Abstract
(Cancer Sci 2010; 101: 751–758)
The present gold standard for bladder cancer is Mycobacterium bovis, Bacillus Calmette Guerin (BCG) immunotherapy. But it has a non‐responder rate of 30–50% and side effects are common. Lactobacillus casei strain Shirota has been reported to reduce the incidence of recurrence in bladder cancer patients and to cure tumor‐bearing mice. Our aim was to determine if Lactobacillus rhamnosus GG (LGG) could be as efficacious as BCG in a murine model of bladder cancer. MB49 bladder cancer cells secreting human prostate‐specific antigen were implanted orthotopically in female C57BL/6 mice and urinary prostate‐specific antigen levels were used as a marker of tumor growth. Mice were treated with either live or lyophilized LGG given via intravesical instillation, or both oral and intravesical LGG given once a week for a period of 6 weeks starting at day 4 after tumor implantation. A comparison of LGG and BCG immunotherapy was also carried out. LGG therapy (live or lyophilized) significantly (P = 0.006) increased the number of cured mice. Cytokine arrays and immune cell recruitment analysis revealed differences between untreated, treated, cured, and tumor‐bearing mice. LGG therapy restored XCL1 levels to those in healthy bladders. LGG also recruited large numbers of neutrophils and macrophages to the tumor site. Intravesical LGG and BCG immunotherapy had cure rates of 89 and 77%, respectively, compared with 20% in untreated mice. LGG has the potential to replace BCG immunotherapy for the treatment of bladder cancer.
Bladder cancer is the seventh most common cancer worldwide. The American Cancer Society estimates that in 2008 there will be approximately 68 810 new cases diagnosed and approximately 14 100 deaths from bladder cancer in the USA. In Singapore it is the tenth most common cancer among men. Bladder cancer is usually superficial at the time of presentation (70%) and the main histopathological type is transitional cell carcinoma (90%). The gold standard therapy consists of transurethral resection of the bladder tumor followed by weekly intravesical BCGinstillations.( 1 , 2 ) Despite successful initial treatment, there is a high local recurrence rate (50–70%) with 10–15% progressing to muscle‐invasive disease.( 3 ) BCG immunotherapy is associated with side effects ranging from cystitis in the majority of patients to septicemia culminating in death in rare cases.( 4 ) Thus there is a need to identify alternative therapies.
Lactobacillus species are generally regarded as safe and have been studied extensively in both people and experimental animals for a wide variety of applications. Aso et al. have shown in clinical trials that oral consumption of Lactobacillus species can reduce bladder cancer recurrence.( 5 , 6 ) The administration of Lactobacillus preparations has also been demonstrated to inhibit chemically induced carcinogenesis and reduce tumor growth in animal models.( 7 , 8 , 9 ) Takahashi et al. showed that LcS intravesical therapy was superior to BCG in reducing tumor growth in C3H mice bearing MBT‐2 tumors.( 10 ) However, the schedule they used consisted of daily instillations for 10 days, which is not the schedule used for BCG immunotherapy in patients (once a week for 6 weeks).
LGG is a probitoic originally isolated from the human gut( 11 ) and has been frequently evaluated for the clinical therapy of intestinal disorders. In vitro studies have shown that Lactobacillus species are more effective than BCG in inducing cytotoxic effects on bladder cancer cells.( 12 ) Using healthy C57BL/6 mice, BCG was found to induce more cytokine production in the bladders after intravesical instillation than LGG, although both recruited similar numbers of NK cells.( 13 )
To confirm the efficacy of LGG for bladder cancer therapy we used a murine bladder cancer model consisting of MB49 cells implanted in C57BL/6 mice. The therapeutic schedule chosen was intravesical instillations once a week for a period of 6 weeks. If LGG is to be used clinically then a lyophilized preparation would ensure better quality control, ease of transport, and storage of the product. Lyophilized preparations of BCG are used for clinical therapy as well. Therefore, an initial comparison of both live and lyophilized preparations of LGG was carried out to determine if there is any loss in efficacy associated with lyophilization. Finally, the efficacy of lyophilized LGG and BCG for the immunotherapy of bladder cancer was compared.
