Sometime during the reign of Tiberius (25-50 ad) Celsus wrote:
A chronic malady may develop in patients who collect water under their skin. The Greeks call this hydrops. There are three species:
(1) Sometimes the belly is tense. The Greeks call this tympanites.
(2) Sometimes the body is rendered uneven by swellings arising here and there and all over. The Greeks call this hyposarka.
(3) Sometimes, the water is all drawn within and is called ascites.1
Oedema has been recognised almost since the earliest recordings of medical history. Even the ancient Egyptians seem to have had a hieroglyphic for “water under the skin.” In the description of case number four in the Edwin Smith papyrus the hieroglyphic appears to be similar to the one used for water that floods from the Nile.2 This ancient familiarity, however, has not yet led to a complete understanding of oedema, and sometimes its treatment remains imperfect. None the less, an improved understanding of the pathophysiology and biophysics of oedema will allow most doctors to develop a more rational approach to treating it.
Summary points
The causes of oedema are not well understood
Three main variables are associated with the formation of oedema: oncotic (colloid osmotic) pressure, hydrostatic pressure, and membrane permeability
Differential diagnosis should be based on an understanding of which of the variables has been altered
Osmotic pressure is a measure of the entropy of the solution
Entropy can be altered without changing the number of molecules in a protein solution if the arrangement of those molecules is changed
Methods
This article is based mostly on our own research and clinical experience.
Pathophysiology
When Bright first described the retention of water and waste by diseased kidneys3 he was deliberately vague about whether he was describing one disease or two since he could not explain water retention without uraemia, nor could he explain uraemia without oedema.4 After more than half a century, Starling defined the physiological forces involved in the production of oedema.5 Those forces included the difference between intracapillary blood pressure and extravascular hydrostatic pressure (ΔP), differences in oncotic (colloid osmotic) pressure (Δπ), and the permeability of the blood vessel wall (Kf). These remain the primary variables used to characterise fluid movement (FM) and the formation of oedema:
FM=Kf(ΔP−Δπ).
Diagnosis
Although up to 4.5 kg of excess total body fluid may be present without physical evidence of oedema, inspection and palpation are usually sufficient to identify it. Compression of the skin with a finger often results in “pitting”; this can be a useful aid in judging the amount of oedema and the type. After 10 seconds’ compression of the affected skin, the resulting depth in millimetres can be used to determine the amount of oedema, which is classed as ranging from trace to +4; the amount of oedema is classed as +4 in cases in which an indentation or “pit” of a depth greater than or equal to the depth of one’s fingers occurs. If the length of time that the pit remains in the skin after the finger is withdrawn is less than 15 seconds then low oncotic pressure is the likely cause of the oedema; if it is greater than 15 seconds a high capillary hydrostatic pressure should be suspected. Localised oedema is most likely to result from a local alteration. Generalised oedema is often most prominent in the lower extremities in ambulatory patients and in the presacral region in those who are confined to bed. Gravitational effects alone usually explain such distributions; however, children with kidney disease sometimes exhibit periorbital oedema (fig 1).
Figure 1.
Child with periorbital oedema
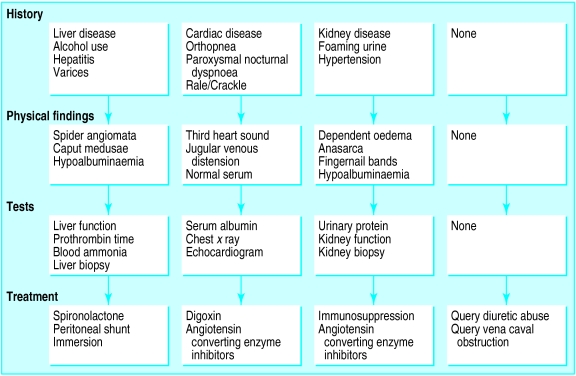
The table presents the clinical classification of the causes of oedema using a rational approach which is based on identification of the underlying physiological alteration. A typical algorithm for the diagnosis and treatment of oedema is shown in figure 2.
Figure 2.
