Abstract
TEL/ETV6 accelerates erythroid differentiation in the murine erythroleukemia cell line. To clarify the effects of TEL on megakaryocytic maturation as well as erythroid differentiation, we chose the human leukemia cell line UT‐7/GM that differentiates into the erythroid and megakaryocytic lineages by treatment with erythropoietin and thrombopoietin, respectively. Upon erythropoietin exposure, overexpressed TEL stimulated hemoglobin synthesis and accumulation of the erythroid differentiation‐specific transcripts such as γ‐globin, δ‐aminolevulinic acid synthase‐erythroid, and erythropoietin receptor. Moreover, the glycophorin A(+)/glycoprotein IIb(–) fraction appeared more rapidly in the TEL‐overexpressing cells. Interestingly, overexpression of TEL was associated with lower levels of the megakaryocytic maturation‐specific glycoprotein IIb and platelet factor 4 transcripts under the treatment with thrombopoietin. Consistently, the glycophorin A(–)/glycoprotein IIb(+) fraction increased more slowly in the TEL‐overexpressing cells. Finally, expression of endogenous TEL proteins in UT‐7/GM cells was down‐regulated following erythropoietin and thrombopoietin exposure. All these data suggest that TEL may decide the fate of human erythrocyte/megakaryocyte common progenitors to differentiate towards the erythroid lineage and against the megakaryocytic lineage. (Cancer Sci 2005; 96: 340–348)
TEL (also known as ETV6) is a member of the E26 transformation‐specific (ETS) family of transcription factors.( 1 ) The highly conserved ETS domain is located at the C‐terminal region, while a distinct domain with weak homology to the well‐described helix‐loop‐helix (HLH) domain (also referred to as the pointed domain) is located at the N‐terminal region. The former serves for DNA binding to the ETS‐binding consensus site (EBS) (GGAA/T) and the latter for homodimerization and heterodimerization with other ETS family members.( 2 , 3 ) Through interacting with relevant corepressors mSin3A, N‐CoR and SMRT, and histone deacetylase‐3,( 4 ) TEL mediates transcriptional repression on its target genes such as FLI‐1,( 2 ) inhibitor of differentiation/DNA binding‐1 (Id‐1),( 5 ) stromelysin‐1( 6 ) and Bcl‐XL .( 7 ) Transcriptional activities of TEL are regulated through phosphorylation with mitogen‐activated protein kinases( 8 , 9 ) and small ubiquitin‐like modifier conjugation.( 10 , 11 )
The TEL gene that is mapped to 12p13 is most frequently rearranged and fused to various partner genes by chromosomal translocations in human leukemias and myelodysplastic syndromes. The partners include receptor type or non‐receptor type tyrosine kinases and transcription factors. Providing tyrosine kinases, such as platelet‐derived growth factor receptor β (PDGFRβ) in t(5;12) (q33;p13),( 12 ) ABL1 in t(9;12) (q34;p13),( 13 ) ARG (ABL2) in t(1;12) (q25;p13),( 14 ) JAK2 in t(9;12) (p24;p13)( 15 ) and Syk in t(9;12) (q22;p13),( 16 ) with the HLH domain, TEL homodimerizes them and thereby stimulates their kinase activities. In contrast, TEL gives corepressor‐binding domains to a transcription factor AML1 in t(12;21) (p13;q22) and interferes with its transcriptional abilities.( 17 ) Therefore, dysregulation of the partner proteins by TEL functional domains seems to cause leukemia in patients with 12p13 translocations. Moreover, inactivation of the TEL gene is speculated to be the second hit in t(12;21) (p13;q22) type leukemia, because the wild‐type‐TEL allele is deleted in the vast majority of the patients.( 18 , 19 ) Thus, TEL appears to be a tumor suppressor. Consistent with its roles as a putative tumor suppressor, expression of TEL in Ras‐transformed NIH3T3 cells inhibits cell growth in liquid and soft agar cultures,( 6 ) and in serum‐starved NIH3T3 cells induces apoptosis.( 6 )
TEL is required for mouse development as its inactivation by homologous recombination results in embryonic lethality at E10.5–11.5.( 18 ) The knockout embryos show defects in yolk sac angiogenesis and intraembryonic apoptosis of mesenchymal and neural cells, while they present normal yolk sac hematopoiesis. Analyzing chimeric mice with TEL(–/–) ES cells, an essential role of TEL in establishing hematopoiesis of all lineages in neonatal bone marrow has been uncovered, although TEL(–/–) ES cells contributed to both primary and definitive fetal hematopoiesis.( 19 ) As for lineage‐specific roles in hematopoietic systems, we have reported that TEL accelerates erythroid differentiation of mouse erythroleukemia (MEL) cells induced by hexamethylene bisacetamide (HMBA) or dimethylsulfoxide (DMSO).( 20 ) Because both erythroblasts and megakaryocytes arise from common progenitors, this observation prompted us to search for TEL's roles in lineage commitment of the bi‐potential progenitors.
