Abstract
Breast cancer resistance protein (BCRP/ABCG2) is a half‐molecule ATP‐binding cassette transporter that we have previously suggested might function as a homodimer, bridged by disulfide bonds. In the present study, we carried out cysteine‐scanning mutagenesis, substituting Ser for Cys, and established 12 PA317 transfectants expressing BCRP mutants with possible disruptions to their S–S bonds. Western blot analysis of BCRP from the wild‐type transfectants (PA/WT) confirmed that the wild‐type protein migrates as a 140‐kDa dimer under non‐reducing conditions, but as a 70‐kDa monomer under reducing conditions. However, under non‐reducing conditions the BCRP‐C603S mutant migrated both as a 70‐kDa monomer and a 140‐kDa dimer, whereas all other mutant BCRP migrated only as dimers. PA317 cells transfected with C603S‐BCRP (PA/C603S) showed either similar or only marginally lower SN‐38 resistance than PA/WT cells, despite the reduced levels of BCRP dimer in these cells. Moreover, the degree of SN‐38 resistance in the mutant BCRP transfectants was found to be associated with the monomer expression levels under reducing conditions. Reverse transcription–polymerase chain reaction analysis showed that the BCRP mRNA levels were similar in the transfectants. We subsequently generated six C603X mutants of BCRP (X = D, H, R, Y, A and W) and carried out western blot analysis and drug sensitivity assays. The results were equivalent to those from the PA/C603S cells, with some variations that again corresponded to the monomer levels. Our findings suggest that Cys‐603 is an important residue in the covalent bridge between BCRP monomers but that a functioning unit of BCRP may not necessarily require covalent linkages. (Cancer Sci 2005; 96: 866–872)
Abbreviations:
- DHFR
dihydrofolate reductase
- IRES
internal ribosome entry site.
ATP‐binding cassette (ABC) transporters, such as the MDR1 gene products P‐glycoprotein( 1 , 2 , 3 , 4 ) and MRP1,( 5 ) are known to be involved in multidrug resistance. Breast cancer resistance protein (BCRP/ABCG2/MXR) is a new member of the ABC transporter family,( 6 , 7 , 8 ) with a C‐terminal transmembrane domain and an N‐terminal ATP‐binding domain,( 9 ) consisting of 655 amino acids. BCRP has been studied as a molecular target for anticancer drug resistance because of its ability to confer resistance to mitoxantrone, 7‐ethyl‐10‐hydroxycamptothecin (SN‐38) and topotecan in cells by pumping out these structurally unrelated drugs.( 10 , 11 , 12 , 13 , 14 , 15 , 16 )
We previously showed that BCRP might form a homodimer, bridged by disulfide bonds, and that BCRP function was impaired by dominant‐negative mutants through S–S‐dependent homodimerization.( 17 ) In our present study we removed each of the possible disulfide bonds that may be involved in BCRP dimerization by site‐directed mutagenesis of the cysteine residues in this protein. As shown in Fig. 1, BCRP has six cysteines in the cytoplasm, three of which are intramembranous and three that are extracellular. We constructed 12 BCRP mutants for these analyses, each with a serine substitution in place of a cysteine, using site‐directed mutagenesis of BCRP cDNA, and examined the resulting effects on both protein structure and function. We show from our data that Cys‐603 is likely to be an important residue for the stable oligomerization of BCRP.
Figure 1.
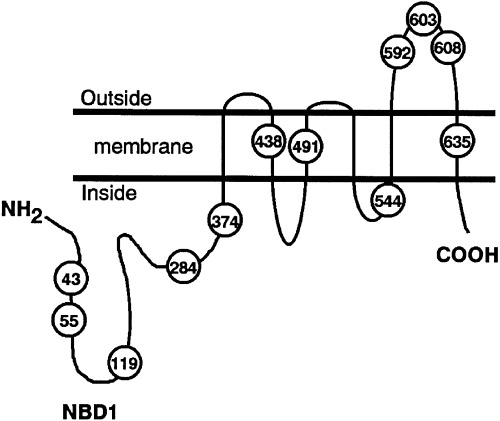
Schematic diagram of a breast cancer resistance protein (BCRP) molecule indicating the position of its 12 cysteine residues. We constructed 12 mutants of BCRP by replacing each of these cysteines with serine by site‐directed mutagenesis of the cDNA template.
