Abstract
As exposure to heterocyclic amines might increase the risk of liver cancer, we investigated the carcinogenic potential of MeIQx under conditions of liver damage caused by TAA. Male, 6‐week‐old F344 rats (n = 280) were divided into 14 groups; groups 1–7 received TAA (0.03% in drinking water) and groups 8–14 received water for the first 12 weeks. Thereafter, the animals received MeIQx at doses from 0, 0.001, 0.01, 0.1, 1, 10 to 100 p.p.m. (groups 1–7 and 8–14, respectively) in pellet basal diet for 16 weeks. All survivors were killed at week 28 for assessment of numbers and areas of GST‐P positive foci, considered to be pre‐neoplastic lesions of the liver. Values were increased significantly in all the groups receiving TAA→MeIQx compared to MeIQx alone (P < 0.01). Numbers of GST‐P positive foci were significantly increased in groups 7 and 14 (treated with 100 p.p.m. MeIQx) as compared to 0 p.p.m.‐MeIQx (groups 1 and 8) (P < 0.01), along with areas in group 14 compared to group 8 (P < 0.01). However, with the maximum likelihood method, the data for numbers of GST‐P positive foci (groups 1–7 and groups 8–14) fitted the hockey stick regression model, representing no differences from groups 1–5 and from groups 8–13, despite a linear dose‐dependent increase of MeIQx‐DNA adducts from 0.1 to 100 p.p.m. We conclude that there is a no effect level for MeIQx hepatocarcinogenicity, even on a background of TAA‐induced liver damage. (Cancer Sci 2006; 97)
Abbreviations:
- 8‐OHdG
8‐hydroxy‐2′‐deoxyguanosine
- GST‐P
glutathione S‐transferase placental form
- MeIQx
2‐amino‐3,8‐dimethylimidazo[4,5‐f]quinoxaline
- TAA
thioacetamide.
Exposure to dietary and environmental carcinogens in our everyday lives may be closely related to the occurrence of cancer.( 1 , 2 ) Of many genotoxic carcinogens occurring naturally in our environment, interest has been concentrated on heterocyclic amines generated during the frying or grilling of foods, especially meat and fish products.( 3 )
It has been generally considered that genotoxic carcinogens have no threshold with regard to their carcinogenic potential. However, this concept is theoretical and has been founded on the limited data available for cancer risk assessment of genotoxic carcinogen exposure.( 4 , 5 ) Therefore it is important to estimate the incidence and severity of adverse effects in human populations at actual exposure levels, which means that the focus should be on relatively low doses.( 6 , 7 , 8 )
MeIQx is a genotoxic heterocyclic amine causing DNA adducts( 9 , 10 ) and tumors with long‐term treatment in mice and rats.( 11 , 12 , 13 ) It is likely that exposure to MeIQx may be particularly harmful in persons suffering from liver disease, such as cirrhosis, and this needs to be taken into account in risk assessment and management. In the present study, we experimentally induced liver damage in rats using TAA, a well‐known hepatotoxin, before feeding MeIQx at doses from low to high levels, and evaluated quantitative data for GST‐P positive foci, which are considered to be pre‐neoplastic lesions of the liver,( 14 , 15 , 16 ) MeIQx DNA adducts, and 8‐OHdG in the liver. Because recent research has indicated that dietary factors can affect DNA methylation,( 17 , 18 ) an examination of this parameter was included.
Materials and Methods
Animals and treatment
Male, 6‐week‐old F344 rats (n = 280) were obtained from Charles River Japan (Atsugi, Japan) and housed in a room with a 12 h light/dark cycle, at constant temperature and humidity. Rats were allowed free access to pellet chow diets (MF‐1, Oriental Yeast, Tokyo, Japan) during the experiment and all procedures were approved by the Institutional Animal Care and Use Committee.
Animals were divided into 14 groups: groups 1–7 received TAA (Sigma‐Aldrich, St Louis, MO, USA) in drinking water at a concentration of 0.03% for 12 weeks, while groups 8–14 were given drinking water without TAA. Thereafter, they received MeIQx (purity 99.9%, Nard Institute, Nishinomiya, Japan) at doses of 0 (groups 1 and 8), 0.001 (groups 2 and 9), 0.01 (groups 3 and 10), 0.1 (groups 4 and 11), 1 (groups 5 and 12), 10 (groups 6 and 13), or 100 p.p.m. (groups 7 and 14) in pellet basal diet for 16 weeks continuously. All survivors were killed at week 28 under ether anesthesia.