A human tri‐factor dependent hematopoietic cell line UT‐7/GM( 21 ) is a subline of UT‐7 that was originally established from a patient of acute megakaryoblastic leukemia.( 22 ) UT‐7/GM cells show absolute dependence for growth and survival on granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), erythropoietin (EPO) or thrombopoietin (TPO). They differentiate into the erythroid or megakaryocytic lineage in the presence of EPO or TPO, while they keep immature phenotypes and proliferate in the presence of GM‐CSF.( 21 ) Thus, UT‐7/GM cells are considered to mimic erythrocyte/megakaryocyte common progenitors and differentiate along two distinct lineages in relatively physiological conditions. We employed this cell line and examined influences of TEL overexpression on erythroid differentiation and megakaryocytic maturation. As judged from higher percentages of benzidine positivity in TEL‐overexpressing cells under treatment with EPO, TEL accelerated erythroid differentiation in UT‐7/GM cells similar as in MEL cells. The TEL‐overexpressing cells showed increased expression of the transcripts for γ‐globin, δ‐aminolevulinic acid synthase‐erythroid (ALAS‐E) and EPO receptor (EPO‐R) during the erythroid differentiation. Moreover, accumulation of the glycophorin A(+)/glycoprotein (GP) IIb(–) fraction was more prompt in these cells. Interestingly, expression levels of the transcripts for GPIIb and platelet factor 4 (PF 4) under the treatment with TPO were lower in the TEL‐overexpressing cells. Consistent with this, accumulation of the glycophorin A(–)/GPIIb(+) fraction was delayed and appearance of platelet peroxidase (PPO)‐positive cells was reduced in these cells. Endogenous TEL proteins disappeared after 14 and 21 days upon EPO and TPO exposure, respectively. We conclude that TEL stimulates erythroid differentiation while opposing megakaryocytic maturation in human hematopoietic system.
Materials and Methods
Cell culture. Parental UT‐7/GM cells, the mock (M‐1 and M‐4) and the TEL‐overexpressing (T‐5 and T‐6) clones were maintained in Isocove's modified Dulbecco's medium (IMDM; Gibco Laboratories, Grand Island, NY) supplemented with 10% fetal calf serum (FCS) and 1 ng/mL of recombinant human (rh) GM‐CSF. To physiologically induce erythroid or megakaryocytic differentiation, these cells were cultured in IMDM supplemented with 10% FCS, and 10 U/mL of rhEPO or 100 ng/mL of rhTPO. Light microscopic examination was performed on Wright‐Giemsa‐stained cytospin preparations. Erythroid differentiation was evaluated by counting percentages of benzidine‐positive cells.
Isolation of stable transfectants. The expression of plasmid pCXN2‐FLAG‐TEL was described in a previous study.( 20 ) To establish stable transfectants, 1 × 107 of UT‐7/GM cells were electroporated with 20 µg of pCXN2‐FLAG‐TEL at 380 V and 975 µF using Gene Pulser (Bio‐Rad, Hercules, CA). Transfected cells were selected with 0.8 mg/mL of G418 (Sigma‐Aldrich, St. Louis, MO) and cloned by limiting dilution. Expression of FLAG tagged‐TEL proteins was confirmed by the western analysis method using anti‐FLAG antibody (Sigma‐Aldrich).
Immunoprecipitation and western analysis. UT‐7/GM cells were lyzed on ice in lysis buffer composed of 20 mM Tris pH 8.0, 50 mM sodium fluoride (NaF), 2 mM ethylenediamine‐N,N,N′,N′‐tetra‐acetic acid (EDTA), 1% NP‐40, 500 U/mL aprotinin, 1 mM sodium orthovanadate (Na3VO4), and 1 mM phenylmethylsulfonyl fluoride (PMSF). Immunoprecipitation and western analysis were performed as described in a previous study,( 20 ) using anti‐TEL (N‐19 for immunoprecipitation and H‐214 for western analysis; Santa Cruz Biotechnology, Santa Cruz, CA) or mouse anti‐FLAG monoclonal (Sigma‐Aldrich) antibodies. The blots were visualized by ProtoBlot AP system (Promega, Madison, WI).
Northern analysis. Total RNA was extracted from the mock and the TEL‐overexpressing cells using ISOGEN (Nippon Gene, Tokyo, Japan) under the manufacturer's instruction. Twenty µg of each RNA sample was resolved by electrophoresis on agarose formaldehyde gels, transferred to Hybond‐N+ nylon membranes (Amersham, Piscataway, NJ) in 20 × standard sodium citrate (SSC) and hybridized to human cDNA fragments for ALAS‐E, EPO‐R, γ‐globin, GPIIb and PF 4 that were labeled with [α‐32P] dCTP using the Megaprime DNA labeling system (Amersham). Human glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) cDNA probe was used as a control. After overnight incubation at 42°C in the presence of 50% formamide, membranes were washed with 0.1 × SSC containing 0.1% sodium dodecyl sulfate (SDS) at 42°C and autoradiographed using Fujix BAS2500 Bio‐image Analyzer (Fuji Photo Film, Tokyo, Japan). Relative expression levels to the level at day 0 in each clone were quantified.
Fluorescence activated cell sorter (FACS) analysis. The mock and the TEL‐overexpressing cells were incubated for 30 min at 4°C with appropriately diluted fluorescein‐labeled antiglycophorin A and anti‐GPIIb (CD41b) antibodies (Beckman Coulter, Fullerton, CA). After washing, cells were analyzed using Becton Dickinson FACS Calibur.
