Abstract
Fatty acid synthase (FAS) is highly expressed in many kinds of human cancers, including colorectal cancer (CRC), and we have investigated the potential use of FAS inhibitors for chemoprevention of liver metastasis of CRC in mice. Expression of FAS was evaluated in murine CRC cell lines Colon 26 and CMT 93. Cerulenin, a natural inhibitor of FAS, induced apoptosis in these cell lines. The ability of cerulenin to prevent development of liver metastatic lesions in Colon 26 was evaluated. The numbers and sizes of liver metastatic CRC tumors were significantly reduced by treating mice with cerulenin. Cerulenin treatment was associated with reduced levels of phosphorylated Akt in Colon 26 cells, suggesting that inhibition of this signal transduction pathway might be involved in the chemopreventive activity of this compound. Based on studies in mouse models, inhibiting FAS would be an effective strategy to prevent and retard growth of liver metastatic tumors of CRC that have high expression of this enzyme. (Cancer Sci 2010; 00: 000–000)
Colorectal cancer (CRC) is one of the most common cancers in the world with more than 1 million new cases.( 1 ) The liver is the commonest site of distant metastasis in CRC, and approximately 50% of patients ultimately develop liver metastasis in the course of CRC.( 1 , 2 ) Despite recent advances, systemic chemotherapy for metastatic disease is considered palliative, and we rarely see long‐term survivors treated only by chemotherapy.( 1 ) Hepatic resection, the only curative treatment for liver metastasis of CRC, has become the standard treatment, but most cases of liver metastases are inoperable and approximately 50% of the patients treated with hepatectomy have a tumor recurrence in the liver.( 3 )
Fatty acid synthase (FAS) is highly expressed in many human cancers including CRC,( 4 , 5 , 6 , 7 ) and previous studies have shown that cancer cell growth can be suppressed by inhibiting the activity of this enzyme with a natural antibiotic cerulenin,( 8 ) orlistat, which is a pancreatic lipase inhibitor developed for obesity treatment,( 9 ) and C75, which is a stable synthetic small molecule developed specifically for inhibiting FAS.( 10 ) But there are no previous studies in which FAS inhibitors suppressed liver metastasis of CRC.
The aim of this study extends the investigation of the potential use of an FAS inhibitor for the chemoprevention of liver metastasis of CRC in mice. In this study, we examined the effect of cerulenin on cell proliferation and apoptosis in murine CRC cell lines Colon 26 and CMT 93. Then, the effect of cerulenin on the prevention of growth of liver metastasis lesions in Colon 26 was investigated.
Materials and Methods
Cerulenin. Cerulenin was obtained from Sigma (St. Louis, MO, USA). For cell culture and i.p. injections, cerulenin was dissolved in acetone at a concentration of 20 mg/mL and stored at −20°C. In in vitro experiment, 50–200 μM of cerulenin was added to the medium, and cell viability assay and western blot experiments were performed 24 h later. In in vivo experiments, treatment with cerulenin at 30 mg/kg was given i.p. at Days 1, 4, and 7 after tumor inoculation in the cerulenin group.
Cell culture. Two murine CRC cell lines, Colon 26 and CMT 93, were used and tested for mycoplasm‐free cell lines. All cancer cell lines were subdivided in multiple tubes for stock in liquid nitrogen immediately after possession. All cell lines were subjected to the present experiment within 6 months of resuscitation. Stock cultures were grown in high‐glucose DMEM containing 10% FBS and 1% antibiotics. The cells were grown in growth medium at 37°C in a 95% air, 5% CO2‐humidified incubator.
Cell viability assay. To measure the cytotoxicity of cerulenin against these cancer cells, 3 × 103 cells were plated per well onto 96‐well plates. Following overnight culture, cerulenin was added at specified concentrations. After 24 h of incubation, cell viability was measured by the mitochondrial activity in reducing 2‐(2‐Methoxy‐4‐nitrophenyl)‐3‐(4‐nitrophenyl)‐5‐(2,4‐disulfophenyl)‐2H‐tetrazolium monosodium salt (WST‐8) to formazan using a Cell Counting kit‐8 (Dojindo Laboratories, Kumamoto, Japan). Cells were incubated with a reagent as per the manufacturer’s instructions. Plates were read at A 450 on a spectrometer.