Bodyweight, food consumption and water intake of all animals were measured every week. At necropsy, liver weight was measured, and liver tissue was fixed in 10% phosphate‐buffered formalin and routinely processed for embedding in paraffin, and staining of 4‐µm sections with hematoxylin and eosin and Azan‐Mallory for histopathological examination. Further sections were applied for immunohistochemical staining. The remaining liver samples from all animals were snap‐frozen in liquid nitrogen for subsequent biochemical analyses.
Immunohistochemical examination of GST‐P
The avidin–biotin complex method was used to demonstrate GST‐P in sections (4 µm) of liver tissue dewaxed with xylene and hydrated through a graded ethanol series. Sections were treated sequentially with 0.3% hydrogen peroxide, normal goat serum, rabbit anti‐GST‐P antibody (MBL, Nagoya, Japan) at 1:1000 dilution, biotin‐labeled goat anti‐rabbit IgG and avidin–biotin–peroxidase complex (ABC kit; Vector Laboratories, Burlingame, CA, USA). Immune complexes were visualized with 3,3′‐diaminobenzidine tetrahydrochloride as a chromogen. As a negative control, normal serum was used instead of primary antibodies. The sections were counterstained with Mayer's hematoxylin to facilitate examination under a light microscope.
Quantification of GST‐P positive foci
GST‐P positive foci (having more than two positive cells) of all animals were counted under a light microscope. Total areas of GST‐P positive foci and of the entire liver sections were measured using a color image processor (IPAP, Sumica Technos, Osaka, Japan) to allow calculation of the number of foci per cm2 and the area (mm2) per cm2 of liver section. Data were the mean ± SD value for all samples per group.
Quantification of MeIQx‐DNA adduct levels in the livers
The levels of MeIQx‐DNA adducts were measured by the 32P‐postlabeling method under modified adduct intensification conditions using frozen samples, as previously reported.( 19 ) Data were the mean ± SD value from three samples per group and three independent experiments.
Quantification of 8‐OHdG formation
DNA samples isolated from pieces of frozen liver weighing 500 mg were digested into deoxynucleosides by combined treatment with nuclease P1 and alkaline phosphatase. Levels of 8‐OHdG were determined by high‐performance liquid chromatography (HPLC), as described earlier,( 20 ) and results were expressed as the number of 8‐OHdG residues/105 total deoxyguanosines. Data were the mean ± SD value for 10 samples per group and four independent experiments.
Immunohistochemical staining of 5‐methylcytosine
The avidin–biotin complex method was used to demonstrate 5‐methylcystosine in sections (4 µm) of liver tissue dewaxed with xylene and hydrated through a graded ethanol series. Sections in sodium citrate buffer (pH 6.0) were boiled in an autoclave for 25 min and then treated with 3.5 N HCl and 3% hydrogen peroxide, normal horse serum, and anti‐5‐methylcytosine antibody (Calbiochem, La Jolla, CA, USA) at 1:1000 dilution, followed by ABC‐peroxidase procedures (ABC kit). Immune complexes were visualized with 3,3′‐diaminobenzidine tetrahydrochloride as a chromogen. As a negative control, normal serum was used instead of primary antibodies. The sections were counterstained with Mayer's hematoxylin to facilitate examination under a light microscope.
Statistical analysis
The Tukey–Kramer method was applied to the data of GST‐P positive foci, MeIQx‐DNA adducts and 8‐OHdG levels using the JMP program (SAS Institute, Cary, NC, USA). For all comparisons, P‐values less than 5% (P < 0.05) were considered to be statistically significant. For analysis of the dose–response relationship for the data of GST‐P positive foci, the data of numbers of GST‐P were divided into two categories (groups 1–7 and groups 8–14), and the maximum likelihood method( 21 ) was used to obtain optimally fitted models using the SAS/IML procedure (SAS Institute).
Results
Body and relative liver weights, and histopathological examination of liver
Marked growth retardation was noted during TAA treatment, with a significant decrease in final bodyweights compared with the corresponding non‐TAA treatment groups (Table 1, P < 0.01). There was a significant increase of relative liver and spleen weight in groups 1, 2 and 3 compared with that of groups 8, 9 and 10 (P < 0.05). Liver of animals treated with TAA showed cirrhosis and/or fibrosis, with nodular areas of hepatocytes, separated by fibrous septa with a collagenous matrix and myofibroblast‐like cells and fibroblasts. There was one unscheduled death in group 7 during the experiment.