Electron microscopic analysis. Ultrastructural PPO activity was detected by a conventional method.( 23 )
Results
TEL accelerates erythroid differentiation upon EPO treatment in UT‐7/GM cells. Human leukemia UT‐7/GM cells differentiate into either erythroblasts or megakaryocytes upon cytokine exposure. Thus, this cell line provides a useful tool to analyze the effects of TEL on erythroid differentiation and megakaryocytic maturation in human hematopoietic cells. We established UT‐7/GM clones stably overexpressing FLAG‐tagged TEL by electroporating the expression plasmid containing TEL cDNA and selecting cells with G418 resistance. Western analysis with anti‐FLAG antibody demonstrated that representative clones T‐5 and T‐6 expressed TEL proteins at high levels (Fig. 1). Mock clones M‐1 and M‐4 were also isolated by introducing the empty expression plasmid. Overexpression of TEL slightly retarded growth of the cells under treatment with EPO or TPO, but did not influence proliferation of the cells maintained in GM‐CSF (data not shown).
Figure 1.
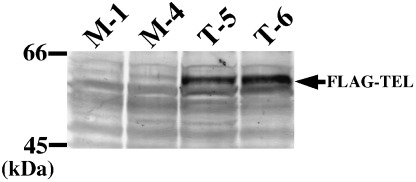
Establishment of UT‐7/GM sublines overexpressing FLAG‐tagged TEL proteins. Clones T‐5 and T‐6 were obtained from UT‐7/GM cells that were transfected with pCXN2‐FLAG‐TEL and selected by G418 resistance. Clones M‐1 and M‐4 were established from UT‐7/GM cells that were transfected with the empty pCXN2 vector and selected by G418 resistance. Expression of FLAG‐tagged TEL proteins was confirmed by western analysis with anti‐FLAG antibody. An arrow indicates overexpressed FLAG‐TEL proteins.
We previously reported that TEL acts as an accelerator of erythroid differentiation induced by chemical compounds such as HMBA and DMSO in MEL cells.( 20 ) To confirm this effect of TEL under a more physiological condition in human hematopoietic cells, we treated the mock and the TEL‐overexpressing clones with EPO. We observed no morphological differences between them, except a faint color difference in the cytoplasm. Figure 2 indicates time courses of hemoglobin synthesis estimated by proportions of benzidine‐positive cells in these UT‐7/GM clones. In the mock clones, proportions of benzidine‐positive cells reached to 80% within two weeks. Interestingly, the TEL‐overexpressing clones showed rapid onset and higher saturation of benzidine positivity in comparison with the mock clones. Eighty percent of the cells became positive for benzidine staining after 10 days of culture and 90% after 14 days. We thus conclude that TEL is also an accelerator for erythroid differentiation upon cytokine stimulation in human hematopoietic cells.
Figure 2.
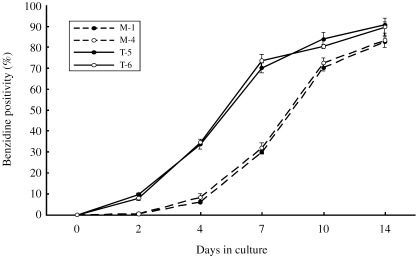
TEL accelerates hemoglobin synthesis induced by treatment with erythropoietin (EPO) in the UT‐7/GM clones. The mock (M‐1 and M‐4) and TEL‐overexpressing (T‐5 and T‐6) UT‐7/GM clones were cultured in the presence of EPO (10 U/mL). Hemoglobin synthesis was evaluated by the proportions of benzidine‐positive cells and their averages in three independent experiments were indicated with standard deviations.
To further obtain evidence for erythroid differentiation exaggerated by TEL in UT‐7/GM cells, erythroid differentiation‐specific transcripts were analyzed using northern analysis. As shown in Fig. 3, transcripts for γ‐globin, ALAS‐E and EPO‐R increased upon EPO exposure in both cell types. However, even before the treatment (at day 0), expression of these genes appeared to be stimulated by overexpressed TEL proteins. This tendency was maintained at all the time points examined. Next, we performed flow cytometric analysis to assess expression levels of erythrocyte‐specific glycophorin A and megakaryocyte‐specific GPIIb in the cell surface during the course of erythroid differentiation. Proportions of the glycophorin A(+)/GPIIb(–) fractions were significantly higher at days 4 and 7 in the TEL‐overexpressing cells than in the mock cells (Fig. 4). The glycophorin A(–)/GPIIb(+) fractions disappeared more rapidly in the TEL‐overexpressing cells. These results collectively confirm the TEL functions as an erythroid differentiation stimulator and indicate the possibility that TEL might concomitantly accelerate erythroid differentiation and repress megakaryocytic maturation.
Figure 3.