Materials and Methods
Cell culture and drug sensitivity assay
PA317 amphotropic retrovirus packaging cells were grown in Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum, at 37°C in a humidified incubator with 5% CO2. The sensitivity of the mutant BCRP‐transfected cells to SN‐38 was evaluated by the inhibition of cell growth after incubation at 37°C in the presence of various concentrations of SN‐38. After a 5‐day incubation of cells with these agents, cell numbers were determined using a Coulter cell counter and drug concentrations that inhibited cell growth by 50% (IC50) were determined from growth inhibition curves. Statistical analysis was carried out using the Student's t‐test.
Generation of wild‐type and mutant BCRP vectors, and transfections
Mutant BCRP cDNAs containing cysteine‐to‐serine substitutions were synthesized using a site‐directed mutagenesis kit (Takara, Kyoto, Japan), according to the manufacturer's instructions. Our previously described BCRP cDNA( 17 ) was used as a template for these reactions. The nucleotide sequences of the mutant BCRP cDNA clones were confirmed using an automated DNA sequencer. The wild‐type and mutant BCRP cDNAs were then cloned into pHaL‐IRES‐DHFR bicistronic retrovirus vectors. PA317 cells were transfected with the vectors according to the method of Chen and Okayama.( 18 ) In cells transfected with pHa‐BCRP‐IRES‐DHFR, a single mRNA is transcribed under the control of a retroviral long terminal repeat of Harvey murine sarcoma virus (LTR) promoter, and two gene products are translated independently from a bicistronic mRNA. It has been shown previously that cells expressing DHFR are resistant to methotrexate.( 19 ) The transfectants were therefore selected by exposure to 120 ng/mL methotrexate for 5 days and by subsequent exposure to 1 ng/mL mitoxantrone for an additional 5 days to eliminate residual BCRP‐non‐expressing cells. The resulting BCRP transfectants were pooled, and were named using the prefix ‘PA’ (parental).
Western blot analysis under both reducing and non‐reducing conditions
Exponentially growing cells were harvested, washed and solubilized in T buffer (10 mM Tris‐HCl, pH 8.0, 0.1% Triton‐X 100, 10 mM MgSO4, 2 mM CaCl2, 1 mM 4‐[2‐aminoethyl]‐benzenesulfonyl fluoride) with or without 1 mM dithiothreitol. Cellular debris was removed by centrifugation and the supernatant was subjected to immunoblotting. The protein concentrations of the cell lysates were measured by the Bradford method using Bio‐Rad Protein Assay reagent (Bio‐Rad, Hercules, CA, USA). Protein samples were then solubilized with 2% sodium dodecylsulfate (SDS), 50 mM Tris‐HCl, pH 7.5, in the presence or absence of 5% 2‐mercaptoethanol. Before loading, samples treated under reducing conditions were heated at 70°C for 10 min and samples subjected to non‐reducing conditions were heated at 20°C for 5 min. Samples (20 µg or the appropriate amount of protein per lane) were resolved by 5–20% SDS‐polyacrylamide gel electrophoresis (SDS‐PAGE) and electroblotted onto nitrocellulose membranes. The blots were then incubated with mouse anti‐BCRP monoclonal antibody (BXP‐21, Chemicon International, Temecula, CA, USA) for 1 h. BXP‐21 recognizes an internal epitope of a 126‐amino acid region of BCRP (amino acids 271–396). After washing, the blots were incubated with peroxidase‐conjugated antimouse immunoglobulins (Amersham Pharmacia Biotech, Buckinghamshire, UK). Membrane‐bound peroxidase was visualized on Hyperfilm ECL Plus after enhancement with a chemiluminescence detection kit (Amersham Pharmacia Biotech).
Fluorescence‐activated cell sorter analysis
After selection by methotrexate and mitoxantrone, the cell‐surface expression levels of the BCRP protein were analyzed by fluorescence‐activated cell sorter (FACS). Briefly, trypsinized cells (5 × 105) were incubated with 20 µg/mL of biotinylated antihuman BCRP (5D3) (eBioscience, San Diego, CA, USA), washed and incubated with R‐phycoerythrin‐conjugated streptavidin (BD Biosciences, San Jose, CA, USA). The epitope for this antibody is localized in one of the extracellular domains of BCRP, which corresponds to amino acids 417–428, 500–504 or 567–628. Fluorescence staining was then analyzed using FACSCalibur (BD Biosciences).
