Abstract
Tyrosine analogs are good candidates for developing melanoma chemotherapies because melanogenesis is inherently toxic and expressed uniquely in melanocytic cells. The sulfur homolog of tyrosine, 4‐S‐cysteaminylphenol (4‐S‐CAP), was shown to be a substrate of melanoma tyrosinase and can cause selective cytotoxicity of melanocytes and melanoma cells. Previously, in order to improve the adsorption of magnetite nanoparticles to target cell surfaces, and generate heat in an alternating magnetic field (AMF) for cancer hyperthermia, we produced hyperthermia using magnetite cationic liposomes (MCL) that have a positive charge at the liposomal surface. In the present study, we constructed 4‐S‐CAP‐loaded MCL (4‐S‐CAP/MCL), which act as a novel modality, combining melanoma‐specific chemotherapy by 4‐S‐CAP with intracellular hyperthermia mediated by MCL. The 4‐S‐CAP/MCL exerted 4‐S‐CAP‐mediated anticancer effects on B16 melanoma cells in vitro and in vivo. Moreover, after intratumoral injection of 4‐S‐CAP/MCL in vivo, the melanoma nodules were heated to 45°C under an AMF. Significantly higher therapeutic effects were observed in mice treated with the combination therapy mediated by 4‐S‐CAP/MCL plus AMF irradiation compared with mice treated with 4‐S‐CAP/MCL alone (without AMF) or mice treated with hyperthermia alone (MCL + AMF irradiation). These results suggest that this novel therapeutic tool is applicable to the treatment of malignant melanoma. (Cancer Sci 2007; 98: 424–430)
Hyperthermia is a promising approach to cancer therapy and has been used for many years to treat a wide variety of tumors in both experimental animals and patients.( 1 ) In Japan, the most commonly used heating method in clinical settings is capacitive heating using a radiofrequency electric field.( 2 ) An unavoidable technical problem with hyperthermia is the difficulty in heating only the local tumor region to the intended temperature without damaging the surrounding healthy tissue. Specifically, heating tumors by capacitive heating using a radiofrequency electric field is difficult because various factors, such as tumor size, position of electrodes, and adhesion of electrodes at uneven sites, influence the heating characteristics. Hyperthermia produced by heating mediators is a promising approach for specifically heating tumors without damaging normal tissues.( 3 ) Magnetite nanoparticles have been used for hyperthermia treatment in an attempt to overcome this obstacle.( 4 , 5 , 6 ) If magnetite nanoparticles can be made to accumulate only in tumor tissue, cancer‐specific hyperthermia can be achieved by generating heat in an alternating magnetic field (AMF) due to hysteresis loss. In order to test this hypothesis, we developed magnetite cationic liposomes (MCL) as mediators of intracellular hyperthermia.( 7 , 8 , 9 ) These cationic liposomes exhibit improved adsorption and accumulation in tumor cells, and have 10‐fold higher affinity for tumor cells than neutrally charged magnetoliposomes,( 7 ) thus suggesting that MCL are superior mediators of hyperthermia. We previously demonstrated the efficacy of MCL‐mediated hyperthermia in animals with several cell lines, including T‐9 rat glioma,( 9 ) Os515 hamster osteosarcoma,( 10 ) MM46 mouse mammary carcinoma,( 11 ) PLS 10 rat prostate cancer,( 12 ) and VX‐7 squamous cell carcinoma in rabbit tongue.( 13 ) Although MCL‐mediated hyperthermia was found to be very effective for inducing complete tumor regression in transplantable tumor models, more powerful therapies are highly desired for cancer patients with malignancy.
Among the various forms of neoplasmas of the skin, malignant melanoma is the most invasive tumor. Various medical therapies, such as surgery, chemotherapy, radiotherapy and immunotherapy, are commonly used in the treatment of melanoma patients; however, none of these has proved sufficiently effective. Therefore, an effective protocol for the prevention and treatment of melanoma is needed urgently. Given this background, the combination of chemotherapy with MCL‐mediated hyperthermia can be considered a possible strategy, because both magnetite nanoparticles and anticancer drugs can be encapsulated simultaneously into a liposome.
Tyrosine analogs are good candidates for developing melanoma chemotherapy because melanogenesis is inherently toxic and uniquely expressed in melanocytic cells. We introduced the use of phenolic thioether analogs of tyrosine for targeted melanoma chemotherapy based on the idea that the incorporation of sulfur would render the phenolics more cytotoxic by increased lipophilicity leading to increased uptake by cells, thus making them better substrates for tyrosinase.( 14 ) Therefore, we synthesized the sulfur homolog of tyrosine, 4‐S‐cysteaminylphenol (4‐S‐CAP), in order to develop a targeted chemotherapy for malignant melanoma.( 14 , 15 ) 4‐S‐CAP was found to be a substrate of melanoma tyrosinase and can cause selective cytotoxicity of melanocytes and melanoma cells.( 16 ) In the present study, we constructed 4‐S‐CAP‐loaded magnetite cationic liposomes (4‐S‐CAP/MCL) and investigated the feasibility of using them for combined melanoma‐targeted chemotherapy and tumor‐specific hyperthermia.
Materials and Methods
Cell lines and animal models. Mouse B16 melanoma cells were cultured in Dulbecco's modified Eagle's medium (Gibco BRL, Gaithersburg, MD, USA), supplemented with 10% fetal calf serum, 0.1 mg/mL streptomycin sulfate and 100 IU/mL potassium penicillin G. Normal human dermal fibroblasts (NHDF) were provided as frozen cells after primary culture by the supplier (Kurabo, Osaka, Japan), and were cultured in commercially available growth media (Medium106S; Kurabo) at 37°C in a humidified atmosphere of CO2 and 95% air.