Algorithm for diagnosing and treating oedema
Increased permeability of the blood vessel wall
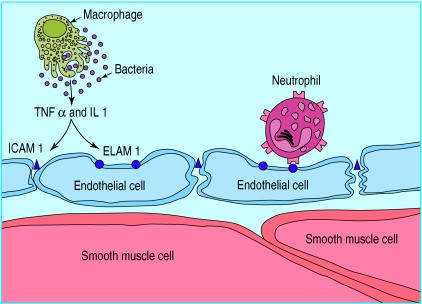
Changes in the permeability of the capillary wall are common. When Celsus described the classic signs of inflammation (redness, heat, swelling, and pain), he was describing an increase in permeability.1 Today we know that cytokines, such as tumour necrosis factor, interleukin 1, and interleukin 10 (fig 3) mediate increases in vascular permeability.
Figure 3.
Cytokines acting on adhesion molecules to increase vascular permeability. TNF=tumour necrosis factor; IL=interleukin; ELAM=endothelial linked adhesion molecule; ICAM=intercellular adhesion molecule
In addition to the localised oedema of inflammation, childhood nephrotic syndrome caused by minimal change disease results from an altered permeability of the glomerular capillary bed and the ensuing proteinuria. The loss of plasma proteins causes a decrease in oncotic (colloid osmotic) pressure which promotes further fluid movement. Similarly, although the difference between intracapillary blood pressure and extravascular hydrostatic pressure, and the difference in oncotic pressure probably play a greater part in the development of oedema in patients with cirrhosis, an increase in circulating vasodilatory prostaglandins, nitric oxide, and various gut hormones also alters vascular permeability.
Increased difference between intracapillary and extravascular pressure
Raised capillary venous pressure occurs in cases of salt and water retention; renal failure with oliguria may be one cause. The most discriminating diagnostic finding is the presence of increased circulating concentrations of urea and creatinine. Ultrasound examination of the kidneys may be useful in distinguishing acute, and often reversible, renal failure from chronic, and irreversible, renal insufficiency.
Venous and lymphatic obstruction may cause localised oedema. Deep venous thrombosis often presents with a palpable vein or occasionally a positive Homans’s sign but without oedema caused by collateral drainage. Phlegmasia cerulea dolens (blue phlebitis) occurs when the major deep veins are occluded with a thrombus and thus results in a marked increase in hydrostatic pressure within the venous system.
Left sided heart failure usually leads to pulmonary oedema as a result of increased pulmonary capillary wedge pressures. Once the right side of the heart begins to fail, increased pressures result in peripheral oedema (fig 4).
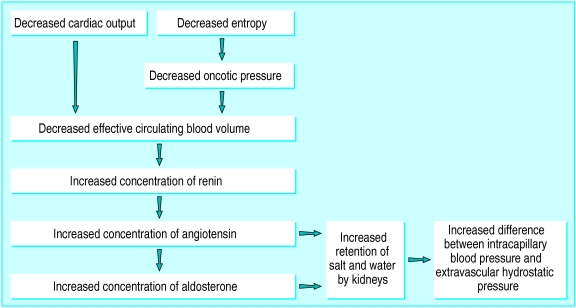
Figure 4.
Factors influencing the increased difference between intracapillary blood pressure and extravascular hydrostatic pressure
Raised portal venous pressure can cause ascites. This type of increase is primarily the result of scarring of the hepatic parenchyma which increases the vascular resistance in postsinusoidal venules; there are also increased circulating concentrations of renin, angiotensin, and aldosterone that are thought to be the result of a decrease in the effective circulating blood volume. However, the nebulous concept of a “decreased effective circulating blood volume” is often little more than a consequence of reduced oncotic pressure (fig 4). When the elevated portal pressure has been caused by cirrhosis of the liver, lower oncotic pressure may occur as a result of the loss of the ability of the liver to synthesise plasma proteins, a process which acts through the renin-angiotensin-aldosterone axis to further increase the difference between intracapillary blood pressure and extravascular hydrostatic pressure.