Materials and Methods
Bacteria. LGG was cultured in MRS (Merck & Co., Whitehouse Station, NJ, USA) media at 37°C. An overnight culture of LGG consistently yielded 3 × 109 cfu/mL. For lyophilisation, 10 mL PBS was added to 1 mL LGG and the suspension was centrifuged at 1699g for 10 min. The bacterial pellet was frozen at −80°C then lyophilized overnight in a freeze dryer at −40°C with a vacuum pressure of 400 mbar (Thermo Savant; Thermo Fisher Scientific, Waltham, MA, USA). Lyo LGG was stored at −80°C until ready for use. The viability of the lyophilized preparation was approximately 15–60% at −80°C for up to 6 weeks. The ability of bacteria to induce cytokine production was assessed by co‐incubation with murine splenocytes for 6 and 12 h.
Cell line. MB49‐PSA cells( 14 ) were maintained in RPMI media supplemented with 2 mM l‐glutamine (Sigma Aldrich, St Louis, MO, USA), 10% v/v foetal bovine serum (Hyclone, Thermo Fisher Scientific, Rockford, IL, USA), penicillin–streptomycin (5000 U/mL and 5 mg/mL), and 0.2 mg/mL hygromycin (Invitrogen Corporation, Carlsbad, CA, USA). PSA is a surrogate marker of disease in mice with implanted tumor cells. It differentiates mice that are cured from those that have small tumors better than measuring bladder weight. Although immunohistochemistry of bladder tissue can identify small tumors, it limits the type of assays and analysis that can be carried out with the bladder. MB49‐PSA cells were incubated with LGG for 2 and 24 h at a 1:100 ratio (cells:bacteria) and the number of bacteria internalized was determined by lysing the cells (1% Triton‐X 100 in water) and plating the bacteria on MRS agar plates.
In vitro stimulation of splenocytes with LGG. Splenocytes (2 × 106/well) were seeded in penicillin G media in 24‐well culture dishes and allowed to settle overnight. Live LGG (2 × 108 cfu) and Lyo LGG were added to the treatment wells the following day. After 6, 12, 24, 48, and 72 h post‐stimulation the following cytokines (IL12p40, TNFα, and IL10) were measured in cell culture supernatants by ELISA (BD Biosciences San Jose, CA, USA).
Orthotopic tumor model. All animal studies were conducted according to Institutional Animal Care and Use Committee guidelines at the National University of Singapore. C57BL/6 female mice aged 4–6 weeks were allowed to acclimatize for 1 week prior to experimentation. Tumor implantation was carried out as described by Ninalga et al. ( 15 ) using 0.1 mg/mL poly‐l‐Lysine (Sigma Aldrich) to enable attachment of MB49‐PSA (1 × 105cells/0.1 mL) cells to the bladder wall. Mice were anaesthetized during orthotopic procedures and were kept under anesthesia for 2 h following treatment to minimize voiding.
The day of tumor implantation was taken to be day 0. The mice were divided into two groups. Control mice were instilled with 100 μL PBS, while the mice treated with LGG were instilled with 100 μL Lyo LGG (n = 10). The amount of Lyo LGG instilled was equivalent to 1 × 108 cfu prior to lyophilization. Both groups were instilled once a week for 6 weeks. The experiment was terminated 1 day after the last instillation (day 40) and bladders were resected. This experiment was carried out three times.
The efficacy of Lyo LGG (n = 10) instillations was compared to that of live LGG (n = 10) and to ascertain if oral feeding of live LGG would augment the antitumor effects of intravesical Lyo LGG, another group of mice was fed 1 × 108 live LGG 1 day before their scheduled Lyo LGG intravesical instillation (O + I group, n = 10). Control mice received PBS. These experiments were repeated twice. In a final set of experiments, tumor‐bearing mice were treated with 100 μL Lyo LGG (1 × 108 cfu, n = 9) or lyophilized BCG (1 × 107 cfu, n = 15; Immucyst; Sanofi Pasteur, Toronto, ON, Canada) or PBS (n = 10) weekly for a period of 6 weeks. Live BCG was cultured as described previously.( 13 )
Monitoring tumor implantation and response to therapy. Mice were transferred to metabolic cages 1 day before the scheduled treatment (tumor implantation or intravesical therapy) for urine collection. Protease inhibitors (Roche Diagnostics, Basel, Switzerland) were added to urine containers to prevent protein degradation. Urinary PSA was measured using the free‐PSA chemiluminescence kit (Autobio Diagnostics, Zhengzhou City, China). Only tumor‐bearing mice tested positive for PSA in their urine. The urinary PSA levels were normalized against creatinine (Wako Pure Chemical Industries, Osaka, Japan). Mice without detectable free‐PSA in their urine by day 11 were excluded from the experiment. For the BCG‐treated mice only end‐point analysis was done as we could not collect interim urine samples due to Institutional Animal Care and Use Committee regulations. Tumor presence was confirmed at the end point (day 40) by measuring PSA levels in bladder homogenates (50 μg total protein).