Table 1.
Final bodyweights and relative liver and spleen weights
| Group | TAA | MeIQx (p.p.m.) | Number of rats | Initial bodyweight (g) | Final bodyweight (g) | Relative organ weight (%) | |
|---|---|---|---|---|---|---|---|
| Liver | Spleen | ||||||
| 1 | + | 0 | 30 | 120.9 ± 5.4 | 331.1 ± 24.4** | 3.2 ± 1.1* | 0.2 ± 0.1* |
| 2 | + | 0.001 | 30 | 118.8 ± 5.2 | 334.4 ± 23.3** | 3.2 ± 1.1* | 0.3 ± 0.1* |
| 3 | + | 0.01 | 30 | 120.5 ± 5.6 | 331.5 ± 20.9** | 3.4 ± 1.3** | 0.3 ± 0.1** |
| 4 | + | 0.1 | 30 | 121.9 ± 5.2 | 339.2 ± 21.0** | 3.0 ± 0.8 | 0.2 ± 0.1 |
| 5 | + | 1 | 30 | 120.7 ± 5.5 | 337.6 ± 20.8** | 2.9 ± 0.8 | 0.2 ± 0.1 |
| 6 | + | 10 | 30 | 120.2 ± 4.9 | 343.6 ± 19.7** | 2.9 ± 0.6 | 0.2 ± 0.0 |
| 7 | + | 100 | 29 | 121.2 ± 5.0 | 326.0 ± 16.3** | 3.3 ± 0.7 | 0.3 ± 0.1* |
| 8 | – | 0 | 10 | 119.5 ± 6.8 | 390.8 ± 11.9 | 2.1 ± 0.1 | 0.2 ± 0.0 |
| 9 | – | 0.001 | 10 | 119.0 ± 6.0 | 404.8 ± 19.2 | 2.1 ± 0.1 | 0.2 ± 0.0 |
| 10 | – | 0.01 | 10 | 117.9 ± 4.9 | 403.9 ± 14.8 | 2.1 ± 0.1 | 0.2 ± 0.0 |
| 11 | – | 0.1 | 10 | 118.1 ± 5.5 | 399.5 ± 27.2 | 2.1 ± 0.2 | 0.2 ± 0.0 |
| 12 | – | 1 | 10 | 118.9 ± 3.1 | 399.5 ± 18.8 | 2.1 ± 0.1 | 0.2 ± 0.0 |
| 13 | – | 10 | 10 | 119.3 ± 5.9 | 394.5 ± 18.1 | 2.1 ± 0.1 | 0.2 ± 0.0 |
| 14 | – | 100 | 10 | 118.1 ± 6.2 | 389.9 ± 21.3 | 2.3 ± 0.1 | 0.2 ± 0.0 |
Data represent the mean ± SD. *,** Significantly different from the corresponding control group without thioacetamide (TAA) at P < 0.05 and P < 0.01, respectively. MeIOx, 2‐amino‐3,8‐dimethylimidazo[4,5‐f]quinoxaline.
Quantitative data for GST‐P positive foci
TAA treatment induced bigger GST‐P positive foci compared to MeIQx alone (Table 2 and Fig. 1A,B). Numbers and area of GST‐P positive foci increased significantly in all groups receiving TAA→MeIQx compared to MeIQx alone (P < 0.01). Moreover, the numbers of GST‐P positive foci were significantly increased in groups 7 and 14 (treated with 100 p.p.m. MeIQx) as compared to 0 p.p.m.‐MeIQx (groups 1 and 8) (P < 0.01), while the areas were significantly increased in group 14 compared to group 8 (P < 0.01). However, there were no differences at 10 p.p.m. or below as compared to control levels, with or without TAA.
Table 2.