To prepare tumor‐bearing mice, 8 × 105 B16 melanoma cells were injected subcutaneously into the right flank of C57BL/6 mice, which were anesthetized by intraperitoneal injection of pentobarbital (50 mg/kg bodyweight). Melanoma nodules that had grown to 5 mm in diameter were used for experiments. Tumor diameter was measured using calipers and the average size was determined by applying the following formula:
| Tumor size = 0.5 × (length + width), |
where length and width were measured in mm.
Animal experiments were carried out according to the principles described in the ‘Guide for the Care and Use of Laboratory Animals’ prepared under the direction of the Prime Minister of Japan.
Analysis of the combined effect of 4‐S‐CAP and hyperthermic treatment. B16 cells were plated in six‐well cell culture plates at 4 × 104 cells/well with experimental media containing 4‐S‐CAP at a concentration of 50 µM. The cells were then treated with hyperthermic treatment using a water bath. Hyperthermic treatment of cultured cells was carried out as in our previous report.( 17 ) Briefly, the cells were heated to 42.5°C for 60 min by direct immersion of cell culture dishes in a temperature‐controlled water bath. The temperature of the medium increased quickly and reached the intended temperature within 5 min. The temperature of the medium was monitored using a fiber optic thermometer probe (FX‐9020; Anritsu Meter, Tokyo, Japan). Control cells were not treated with 4‐S‐CAP or hyperthermia.
The antiproliferative effect of the treatment was determined after a 2‐day incubation period. The number of viable cells was evaluated by the trypan blue dye‐exclusion method using a hemocytometer, and the relative cell number was calculated as follows:
| Relative cell number (%) = (number of viable experimental cells/number of viable control cells) × 100. |
The combined effect of 4‐S‐CAP and hyperthermia was evaluated using a method reported previously.( 18 ) With [A], [B] and [A + B] representing the percentage cell viability for treatments A and B and the combination treatment A + B, the combined effects were defined as follows: synergistic, [A + B] < [A] ×[B]/100; additive, [A + B] = [A] × [B]/100; subadditive, [A] × [B]/100 < [A + B] < [A], if [A] < [B]; interference, [A] < [A + B] < [B], if [A] < [B]; and antagonistic, [B] < [A + B], if [A] < [B].
Preparation of 4‐S‐CAP/MCL. Magnetite nanoparticles (Fe3O4; average particle size, 10 nm) were the kind gift of Toda Kogyo (Hiroshima, Japan). 4‐S‐CAP was synthesized as described by Padgette et al.( 19 ) For the preparation of 4‐S‐CAP/MCL, 0–32 mg of 4‐S‐CAP was added to a lipid mixture consisting of N‐(α‐trimethylammonioacetyl)‐didodecyl‐d‐glutamate chloride (2.78 mg; Sogo Pharmaceutical, Tokyo, Japan), dilauroylphosphatidylcholine (6.64 mg; Sigma Chemical, St Louis, MO, USA) and dioleoylphosphatidyl‐ethanolamine (5.58 mg; Sigma Chemical) dissolved in methanol (2 mL), and the mixture was dried down by evaporation for a minimum of 5 h. Then, the lipids containing 4‐S‐CAP were hydrated by vortexing with colloidal magnetite nanoparticles (20 mg/mL, 2 mL) and the liposomes were sonicated for 20 min (28 W). The size of the 4‐S‐CAP/MCL was measured using a dynamic light scattering spectrophotometer (FRAR 1000; Otsuka Electronics, Osaka, Japan).
Antiproliferative activity of 4‐S‐CAP in 4‐S‐CAP/MCL. B16 cells, or NHDF cells as a non‐melanocytic control, were plated in six‐well cell culture plates at 5 × 104 cells/well with experimental media containing 4‐S‐CAP/MCL at the indicated concentration. The antiproliferative activity was determined after the 2‐day incubation period. The number of viable cells was measured by the trypan blue dye‐exclusion method using a hemocytometer, and the relative cell number was calculated.
Uptake of 4‐S‐CAP/MCL by B16 cells. B16 cells were cultured to approximately 80% confluence before treatment with 4‐S‐CAP/MCL. Cells were then incubated with experimental media containing 4‐S‐CAP/MCL at a concentration of 43 µg magnetite/mL (4‐S‐CAP, 100 µM), and cells were incubated at 37°C with gentle shaking using a rotary shaker (SHK‐320; Asahi Technoglass, Tokyo, Japan). After incubation for 24 h, the cells were washed twice with phosphate‐buffered saline and harvested and the magnetite concentration was measured in accordance with our reported method.( 7 ) Briefly, the harvested cells were dissolved completely in 0.2 mL of concentration HCl, after which 1 mL of 5% trichloroacetic acid was added. These mixtures were centrifuged in order to remove aggregates and the supernatants were measured by the potassium thiocyanate method.( 20 )
In vitro magnetite nanoparticle‐induced hyperthermia. An in vitro hyperthermia experiment using magnetite nanoparticles was carried out using our previously described method.( 7 ) Briefly, B16 cells were cultured to approximately 80% confluence. Cells were then incubated with experimental media containing 4‐S‐CAP/MCL at a concentration of 43 µg magnetite/mL (4‐S‐CAP, 100 µM). At 24 h after the magnetite incorporation, the cells were collected in a microcentrifuge tube and centrifuged gently in order to form a cell pellet. The tube was then placed at the center of the coil of a high‐frequency magnetic field generator (360 kHz, 120 Oe; Daiichi High Frequency, Tokyo, Japan). The temperature of the cell pellet was measured by inserting an optical fiber probe into its center, and the pellet was maintained at a constant temperature by manually tuning the strength of the magnetic field. The duration of the AMF irradiation was 30 min. During AMF irradiation, the temperature of the environment was maintained at 37°C. The treated cells were reseeded in a six‐well cell culture plate at 2 × 104 cells/well. The number of viable cells was measured by the trypan blue exclusion method using a hemocytometer.