Cell proliferation assay. To measure the cell proliferation activity of cerulenin against these cancer cells, 3 × 103 cells were plated per well onto 96‐well plates. Following overnight culture, cerulenin was added at specified concentrations. After 24 h of incubation, cell proliferation was measured with a BrdU assay kit (Roche Diagnostics, Penzberg, Germany). Cells were incubated with a reagent as per the manufacturer’s instructions. Plates were read at A 450 on a spectrometer.
Apoptosis assay. The In situ Cell Death Detection kit (Roche diagnostics, Basel, Switzerland) was used for the demonstration of apoptotic cell death of cell culture and liver tissue. 3 × 104/mL cells were plated per well onto Lab‐Tek II Chamber Slides (Nalge Nunc International, Tokyo, Japan) and paraffin‐embedded liver samples were incubated with the terminal deoxynucleotidyl transferase‐mediated dUTP nick end labeling (TUNEL) reaction mixture according to the manufacturer’s recommendations.
Western blot analysis. For western blot analysis, total protein extracts of Colon 26 and CMT 93 were obtained 24 h after cerulenin treatment, and separated by 10% SDS‐PAGE and transferred to nitrocellulose membrane (Millipore, Bedford, MA, USA). The following antibodies were used as primary antibodies: total Akt (9272), phosphoserine 473 Akt (9271), p mTOR, cleaved caspase 3, and GAPDH (2118) (Cell Signaling, Beverly, MA, USA). Purified mouse anti‐FAS antibody (610962) was purchased from BD Biosciences (San Jose, CA, USA). Secondary goat antirabbit antibody conjugated with horseradish peroxidase was purchased from Zymed Laboratories (San Francisco, CA, USA). Immunoblots were analyzed by enhanced chemiluminescence.
Animals. Eight‐week‐old male BALB/c mice (Clea, Tokyo, Japan), weighing 24–28 g were utilized. The mice were kept in a temperature‐controlled room on a 12‐h light–dark cycle. They had free access to water and standard chow throughout the experiment. After an acclimation period of at least 7 days, the mice were separated into two groups as follows: control group, mice without any treatment (n = 8); and cerulenin group, mice with cerulenin treatment (n = 6). All animal experiments were carried out in a humane manner after receiving approval from the Institutional Animal Experiment Committee of the University of Tsukuba and in accordance with the Regulation for Animal Experiments of the University and Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Liver metastasis. Under ether anesthesia, laparotomy was performed with midline incision, and hepatic ischemia was induced by clamping the portal triad (hepatic artery, portal vein, and bile duct) with a microclip (Aesculap, Tuttlingen, Germany) for 1 min. After 1‐min reperfusion of the liver, 1 × 105 cells of Colon 26 cells were injected into the lower splenic pole with a 27‐gauge needle. Nine days after inoculation, mice were sacrificed and livers were removed for examination.
Histochemical examination. To assess the liver metastatic area, left and middle lobes of liver were divided in 5 mm slices, fixed with 10% formaldehyde, and embedded in paraffin. Thin sections (4 μm) were stained with hematoxylin–eosin, and the percentage of liver metastatic area of Colon 26 cells was calculated using the image‐processing software WinROOF (Mitani, Fukui, Japan).
Serum parameter. Blood was collected from the plexus of the retro‐orbital vein of mice. Blood was centrifuged for 10 min at 4°C at 1200g. Supernatants were collected and stored at −80°C until tested by a serum multiple biochemical analyzer (Fuji Drichem; Fuji Film, Tokyo, Japan) for measuring triglyceride, cholesterol, and alanine aminotransferase (ALT) levels.
Statistical analysis. All data are expressed as the mean ± SD of samples. Unpaired t‐test was used for the comparison between the two groups. Comparisons between various points were made using one‐way anova. Significant data were examined by the Bonferroni–Dunn multiple comparisons post‐hoc test. In all cases, a P‐value <0.05 was considered significant.