Number and area of glutathione S‐transferase placental (GST‐P) positive foci
| Group | TAA | MeIQx (p.p.m.) | Number of rats | Number (no./cm2) | Area (mm2/cm2) |
|---|---|---|---|---|---|
| 1 | + | 0 | 30 | 21.836 ± 5.615* | 6.291 ± 4.065* |
| 2 | + | 0.001 | 30 | 21.074 ± 7.218 | 6.558 ± 3.651* |
| 3 | + | 0.01 | 30 | 19.626 ± 6.597* | 5.845 ± 3.856* |
| 4 | + | 0.1 | 30 | 19.734 ± 6.708* | 4.521 ± 3.246* |
| 5 | + | 1 | 30 | 20.237 ± 6.115* | 3.841 ± 1.261* |
| 6 | + | 10 | 30 | 25.740 ± 7.942* | 5.033 ± 2.771* |
| 7 | + | 100 | 29 | 40.671 ± 12.187*,** | 6.291 ± 2.529* |
| 8 | – | 0 | 10 | 0.698 ± 0.844 | 0.002 ± 0.003 |
| 9 | – | 0.001 | 10 | 0.778 ± 0.746 | 0.001 ± 0.002 |
| 10 | – | 0.01 | 10 | 0.679 ± 0.441 | 0.001 ± 0.001 |
| 11 | – | 0.1 | 10 | 0.735 ± 0.894 | 0.002 ± 0.003 |
| 12 | – | 1 | 10 | 0.538 ± 0.475 | 0.002 ± 0.002 |
| 13 | – | 10 | 10 | 1.010 ± 0.981 | 0.001 ± 0.001 |
| 14 | – | 100 | 10 | 7.504 ± 1.977** | 0.024 ± 0.012** |
Data represent the mean ± SD. *Significantly different from the corresponding control group without thioacetamide (TAA) at P < 0.01. **Significantly different from control group (group 1 or 8) at P < 0.01. MeIOx, 2‐amino‐3,8‐dimethylimidazo[4,5‐f]quinoxaline.
Figure 1.
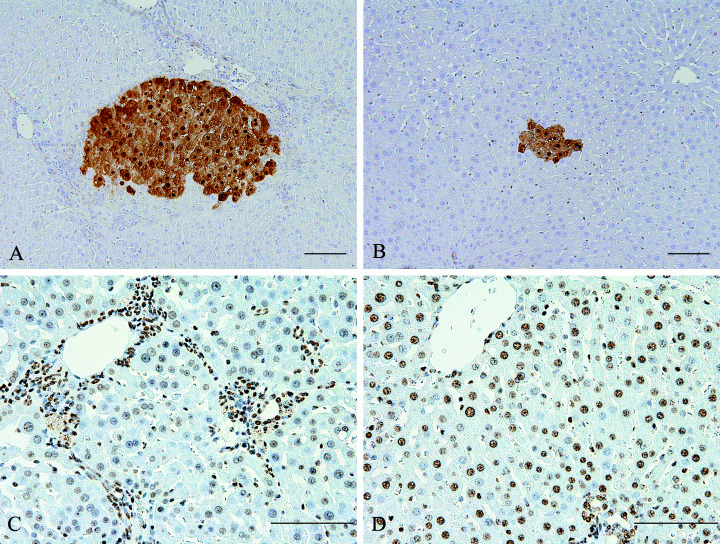
Immunohistochemical staining of glutathione S‐transferase placental form (GST‐P) positive foci and 5‐methylcytosine in liver of rats. (A) GST‐P positive foci in a rat treated with 0.03% thioacetamide (TAA) in the drinking water for 12 weeks then 2‐amino‐3,8‐dimethylimidazo[4,5‐f]quinoxaline (MeIQx) 100 p.p.m. for 16 weeks. (B) GST‐P positive foci in a rat treated with MeIQx 100 p.p.m. for 16 weeks. (C) 5‐methylcytosine in the liver from a rat treated with 0.03% TAA in the drinking water for 12 weeks then MeIQx 100 p.p.m. for 16 weeks; note many hepatic cells in hepatic nodules show negative staining, but non‐parenchymal cells are positive. (D) 5‐methylcytosine in the liver from a rat treated with normal diet (MeIQx 0 p.p.m.) for 16 weeks; most hepatic cells show positive staining; bar = 100 µm.
With the maximum likelihood method, the data of numbers of GST‐P positive foci (groups 1–7 and groups 8–14) fitted the hockey stick regression model, with no differences from groups 1–5 and from groups 8–13 (Fig. 2A and 2B, respectively).
Figure 2.