Semi‐quantitative reverse transcription–polymerase chain reaction analysis
BCRP mRNA expression in wild‐type and mutant BCRP transfectants was examined by reverse transcription–polymerase chain reaction (RT‐PCR). Total RNA was extracted from 8 × 106 cells using an RNeasy Mini kit (Qiagen, Valencia, CA, USA) and subsequent RT‐PCR was carried out using an LA‐RT‐PCR kit (Takara), according to the manufacturer's instructions. The primers for PCR described below were previously reported.( 20 ) First strand cDNA was synthesized with 0.3 µg of total RNA and a 315‐bp BCRP cDNA fragment, and then amplified with the primers 5′‐CAG GTG GAG GCA AAT CTT CGT‐3′ (forward) and 5′‐A CAC ACC ACG GAT AAA CTG A‐3′ (reverse). As an internal control, amplification of GAPDH mRNA (551‐bp fragment) was carried out with the primers 5′‐ATC ACC ATC TTC CAG GAG CGA‐3′ (forward) and 5′‐GCT TCA CCA CCT TCT TGA TGT‐3′ (reverse). The PCR conditions were as follows: 95°C for 9 min, then increasing cycle numbers of 95°C for 30 s, 55°C for 30 s and 72°C for 30 s.
Results
Expression of BCRP in mutant BCRP transfectants
The expression of BCRP in the PA317 transfectants was evaluated by both western blotting and FACS analyses. BCRP protein in PA/WT (BCRP‐WT) cells migrated as a 70‐kDa monomer under reducing conditions and as a 140‐kDa dimer protein under non‐reducing conditions. Under reducing conditions each of the mutants migrated as a 70‐kDa species, as observed for the wild‐type protein (Fig. 2a). Under non‐reducing conditions, however, the BCRP‐C603S mutant was found to migrate as both a 70‐kDa monomer and a 140‐kDa dimer, whereas the BCRP‐WT and each of the other mutant proteins migrated only as 140‐kDa dimers (Fig. 2b). Also under reducing conditions, the BCRP protein levels in PA/C43S, PA/C55S, PA/C284S and PA/C603S cells were slightly lower than the levels in the wild‐type transfectants. Because BCRP protein expression levels in PA/C438S, PA/C592S and PA/C608S cells were found to be remarkably decreased under non‐reducing conditions (Fig. 2b), they were further examined by overexposing the immunoblot (Fig. 2c). Following this increased exposure time, these mutants could also be detected as both a 70‐kDa monomer and a 140‐kDa dimer, as is the case for BCRP‐C603S, although their expression levels were less than 10% of wild‐type. The ratio of monomer to dimer in these mutants was also far higher than PA/WT, as observed for PA/C603S.
Figure 2.
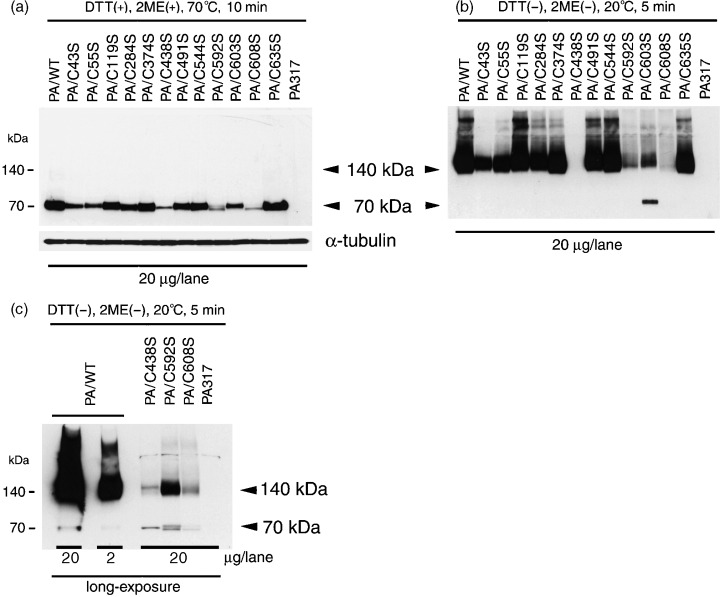
Detection of mutant breast cancer resistance proteins (BCRP), containing serine substitutions, by western blot analysis. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS‐PAGE) was carried out (a) under reducing conditions with heating at 70°C for 10 min, or (b) under non‐reducing conditions with heating at 20°C for 5 min. α‐Tubulin expression was analyzed as a loading control. (c) Western blot analysis of three BCRP mutants with a long exposure showing weak band intensities under non‐reducing conditions. Cellular protein (20 µg/lane) was separated by 5–20% SDS‐PAGE and then transferred onto nitrocellulose membranes. BCRP was detected using mouse anti‐BCRP monoclonal antibody (BXP‐21).