Decreased oncotic (colloid osmotic) pressure
The nephrotic syndrome, liver disease, and kwashiorkor are the most common examples of disorders that cause oedema when there is a loss of oncotic pressure. The nephrotic syndrome encompasses a constellation of signs and symptoms (proteinuria >3.5 g per 24 hours, hypoalbuminaemia, lipiduria, hypercholesterolaemia, and oedema), but many clinicians often incorrectly use “nephrotic range proteinuria” as a synonym for the nephrotic syndrome. Nevertheless, proteinuria remains the most salient finding in establishing the diagnosis.
Although a decreased serum concentration of albumin may indicate excessive renal loss and paired white lines in the fingernails are often present on examination, it may also have been caused by a reduction in the capability of the liver to synthesise plasma proteins. In such cases a prolonged prothrombin time combined with other evidence of liver disease may lead to a confirmatory diagnosis of cirrhosis by biopsy (table).
A number of formulas have been derived to predict oncotic pressure from serum protein concentrations. However, we have not been able to adequately predict which patients would develop oedema using a given concentration of serum proteins.6 Moreover, there have been reports of patients with AIDS nephropathy who have not developed oedema, despite severely decreased concentrations of plasma proteins.7 One study showed that protein from patients with kidney disease did not behave similarly to protein from normal controls; it often exerted a much lower oncotic pressure when measured in vitro by a synthetic membrane in a colloid osmometer.8 Furthermore, when the plasma proteins were concentrated or diluted, plasma from patients with kidney disease showed a great variation in the predictability of oncotic pressure.8 To understand oedema, doctors must understand oncotic (colloid osmotic) pressure (box).
What is entropy?
Molecular arangements in a solution are random. The more chaotic the arrangement, the less free energy each molecule has. Oedema arises when molecules have increased free energy. Entropy is a measure of the degree of chaos in the solution. Thus, increased entropy also causes less oedema.
The biophysics of osmotic pressure
One hundred years after Starling, argument continues as to the biophysics of the osmotic phenomenon.9 How does osmotic pressure work? A solute dissolving in a solvent disrupts the arrangement of molecules resulting in an increase in entropy and a decrease in free energy. The decrease in free energy results in less random thermal movement and fewer collisions, which causes comparatively more movement of the solute free solvent across membranes until a new equilibrium of free energy and entropy is reached.
Plasma proteins provide most of the oncotic pressure. Polyelectrolyte colloids such as plasma proteins are even more complicated than simple solutions. Because most plasma proteins carry multiple anionic charges, a cloud of cations forms a counterion cloud around these molecules. If a change is made in the plasma that causes a reduction or condensation of the counterion cloud, there will be a decrease in free entropy with corresponding increases in free energy and the activity coefficient with a resulting increase in random thermal motion and a decrease in oncotic pressure. The contribution of that ion atmosphere to an increase in entropy has attracted increasing attention.10
Treatment
Treatment should always focus on correcting the underlying cause of the oedema. Our approach has been to determine which variable (permeability of the blood vessel wall, difference between intracapillary and extravascular pressure, or difference in oncotic pressure) has become deranged and then to treat the disease that has altered that variable. For localised oedema caused by infection, hypothyroidism, or an autoimmune reaction, treatment should be with antibiotics, thyroid replacement, and steroids respectively.
If the oedema is the result of decreased cardiac function then inotropic agents such as digoxin or dobutamine may be useful in conjunction with afterload reduction with angiotensin converting enzyme inhibitors or angiotensin receptor blockers.
If the nephrotic syndrome is identified by renal biopsy as an autoimmune phenomenon, immunosuppression may prove useful. Similarly, if the renal pathology seems to be caused by glomerulosclerosis or hyperfiltration, angiotensin converting enzyme inhibitors may be beneficial. In cases of oliguric renal failure, dialysis may be required for volume removal.
In kwashiorkor and oedema caused by nutritional deficiency, improved nutrition may initially result in “refeeding oedema” because of the effect of increased insulin concentrations on sodium retention.