In vivo magnetite nanoparticle‐induced hyperthermia. When melanoma nodules grew to 5 mm in diameter, the tumor‐bearing mice were separated into five groups (day 0). Mice in group I (control) were the non‐treated control. Mice in groups II (MCL) and IV (MCL + AMF) received intratumoral injection of MCL (0.2 mL, 20 mg magnetite/mL). Mice in groups III (4‐S‐CAP/MCL) and V (4‐S‐CAP/MCL + AMF) received intratumoral injection of 4‐S‐CAP/MCL (0.2 mL; 20 mg/mL magnetite; 100 µM 4‐S‐CAP). At 1 day after the injection of MCL or 4‐S‐CAP/MCL (day 1), mice in groups IV (MCL + AMF) and V (4‐S‐CAP/MCL + AMF) were subjected to AMF for 30 min in a coil with a transistor inverter. Magnetic field frequency and intensity were 118 kHz and 384 Oe, respectively. Tumor and rectal temperatures were measured using an optical fiber probe. Subsequently, after the AMF irradiation, MCL or 4‐S‐CAP/MCL were again injected into the mice in groups II and IV or groups III and V, respectively. At 1 day after the second injection (day 2), mice in groups IV (MCL + AMF) and V (4‐S‐CAP/MCL + AMF) were again subjected to AMF for 30 min. During the injection of MCL or 4‐S‐CAP/MCL and AMF irradiation, tumor‐bearing mice were anesthetized with pentobarbital sodium (50 mg/kg intraperitoneally).
For histological examination, the tumor was resected and fixed in a 10% formalin solution 24 h after hyperthermia. The specimens were fixed in 10% neutrally buffered formalin, and embedded in paraffin. Serial specimens were then prepared for histological examination, and were stained with hematoxylin and eosin (H&E).
Statistical analysis. Statistical analysis was carried out by the Mann–Whitney rank sum test calculated using WinSTAT statistical software (Light Stone International, Tokyo, Japan). Differences were considered to be statistically significant at P < 0.05.
Results
Combined effect of 4‐S‐CAP and hyperthermic treatment. Figure 1 shows the relative cell number for B16 cells on the second day after hyperthermic treatment using a 42.5°C water bath for 60 min. For hyperthermic treatment alone, the relative cell number was 52.5 ± 6.6%. For 4‐S‐CAP (50 µM) treatment alone, the relative cell number was 64.4 ± 9.9%. When hyperthermic treatment was combined with 4‐S‐CAP treatment, the relative cell number decreased to 33.7 ± 10.6%. With [A], [B] and [A + B] representing the percentage cell viability for treatments of 4‐S‐CAP, heat and the combination treatment 4‐S‐CAP + heat, respectively, the combined effects were defined as follows: synergistic, [A + B] < [A] × [B]/100; additive, [A + B] = [A] × [B]/100; subadditive, [A] × [B]/100 < [A + B] < [A], if [A] < [B]; interference, [A] < [A + B] < [B], if [A] < [B]; and antagonistic, [B] < [A + B], if [A] < [B]. Here, [A + B] and [A] × [B]/100 were 33.7 ± 10.6% and 34.1 ± 9.5%, respectively. Because there was no significant difference (P = 0.56) between [A + B] and [A] × [B]/100, the combined effect of 4‐S‐CAP with heat was evaluated as additive effects.
Figure 1.
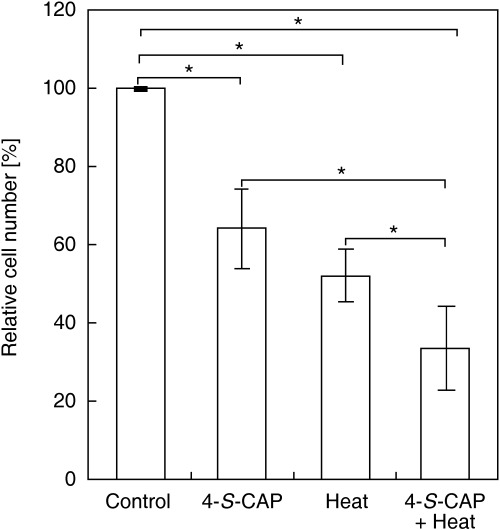
Combined effects of 4‐S‐cysteaminylphenol (4‐S‐CAP) and heat treatment on B16 cell viability. B16 cells were treated with 4‐S‐CAP at 50 µM. Simultaneously, the cells were heated using a water bath at 42.5°C for 1 h. After the 2‐day incubation period, antiproliferative effects were assessed as the relative cell number (%). Data and bars are mean ± SD of two independent experiments. *P < 0.05.