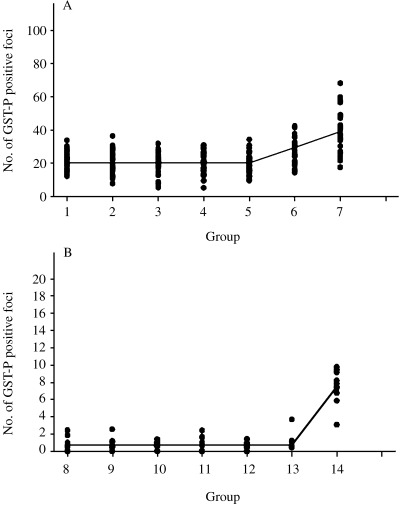
Analysis of the dose–response relationship for the number of glutathione S‐transferase placental form (GST‐P) positive foci. Statistical analyses were carried out as detailed in the Materials and Methods section. (A) Numbers of GST‐P positive foci demonstrated no differences from groups 1–5. (B) Numbers of GST‐P positive foci demonstrated no significant variation from groups 8–13.
MeIQx‐DNA adduct levels in liver
Linear dose‐dependent increase of MeIQx‐DNA adducts was evident from 0.1 to 100 p.p.m. (Fig. 3). However, there were no differences between the groups treated with TAA→ΜeIQx and those exposed to MeIQx alone. With the low MeIQx intake of groups below 0.01 p.p.m., no adducts could be detected in either TAA→MeIQx or MeIQx alone treated animals; their levels might be under the detection limit (<5 × 10−10).
Figure 3.
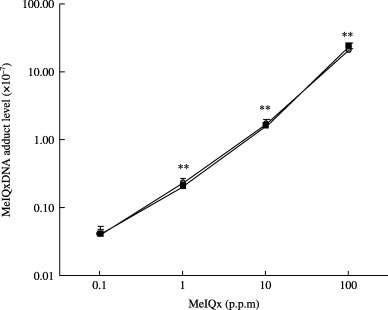
2‐amino‐3,8‐dimethylimidazo[4,5‐f]quinoxaline (MeIQx)‐DNA adduct levels in the livers of rats treated with MeIQx, with or without thioacetamide (TAA). Levels of MeIQx‐DNA adducts in the liver were measured as detailed in the Materials and Methods section. Data are the mean ± SD values from three samples per group and three independent experiments. (O) TAA and MeIQx combined treatment groups; (□) MeIQx alone groups. **Significantly different from MeIQx 0.1 p.p.m. (P < 0.01).
Quantitative data for 8‐OHdG
HPLC analysis of 8‐OHdG formation showed that there were no differences among the groups (Fig. 4), including the TAA→MeIQx and MeIQx alone groups.
Figure 4.
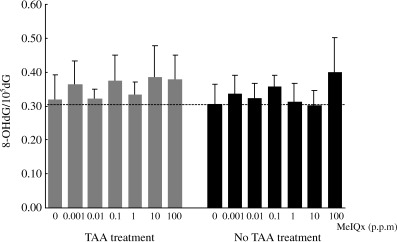
8‐OHdG formation levels in the livers of rats treated with 2‐amino‐3,8‐dimethylimidazo[4,5‐f]quinoxaline (MeIQx), with or without thioacetamide (TAA). Levels of 8‐OHdG were determined as detailed in the Materials and Methods section, and expressed as numbers of residues/105 total deoxyguanosines. Data are the mean ± SD values from 10 samples per group and four independent experiments.
Immunohistochemical staining of 5‐methylcytosine in liver
Around nodules, non‐parenchymal cells such as fibroblasts or myofibroblasts and bile ductular cells showed positive staining, but many cells in hepatic nodules were negative (Fig. 1C). However, most nuclei in livers from rats treated with normal diet (MeIQx 0 p.p.m.) showed positive staining (Fig. 1D).
Discussion
We have shown earlier that low‐dose treatment with MeIQx results in elevation of DNA adducts, but no induction of GST‐P positive foci, 8‐OHdG and mutation frequency in rat liver.( 6 , 7 , 22 ) These experimental results are line with the data for several genotoxic carcinogens such as N‐nitrosodiethylamine, N‐nitrosodimethylamine, 2‐amino‐1‐methyl‐6‐phenolimidazo [4,5‐b]pyridine and 2‐acetylaminofluorene.( 6 , 7 , 8 , 23 , 24 , 25 ) In the present study, the modifying effect of MeIQx on GST‐P positive foci was more marked with numbers than areas, but in both cases there were no effect levels at lower doses regarding induction of GST‐P positive foci, with or without TAA treatment.
The dose–response pattern analysis of the foci using the maximum likelihood method showed numbers to fit the hockey stick regression model, with no differences from groups 1–5 and from groups 8–13. Although shortening of no effects levels, it is particularly important that there were no effects even on a background of liver damage.