FACS analysis of our mutant BCRP transfectants was undertaken to examine the levels of exogenous protein expressed on the cell surfaces of these cells (Fig. 3). For the PA/C119S, PA/C374S, PA/C491S, PA/C544S and PA/C635S species, these exogenous mutant protein levels were equivalent to PA/WT. In contrast, the PA/C43S, PA/C55S, PA/C284S and PA/C603S mutants expressed intermediate amounts of BCRP and, in PA/C438S cells, little surface expression of BCRP was observed. In addition, the results of our FACS analyses of these 10 BCRP mutants were in accordance with the western blot analyses carried out under reducing conditions (Fig. 2a). For the PA/C592S and PA/C608S species, which may have mutations in the epitope for the 5D3 antibody located on a extracellular domain of BCRP, BCRP expression on the cell surface was undetectable by FACS, but protein could be detected at low levels by western blotting.
Figure 3.

Expression analysis of mutant breast cancer resistance proteins (BCRP) on the cell surfaces of PA317 transfectants by FACS. Trypsinized cells were incubated with (bold line) or without (fine line) the biotinylated antihuman BCRP monoclonal antibody 5D3, followed by incubation with R‐phycoerythrin‐conjugated streptavidin.
Semi‐quantitative reverse transcription–polymerase chain reaction
Reverse transcription–polymerase chain reaction revealed that each of the transfectants expressed similar levels of BCRP mRNA (Fig. 4). Hence, the observed differences in BCRP expression, particularly in the PA/C438S, PA/C592S, PA/C603S and PA/C608S cells, are not attributable to low transcript levels.
Figure 4.
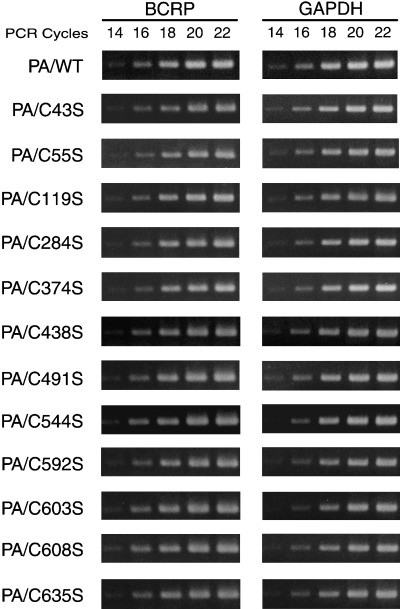
Semi‐quantitative reverse transcription–polymerase chain reaction of mRNA in wild‐type and mutant breast cancer resistance protein (BCRP) transfectants. First‐strand cDNA was synthesized with 0.3 µg of total RNA and a BCRP cDNA fragment (315 bp) was amplified by PCR using the indicated cycle numbers. Amplification of glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) mRNA (551 bp fragment) was carried out as an internal control.
SN‐38 sensitivity of the mutant BCRP transfectants
The results of our SN‐38 sensitivity measurements in the mutant BCRP transfectants, in which the exogenous BCRP expression levels were almost equivalent to PA/WT cells, are shown in Fig. 5a. Transfectants expressing a high level of exogenous BCRP mutant, including PA/C544S and PA/C635S, showed almost the same degree of resistance as PA/WT cells. The SN‐38 sensitivity levels of the mutant BCRP transfectants that expressed either intermediate or small amounts of exogenous protein were also determined and are shown in Fig. 5b,c. Significantly, the degree of drug resistance (IC50 of mutant BCRP transfectants/IC50 of PA/WT) was found to be coincident with the levels of BCRP monomer detected under reducing conditions (Fig. 2a), with a correlation coefficient of 0.88.
Figure 5.