Results
Dose‐dependent inhibition of proliferation of CRC cell lines by cerulenin. We initially determined whether cerulenin treatment led to the inhibition of CRC cell proliferation. Colorectal cancer (CRC) cells were treated with various doses of cerulenin for 24 h, and cell viability was assayed using WST‐8 assay (Fig. 1a) and BrdU assay (Fig. 1b). Figure 1(a,b) shows that as the dose of cerulenin increased from 100 to 200 μM, cell growth inhibition increased in a dose‐dependent fashion in CRC cell lines. Cerulenin‐induced growth inhibition was found to be statistically significant (P < 0.05) (one‐way anova) in 100, 150, and 200 μM of cerulenin compared to 0 μM.
Figure 1.
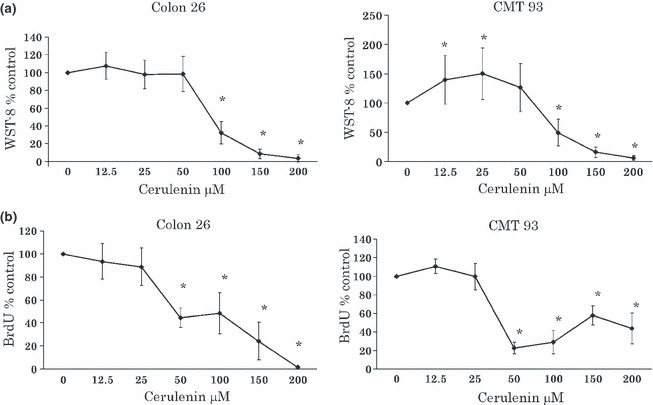
(a) Effect of cerulenin on the 2‐(2‐Methoxy‐4‐nitrophenyl)‐3‐(4‐nitrophenyl)‐5‐(2,4‐disulfophenyl)‐2H‐tetrazolium monosodium salt (WST‐8) assay of murine colorectal cancer (CRC) cell lines. Colorectal cancer (CRC) cell lines were treated with 0–200 μM cerulenin for 24 h. Left, Colon 26; right, CMT 93. *P < 0.01 compared to 0 μM cerulenin. The values indicate ratio compared to 0 μM cerulenin as 100%. (b) Effect of cerulenin on the BrdU assay of murine CRC cell lines. Colorectal cancer (CRC) cell lines were treated with 0–200 μM cerulenin for 24 h. Left, Colon 26; right, CMT 93. *P < 0.01 compared to 0 μM cerulenin. The values indicate ratio compared to 0 μM cerulenin as 100%.
Induction of apoptosis via activation of caspase‐dependent pathway by cerulenin. In subsequent experiments, we determined the mechanism of the observed suppressive effect of cerulenin by WST‐8 assays. The overexpression of FAS has been observed to cooperate with survival pathways, including the phosphatidylinositol‐3‐kinase (PI3K)/Akt pathway. These two cell lines expressed FAS and p‐Akt constitutively, and treatment of CRC cells with cerulenin suppressed FAS expression, dephosphorylated constitutive activated Akt, and increased cleaved caspase‐3 in both Colon 26 and CMT 93 cells (Fig. 2a). TUNEL staining of CRC cells revealed that cerulenin induced apoptosis both in Colon 26 and CMT 93 cells in 50–100 μM (Fig. 2b).
Figure 2.