Preparation of 4‐S‐CAP‐loaded MCL. Because the combination of 4‐S‐CAP and hyperthermia has an additive effect, we constructed 4‐S‐CAP/MCL (Fig. 2) and investigated whether 4‐S‐CAP/MCL can combine 4‐S‐CAP‐mediated chemotherapy with MCL‐induced hyperthermia. The size distribution of 4‐S‐CAP/MCL with various 4‐S‐CAP contents was measured using a dynamic light‐scattering spectrophotometer (Fig. 3). For MCL without 4‐S‐CAP, the peak in the particle size distribution was 124.5 ± 0.95 nm (the mean ± SD of three independent experiments). The average size of 4‐S‐CAP/MCL increased drastically when the 4‐S‐CAP concentration exceeded 100 µM. When the 4‐S‐CAP concentration exceeded 100 µM, the dispersibility of 4‐S‐CAP/MCL was extremely low and precipitation of 4‐S‐CAP/MCL was observed. Therefore, 4‐S‐CAP/MCL at 100 µM of 4‐S‐CAP concentration was used in the following in vivo experiments.
Figure 2.
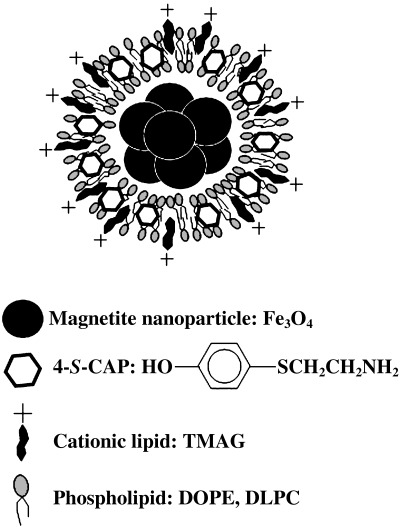
Preparation of 4‐S‐cysteaminylphenol (4‐S‐CAP)/magnetite cationic liposomes (MCL). Illustration of 4‐S‐CAP/MCL is shown.
Figure 3.
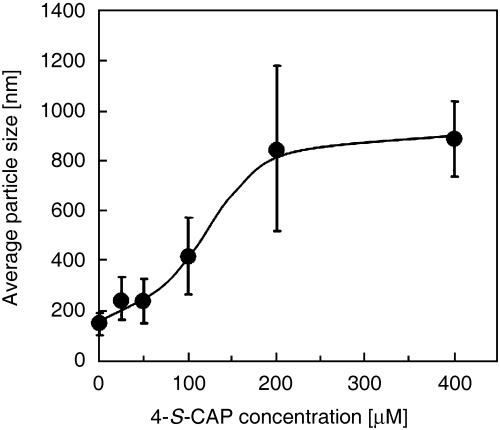
Relationship between 4‐S‐cysteaminylphenol (4‐S‐CAP) concentration and liposome size of 4‐S‐CAP/magnetite cationic liposomes (MCL). The size of the 4‐S‐CAP/MCL was measured using a dynamic light scattering spectrophotometer. Data and bars are mean ± SD of three independent experiments.
Antiproliferative activity of 4‐S‐CAP in 4‐S‐CAP/MCL. Figure 4a shows the antiproliferative effects of different concentrations of 4‐S‐CAP/MCL on B16 cells. The dose–response curve showed a dose‐dependent antiproliferative effect for 4‐S‐CAP in 4‐S‐CAP/MCL, with the maximum effects achieved using a concentration of 400 µM (46.6 ± 0.9% of relative cell number), which was comparable to the cytotoxicity of free 4–S‐CAP (inhibition concentration [IC50] IC50 of 507 µM) toward B16 melanoma cells.( 21 ) Figure 4b shows the time course of the relative cell number when B16 or NHDF cells were treated with 100 µM 4‐S‐CAP/MCL. The relative cell number for B16 cells decreased linearly to 60% during the 2‐day culture period. In contrast, non‐melanocytic NHDF cells showed no decrease in relative cell number 1 day after 4‐S‐CAP/MCL treatment, with only slight antiproliferative effects (15% decrease in the relative cell number) observed on day 2. These results indicate that 4‐S‐CAP in 4‐S‐CAP/MCL had an antiproliferative effect on B16 cells, although 4‐S‐CAP was less toxic to non‐melanocytic NHDF cells, an observation that corresponds to previous reports.( 22 )
Figure 4.
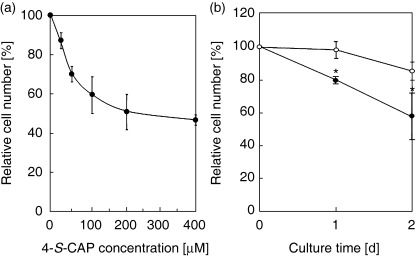
Antiproliferative activity of 4‐S‐cysteaminylphenol (4‐S‐CAP) mediated by 4‐S‐CAP/magnetite cationic liposomes (MCL). (a) B16 cells were treated with different concentrations of 4‐S‐CAP/MCL (4‐S‐CAP, 0–400 µM). After the 2‐day incubation period, antiproliferative effects were assessed as the relative cell number (%). Data and bars are mean ± SD of three independent experiments. (b) B16 (•) and normal human dermal fibroblasts (NHDF) (○) cells were treated with 4‐S‐CAP/MCL at 100 µM 4‐S‐CAP, and the relative cell number was assessed on days 1 and 2. Data and bars are mean ± SD of three independent experiments. *P < 0.05.
Uptake of magnetite nanoparticles by B16 cells and in vitro hyperthermia. Next, in order to assess the feasibility of magnetite nanoparticle‐mediated hyperthermia in vitro, we investigated whether 4‐S‐CAP/MCL were incorporated into B16 cells. The uptake of magnetite nanoparticles by B16 cells is shown in Fig. 5a. Magnetite nanoparticles in 4‐S‐CAP/MCL were incorporated into B16 cells, and the amount of uptake was 18.4 ± 1.0 pg magnetite/cell after 24 h, which was comparable to that of MCL (15.0 ± 0.4 pg magnetite/cell).
