4. [DOI] [PubMed] [Google Scholar]
- 21. Dustin ML, Miller JM, Ranganath S et al. TCR‐mediated adhesion of T‐cell hybridomas to planar bilayers containing purified MHC class II/peptide complexes and receptor shedding during detachment. J Immunol 1996; 157: 2014–21. [PubMed] [Google Scholar]
- 22. Valitutti S, Müller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T‐cell receptors by a few peptide‐MHC complexes. Science 1995; 375: 148–51. [DOI] [PubMed] [Google Scholar]
- 23. Itoh Y, Hemmer B, Martin R, Germain RN. Serial TCR engagement and down‐modulation by peptide : MHC molecule ligands: relationship to the quality of individual TCR signaling events. J Immunol 1999; 162: 2073–80. [PubMed] [Google Scholar]
- 24. Lee KH, Wang E, Nielsen MB et al. Increased vaccine‐specific T cell frequency after peptide‐based vaccination correlates with increased susceptibility to in vitro stimulation but does not lead to tumor regression. J Immunol 1999; 163: 6292–300. [PubMed] [Google Scholar]