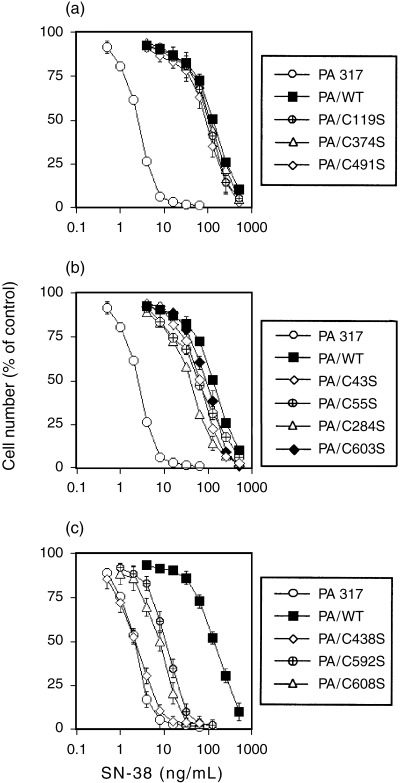
SN‐38 sensitivity assay of mutant breast cancer resistance protein (BCRP)‐transfected PA317 cells. Drug sensitivity to SN‐38 was examined by cell growth inhibition assays as described in Materials and Methods. All data are representative of the mean values ± SD from triplicate determinations. (a) SN‐38 sensitivity of the indicated mutant BCRP transfectants, which expressed similar levels of BCRP protein to PA/WT. (b) SN‐38 sensitivity of four mutant BCRP transfectants, which expressed lower levels of BCRP protein than PA/WT. PA/C43S, PA/C55S, PA/C284S and PA/C603S transfectants acquired similar or somewhat lower degrees of SN‐38 resistance than PA/WT cells. (c) SN‐38 sensitivity of mutants that expressed very small amounts of monomer BCRP. PA/C592S and PA/C608S showed decreased drug resistance and the sensitivity of PA/C438S cells was similar to that of the parental PA317 cells.
Protein expression and SN‐38 sensitivity levels of PA317 cells transfected with mutant BCRP containing Cys‐603 substitutions
Because the monomeric form of BCRP was detectable in PA/C603S cells under non‐reducing conditions (Fig. 2b), we chose to further analyze this specific cysteine residue by generating additional amino acid substitutions at this position. The resulting PA/C603X mutants (X = D, H, R, S, Y, A and W) were also investigated by western blotting under the same conditions used for PA/C603S. Even without the use of reducing agents, both a monomeric band and decreased amounts of the BCRP dimer could be observed for each of these mutants, whereas BCRP‐WT was again present only as a dimer (Fig. 6b). Under reducing conditions, the intensities of the 70‐kDa BCRP monomeric mutant bands were similar to wild‐type with the exception of PA/C603S and PA/C603Y, which had somewhat lower expression levels (Fig. 6a). The SN‐38 resistance measurements in the PA/C603X cells were also found to be slightly lower than PA/WT, and correlated with the levels of the BCRP monomer in each case (Fig. 6b,c). Similar results were found for PA/C603H, PA/C603R, PA/C603G and PA/C603W cells (data not shown). Taken together, these findings suggest that Cys‐603 is directly involved in the covalent attachment of BCRP monomers.
Figure 6.
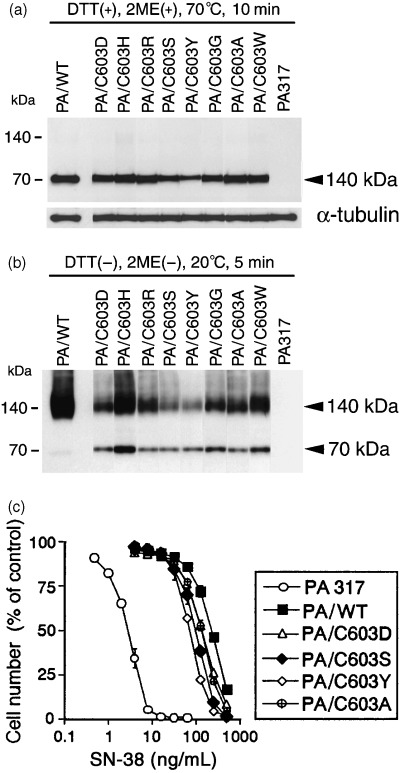
Western blot analysis of breast cancer resistance protein (BCRP) expression and SN‐38 drug sensitivity in PA/C603X mutants. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis was carried out (a) under reducing conditions with heating at 70°C for 10 min and (b) under non‐reducing conditions with heating at 20°C for 5 min. α‐Tubulin expression was analyzed as a loading control. BCRP was detected using the mouse anti‐BCRP monoclonal antibody BXP‐21. Cellular protein (20 µg) was loaded in each lane. (c) SN‐38 sensitivity of parental PA317, PA/WT, PA/C603D, PA/C603Y, PA/C603S and PA/C603A. Drug sensitivity was examined by cell growth inhibition assays as described in Materials and Methods. All data are representative of the mean values ± SD from triplicate determinations.