Bladder protein isolation and array analysis. Mice bladders were harvested 24 h after the last instillation and snap frozen in liquid nitrogen and stored at −80°C. The bladders (total) were homogenized in 1 mL buffer as previously described( 14 ) and the protein concentration was determined with a Micro BCA Kit (Thermo Fisher Scientific). The bladder lysate (1 mg) was used to probe an antibody‐based cytokine array (C Series 1000; Raybiotech, Norcross, GA, USA) that can detect 96 proteins (array 3, Axl, BLC,CD30L,CD30T,CD40, CRG‐2, CTACK, CXCL16, Eotaxin, Eotaxin‐2, Fas‐Ligand, Fractalkine, GCSF, GMCSF, IFNγ, IGFBP3, IGFBP5, IGFBP6, IL1α, IL1β, IL2, IL3, IL3Rb, IL4, IL5, IL6, IL9, IL10, IL12p40/p70, IL12p70, IL13, IL17, KC, Leptin R, Leptin, LIX, L‐Selectin, Lymphotactin, MCP1, MCP5, MCSF, MIG, MIP‐1α, MIP‐1γ, MIP‐2, MIP‐3β, MIP‐3α, PF4, P‐Selectin, RANTES, SCF, SDF‐1α, TARC, TCA‐3, TECK, TIMP‐1, TNFα, sTNFα RI, sTNFα RII, TPO, VCAM‐1, VEGF; and array 4, bFGF, DPPIV/CD26, Dtk, E‐Selectin, Fcγ RIIB, Flt‐3 Ligand, GITR, HGF R, ICAM‐1, IGFBP‐2, IGF‐I, IGF‐II, IL‐15, IL17B R, IL7, I‐TAC, Lungkine, MDC, MMP‐2, MMP‐3, OPN, Osteoporotegerin, Pro‐MMP‐9, Resistin, Shh‐N, Thymus CK‐1, TIMP‐2, TRANCE, TROY, TSLP, VEGF R1, VEGF R2, VEGF‐D). The array was processed according to the manufacturer’s instructions and the chemiluminescence image was analyzed with the SynGene GeneTools software (Synoptics Ltd., Cambridge, UK). Differentially expressed proteins identified on the array were confirmed by analyzing 50 μg total bladder proteins using commercial ELISA kits (R&D Systems, Minneapolis, MN, USA; and BD Biosciences). Proteins from healthy mice bladders and MB49‐PSA cells were also analyzed.
Immunohistochemistry. Paraffin‐embedded bladder tissues (n = 5 per experimental group) were sectioned and the slides were dewaxed and rehydrated in deionised H2O then washed three times in PBS (5 min per wash). The tissues were blocked in 3% H2O2 in methanol for 15 min, then washed and blocked with 5% normal rabbit serum for 60 min. The slides were incubated with anti‐Mac‐3 (1:10 dilution; BD Biosciences) or anti‐neutrophil (1:10 dilution; Cedarlane, Burlington, ON, Canada) antibodies overnight. Slides were incubated with anti‐rat‐IgG (1:200 dilution) for 1 h followed by incubation with avidin–biotin complex for 1 h, then washed three times in PBS (5 min per wash). Finally, the slides were rinsed with Tris‐buffered saline, developed with DAB, and counterstained with methyl green. The slides were examined on an Olympus BX51 microscope and photos taken with an Olympus PM C35DX camera (Olympus, Tokyo, Japan). The Acquisition software used was the Viewfinder Lite Version 1.0 (Pixera Corporation, San Jose, CA, USA). The extent of neutrophil infiltration into bladder tumors was estimated under 400× magnification.