Figure 5.
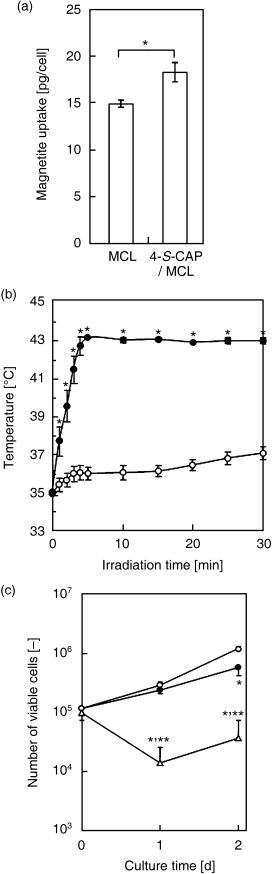
In vitro hyperthermia induced by 4‐S‐cysteaminylphenol (4‐S‐CAP)/magnetite cationic liposomes (MCL). (a) Uptake of magnetite nanoparticles into B16 cells at 24 h after addition of MCL or 4‐S‐CAP/MCL. Percentage magnetite uptake was evaluated. Data and bars are mean ± SD of three independent experiments (*P < 0.05). (b) Temperature increase in cell pellets treated with 4‐S‐CAP/MCL during alternating magnetic field (AMF) irradiation. B16 cells with (•) or without (○) 4‐S‐CAP/MCL were irradiated with AMF for 30 min. Data and bars are mean ± SD of three independent experiments (*P < 0.05). (c) In vitro antiproliferation effects of 4‐S‐CAP/MCL after AMF irradiation. After the AMF irradiation, cells were reseeded and the number of viable cells was measured on the indicated day by the trypan blue exclusion method using a hemocytometer. (○) Control B16 cells; (•) B16 cells with 4‐S‐CAP/MCL; and (▵) B16 cells treated with 4‐S‐CAP/MCL and AMF irradiation. Data and bars are mean ± SD of three independent experiments. *P < 0.05, significantly different from control group (non‐treated B16 cells); **P < 0.05, significantly different from 4‐S‐CAP/MCL group (4‐S‐CAP/MCL alone).
We then investigated whether AMF irradiation generated heat in B16 cells treated with 4‐S‐CAP/MCL, and observed intracellular hyperthermia in vitro. Figure 5b shows the temperature profile of cell pellets treated with 4‐S‐CAP/MCL during AMF irradiation at 360 kHz and 120 Oe. Heat was generated in B16 cells incorporating magnetite nanoparticles. The temperature of these cells rose quickly and reached 43.0°C, which is an effective temperature for hyperthermia treatment, and was then maintained at that temperature for 30 min by controlling the intensity of the AMF. In contrast, in non‐treated cells (0 pg magnetite/cell), the temperature increased only 2°C during the AMF irradiation. Figure 5c shows the viable cell number after the AMF irradiation. When the cells were treated with 4‐S‐CAP/MCL alone (without irradiation; 4‐S‐CAP/MCL, 100 µM), the viable cell number decreased to approximately half of that for non‐treated cells. Moreover, when the cells were treated with 4‐S‐CAP/MCL plus AMF irradiation, the viable cell number decreased drastically for 1 day and thereafter cells grew again, resulting in an apparently smaller number than with 4‐S‐CAP/MCL alone (without irradiation).
In vivo hyperthermic treatment using 4‐S‐CAP/MCL. For hyperthemic treatment in vivo, 4‐S‐CAP/MCL was injected into the tumor and AMF was applied to the whole body of the mouse. Figure 6a shows the tumor surface and rectal temperatures during AMF irradiation. Tumor temperature increased rapidly to 45°C within 3 min and was then maintained for 30 min by controlling the AMF intensity. In contrast, the temperature in the rectum showed only a limited increase during irradiation. These temperature profiles for 4‐S‐CAP/MCL‐induced hyperthermia were comparable to those of MCL‐induced hyperthermia (data not shown). No serious burns or damage were observed in all mice treated with magnetite‐mediated hyperthermia.
Figure 6.
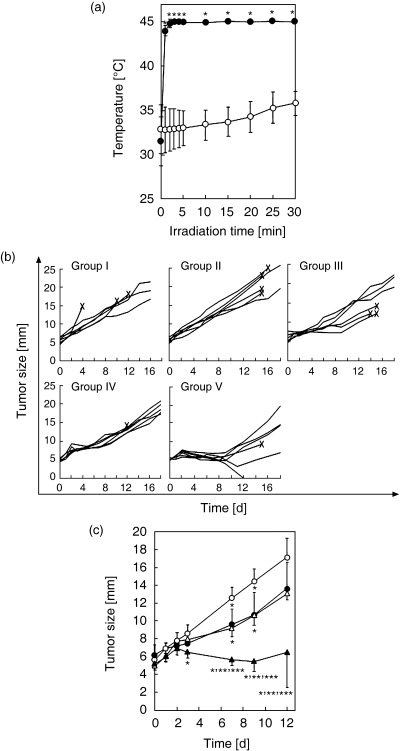
In vivo hyperthermia using 4‐S‐cysteaminylphenol (4‐S‐CAP)/magnetite cationic liposomes (MCL). Schema of the therapeutic experiment. (a) Temperature increase in melanoma nodules treated with 4‐S‐CAP/MCL during alternating magnetic field (AMF) irradiation. 4‐S‐CAP/MCL containing 4 mg magnetite were injected directly into subcutaneous B16 tumors with diameters of 5 mm, which were then irradiated with an AMF for 30 min. Tumor and rectal temperatures were measured using optical fiber probes. (•) Tumor; (○) rectum. Each point represents the mean ± SD of five mice. *P < 0.01. (b) Therapeutic effects of 4‐S‐CAP/MCL on B16 tumors. Each line represents tumor growth in a single mouse (n = 6). Crosses (×) indicate when each mouse died. (c) Comparison of each group in the first 12 days after treatment. (○) Group II; (•) group III; (△) group IV; (s) group V. Each point represents the mean ± SD of six mice. *P < 0.05, significantly different from group II (MCL alone); **P < 0.05, significantly different from group III (4‐S‐CAP/MCL alone); ***P < 0.05, significantly different from group IV (MCL + AMF).