No effect levels with biological responses may fluctuate with health status and other exposures,( 26 ) and may be associated with repair of DNA damage( 27 , 28 ) and homeostatic mechanisms to maintain cellular equilibrium and normal function.( 29 ) However, as there may be a particular individual susceptibility at a low dose,( 30 ) intermittent exposure of genotoxic carcinogens may be related to greater risk of genotoxic damage.( 31 ) Therefore, it is important to assay potential damage induced by genotoxic carcinogens under complex conditions in further studies.
Our previous findings on cotreatment of MeIQx with CCl4 also showed no effect levels for the induction of GST‐P positive foci in liver of rats with mild fibrosis.( 32 ) Compared with the CCl4 method, continuous administration of TAA in the drinking water is a non‐invasive approach for chronic induction of fibrosis and/or cirrhosis in large numbers of animals.( 33 , 34 ) In this study, we could evaluate the carcinogenic potential of MeIQx without any chemical interference, and found treatment of TAA induced liver fibrosis and/or cirrhosis without mortality.
It is considered that DNA adduct formation is a good marker for exposure to several carcinogens.( 35 , 36 ) While an increase in MeIQx‐DNA adducts was noted with dose‐dependence, the additional liver disease did not exert any influence in the present study. Furthermore, formation of 8‐OHdG showed no differences among the groups. Compared with previous reports,( 7 , 37 , 38 ) it seems older animals show higher background levels of 8‐OHdG and there is low sensitivity to MeIQx treatment.
Immunohistochemical staining of 5‐methylcytosine provided evidence that hepatic nodules were characterized by hypomethylation status. Interestingly, around nodules, non‐parenchymal cells such as fibroblasts or myofibroblasts and bile ductular cells showed positive staining. As involvement of global hypomethylation and regional hypermethylation of tumor suppressor genes in carcinogenesis has been proposed,( 39 , 40 ) alteration of methylation in genotoxic carcinogen‐induced tumors warrants further investigation.
In conclusion, in the present study, there was a no effect level regarding hepatocarcinogenicity of MeIQx, even on a background of TAA‐induced liver damage.
Acknowledgments
We would like to thank Ms Kaori Touma, Masayo Imanaka and Shoko Araki for their technical assistance, and Ms Mari Dokoh, Yuko Onishi and Yoko Shimada for their help during the preparation of this manuscript. This research was supported by Grants‐in‐Aid for Cancer Research from the Ministry of Health, Labour and Welfare, and by Grants‐in‐Aid from the Ministry of Economy, Trade and Industry of Japan.
References
- 1. Sugimura T. Studies on environmental chemical carcinogenesis in Japan. Science 1986; 233: 312–8. [DOI] [PubMed] [Google Scholar]
- 2. Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci 2004; 95: 290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johansson MA, Jagerstad M. Occurrence of mutagenic/carcinogenic heterocyclic amines in meat and fish products, including pan residues, prepared under domestic conditions. Carcinogenesis 1994; 15: 1511–8. [DOI] [PubMed] [Google Scholar]
- 4. Littlefield NA, Farmer JH, Gaylor DW, Sheldon WG. Effects of dose and time in a long‐term, low‐dose carcinogenic study. J Environ Pathol Toxicol 1980; 3: 17–34. [PubMed] [Google Scholar]
- 5. Peto R, Gray R, Brantom P, Grasso P. Effects on 4080 rats of chronic ingestion of N‐nitrosodiethylamine or N‐nitrosodimethylamine: a detailed dose‐response study. Cancer Res 1991; 51: 6415–51. [PubMed] [Google Scholar]