Immune cell population changes after LGG intravesical instillations. The bladder, spleen, and draining lymph nodes were resected and single cells isolated for flow cytometry as previously described( 13 ) from tumor‐bearing and healthy mice instilled with Lyo LGG or PBS (treated as described for the tumor‐bearing mice except no tumor implantation was carried out). Samples were assessed for: T cells (anti‐CD3‐FITC, anti‐CD4‐PE and anti‐CD8a‐PE); macrophages (anti‐Mac3‐FITC); NK cells (anti‐Pan NK‐PE); dendritic cells (anti‐CD11c‐FITC and anti‐CD83‐PE or anti‐CD86‐PE); B‐cells (anti‐B220‐PE or anti‐CD19‐FITC); and granulocytes (anti‐Ly6G‐FITC). Appropriate isotype controls were also included in the experiments. Immune cells were identified based on forward and side scatter characteristics and their binding with anti‐CD45‐PE and this was used to gate the population of cells for further analysis. Only viable cells were analyzed. All antibodies were from BD Biosciences, and flow cytometry was carried out on a CyAnADP (Dako, Glostrup, Denmark). Data were analyzed with the Summit software (Dako, Glostrup, Denmark).
Neutrophil isolation and stimulation with microbes. The femurs of 8–10‐week‐old female C57BL/6 mice were flushed with a 22‐G needle to remove the bone marrow cells, which were passed through a 70‐μm cell strainer (BD Bioscience). Red blood cells were lysed by incubation in red blood cell lysis buffer (150 mm NH4Cl, 10 mm KHCO3 , and 0.1 mm EDTA) at room temperature for 5 min. The cells were centrifuged at 453 g for 5 min and washed with PBS twice and positively selected with an anti‐Ly6G microbead kit (Miltenyi Biotech, GmbH, Bergish Gladbach, Germany) according to manufacturer’s protocol. The purity of neutrophils was at least 95% by flow cytometry. Neutrophils (5 × 105) were cocultured with 2.5 × 106 cfu of live LGG or BCG in DMEM (supplemented with 10% fetal bovine serum, 2 mm l‐glutamine, 50 μg/mL penicillin G, and 50 μmβ‐mercaptoethanol [Merck & Co. Inc., Whitehouse Station, NJ, USA]) for 2 h at 37°C after which 200 μg/mL gentamicin (Sigma‐Aldrich) was added for a further 2 h to kill extracellular bacteria. Neutrophils were washed three times with PBS to remove extracellular bacteria and placed in fresh media for another 18 h. The supernatant was collected for cytokine analysis.
To monitor bacterial internalization the neutrophils were treated as above except that after killing the external bacteria the cells were lysed with 1% Trition‐X 100 and the intracellular bacteria were collected by centrifugation (1699 g for 10 min), diluted in PBS, and plated on MRS plates.
Statistical methods. All graphical data was expressed as the mean ± SD of combined data from the replicate experiments. The live and Lyo LGG data were subjected to a one‐way analysis of variance (ANOVA) followed by a post‐hoc Scheffe test. P‐values less than 0.05 were considered statistically significant. Bladder protein level data from the replicate experiments were subjected to Dunnett analysis with the healthy mice and control tumor‐bearing mice as the reference groups. Logistic regression was used to compare the mortality rates of live and Lyo LGG groups with their control; and for Immucyst, Lyo LGG with their controls. Statistical significance was set at P < 0.05. Neutrophil cytokine production was analyzed with one‐way ANOVA, post‐hoc test Bonferroni.
Results
Comparison of live and Lyo LGG using splenocytes and healthy mice. In vitro studies were carried out using splenocytes to determine the ability of live and Lyo LGG to stimulate cytokine production. Both preparations produced increased TNF‐α (control, 72.35 ± 11.12 pg/mL; live LGG, 414.27 ± 251.96 pg/mL; and Lyo LGG, 318.46 ± 208.28* pg/mL; *P < 0.05 when compared with control, maximal at 6 h), IL‐12p40 (control, 55.11 ± 8.74 pg/mL; live LGG, 76.45 ± 2.97 pg/mL; and Lyo LGG, 102.30 ± 31.64* pg/mL; *P < 0.05 when compared with control, maximal at 12 h), and IL‐10 (control, 44.18 ± 18.38 pg/mL; live LGG, 193.14 ± 93.35 pg/mL; and Lyo LGG, 393.42 ± 225.19* pg/mL; *P < 0.05 when compared with control, maximal at 12 h) but only Lyo LGG induced significantly more cytokine production.