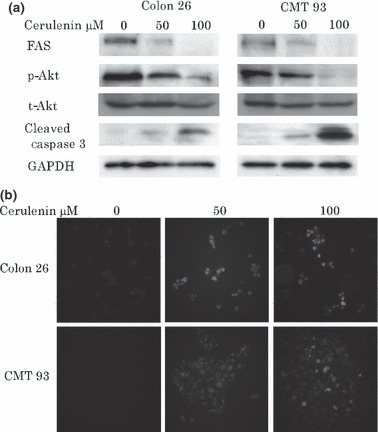
(a) Cerulenin treatment causes down regulation of fatty acid synthase (FAS) and dephosphorylation of constitutive phosphorylation of Akt in colorectal cancer (CRC) cell lines. Colon 26 and CMT 93 cell lines were treated with 0, 50, and 100 μM cerulenin for 24 h. After cell lysis, equal amounts of proteins were separated by SDS‐PAGE, transferred to Immobilon membrane, and immunoblotted with antibodies against FAS, p‐Akt, cleaved caspase‐3, and GAPDH as indicated. (b) Cerulenin treatment causes apoptosis in CRC cell lines. Terminal deoxynucleotidyl transferase‐mediated dUTP nick end labeling (TUNEL) staining of Colon 26 and CMT 93 cell lines which were treated with 0, 50, and 100 μM cerulenin for 24 h.
Inhibition of general lipogenesis by cerulenin. To investigate the physiological consequences of in vivo inhibition of FAS, we administered cerulenin (30 mg/kg body weight every 3 days) to mice by i.p. injection. We observed slight weight loss following treatment with no significant difference in comparison to the control group (Fig. 3). Serum triglyceride was significantly decreased in the cerulenin group compared to the control group. This suggests general lipogenesis in the liver was reduced by cerulenin (Fig. 4).
Figure 3.
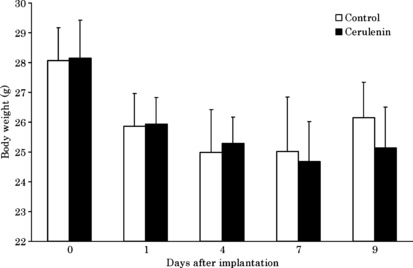
Weight of animals for cerulenin and control groups in the experiment. Columns, mean; bars, SD. White bar, control group; black bar, cerulenin group.
Figure 4.
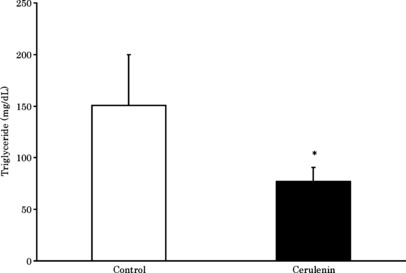
Serum triglyceride 9 days after tumor inoculation. Columns, mean; bars, SD. White bar, control group; black bar, cerulenin group. *P < 0.05 versus control group, t‐test.
Inhibition of tumor growth of liver metastasis of CRC by cerulenin. We evaluated the potential effectiveness of cerulenin for metastatic liver tumors of the CRC cell line. Figure 5(a) shows histological cross sections of livers removed from the representative control and cerulenin groups. Growth reduction of metastatic liver tumors was recognized in the cerulenin group. Figure 5(b) indicates tumor areas of the liver in both groups. Tumor growth was significantly reduced by cerulenin administration. Figure 5(c) shows TUNEL staining of liver sections from the control and cerulenin groups. In the cerulenin group, apoptotic tumor cells were observed in the metastatic liver tumor.
Figure 5.
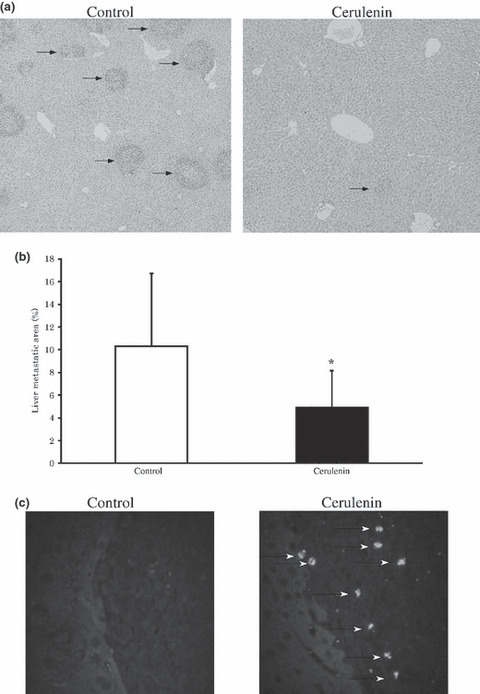
Cerulenin inhibits tumor growth of liver metastasis of colorectal cancer (CRC) cell line in mice. (a) Histologic cross sections of livers of control group (left) and cerulenin group (right). Arrows, metastatic tumors. Original magnification: × 100. (b) Tumor area in the liver of all animals in the experiment. Columns, mean; bars, SD. White bar, control group; black bar, cerulenin group. *P < 0.05 versus control group, t‐test. (c) Terminal deoxynucleotidyl transferase‐mediated dUTP nick end labeling (TUNEL) staining of tumor tissues. Arrows indicate apoptotic tumor cells in cerulenin group. Original magnification: × 200.