Discussion
In the present study, we undertook cysteine‐scanning mutagenesis of BCRP to investigate the involvement of specific cysteine residues in the conformation of the functioning BCRP molecule. In a previous study we used a bicistronic vector, pHa‐BCRP‐IRES‐DHFR, to examine the function of BCRP single nucleotide polymorphism (SNP).( 21 ) It has been shown that cells expressing DHFR are resistant to methotrexate.( 19 ) Cells were therefore transfected with wild‐type, G34A, C421A or 944–949‐deleted BCRP cDNAs and were then selected with methotrexate. The methotrexate‐resistant colonies were mixed and then analyzed. In cells transfected with pHa‐BCRP‐IRES‐DHFR, a single mRNA is transcribed under control of a retroviral LTR promoter, and two gene products are translated independently from a bicistronic mRNA. The upstream BCRP cDNA is translated cap‐dependently, and the downstream DHFR cDNA is translated under control of the IRES. Because only one mRNA is transcribed, cells expressing DHFR theoretically always coexpress the BCRP cDNA. Consequently, when the transfected cells are selected with methotrexate, most methotrexate‐resistant cells coexpress BCRP at similar levels and the mRNA levels of the mixed population will closely reflect the levels in individual clones. We subsequently showed from mixed populations of resistant clones that these four BCRP transfectants expressed similar levels of exogenous BCRP mRNA. Moreover, subsequent FACS analysis showed that almost all of the methotrexate‐selected cells expressed BCRP on the cell surface. We then showed that BCRP protein expression from C421A BCRP cDNA was markedly lower than wild‐type. In our current study we used the same strategy to examine amino acid substitution of Cys residues in the BCRP protein. Cells transfected with pHa‐BCRP‐IRES‐DHFR that carried wild‐type or mutant BCRP cDNAs were used for further study after drug selection.
Under non‐reducing conditions, BCRP‐WT and 8/12 Cys–Ser mutant BCRP species were found to migrate as a 140‐kDa dimer. In contrast, considerable levels of the monomeric form of BCRP were observed for the PA/C603S mutant, which correspondingly showed decreased dimer levels (Fig. 2b). These findings suggest that Cys‐603 is significantly involved in covalent bond formation between BCRP monomers. Under non‐reducing conditions, small quantities of both monomeric and dimeric forms of BCRP could be observed for the PA/C438S, PA/C592S and PA/C608S transfectants as well as in PA/C603S cell extracts following overexposure of the blot (Fig. 2c). The Cys‐592 and Cys‐608 residues, located on the same extracellular domain, possibly participate in crosslinking with Cys‐603. In order to elucidate the possible involvement of Cys‐592 and Cys‐608 in BCRP dimerization, and to confirm the protein structure, we are now preparing mutant BCRP with double or triple mutations in cysteines 592, 603 and 608, which may completely inhibit dimer formation. The intensity of the BCRP‐C603S monomer band under non‐reducing conditions was far stronger than the other mutants (Fig. 2b). This suggests that the loss of possible disulfide bridges involving Cys‐603 does not alter the stability of the corresponding mutant BCRP. In the case of BCRP‐C438S, which has a mutation in the second transmembrane domain, little expression of BCRP was observed by either western blotting or FACS. In addition, the SN‐38 sensitivity of PA/C438S cells was equivalent to the parental PA317 cells (Fig. 5c). Hence, the mutation in Cys‐438 is likely to result in a remarkable loss of BCRP activity. In contrast, C43S, C55S and C284S mutations did not interfere with the overall function of BCRP, as these mutants conferred high levels of resistance to SN‐38 (Fig. 5b).