Lyo LGG instillations into a healthy bladder also attracted significantly more CD11c+CD83+ (control 0.78 ± 0.58, Lyo LGG 5.74 ± 3.37, P < 0.05) and CD11c+CD86+ (control 0.58 ± 0.2, Lyo LGG 3.65 ± 2.32, P < 0.05) dendritic cells to the bladder. There was a corresponding decrease of these cell types in the local lymph nodes (CD11c+CD83+, control 5.65 ± 3.79 and Lyo LGG 0.19 ± 0.13, P < 0.05; and CD11c+CD86+, control 3.46 ± 2.83 and Lyo LGG 0.33 ± 0.4, P < 0.05). There were more B220+ cells in the lymph nodes (control 43.59 ± 8.21 and LGG 69.93 ± 12.42, p < 0.05) after LGG instillation in the bladder. Therefore, intravesical Lyo LGG, like live LGG( 13 ), induces immune cell recruitment into the bladder.
LGG confers a survival advantage and cures bladder cancer. The modified syngenic tumor cell line MB49‐PSA was used for tumor implantation and PSA served as a surrogate marker of disease. There were three treatment groups receiving either intravesical live or Lyo LGG, or oral live LGG 1 day before intravesical Lyo (O + I) LGG. There were also two control groups receiving either intravesical instillations of PBS or oral PBS 1 day before intravesical PBS. Therapy began on day 4 after tumor implantation and was given once a week for a period of 6 weeks. The presence of tumors was detected by monitoring PSA levels in the urine. Weekly instillations of Lyo or live LGG or O + I LGG doubled the number of cured mice (Table 1). Although all mice were positive for PSA at day 11 some mice were spontaneously cured as shown by the decreased number of tumor‐bearing mice in the control groups at the end of the experiment. Tumor presence at the end point was confirmed visually and by PSA ELISA carried out on bladder tissue homogenates.
Table 1.
Efficacy of Lactobacillus rhamnosus GG immunotherapy for bladder cancer
| Group | n | Cured§ (%) | OR | 95% CI | P‐value |
|---|---|---|---|---|---|
| Control | 37 | 14 (38) | 1 | — | — |
| Live† | 19 | 13 (68) | 0.195 | 0.059–0.646 | 0.006* |
| Lyo† | 43 | 26 (60) | 0.277 | 0.109–0.703 | 0.006* |
| Control O + I | 5 | 2 (40) | 1 | — | — |
| O + I Lyo‡ | 20 | 13 (65) | 0.359 | 0.048–2.683 | 0.358 |
*Statistical significance at P < 0.05. †Chi‐square test compared to control. ‡O + I groups analyzed using the Fisher’s exact test. §All mice were killed at day 40 and prostate‐specific antigen levels in the bladder homogenates were examined by ELISA to identify tumor‐bearing and cured mice.
Bladder protein analysis of cured and tumor‐bearing mice. To determine how LGG induced tumor regression, 1 mg total bladder proteins from both cured and tumor‐bearing mice were analyzed on antibody arrays. These arrays allow a panel of proteins to be rapidly screened and by using whole bladders the overall effect of therapy in this tissue could be evaluated.