For B16 melanoma, the therapeutic effects of the 4‐S‐CAP/MCL‐induced combination of chemotherapy and hyperthermia were assessed. Figure 6b shows the time course of tumor growth in each mouse, and the comparison of tumor growth kinetics in each group in the first 12 days is shown in Fig. 6c. In groups I (control) and II (MCL), tumors grew progressively and some mice died from pulmonary metastases (data not shown). In groups III (4‐S‐CAP/MCL), in which mice received 4‐S‐CAP/MCL alone (without AMF irradiation), and IV (MCL + AMF), in which mice received MCL‐induced hyperthermia, tumor growth was slightly but significantly suppressed on days 7 and 9, and thereafter tumors grew progressively. In contrast, in group V (4‐S‐CAP/MCL + AMF), in which mice received 4‐S‐CAP/MCL and AMF irradiation, tumor growth was strongly suppressed in the first 12 days (Fig. 6c), and 17% (1/6) of subcutaneous tumors regressed completely (Fig. 6b). Histological examination of the tumors was done by H&E staining (Fig. 7). Severe necrosis with hemorrhage was observed, and many dead cells with condensed nuclei were seen, as shown in Fig. 7.
Figure 7.
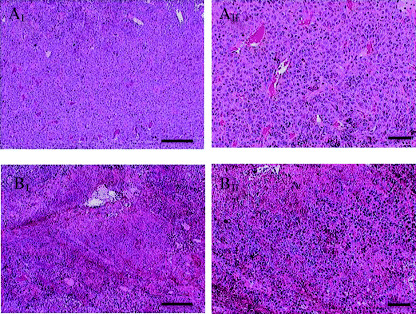
Pathological features of the tumor in representative mice treated with hyperthermia using 4‐S‐cysteaminylphenol (4‐S‐CAP)/magnetite cationic liposomes (MCL). Tumors of mice in the control group (groups I, A‐I and A‐II) and 4‐S‐CAP/MCL‐induced hyperthermia treatment group (groups IV, B‐I and B‐II) were stained with hematoxylin and eosin. Scale bars in I and II are 200 µm and 50 µm, respectively.
Discussion
In the present study, we examined the combination effect of 4‐S‐CAP treatment and hyperthermia on B16 melanoma cells, and demonstrated that the combination therapy has an additive effect (Fig. 1). In normal melanocytes and malignant melanoma cells, the specific enzyme tyrosinase catalyzes the oxidative conversion of l‐tyrosine to melanin pigments.( 23 ) 4‐S‐CAP is a good substrate for tyrosinase, and an oxidized form binds to sulphydryl enzymes.( 24 ) With respect to the mechanism of cytotoxicity, dihydro‐1,4‐benzothiazine‐6,7‐dione, a metabolite of 4‐S‐CAP, deprives melanoma cells of reduced glutathione and may inactivate SH enzymes essential for DNA synthesis and cell proliferation by covalent binding through their cysteine residues, thereby exerting melanocytotoxicity.( 21 ) Thus, cytotoxicity of 4‐S‐CAP depends mostly on reactive oxygen species (ROS). Similarly to 4‐S‐CAP activities in melanoma cells, hyperthermia can promote the formation of free radicals and related ROS from metabolic pathways, and these ROS may result in some cellular injury.( 25 ) We speculate that ROS played an important role in the additive effect of the combined 4‐S‐CAP treatment and hyperthermia on melanoma cells; therefore, further research is required in order to elucidate the mechanism.
In the case of a superficial tumor, such as melanoma, a simple heat mediator is desirable for the clinical application of hyperthermia. Previously, we confirmed hyperthermic effects on B16 melanoma using MCL injected directly into the tumor.( 26 ) MCL uptake by cells in the injection site was much higher than that of magnetoliposomes whose liposomal surface had neutral charge, because MCL can electrostatically interact with the negative‐charged phospholipid membrane of cells,( 7 ) resulting in retention at the injection site. Thus, in a drug‐targeting modality for melanoma, the use of 4‐S‐CAP/MCL is appropriate because superficial tumors can be treated with intratumoral injection of 4‐S‐CAP/MCL. Therefore, we used 4‐S‐CAP/MCL in order to heat the tumoral region and minimize heating of the surrounding healthy tissue. As shown in Fig. 6a, during hyperthermia using 4‐S‐CAP/MCL, tumor temperature reached 45°C rapidly, whereas rectal temperature remained at 38°C. These results indicate that using 4‐S‐CAP/MCL allows the application of hyperthermia to specific tumor tissue and that accurate control of the tumor temperature is possible by manipulating the magnetic field intensity. In this study, we set the tumor temperature at 45°C; however, this was insufficient to completely destroy the malignant melanoma. Our hyperthermia system can be carried out at higher temperatures and can be conducted repeatedly without damaging healthy tissue. For example, complete regression of B16 melanoma was observed in 90% of mice using MCL‐induced hyperthermia at 46°C applied once daily for 2 days.( 26 ) In the present study, we set the temperature at 45°C in order to examine the effects of combining hyperthermia with 4‐S‐CAP‐madiated chemotherapy.