The drug resistance levels induced by exogenous PA/C603S were almost equivalent to PA/WT (Fig. 5b), although the intensity of the 140‐kDa BCRP‐C603S band under non‐reducing conditions was far less than BCRP‐WT (Fig. 2b). Significantly, the degree of SN‐38 resistance was found to be associated with the expression levels of the BCRP monomer, detected under reducing conditions by western blotting, as shown in Fig. 2a and Fig. 5. This finding suggests that a functioning unit of BCRP may not necessarily require a covalent bond. This hypothesis is supported by the recent study of Mitomo et al., which reported that the methotrexate transport ability of BCRP was little affected by disruption of interpeptide disulfide bonds following treatment with 2‐mercaptoethanol.( 22 ) It is known that typical ABC transporter proteins have two transmembrane segments and two ATP‐binding sites,( 23 , 24 , 25 ) and that some half‐molecule ABC transporters form homodimers or heterodimers.( 26 , 27 , 28 , 29 ) Some studies have also reported that BCRP‐WT forms either a homodimer or a homo‐oligomer,( 30 , 31 ) and Xu and coworkers have recently suggested that BCRP exists and functions as a homotetramer.( 32 ) In a previous study,( 17 ) we showed that a 140‐kDa BCRP complex that forms in cells coexpressing Myc‐tagged BCRP and hemagglutinin (HA)‐tagged BCRP could be immunoprecipitated with anti‐Myc antibodies. The precipitate also reacted with anti‐HA, and a 70‐kDa HA‐BCRP monomer was detectable under reducing conditions. These results suggest that BCRP forms a homodimer bridged by disulfide bonds and that it may form other homo‐oligomers. Dominant‐negative inhibition of BCRP function was also demonstrated, suggesting that homodimerization is essential for BCRP function. So far, however, no studies have directly shown the existence of covalent bonds between monomers and counterpart molecules in a BCRP functioning unit. Hence, further studies are needed to confirm the structure of BCRP.
In conclusion, the BCRP cysteine residue at position 603 is significantly involved in a covalent bridge between BCRP monomers, but a functioning unit of BCRP may not necessarily require these covalent bonds.
Acknowledgments
We thank Dr Y. Imai and S. Tsukahara for their expert technical assistance in the laboratory and for their helpful discussions. This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labour and Welfare, Japan.
References
- 1. Chen C‐J, Chin JE, Ueda K et al. Internal duplication and homology with bacterial transport proteins in the mdr1 (P‐glycoprotein) gene from multidrug‐resistant human cells. Cell 1986; 47: 381–9. [DOI] [PubMed] [Google Scholar]
- 2. Ueda K, Clark DP, Chen C‐J, Roninson IB, Gottesman MM, Pastan I. The human multidrug resistance (mdr1) gene. cDNA cloning and transcription initiation. J Biol Chem 1987; 262: 505–8. [PubMed] [Google Scholar]
- 3. Pastan I, Gottesman MM, Ueda K, Lovelace E, Rutherford AV, Willingham MC. A retrovirus carrying an MDR1 cDNA confers multidrug resistance and polarized expression of P‐glycoprotein in MDCK cells. Proc Natl Acad Sci USA 1988; 85: 4486–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gottesman MM, Hrycyna CA, Schoenlein PV, Germann UA, Pastan I. Genetic analysis of the multidrug transporter. Annu Rev Genet 1995; 29: 607–49. [DOI] [PubMed] [Google Scholar]
- 5. Cole SPC, Bhardwaj G, Gerlach JH et al. Overexpression of a transporter gene in a multidrug‐resistant human lung cancer cell line. Science 1992; 258: 1650–4. [DOI] [PubMed] [Google Scholar]
- 6. Doyle LA, Yang W, Abruzzo LV et al. A multidrug resistance transporter from human MCF‐7 breast cancer cells. Proc Natl Acad Sci USA 1998; 95: 15 665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allikmets R, Schriml LM, Hutchinson A, Romano‐Spica V, Dean M. A human placenta‐specific ATP‐binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res 1998; 58: 5337–9. [PubMed] [Google Scholar]
- 8. Miyake K, Mickley L, Litman T et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone‐resistant cells: Demonstration of homology to ABC transport genes. Cancer Res 1999; 59: 8–13. [PubMed] [Google Scholar]
- 9. Litman T, Brangi M, Hudson E et al. The multidrug‐resistant phenotype associated with overexpression of the new ABC half‐transporter, MXR (ABCG2). J Cell Sci 2000; 113: 2011–21. [DOI] [PubMed] [Google Scholar]