- 6. Fukushima S. Low‐dose carcinogenicity of a heterocyclic amine, 2‐amino‐3,8‐dimethylimidazo[4,5‐f]quinoxaline: relevance to risk assessment. Cancer Lett 1999; 143: 157–9. [DOI] [PubMed] [Google Scholar]
- 7. Fukushima S, Wanibuchi H, Morimura K et al. Lack of a dose‐response relationship for carcinogenicity in the rat liver with low doses of 2‐amino‐3,8‐dimethylimidazo[4,5‐f]quinoxaline or N‐nitrosodiethylamine. Jpn J Cancer Res 2002; 93: 1076–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fukushima S, Wanibuchi H, Morimura K et al. Existence of a threshold for induction of aberrant crypt foci in the rat colon with low doses of 2‐amino‐1‐methyl‐6‐phenolimidazo[4,5‐b]pyridine. Toxicol Sci 2004; 80: 109–14. [DOI] [PubMed] [Google Scholar]
- 9. Rich KJ, Murray BP, Lewis I et al. N‐hydroxy‐MeIQx is the major microsomal oxidation product of the dietary carcinogen MeIQx with human liver. Carcinogenesis 1992; 13: 2221–6. [DOI] [PubMed] [Google Scholar]
- 10. Turesky RJ, Lang NP, Butler MA, Teitel CH, Kadlubar FF. Metabolic activation of carcinogenic heterocyclic aromatic amines by human liver and colon. Carcinogenesis 1991; 12: 1839–45. [DOI] [PubMed] [Google Scholar]
- 11. Kato T, Ohgaki H, Hasegawa H, Sato S, Takayama S, Sugimura T. Carcinogenicity in rats of a mutagenic compound, 2‐amino‐3,8‐dimethylimidazo[4,5‐f]quinoxaline. Carcinogenesis 1988; 9: 71–3. [DOI] [PubMed] [Google Scholar]
- 12. Kushida H, Wakabayashi K, Sato H, Katami M, Kurosaka R, Nagao M. Dose‐response study of MeIQx carcinogenicity in F344 male rats. Cancer Lett 1994; 83: 31–5. [DOI] [PubMed] [Google Scholar]
- 13. Ohgaki H, Hasegawa H, Suenaga M, Sato S, Takayama S, Sugimura T. Carcinogenicity in mice of a mutagenic compound, 2‐amino‐3,8‐dimethylimidazo[4,5‐f]quinoxaline (MeIQx) from cooked foods. Carcinogenesis 1987; 8: 665–8. [DOI] [PubMed] [Google Scholar]
- 14. Sato K. Glutathione S‐transferases and hepatocarcinogenesis. Jpn J Cancer Res 1988; 79: 556–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ito N, Imaida K, Asamoto M, Shirai T. Early detection of carcinogenic substances and modifiers in rats. Mutat Res 2000; 462: 209–17. [DOI] [PubMed] [Google Scholar]
- 16. Tsuda H, Fukushima S, Wanibuchi H et al. Value of GST‐P positive preneoplastic hepatic foci in dose‐response studies of hepatocarcinogenesis: evidence for practical thresholds with both genotoxic and nongenotoxic carcinogens. A review of recent work. Toxicol Pathol 2003; 31: 80–6. [DOI] [PubMed] [Google Scholar]
- 17. Davis CD, Uthus EO. DNA methylation, cancer susceptibility, and nutrient interactions. Exp Biol Med (Maywood) 2004; 229: 988–95. [DOI] [PubMed] [Google Scholar]
- 18. Pogribny IP, Miller BJ, James SJ. Alterations in hepatic p53 gene methylation patterns during tumor progression with folate/methyl deficiency in the rat. Cancer Lett 1997; 115: 31–8. [DOI] [PubMed] [Google Scholar]
- 19. Totsuka Y, Fukutome K, Takahashi M et al. Presence of N 2‐(deoxyguanosin‐8‐yl)‐2‐amino‐3,8‐dimethylimidazo[4,5‐f]quinoxaline (dG‐C8‐MeIQx) in human tissues. Carcinogenesis 1996; 17: 1029–34. [DOI] [PubMed] [Google Scholar]
- 20. Nakae D, Kobayashi Y, Akai H et al. Involvement of 8‐hydroxyguanine formation in the initiation of rat liver carcinogenesis by low dose levels of N‐nitrosodiethylamine. Cancer Res 1997; 57: 1281–7. [PubMed] [Google Scholar]
- 21. Piegorsch WW, Bailer AJ. Dose‐response Modeling and Analysis. Statistics for Environmental Biology and Toxicology. London: Chapman & Hall, 1997: 297–305. [Google Scholar]
- 22. Hoshi M, Morimura K, Wanibuchi H et al. No‐observed effect levels for carcinogenicity and for in vivo mutagenicity of a genotoxic carcinogen. Toxicol Sci 2004; 81: 273–9. [DOI] [PubMed] [Google Scholar]