Non-specific considerations
Restriction of salt and water intake has been used to treat generalised oedema since the days of Celsus for cases in which the underlying cause cannot be treated. In such cases diuretics are usually used; however, they should not be used for reasons that are merely cosmetic. Because they stimulate the production of aldosterone, diuretics have been implicated in the aggravation of the cyclical oedema of women (a disorder of periodic oedema associated with menstruation and often called idiopathic or diuretic induced oedema). Similarly, the complications of loop diuretics may make their use counterproductive. In fact, the metabolic alkalosis that arises from the inhibition of chloride reabsorption may actually result in a worsening of the oedema because of a decrease in oncotic pressure. This may be a contributing factor in the production of the idiopathic oedema associated with the use of diuretics.
Albumin infusions create an ephemeral improvement and are of little value11; they carry the risks associated with the infusion of any blood product. Ascites reinfusion, in which ascitic fluid is transferred back into the vascular space either by removal and subsequent reinfusion through a peripheral vein or by continuous reinfusion with a peritoneal-atrial, or Leveen, shunt can be useful in treating cirrhosis, particularly when a peritoneal-atrial or peritoneal-venous shunt is surgically created. “Head out immersion” treatment can increase extravascular hydrostatic pressure thereby decreasing the difference between intracapillary pressure and extravascular hydrostatic pressure, but the effect will last only as long as the body is immersed under water, so it is not a practical solution. Support stockings and limb elevation provide the same effect. Captopril may be useful in treating the cyclical oedema of women because it inhibits production of aldosterone and bradykinin.12
Future considerations
Can alterations be made to plasma to restore free entropy and avoid the need for diuretics or albumin infusions? Theoretically, the answer is “yes.” The addition of a solitary carbon atom to the tail of a variable alkyl chain, a colloid, has been shown to alter its osmotic coefficient.13 Antioxidants might help, since oxidation stress results in greater surface hydrophobicity. Work is just beginning into many of the theoretical areas of intervention.
Some day we may be able to treat oedema caused by the nephrotic syndrome by restoring free entropy to the plasma rather than by using diuretics. Perhaps we might be able to maintain oncotic pressure even in the presence of decreased concentrations of plasma proteins, in a manner similar to the Nargase analbuminaemic rats, a strain of rat that cannot synthesise albumin.14 Then, for the first time since Celsus, we would actually be addressing the problem rather than merely the symptom of water retention.
Table.
Physiological mechanisms and diagnosis of diseases that cause oedema
| Clinical finding | Altered physiology | Mechanism | History and physical findings | Tests |
|---|---|---|---|---|
| Localised oedema | ||||
| Inflammation | High Kf | Cytokine mediated | Redness, heat, swelling, pain | |
| Deep venous thrombosis | Elevated ΔP | Venous obstruction | Phlegmasia cerulea dolens | Doppler venogram |
| Lymphatic obstruction | Lymphadenopathy | Lymphogram | ||
| Generalised oedema | ||||
| High Kf | Cytokine mediated | “Foaming urine” | Urinary protein | |
| Nephrotic syndrome | Elevated ΔP | Aldosterone mediated | Dependent oedema, hypertension | Serum albumin, serum cholesterol |
| Depressed Δπ | Decreased free entropy | Anasarca | ||
| Oliguric renal failure | Elevated ΔP | Excess intravascular volume | Orthopnoea, nausea, hypertension, dependent oedema | Blood urea nitrogen, chest x ray, ultrasound or computed tomography scan of kidneys, biopsy of kidneys |
| Congestive heart failure | Elevated ΔP | Decreased cardiac output, renin mediated, angiotensin mediated, aldosterone mediated | Orthopnoea, paroxysmal nocturnal dyspnoea, dependent oedema | Chest x ray, echocardiogram |
| Elevated ΔP | Portal hypertension, aldosterone mediated | Varices, caput medusae, ascites | Ultrasound scan of the liver, liver biopsy | |
| Cirrhosis | Depressed Δπ | Decreased free entropy | Dependent oedema | Serum albumin, prothrombin time |
| High Kf | Prostaglandin mediated, nitric oxide mediated | Palmar erythema, spider angiomata | ||
| Kwashiorkor | Depressed Δπ | Decreased free entropy | Dependent oedema | Serum albumin |
| Idiopathic oedema | Elevated ΔP | Renin mediated, angiotensin mediated, or aldosterone mediated | Dependent oedema | Serum albumin |
Kf=permeability of blood vessel wall; ΔP=difference between intracapillary blood pressure and extravascular hydrostatic pressure; Δπ=difference in oncotic (colloid osmotic) pressure; blood urea nitrogen is a measure of excretory function.