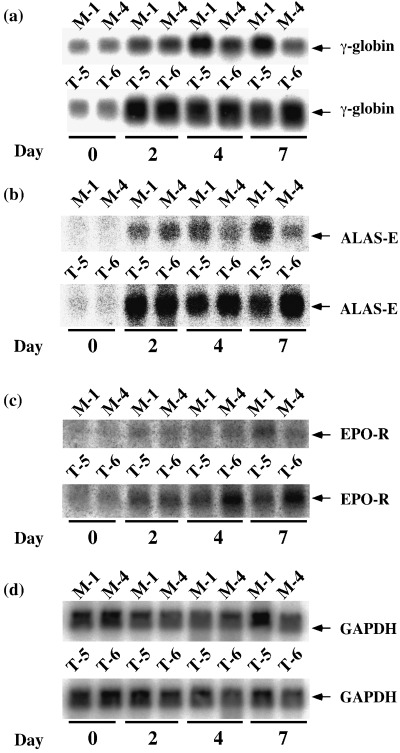
Erythroid lineage‐specific gene transcription in the UT‐7/GM clones under treatment with erythropoietin (EPO). The mock (M‐1 and M‐4) and TEL‐overexpressing (T‐5 and T‐6) UT‐7/GM clones cultured in the presence of EPO (10 U/mL) were harvested at each time point indicated (days 0, 2, 4, 7). Total mRNA was extracted and subjected to northern analysis with γ‐globin (A), ALAS‐E (B), EPO‐R (C) and GAPDH (D) probes.
Figure 4.
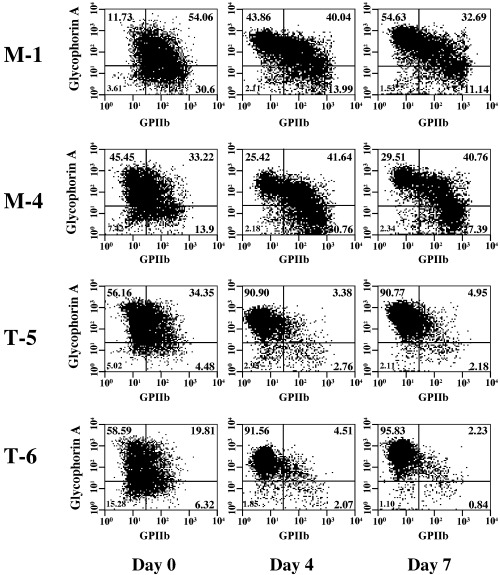
Erythroid and megakaryocytic lineages‐specific surface antigen expression in the UT‐7/GM clones under the treatment with erythropoietin (EPO). The mock (M‐1 and M‐4) and TEL‐overexpressing (T‐5 and T‐6) UT‐7/GM clones cultured in the presence of EPO (10 U/mL) were harvested at each time point indicated (days 0, 4, 7) and subjected to flow‐cytometric analysis. GPIIb on X axis and glycophorin A on Y axis were megakaryocyte‐ and erythrocyte‐specific markers, respectively.
TEL inhibits megakaryocytic maturation upon TPO treatment in UT‐7/GM cells. To clarify the roles of TEL in megakaryocytic maturation of human hematopoietic cells, we induced megakaryocytic maturation by treatment with TPO in the mock and the TEL‐overexpressing clones and first analyzed their morphological changes. Differing from the mock clones, the TEL‐overexpressing clones hardly maturated into megakaryocyte‐containing multilobulated nuclei even after 28 days of culture with TPO (Fig. 5a). Expression of megakaryocytic maturation‐specific genes such as GPIIb and PF 4 was also examined using northern analysis. The TEL‐overexpressing cells expressed these transcripts at almost comparable levels to mock cells before the treatment (Fig. 5b–d). As expected, they increased upon TPO exposure in both cell types. It is interesting to note that levels of these transcripts were lower in the TEL‐overexpressing cells than in the mock cells at least until day 14. We again examined cell surface expression of glycophorin A and GPIIb during the course of megakaryocytic maturation. Proportions of the glycophorin A(–)/GPIIb(+) fractions were markedly lower until day 14 in the TEL‐overexpressing cells than in the mock cells, whereas proportions of the glycophorin A(+)/GPIIb(–) fractions higher (Fig. 6). Furthermore, fewer percentages of the cells became positive for electron microscopic PPO in the TEL‐overexpressing clones after 14 days treatment with TPO (Fig. 7). We hypothesize that TEL could prevent megakaryocytic maturation and maintain expression of erythroid markers in erythrocyte/megakaryocyte common progenitors even when induced towards the megakaryocytic lineage.
Figure 5.
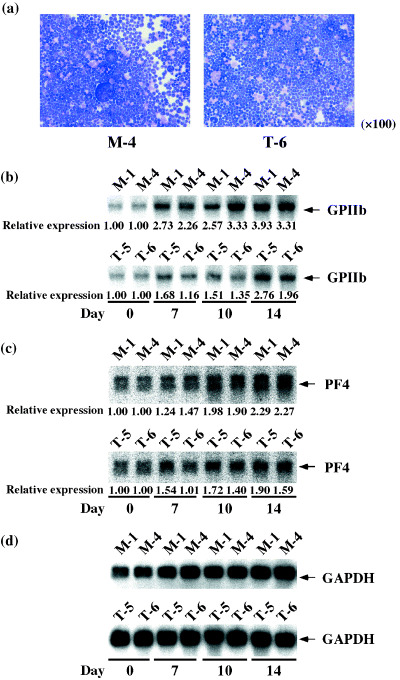
Morphology and megakaryocytic lineage‐specific gene transcription in the UT‐7/GM clones under treatment with thrombopoietin (TPO). The mock (M‐1 and M‐4) and TEL‐overexpressing (T‐5 and T‐6) UT‐7/GM clones cultured in the presence of TPO (100 ng/mL) were harvested at each time point indicated (days 0, 7, 10, 14, 28). (a) Cytospin preparations of M‐4 and T‐5 at day 28. Wright‐Giemsa staining, ×100. (b–d) Total mRNA was extracted and subjected to northern analysis with GPIIb (b), PF 4 (c) and GAPDH (d) probes. Signal ratios between day 0 and the indicated time points were quantified and presented below each lane.