- 23. Williams GM, Iatropoulos MJ, Jeffrey AM. Mechanistic basis for nonlinearities and thresholds in rat liver carcinogenesis by the DNA‐reactive carcinogens 2‐acetylaminofluorene and diethylnitrosamine. Toxicol Pathol 2000; 28: 388–95. [DOI] [PubMed] [Google Scholar]
- 24. Williams GM, Iatropoulos MJ, Jeffrey AM. Thresholds for the effects of 2‐acetylaminofluorene in rat liver. Toxicol Pathol 2004; 32 (Suppl. 2): 85–91. [DOI] [PubMed] [Google Scholar]
- 25. Fukushima S, Wanibuchi H, Morimura K et al. Lack of potential of low dose N‐nitrosodimethylamine to induce preneoplastic lesions, glutathione S‐transferase placental form‐positive foci, in rat liver. Cancer Lett 2005; 222: 11–5. [DOI] [PubMed] [Google Scholar]
- 26. Tomatis L, Huff J, Hertz‐Picciotto I et al. Avoided and avoidable risks of cancer. Carcinogenesis 1997; 18: 97–105. [DOI] [PubMed] [Google Scholar]
- 27. Sofuni T, Hayashi M, Nohmi T, Matsuoka A, Yamada M, Kamata E. Semi‐quantitative evaluation of genotoxic activity of chemical substances and evidence for a biological threshold of genotoxic activity. Mutat Res 2000; 464: 97–104. [DOI] [PubMed] [Google Scholar]
- 28. Lutz WK. Dose‐response relationships in chemical carcinogenesis. Super position of different mechanisms of action, resulting in linear‐nonlinear curves, practical thresholds, J‐shapes. Mutat Res 1998; 405: 117–24. [DOI] [PubMed] [Google Scholar]
- 29. Dybing E, Doe J, Groten J et al. Hazard characterisation of chemicals in food and diet. dose‐response, mechanisms and extrapolation issues. Food Chem Toxicol 2002; 40: 237–82. [DOI] [PubMed] [Google Scholar]
- 30. Lutz WK. Susceptibility differences in chemical carcinogenesis linearize the dose‐response relationship: threshold doses can be defined only for individuals. Mutat Res 2001; 482: 71–6. [DOI] [PubMed] [Google Scholar]
- 31. Hofseth LJ. The adaptive imbalance to genotoxic stress: genome guardians rear their ugly heads. Carcinogenesis 2004; 25: 1787–93. [DOI] [PubMed] [Google Scholar]
- 32. Iwai S, Karim R, Kitano M et al. Role of oxidative DNA damage caused by carbon tetrachloride‐induced liver injury – enhancement of MeIQx‐induced glutathione S‐transferase placental form positive foci in rats. Cancer Lett 2002; 179: 15–24. [DOI] [PubMed] [Google Scholar]
- 33. Li X, Benjamin IS, Alexander B. Reproducible production of thioacetamide‐induced macronodular cirrhosis in the rat with no mortality. J Hepatol 2002; 36: 488–93. [DOI] [PubMed] [Google Scholar]
- 34. Muller A, Machnik F, Zimmermann T, Schubert H. Thioacetamide‐induced cirrhosis‐like liver lesions in rats – usefulness and reliability of this animal model. Exp Pathol 1988; 34: 229–36. [DOI] [PubMed] [Google Scholar]
- 35. Poirier MC. Chemical‐induced DNA damage and human cancer risk. Nat Rev Cancer 2004; 4: 630–7. [DOI] [PubMed] [Google Scholar]
- 36. Schut HA, Snyderwine EG. DNA adducts of heterocyclic amine food mutagens: implications for mutagenesis and carcinogenesis. Carcinogenesis 1999; 20: 353–68. [DOI] [PubMed] [Google Scholar]
- 37. Kang JS, Morimura K, Salim EI, Wanibuchi H, Yamaguchi S, Fukushima S. Persistence of liver cirrhosis in association with proliferation of nonparenchymal cells and altered location of alpha‐smooth muscle actin‐positive cells. Toxicol Pathol 2005; 33: 329–35. [DOI] [PubMed] [Google Scholar]
- 38. Kato T, Hasegawa R, Nakae D et al. Dose‐dependent induction of 8‐hydroxyguanine and preneoplastic foci in rat liver by a food‐derived carcinogen, 2‐amino‐3,8‐dimethylimidazo[4,5‐f]quinoxaline, at low dose levels. Jpn J Cancer Res 1996; 87: 127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer 2004; 4: 143–53. [DOI] [PubMed] [Google Scholar]
- 40. Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med 2003; 349: 2042–54. [DOI] [PubMed] [Google Scholar]