- 10. Allen JD, Brinkhuis RF, Wijnholds J, Schinkel AH. The mouse Bcrp1/Mxr/Abcp gene: Amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res 1999; 59: 4237–41. [PubMed] [Google Scholar]
- 11. Ross DD, Yang W, Abruzzo LV et al. Atypical multidrug resistance: Breast cancer resistance protein messenger RNA expression in mitoxantrone‐selected cell lines. J Natl Cancer Inst 1999; 91: 429–33. [DOI] [PubMed] [Google Scholar]
- 12. Maliepaard M, van Gastelen MA, de Jong LA et al. Overexpression of the BCRP/MXR/ABCP gene in a topotecan‐selected ovarian tumor cell line. Cancer Res 1999; 59: 4559–63. [PubMed] [Google Scholar]
- 13. Kawabata S, Oka M, Shiozawa K et al. Breast cancer resistance protein directly confers SN‐38 resistance of lung cancer cells. Biochem Biophys Res Commun 2001; 280: 1216–23. [DOI] [PubMed] [Google Scholar]
- 14. Yang C‐H, Schneider E, Kuo M‐L, Volk EL, Rocchi E, Chen Y‐C. BCRP/MXR/ABCP expression in topotecan‐resistant human breast carcinoma cells. Biochem Pharmacol 2000; 60: 831–7. [DOI] [PubMed] [Google Scholar]
- 15. Jonker JW, Smit JW, Brinkhuis RF et al. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst 2000; 92: 1651–6. [DOI] [PubMed] [Google Scholar]
- 16. Sugimoto Y, Tsukahara S, Ishikawa E, Mitsuhashi J. Breast cancer resistance protein: Molecular target for anticancer drug resistance and pharmacokinetics/pharmacodynamics. Cancer Sci 2005; 96: 457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kage K, Tsukahara S, Sugiyama T et al. Dominant‐negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S–S dependent homodimerization. Int J Cancer 2002; 97: 626–30. [DOI] [PubMed] [Google Scholar]
- 18. Chen C, Okayama H. High efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 1987; 7: 2745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hock RA, Miller AD. Retrovirus‐mediated transfer and expression of drug resistance genes in human haematopoietic progenitor cells. Nature 1986; 320: 275–7. [DOI] [PubMed] [Google Scholar]
- 20. Imai Y, Ishikawa E, Asada S, Sugimoto Y. Estrogen‐mediated post transcriptional down‐regulation of breast cancer resistance protein/ABCG2. Cancer Res 2005; 65: 596–604. [PubMed] [Google Scholar]
- 21. Imai Y, Nakane M, Kage K et al. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low‐level drug resistance. Mol Cancer Ther 2002; 8: 611–16. [PubMed] [Google Scholar]
- 22. Mitomo H, Kato R, Ito A et al. A functional study on polymorphism of the ATP‐binding cassette transporter ABCG2: critical role of arginine‐482 in methotrexate transport. Biochem J 2003; 373: 767–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ross DD. Novel mechanisms of drug resistance in leukemia. Leukemia 2000; 14: 467–73. [DOI] [PubMed] [Google Scholar]
- 24. Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 1999; 39: 361–98. [DOI] [PubMed] [Google Scholar]
- 25. Higgins CF. ABC transporters: From microorganisms to man. Annu Rev Cell Biol 1992; 8: 67–113. [DOI] [PubMed] [Google Scholar]
- 26. Hyde SC, Emsley P, Hartshorn MJ et al. Structural model of ATP‐binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature 1990; 346: 362–5. [DOI] [PubMed] [Google Scholar]
- 27. Puglielli L, Mandon EC, Hirschberg CB. Identification, purification, and characterization of the rat liver golgi membrane ATP transporter. J Biol Chem 1999; 274: 12 665–9. [DOI] [PubMed] [Google Scholar]
- 28. van Veen HW, Venema K, Bolhuis H et al. Multidrug resistance mediated by a bacterial homolog of the human multidrug transporter MDR1. Proc Natl Acad Sci USA 1996; 93: 10 668–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shani N, Valle D. A Saccharomyces cerevisiae homolog of the human adrenoleukodystrophy transporter is a heterodimer of two half ATP‐binding cassette transporters. Proc Natl Acad Sci USA 1996; 93: 11 901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ozvegy C, Litman T, Szakacs G et al. Functional characterization of the human multidrug transporter, ABCG2, expressed in insect cells. Biochem Biophys Res Commun 2001; 285: 111–17. [DOI] [PubMed] [Google Scholar]
- 31. Litman T, Jensen U, Hansen A et al. Use of peptide antibodies to probe for the mitoxantrone resistance‐associated protein MXR/BCRP/ABCP/ABCG2. Biochim Biophys Acta 2002; 1565: 6–16. [DOI] [PubMed] [Google Scholar]
- 32. Xu J, Liu Y, Yang Y, Bates S, Zhang J‐T. Characterization of oligomeric human half‐ABC transporter ATP‐binding cassette G2. J Biol Chem 2004; 279: 19 781–9. [DOI] [PubMed] [Google Scholar]