Figure 6.
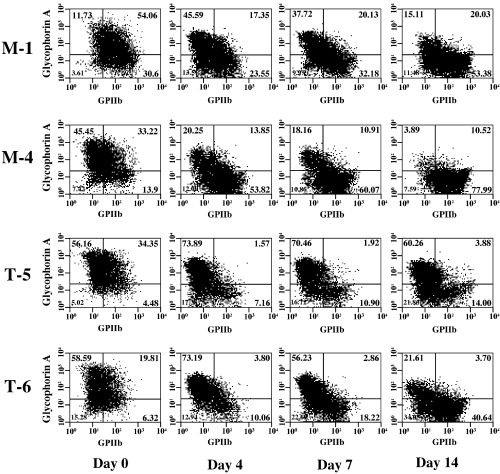
Erythroid and megakaryocytic lineage‐specific surface antigen expression in the UT‐7/GM clones under the treatment with thrombopoietin (TPO). The mock (M‐1 and M‐4) and TEL‐overexpressing (T‐5 and T‐6) UT‐7/GM clones cultured in the presence of TPO (100 ng/mL) were harvested at each time point indicated (days 0, 4, 7, 14) and subjected to flow‐cytometric analysis. GPIIb on X axis and glycophorin A on Y axis were megakaryocyte‐ and erythrocyte‐specific markers, respectively.
Figure 7.
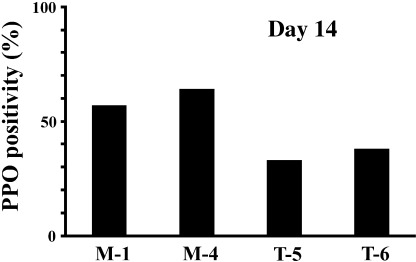
TEL represses ultrastructural platelet peroxidase (PPO) reactions after 14 days of treatment with thrombopoietin (TPO) in the UT‐7/GM clones. The mock (M‐1 and M‐4) and TEL‐overexpressing (T‐5 and T‐6) UT‐7/GM clones were cultured in the presence of TPO (100 ng/mL) for 14 days. PPO reactions were evaluated by electron microscopic analysis.
Expression of endogenous TEL proteins decreases upon both EPO and TPO treatments in UT‐7/GM cells. Finally, we examined changes of endogenous TEL expression during both the courses of erythroid and megakaryocytic differentiation in parental UT‐7/GM cells to further obtain findings for the physiological roles of TEL. Under the presence of GM‐CSF, endogenous TEL proteins were detected at almost the same size as overexpressed TEL proteins in the T‐5 clone (lane 4, Fig. 8a) using western analysis (lane 3) and self‐immunoprecipitation assay (lane 1). When the cells were induced to erythroid differentiation by treatment with EPO, endogenous TEL proteins maintained steady expression until 3 days of culture and then began to decline (Fig. 8b). At day 14, endogenous TEL proteins almost completely disappeared. When induced to megakaryocytic maturation by treatment with TPO, UT‐7/GM cells kept constant expression of endogenous TEL proteins until 14 days of culture and lost their expression at day 21 (Fig. 8c). These data suggest that endogenous TEL may work in the early phase of differentiation to either lineage and accelerate erythroid differentiation and actively repress megakaryocytic maturation.
Figure 8.
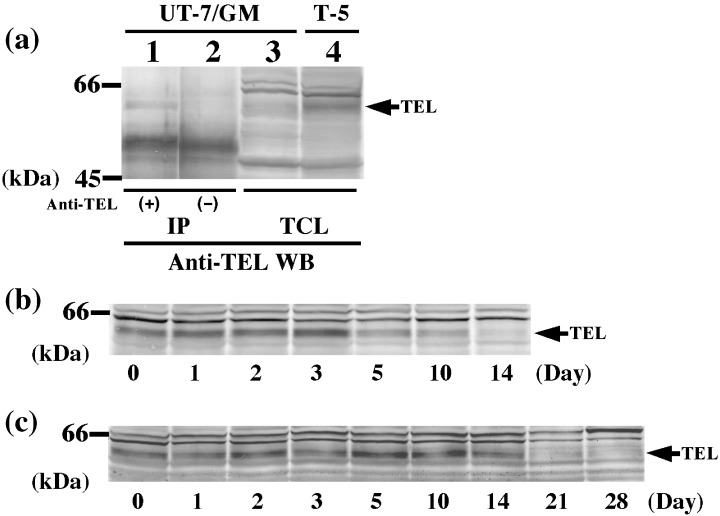
Expression of endogenous TEL proteins in parental UT‐7/GM cells. (a) Expression of endogenous TEL proteins in parental UT‐7/GM cells was confirmed under the presence of GM‐CSF (1 ng/mL) by western analysis (lane 3) or immunoprecipitation assay (lane 1) with anti‐TEL antibody. Overexpressed FLAG‐tagged TEL proteins in clone T‐5 were shown in lane 4. An arrow indicates endogenous TEL or overexpressed FLAG‐tagged TEL proteins; (b,c) Parental UT‐7/GM cells cultured in the presence of erythropoietin (10 U/mL); (b) or thrombopoietin (100 ng/mL); (c) were harvested at each time point indicated (days 0, 1, 2, 3, 5, 10, 14, 21, 28). Cell lysates were extracted and subjected to western analysis with anti‐TEL antibody. Arrows indicate endogenous TEL proteins.