Discussion
The present study extends the investigation of the potential use of an FAS inhibitor for chemoprevention of liver metastasis of CRC in mice. In this study, we revealed the effect of cerulenin on the murine CRC cell lines Colon 26 and CMT 93 on growth inhibition by inducing apoptosis. Also, the effect of cerulenin on the prevention of growth of liver metastasis lesions in Colon 26 was revealed. This is the first study on inhibiting CRC liver metastasis by administering the FAS inhibitor, cerulenin. Many kinds of human cancer cells have high activities of FAS and numerous studies have described the cytotoxic effects of FAS both in vitro and in vivo.( 4 ) The role of increased FAS in cancer cells and the mechanisms of cell killing by inhibitors of FAS are still not fully understood.( 11 )
The effect of an intermediate metabolite of fatty acid synthesis on cancer cells is likely mediated through cell signaling pathways.( 12 ) For example, inhibiting FAS decreases phosphorylation of Akt in ovarian cancer cells and suppresses human epidermal growth factor receptor 2 (HER2) overexpression in breast cancer cells.( 12 , 13 ) The mechanism by which FAS inhibitor decreases Akt activation is still unclear, but one mechanism that is advocated is that fatty acids synthesized by FAS are incorporated into membrane phospholipids, which are known modulators of Akt activation.( 14 , 15 ) FAS has a major role in the synthesis of phospholipids.( 16 ) When FAS expression is decreased by cerulenin treatment, less fatty acid will be synthesized and less phospholipid will be available. One of the important phospholipids is phosphatidylinositol trisphosphate (PIP3). PIP3 binds to Akt and its activating kinase phosphoinositide‐dependent protein kinase‐1(PDK‐1) with high affinity, and the phosphorylation of Akt is dependent on PIP3.( 17 ) In this study, a decrease of phospholipids may have resulted in inhibition of Akt activity in Colon 26 and CMT 93 cell lines after cerulenin administration. Recently, Liu et al. ( 18 ) reported that inhibition of PI3K/Akt by cerulenin induces apoptosis in breast cancer cell lines via release of cytochrome and activation of caspase. In our study it was clarified that inhibition of PI3K/Akt by cerulenin induces apoptosis in CRC cell lines via activation of caspase.
Colon 26 is chemically derived colon cancer from BALB/c mice.( 19 ) It is highly metastatic and many researchers use this cell line for study of liver metastasis of CRC in mice.( 20 ) Based on these reports, we used Colon 26 for this study.P53 expression of Colon 26 is not fully understood, but reacts to interferon (IFN)‐α to increase p53 and induce apoptosis.( 21 ) Functional p53 reacts to IFN‐α or ‐β.( 22 ) That means p53 in Colon 26 would be functional. Further investigation of p53 in Colon 26 is required.