Footnotes
Competing interests: None declared.
References
- 1.Spencer WG. De medicina. Cambridge, MA: Harvard University Press; 1938. Translation of: Celsus. book 3:180-1. [Google Scholar]
- 2.Breasted JH. Chicago: University of Chicago Press; 1930. Translation of: The Edwin Smith surgical papyrus [facsimile hieroglyphic transliteration] pp. 140–155. [Google Scholar]
- 3.Bright R. Report of medical cases with a selected view of illustrating the symptoms and cure of medical diseases by reference to morbid anatomy. London: Longman; 1827. [PMC free article] [PubMed] [Google Scholar]
- 4.Richet G. Edema and uremia from 1827 to 1905: the first faltering steps of renal pathophysiology. Kidney Int. 1993;43:1385–1396. doi: 10.1038/ki.1993.195. [DOI] [PubMed] [Google Scholar]
- 5.Starling EH. Physiologic forces involved in the causation of dropsy. Lancet. 1896;i:1267–1270. [Google Scholar]
- 6.Diskin CJ, Gupta RB, Ravis W, Stokes TJ, Dansby LM, Carter TB, et al. Edema, oncotic pressure and free entropy: novel considerations for the treatment of edema through attention to thermodynamics. Nephron. 1998;78:131–138. doi: 10.1159/000044900. [DOI] [PubMed] [Google Scholar]
- 7.Guardia JA, Ortiz-Butcher C, Bourgornie JJ. Oncotic pressure and edema formation in hypoalbuminemic HIV patients with proteinuria. Am J Kidney Dis. 1997;30:822–828. doi: 10.1016/s0272-6386(97)90088-3. [DOI] [PubMed] [Google Scholar]
- 8.Canaan-Kuhl S, Venkatramen ES, Ernst SIB, Olshen RA, Myers BD. Relationships among proteins and albumin concentrations in nephrotic plasma. Am J Physiol. 1993;264:1052–9F. doi: 10.1152/ajprenal.1993.264.6.F1052. [DOI] [PubMed] [Google Scholar]
- 9.Hammel HT. Role of colloid proteins in Starling’s hypothesis and in returning interstitial fluid to the vasa recta. Am J Physiol. 1995;268:2133–44H. doi: 10.1152/ajpheart.1995.268.5.H2133. [DOI] [PubMed] [Google Scholar]
- 10.Sharp KA. Polyelectrolyte electrostatics: salt dependence, entropic, and enthalpic contributions to free energy in nonlinear Poisson-Boltzmann Model. Biopolymers. 1995;36:227–243. [Google Scholar]
- 11.Smorenburg CH, ter Wee PM, Gans RO. Clinical applications of albumin: a closer look at indications. Ned Tijdschr Geneeskd. 1997;141:719–723. [PubMed] [Google Scholar]
- 12.Mimran A, Targhetta R. Captopril treatment of idiopathic edema. N Engl J Med. 1979;301:1289–1290. [PubMed] [Google Scholar]
- 13.Milroto M, Baksi MS, Crisantino R, Delisi R. N N N alkyloctyldimethylammonium chloride in water: a thermodynamic investigation. Journal of Colloid and Interface Sciences. 1993;159:354–365. [Google Scholar]
- 14.Joles JA, Willekes-Koolshlin N, Braam B, Kortlandt W, Koomans HA, Dorhout Mees EJ. Colloid oncotic pressure in young analbuminemic rats. Am J Physiol. 1989;257:23–8F. doi: 10.1152/ajprenal.1989.257.1.F23. [DOI] [PubMed] [Google Scholar]