Discussion
We demonstrated in the present study that TEL accelerates erythroid differentiation induced by a physiological cytokine EPO in human leukemia cell line UT‐7/GM. Associated with increased expression of erythroid differentiation‐specific transcripts γ‐globin, ALAS‐E and EPO‐R, and a surface antigen glycophorin A, the TEL‐overexpressing cells accumulate hemoglobin more rapidly than the mock cells. A megakaryocyte maturation‐specific surface marker GPIIb disappears more quickly during the course of erythroid differentiation in the TEL‐overexpressing cells. Importantly, morphological maturation towards megakaryocyte with multilobulated nuclei and induction of megakaryocyte maturation‐specific transcripts GPIIb and PF 4 after TPO treatment are weaker when TEL is overexpressed. Moreover, GPIIb accumulates and glycophorin A disappears more slowly in the cell surface of these cells. Electron microscopic PPO reaction is detected at fewer ratios. All these data collectively suggest that TEL might drive erythroid differentiation and suppress megakaryocytic maturation in erythrocyte/megakaryocyte common progenitors. Consistently, endogenous TEL proteins are expressed only in the early phase of either differentiation in which TEL is expected to function, and thereafter disappear. This paper is the first describing the unique role of TEL in the megakaryocytic lineage.
Because TEL is a transcriptional regulator for EBS‐containing promoters, it is interesting to know whether the cis‐regulatory elements actually exist in the erythrocyte or megakaryocyte‐specific genes, the expression of which was found in this study to be altered by overexpressed TEL. Numerous megakaryocyte‐specific genes contain EBS and GATA‐1 binding sites in their promoters.( 24 ) Of note, both ETS‐1 and GATA‐1 are reported essential for positive regulation of GPIIb and PF 4 gene transcription.( 25 ) Moreover, ETS‐1 is demonstrated to directly bind to their promoters by chromatin precipitation assays.( 26 ) Although it remains undetermined whether TEL binds to EBS in the promoters of GPIIb and PF 4 genes, overexpressed TEL could repress it directly or indirectly. In the latter case, TEL may dominantly suppress functions of other ETS family members such as ETS‐1 that show transactivation abilities on the promoters through heterodimerizing with them by the HLH domain. However, because EBS is not identified in the promoters of the erythrocyte‐specific genes examined in this study, we have no ground to speculate that TEL could be involved in their transcriptional regulation.
We hypothesize that TEL could trigger erythroid differentiation and prevent megakaryocytic maturation through repressing transcription of its target genes that play key roles in hematopoietic differentiation. Among the known target genes of TEL, FLI‐1 and Id‐1 are shown to have functions in erythrocyte/megakaryocyte differentiation. The FLI‐1 gene was first isolated as a common site for retroviral integration in Friend virus‐induced erythroleukemia cells,( 27 ) and also encodes a member of the ETS family of transcription factors. FLI‐1 suppresses erythroid differentiation partly through inhibiting transcription of the genes such as GATA‐1,( 28 ) Rb ( 29 ) and β‐globin ( 30 ) that promote erythroid differentiation. Moreover, FLI‐1 knockout mice are embryonic lethal around mid‐gestation and display a marked reduction of megakaryocytes in the fetal liver as well as a vascular developmental aberration,( 31 ) suggesting a critical role of FLI‐1 in megakaryocytic maturation. FLI‐1 binds and transactivates the promoters from megakaryocyte‐specific genes including GPIX,( 32 ) GPIIb ( 32 ) and TPO receptor.( 33 ) Therefore, FLI‐1 appears to play opposite roles in erythroid differentiation and megakaryocytic maturation. We analyzed expression levels of FLI‐1 proteins before and after induction of erythroid differentiation or megakaryocytic maturation in the mock and TEL‐overexpressing UT‐7/GM cells. However, overexpressed TEL proteins did not affect the expression of FLI‐1 in UT‐7/GM cells (data not shown). In spite of this, there still remains the possibility that TEL could repress molecular functions of FLI‐1 in these cells, because TEL has been proved to exert a dominant‐negative effect on FLI‐1 in reporter assays.( 2 ) The Id‐1 gene was initially cloned from MEL cells by virtue of homology to the helix 2 subdomain in c‐myc, MyoD and myogenin, and codes for the first member of Id‐family that has the HLH domain.( 34 ) Id‐1 has been reported to be functionally implicated in differentiation of specific hematopoietic lineages including erythroid,( 35 ) myeloid,( 36 ) and B cells( 37 ) and negatively control erythroid differentiation. We observed that expression of Id‐1 proteins slightly increased after induction of erythroid differentiation or megakaryocytic maturation in UT‐7/GM cells, but that overexpressed TEL proteins did not influence its expression levels. Therefore, we failed to obtain evidence that FLI‐1 or Id‐1 could be targets of TEL‐induced transcriptional repression in UT‐7/GM cells. Identification of novel target genes for TEL that regulate erythroid and/or megakaryocytic differentiation should provide new insights into molecular mechanisms in hematopoietic cell differentiation. Studies to determine the target genes of TEL in differentiating MEL and UT‐7/GM cells are now in progress in our laboratory.