Many efforts to treat xenograft cancers with cerulenin or its derivative of C75 were reported in ovary,( 23 ) prostate,( 24 ) mesothelioma,( 25 ) breast cancer,( 26 ) and CRC.( 27 , 28 ) But there has been no study of treatment of CRC liver metastasis using cerulenin. In this study, we report that cerulenin inhibits liver metastasis of CRC in a mouse model. In the prior studies, the use of cerulenin or C75 was hampered by transient but severe anorexia and weight loss. Therefore, this compound could also limit use in the clinical setting.( 29 , 30 ) Loftus et al. ( 29 ) reported that cerulenin treatment 60 mg/kg daily for 7 days caused severe weight loss. Pizer et al. ( 23 ) reported that cerulenin treatment 80 mg/kg per daily for 7 days caused severe weight loss. Uddin et al. ( 15 ) reported that C‐75 treatment 10 mg/kg or 20 mg/kg twice weekly did not cause severe weight loss. In our experiments, we obtained adequate liver metastasis 9 days after tumor injection. We treated cerulenin 10 mg/kg or 30 mg/kg twice weekly (every 3 days). In our preliminary study, 10 mg/kg cerulenin treatment had no effect (data not shown). By treatment with 30 mg/kg of cerulenin every 3 days, inhibition of liver metastasis was observed. At this amount, serum triglyceride was significantly decreased in the cerulenin group. That means that FAS was really inhibited in our animal model. But we could not observe significant weight loss in this group compared to control group. This result indicated that the amount of cerulenin in our experiment was appropriate for the prevention of side effects of FAS inhibitor. We could not promote complete remission of metastatic tumors by cerulenin at this dose and the appropriate dose of cerulenin or other FAS inhibitors needs to be studied. Also, the effect of FAS inhibitors on chemically induced colon cancer should be evaluated.
One of the reasons for anorexia brought on by cerulenin or C75 is fatty acid oxidation by activating carnitine O palmitoyltransferase 1.( 30 ) In recent years, it was reported that C93, which inhibits FAS but has no significant effect on fatty acid oxidation, is effective for treatment of human lung cancer xenografts. As a result, C93 does not cause anorexia or weight loss.( 11 ) In the near future, analogues of FAS inhibitors such as C93 may represent promising new treatments for cancer, including liver metastasis of CRC.
In conclusion, an FAS inhibitor of cerulenin inhibits CRC cell line survival in in vitro and in vivo models with liver metastasis. Fatty acid synthase (FAS) may be a new drug target for liver metastasis of CRC.
Acknowledgment
The authors thank Yuko Jinzenji and Ako Takahashi for technical assistance.
Reference
- 1. Leonard GD, Brenner B, Kemeny NE. Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. J Clin Oncol 2005; 23: 2038–48. [DOI] [PubMed] [Google Scholar]
- 2. Geoghegan JG, Scheele J. Treatment of colorectal liver metastasis. Br J Surg 1999; 86: 158–69. [DOI] [PubMed] [Google Scholar]
- 3. Tachimori A, Yamada N, Amano R et al. Combination therapy of S‐1 with selective cyclooxygenase‐2 inhibitor for liver metastasis of colorectal carcinoma. Anticancer Res 2008; 28: 629–38. [PubMed] [Google Scholar]
- 4. Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res 2006; 66: 5977–80. [DOI] [PubMed] [Google Scholar]
- 5. Menendez JA, Lupu R. Oncogenic properties of the endogenous fatty acid metabolism: molecular pathology of fatty acid synthase in cancer cells. Curr Opin Clin Nutr Metab Care 2006; 9: 346–57. [DOI] [PubMed] [Google Scholar]
- 6. Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care 2006; 9: 358–65. [DOI] [PubMed] [Google Scholar]
- 7. Ogino S, Nosho K, Meverhardt JA et al. Cohort study of fatty acid synthase expression and patient survival in colon cancer. J Clin Oncol 2008; 26: 5713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuhajda FP, Jenner K, Wood FD et al. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc Natl Acad Sci USA 1994; 91: 6379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW. Orlistat is a novel inhibitor of fatty acid synthase with anti‐tumor activity. Cancer Res 2004; 64: 2070–5. [DOI] [PubMed] [Google Scholar]