A major advantage of hyperthermia is that it has few side effects. In contrast to chemotherapy, hyperthermic effects are independent of cell lines and animals.( 9 , 10 , 11 , 12 , 13 ) Furthermore, hyperthermic effects on cancer cells are caused mainly by physical damage, and differences in sensitivities to hyperthermia are negligible, especially at higher temperatures.( 27 , 28 ) Therefore, when magnetite nanoparticles are internalized into tumor cells, the hyperthermic effect should be independent of cell type. However, in the case of repeated injection of 4‐S‐CAP/MCL, the toxicity of 4‐S‐CAP/MCL may become an important issue in a clinical trial. For MCL, in our preliminary study, the toxicity of a single administration of MCL solution (33 mg magnetite, intraperitoneally) was investigated. MCL accumulated mostly in the liver and spleen of mice, but none of the five observed mice died after MCL injection.( 29 ) In the present study, 8 mg magnetite was used, which was much less than that used in the preliminary examination (33 mg). The 4‐S‐CAP was synthesized as an antimelanoma agent that is selectively toxic to the in vivo melanocytes engaged in melanin synthesis,( 30 , 31 ) but not to the melanocytes and keratinocytes of albino mice.( 31 ) However, a study by Padgette et al. has shown that 4‐S‐CAP is a good substrate of plasma monoamine oxidase, as well as tyrosinase.( 19 ) The cytotoxicity of 4‐S‐CAP to cultured cells may be partly mediated by the aldehyde formed by oxidative deamination.( 32 ) In the present study, non‐melanocytic NHDF cells showed a slight decrease in relative cell number (decrease of 15%; Fig. 4b), suggesting that 4‐S‐CAP induced tyrosinase‐independent toxicity. However, the mechanism of this tyrosinase‐independent toxicity remains to be clarified. Taken together, these findings indicate that 4‐S‐CAP/MCL toxicity should be fully investigated before clinical application.
In conclusion, the promising results of the present study warrant further investigation of new modalities using 4‐S‐CAP/MCL, with the long‐term goal of future application in the treatment of malignant melanoma in humans.
Acknowledgments
The authors would like to thank Toda Kogyo Co. for supplying the magnetite. This work was partially supported by a Health and Labour Sciences Research Grant‐in‐Aid for Research on Advanced Medical Technology from the Ministry of Health, Labour and Welfare, a Grant‐in‐Aid for University Start‐Up Creation Support System, and a 21st Century COE Program ‘Nature‐Guided Materials Processing’ grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- 1. Van der Zee J. Heating the patient: a promising approach? Annu Oncol 2002; 13: 1173–84. [DOI] [PubMed] [Google Scholar]
- 2. Abe M, Hiraoka M, Takahashi M et al. Multi‐institutional studies on hyperthermia using an 8‐MHz radiofrequency capacitive heating device (Thermotron RF‐8) in combination with radiation for cancer therapy. Cancer 1986; 58: 1589–95. [DOI] [PubMed] [Google Scholar]
- 3. Moroz P, Jones SK, Gray BN. Magnetically mediated hyperthermia: current status and future directions. Int J Hyperthermia 2002; 18: 267–84. [DOI] [PubMed] [Google Scholar]
- 4. Jordan A, Wust P, Fahling H, John W, Hinz A, Felix R. Inductive heating of ferrimagnetic particles and magnetic fluids: physical evaluation of their potential for hyperthermia. Int J Hyperthermia 1993; 9: 51–68. [DOI] [PubMed] [Google Scholar]
- 5. Minamimura T, Sato H, Kasaoka S et al. Tumor regression by inductive hyperthermia combined with hepatic embolization using dextran magnetite‐incorporated microspheres in rats. Int J Oncol 2000; 16: 1153–8. [DOI] [PubMed] [Google Scholar]
- 6. Shinkai M, Matsui M, Kobayashi T. Heat properties of magnetoliposomes for local hyperthermia. Jpn J Hyperthermic Oncol 1994; 10: 168–77. [Google Scholar]
- 7. Shinkai M, Yanase M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposomes: in vitro study. Jpn J Cancer Res 1996; 87: 1179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yanase M, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposomes: ex vivo study. Jpn J Cancer Res 1997; 88: 630–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yanase M, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposomes: an in vivo study. Jpn J Cancer Res 1998; 89: 463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsuoka F, Shinkai M, Honda H, Kubo T, Sugita T, Kobayashi T. Hyperthermia using magnetite cationic liposomes for hamster osteosarcoma. Biomagn Res Technol 2004; 25: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ito A, Tanaka K, Honda H, Abe S, Yamaguchi H, Kobayashi T. Complete regression of mouse mammary carcinoma with a size greater than 15 mm by frequent repeated hyperthermia using magnetite nanoparticles. J Biosci Bioeng 2003; 96: 364–9. [DOI] [PubMed] [Google Scholar]