Acknowledgments
TEL10/pcDNA3 and pCXN2 are generous gifts from Dr T.R. Golub (Dana‐Farber Cancer Institute, Boston, USA) and Dr J. Miyazaki (University of Osaka, Osaka, Japan), respectively. rhGM‐CSF, EPO and TPO were provided by KIRIN Brewery. We thank Dr M. Eguchi (Dokkyo University School of Medicine, Tochigi, Japan) for ultrastructural analysis of PPO. This work was financially supported in part by Grants‐in‐Aid from ministries in Japan of Education, Culture, Sports, Science and Technology, and Health, Labour and Welfare, The Japan Health Sciences Foundation, and Japanese Society for the Promotion of Science.
References
- 1. Golub TR, Barker GF, Lovett M, Gilliland DG. Fusion of PDGF receptor β to a novel ets‐like gene, Tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell 1994;. 77: 307–316. [DOI] [PubMed] [Google Scholar]
- 2. Kwiatkowski BA, Bastian LS, Bauer TR, Jr , Tsai S, Zielinska‐Kwiatkowska AG, Hickstein DD. The ets family member Tel binds to the Fli‐1 oncoprotein and inhibits its transcriptional activity. J Biol Chem 1998; 273: 17525–30. [DOI] [PubMed] [Google Scholar]
- 3. Potter MD, Buijs A, Kreider B, Van Rompaey L, Grosveld GC. Identification and characterization of a new human ETS‐family transcription factor, TEL2, that is expressed in hematopoietic tissues and can associate with TEL1/ETV6 . Blood 2000; 95: 3341–8. [PubMed] [Google Scholar]
- 4. Wang L, Hiebert SW. TEL contacts multiple co‐repressors and specifically associates with histone deacetylase‐3. Oncogene 2001; 20: 3716–25. [DOI] [PubMed] [Google Scholar]
- 5. Martinez R, Golub TR. Blood 2000; 96: 453a. [Google Scholar]
- 6. Fenrick R, Wang L, Nip J et al. TEL, a putative tumor suppressor, modulates cell growth and cell morphology of ras‐transformed cells while repressing the transcription of Stromelysin‐1 . Mol Cell Biol 2000; 20: 5828–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Irvin BJ, Wood LD, Wang L et al. TEL, a putative tumor suppressor, induces apoptosis and represses transcription of Bcl‐XL . J Biol Chem 2000; 278: 46378–86. [DOI] [PubMed] [Google Scholar]
- 8. Arai H, Maki K, Waga K et al. Functional regulation of TEL by p38‐induced phosphorylation. Biochem Biophys Res Commun 2002; 299: 116–25. [DOI] [PubMed] [Google Scholar]
- 9. Maki K, Arai H, Waga K et al. Leukemia‐related transcription factor TEL is negatively regulated through extracellular signal‐regulated kinase‐induced phosphorylation. Mol Cell Biol 2004; 24: 3227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wood LD, Irvin BJ, Nucifora G, Luce KS, Hiebert SW. Small ubiquitin‐like modifier conjugation regulates nuclear export of TEL, a putative tumor suppressor. Proc Natl Acad Sci USA 2003; 100: 3257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chakrabarti SR, Sood R, Nandi S, Nucifora G. Posttranslational modification of TEL and TEL/AML1 by SUMO‐1 and cell‐cycle‐dependent assembly into nuclear bodies. Proc Natl Acad Sci USA 2000; 97: 13281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jousset C, Carron C, Boureux A et al. A domain of TEL conserved in a subset of ETS proteins defines a specific oligomerization interface essential to the mitogenic properties of the TEL‐PDGFRβ oncoprotein. EMBO J 1997; 16: 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Golub TR, Goga A, Barker GF et al. Oligomerization of the ABL tyrosine kinase by the Ets protein TEL in human leukemia. Mol Cell Biol 1996; 16: 4107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cazzaniga G, Tosi S, Aloisi A et al. The tyrosine kinase abl‐related gene ARG is fused to ETV6 in an AML‐M4Eo patient with a t(1;12) (q25;p13): molecular cloning of both reciprocal transcripts. Blood 1999: 94: 4370–3. [PubMed]
- 15. Lacronique V, Boureux A, Valle VD et al. A TEL‐JAK2 fusion protein with constitutive kinase activity in human leukemia. Science 1997; 278: 1309–12. [DOI] [PubMed] [Google Scholar]
- 16. Kuno Y, Abe A, Emi N et al. Constitutive kinase activation of the TEL‐Syk fusion gene in myelodysplastic syndrome with t(9;12) (q22;p12). Blood 2001: 97 : 1050–5. [DOI] [PubMed] [Google Scholar]