- 10. Kuhajda FP, Pizer ES, Li JN et al. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci USA 2000; 97: 3450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Orita H, Coulter J, Lemmon C et al. Selective inhibition of fatty acid synthase for lung cancer treatment. Clin Cancer Res 2007; 13: 7139–45. [DOI] [PubMed] [Google Scholar]
- 12. Wang HQ, Altomare DA, Skele KL et al. Positive feedback regulation between AKT activation and fatty acid synthase expression in ovarian carcinoma cells. Oncogene 2005; 24: 3574–82. [DOI] [PubMed] [Google Scholar]
- 13. Menendez JA, Vellon L, Mehmi I et al. Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB‐2) oncogene overexpression in cancer cells. Proc Natl Acad Sci USA 2004; 101: 10715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Swinnen JV, Van Veldhoven PP, Timmermans L et al. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent‐resistant membrane microdomains. Biochem Biophys Res Commun 2003; 302: 898–903. [DOI] [PubMed] [Google Scholar]
- 15. Uddin S, Siraj AK, Al‐Rasheed M et al. Fatty acid synthase and AKT pathway signaling in a subset of papillary thyroid cancers. Clin Endocrinol Metab 2008; 93: 4088–97. [DOI] [PubMed] [Google Scholar]
- 16. Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 2007; 7: 763–77. [DOI] [PubMed] [Google Scholar]
- 17. Denley A, Gymnopulos M, Kang S et al. Requirement of phosphatidylinositol(3,4,5)trisphosphate in phosphatidylinositol 3‐kinase‐induced oncogenic transformation. Mol Cancer Res 2009; 7: 1132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu X, Shi Y, Giranda VL, Luo Y. Inhibition of the phosphatidylinositol 3‐kinase/Akt pathway sensitizes MDA‐MB468 human breast cancer cells to cerulenin‐induced apoptosis. Mol Cancer Ther 2006; 5: 494–501. [DOI] [PubMed] [Google Scholar]
- 19. Corbett TH, Griswold DP, Roberts BJ et al. Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res 1975; 35: 2434–9. [PubMed] [Google Scholar]
- 20. Higashijima J, Shimada M, Chikakiyo M et al. Effect of splenectomy on antitumor immune system in mice. Anticancer Res 2009; 29: 385–94. [PubMed] [Google Scholar]
- 21. Kim JS, Yu KN, Noh MS et al. Serum immunoglobin fused interferon‐α inhibited tumor growth in athymic mice bearing colon 26 adenocarcinoma cells. J Vet Sci 2008; 9: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takaoka A, Hayakawa S, Yanai H et al. Integration of interferon‐α/β signaling to p53 responses in tumour suppression and antiviral defense. Nature 2003; 424: 516–23. [DOI] [PubMed] [Google Scholar]
- 23. Pizer ES, Wood FD, Heine HS et al. Inhibition of fatty acid synthesis delays disease progression in a xenograft model of ovarian cancer. Cancer Res 1996; 56: 1189–93. [PubMed] [Google Scholar]
- 24. Pizer ES, Pflug BR, Bova GS et al. Increased fatty acid synthase as a therapeutic target in androgen‐independent prostate cancer progression. Prostate 2001; 47: 102–10. [DOI] [PubMed] [Google Scholar]
- 25. Gabrielson EW, Pinn ML, Testa JR, Kuhajda FP. Increased fatty acid synthase is a therapeutic target in mesothelioma. Clin Cancer Res 2001; 7: 153–7. [PubMed] [Google Scholar]
- 26. Pizer ES, Thupari J, Han WF et al. Malonyl‐coenzyme A is a potential mediator of cytotoxicity induced by fatty‐acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res 2000; 60: 213–18. [PubMed] [Google Scholar]
- 27. Huang P, Zhu S, Lu S et al. Cerulenin inhibits growth of human colonic carcinoma in nude mice. Zhongua Bing Li Xue Za Zhi 2000; 29: 435–8. [PubMed] [Google Scholar]
- 28. Huang P, Zhu S, Lu S et al. Inhibitor of fatty acid synthase induced apoptosis in human colonic cancer cells. World J Gastroenterol 2000; 6: 295–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loftus TM, Jaworsky DE, Frehywot GL et al. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 2000; 288: 2379–81. [DOI] [PubMed] [Google Scholar]
- 30. Thupari JN, Landree LE, Ronnett GV, Kuhajda FP. C75 increases peripheral energy utilization and fatty acid oxidation in diet‐induced obesity. Proc Natl Acad Sci USA 2002; 99: 9498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]