- 12. Kawai N, Ito A, Nakahara Y et al. Anticancer effect of hyperthermia on prostate cancer mediated by magnetite cationic liposomes and immune‐response induction in transplanted syngeneic rats. Prostate 2005; 64: 373–81. [DOI] [PubMed] [Google Scholar]
- 13. Matsuno H, Tohnai I, Mitsudo K et al. Interstitial hyperthermia using magnetite cationic liposomes to inhibit tumor growth of VX‐7 transplanted tumor in rabbit tongue. Jpn J Hyperthermic Oncol 2001; 17: 141–50. [Google Scholar]
- 14. Miura S, Ueda T, Jimbow K, Ito S, Fujita K. Synthesis of cysteinylphenol, cysteaminylphenol, and related compounds, and in vivo evaluation of antimelanoma effect. Arch Dermatol Res 1987; 279: 219–25. [DOI] [PubMed] [Google Scholar]
- 15. Yamada I, Seki S, Matsubara O, Ito S, Suzuki S, Kasuga T. The cytotoxicity of cysteinylcatechols and related compounds to human melanoma cells in vitro . J Invest Dermatol 1987; 88: 538–40. [DOI] [PubMed] [Google Scholar]
- 16. Minamitsuji Y, Toyofuku K, Sugiyama S, Yamada K, Jimbow K. Sulfur containing tyrosine analogs can cause selective melanocytotoxicity involving tyrosinase‐mediated apoptosis. J Invest Dermatol Symp Proc 1999; 4: 130–6. [DOI] [PubMed] [Google Scholar]
- 17. Ito A, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Augmentation of MHC class I antigen presentation via heat shock protein expression by hyperthermia. Cancer Immunol Immunother 2001; 50: 515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Valeriote F, Lin H. Synergistic interaction of anticancer agents: a cellular perspective. Cancer Chemother Rep 1975; 59: 895–900. [PubMed] [Google Scholar]
- 19. Padgette SR, Herman HH, Han JH, Pollock SH, May SW. Antihypertensive activities of phenyl aminoethyl sulfides, a class of synthetic substrates for dopamine β‐hydroxylase. J Med Chem 1984; 27: 1354–7. [DOI] [PubMed] [Google Scholar]
- 20. Owen CS, Sykes NL. Magnetic labeling and cell sorting. J Immunol Methods 1984; 73: 41–8. [DOI] [PubMed] [Google Scholar]
- 21. Hasegawa K, Ito S, Inoue S, Wakamatsu K, Ozeki H, Ishiguro I. Dihydro‐1,4‐benzothiazine‐6,7‐dione, the ultimate toxic metabolite of 4‐S‐cysteaminylphenol and 4‐S‐cysteaminylcatechol. Biochem Pharmacol 1997; 53: 1435–44. [DOI] [PubMed] [Google Scholar]
- 22. Parsons PG, Favier D, McEwan M, Takahashi H, Jimbow K, Ito S. Action of cysteaminylphenols on human melanoma cells in vivo and in vitro: 4‐S‐cysteaminylphenol binds protein disulphide isomerase. Melanoma Res 1991; 1: 97–104. [DOI] [PubMed] [Google Scholar]
- 23. Prota G. Melanins and Melanogenesis. New York: Academic Press, 1992. [Google Scholar]
- 24. Ito S, Kato T, Ishikawa K, Kasuga T, Jimbow K. Mechanism of selective toxicity of 4‐S‐cysteinylphenol and 4‐S‐cysteaminylphenol to melanocytes. Biochem Pharmacol 1987; 36: 2007–11. [DOI] [PubMed] [Google Scholar]
- 25. Venkataraman S, Wagner BA, Jiang X et al. Overexpression of manganese superoxide dismutase promotes the survival of prostate cancer cells exposed to hyperthermia. Free Radic Res 2004; 38: 1119–32. [DOI] [PubMed] [Google Scholar]
- 26. Suzuki M, Shinkai M, Honda H, Kobayashi T. Anticancer effect and immune induction by hyperthermia of malignant melanoma using magnetite cationic liposomes. Melanoma Res 2003; 13: 129–35. [DOI] [PubMed] [Google Scholar]
- 27. Kano E, Miyakoshi J, Sugahara T. Difference in sensitivities to hyperthermia and ionizing radiation of various mammalian cell stress in vitro . In: Streffer C, Van Beuninen D, Dietzel F et al., eds. Cancer Therapy by Hyperthermia and Radiation. Baltimore‐Munich: Urban and Schwarzenberg, 1978: 188–90. [Google Scholar]
- 28. Kano E, Miyakoshi J, Furukawa M et al. Effects of hyperthermia at 50 degrees C on V‐79 cells in vitro . J Radiat Res (Tokyo) 1982; 23: 218–27. [DOI] [PubMed] [Google Scholar]
- 29. Ito A, Nakahara Y, Tanaka K, Kuga Y, Honda H, Kobayashi T. Time course of biodistribution and heat generation of magnetite cationic liposomes in mouse model. Jpn J Hyperthermic Oncol 2003; 19: 151–9. [Google Scholar]
- 30. Ito Y, Jimbow K, Ito S. Depigmentation of black guinea pig skin by topical application of cysteaminylphenol, cysteinylphenol, and related compounds. J Invest Dermatol 1987; 88: 77–82. [DOI] [PubMed] [Google Scholar]
- 31. Ito Y, Jimbow K. Selective cytotoxicity of 4‐S‐cysteaminylphenol on follicular melanocytes of the black mouse: rational basis for its application to melanoma chemotherapy. Cancer Res 1987; 47: 3278–84. [PubMed] [Google Scholar]
- 32. Inoue S, Ito S, Wakamatsu K, Jimbow K, Fujita K. Mechanism of growth inhibition of melanoma cells by 4‐S‐cysteaminylphenol and its analogues. Biochem Pharmacol 1990; 39: 1077–83. [DOI] [PubMed] [Google Scholar]