- 17. Hiebert SW, Sun W, Davis JN et al. The t(12;21) translocation converts AML−1B from an activator to a repressor of transcription. Mol Cell Biol 1996: 16 : 1349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang LC, Kuo F, Fujiwara Y, Gilliland DG, Golub TR, Orkin SH. Yolk sac angiogenic defect and intra‐embryonic apoptosis in mice lacking the Ets‐related factor TEL. EMBO J 1997; 16: 4374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang LC, Swat W, Fujiwara Y et al. The TEL/ETV6 gene is required specifically for hematopoiesis in the bone marrow. Genes Dev 1998; 12: 2392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waga K, Nakamura Y, Maki K et al. Leukemia‐related transcription factor TEL accelerates differentiation of Friend erythroleukemia cells. Oncogene 2003; 22: 59–68. [DOI] [PubMed] [Google Scholar]
- 21. Komatsu N, Kirito K, Shimizu R et al. In vitro development of erythroid and megakaryocytic cells from a UT‐7 subline, UT‐7/GM. Blood 1997; 89: 4021–33. [PubMed] [Google Scholar]
- 22. Komatsu N, Nakauchi H, Miwa A et al. Establishment and characterization of a human leukemic cell line with megakaryocytic features dependency on granulocyte‐macrophage colony‐stimulating factor, interleukin 3, or erythropoietin for growth and survival. Cancer Res 1991; 51: 341–8. [PubMed] [Google Scholar]
- 23. Breton‐Gorius J, Guichard J. Ultrastructural localization of peroxidase activity in human platelets and megakaryocytes. Am J Pathol 1972; 66: 277–93. [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang C, Thornton MA, Kowalska MA et al. Localization of distal regulatory domains in the megakaryocyte‐specific platelet basic protein/platelet factor 4 gene locus. Blood 2001; 98: 610–7. [DOI] [PubMed] [Google Scholar]
- 25. Lemarchandel V, Ghysdael J, Mignotte V, Rahuel C, Romeo PH. GATA and Ets cis‐acting sequences mediate megakaryocyte‐specific expression. Mol Cell Biol 1993; 13: 668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu J, Pazin MJ, Ravid K. Properties of ets‐1 binding to chromatin and its effect on platelet factor 4 gene expression. Mol Cell Biol 2004; 24: 428–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ben‐David Y, Giddens EB, Letwin K, Bernstein A. Erythroleukemia induction by Friend murine leukemia virus: insertional activation of a new member of the ets gene family, Fli‐1, closely linked to c‐ets‐1. Genes Dev 1991; 5: 908–18. [DOI] [PubMed] [Google Scholar]
- 28. Athanasiou M, Mavrothalassitis G, Sun‐Hoffman L, Blair DG. FLI‐1 is a suppressor of erythroid differentiation in human hematopoietic cells. Leukemia 2000; 14: 439–45. [DOI] [PubMed] [Google Scholar]
- 29. Tamir A, Howard J, Higgins RR et al. Fli‐1, an Ets‐related transcription factor, regulates erythropoietin‐induced erythroid proliferation and differentiation: evidence for direct transcriptional repression of the Rb gene during differentiation. Mol Cell Biol 1999; 19: 4452–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Starck J, Cohet N, Gonnet C et al. Functional cross‐antagonism between transcription factors FLI‐1 and EKLF. Mol Cell Biol 2003; 23: 1390–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hart A, Melet F, Grossfeld P et al. Fli‐1 is required for murine vascular and megakaryocytic development and is hemizygously deleted in patients with thrombocytopenia. Immunity 2000; 13: 167–77. [DOI] [PubMed] [Google Scholar]
- 32. Bastian LS, Kwiatkowski BA, Breininger J, Danner S, Roth G. Regulation of the megakaryocytic glycoprotein IX promoter by the oncogenic Ets transcription factor Fli‐1. Blood 1999; 93: 2637–44. [PubMed] [Google Scholar]
- 33. Deveaux S, Filipe A, Lemarchandel V, Ghysdael J, Romeo PH, Mignotte V. Analysis of the thrombopoietin receptor (MPL) promoter implicates GATA and Ets proteins in the coregulation of megakaryocyte‐specific genes. Blood 1996; 87: 4678–85. [PubMed] [Google Scholar]
- 34. Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix‐loop‐helix DNA binding proteins. Cell 1990; 61: 49–59. [DOI] [PubMed] [Google Scholar]
- 35. Lister J, Forrester WC, Baron MH. Inhibition of an erythroid differentiation switch by the helix‐loop‐helix protein Id1. J Biol Chem 1995; 270: 17939–46. [DOI] [PubMed] [Google Scholar]
- 36. Kreider BL, Benezra R, Rovera G, Kadesch T. Inhibition of myeloid differentiation by the helix‐loop‐helix protein Id. Science 1992; 255: 1700–2. [DOI] [PubMed] [Google Scholar]
- 37. Pongubala JM, Atchison ML. Functional characterization of the developmentally controlled immunoglobulin kappa‐3′ enhancer. regulation by Id, a repressor of helix‐loop‐helix transcription factors. Mol Cell Biol 1991; 11: 1040–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