After 6 weeks of therapy, we identified a total of 18 proteins (E‐selectin, Fcg RIIB, ICAM‐1, OPN, Pro‐MMP9, Thymus Ck‐1, VEGF‐R2, VEGF‐R3, VEGF‐D, Eotaxin‐2, IL3Rb, LIX, Lymphotactin (XCL1), MIP2, PF4, P‐Selectin, sTNF RI, and VCAM1) that were twofold or more differentially expressed with respect to control tumor‐bearing bladders (Fig. 1). Of these, only PF4, XCL1, and P‐selectin were increased after LGG therapy while OPN, Pro‐MMP9, Thymus CK‐1, and VEGFR2 were decreased. Subsequently, ELISA was carried out for six of the proteins identified on the array and TNF‐α as well (Table 2). Although TNFα was elevated in both tumor‐bearing and cured mice relative to normal mice, E‐selectin, Pro‐MMP9, and OPN were increased and XCL1 decreased in tumor‐bearing mice compared with normal and cured mice. Both OPN and E‐selectin were produced by the tumor cell line MB49‐PSA in significantly greater quantities than in normal bladders (Table 2). Treatment with LGG resulted in increased XCL1 in tumor‐bearing mice and a reduction in E‐selectin and Pro‐MMP9. Oral LGG therapy boosted bladder VEGF‐D to levels comparable with healthy bladders (Table 2). Therefore, LGG therapy could potentially inhibit metastasis‐related and tissue‐remodeling enzymes.
Figure 1.

X‐ray images of antibody arrays. The 96 proteins analyzed were distributed between two arrays: (A) array 4.1, containing antibodies to 34 proteins and (B) array 3.1, containing antibodies to 62 proteins. Each antibody was represented by two spots on the array. The three panels from left to right are the arrays probed with bladder protein homogenates from control PBS‐treated mice still bearing tumors, and lyophilized (Lyo) Lactobacillus rhamnosus GG (LGG)‐treated mice that were tumor‐bearing and Lyo LGG‐treated mice that were cured at the time of termination of the experiment. Proteins expressed in tumor‐bearing mice treated with LGG with a twofold difference with respect to the control tumor‐bearing mice are identified by having their relative position on the arrays circled in white and labeled. The proteins are thymus chemokine 1 (CK), vascular endothelial growth factor (VEGF), osteopontin (OPN), inter‐cellular adhesion molecule 1 (ICAM‐1), macrophage inflammatory protein (MIP), soluble tumor necrosis factor receptor inhibitor I (sTNFRII), interleukin (IL), vascular cell adhesion molecule 1 (VCAM‐1), LPS induced C‐X‐C chemokine (LIX) and platelet factor (PF).
Table 2.
ELISA analysis of bladder tissue homogenates
| Group Sample | n | Protein† (pg × 102/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| E‐selectin | Pro‐MMP9 | XCL1 | OPN | VEGF‐D | VEGF‐R2 | TNF‐α | ||
| Cell line MB49 cell line | 3 | 33.39 ± 11.61*,** | 20.46 ± 10.38* | 2.63 ± 0.53** | 16.58 ± 4.90** | 0.3 ± 0.17 | 1.00 ± 0.29 | 0.03 ± 0.01 |
| Normal mice | 5 | 3.95 ± 0.06* | 7.97 ± 4.33* | 37.17 ± 4.28* | 3.35 ± 1.03 | 2.60 ± 0.09 | 1.37 ± 0.71 | 0.02 ± 0.01* |
| Tumor‐bearing mice | ||||||||
| Control PBS | ||||||||
| Tumor | 8 | 9.09 ± 4.39 | 186.55 ± 59.36** | 10.99 ± 5.06** | 17.99 ± 2.99** | 1.01 ± 0.22 | 2.43 ± 0.72 | 0.91 ± 0.85 |
| Cured | 6 | 2.69 ± 0.40* | 20.28 ± 13.28* | 18.62 ± 3.35 | 6.92 ± 4.08* | 1.13 ± 0.26 | 2.16 ± 1.14 | 1.55 ± 0.93 |
| Live LGG | ||||||||
| Tumor | 3 | 4.98 ± 3.16 | 183.48 ± 61.94** | 19.48 ± 5.67 | 14.41 ± 8.01** | 0.82 ± 0.28 | 2.20 ± 0.50 | 1.55 ± 0.58 |
| Cured | 7 | 2.08 ± 0.62* | 76.61 ± 22.94* | 23.14 ± 12.89 | 7.93 ± 3.21* | 0.83 ± 0.44 | 2.61 ± 0.68 | 1.42 ± 0.91 |
| Lyo LGG | ||||||||
| Tumor | 4 | 6.22 ± 3.60 | 147.28 ± 76.46** | 25.10 ± 13.06 | 13.72 ± 3.22** | 0.91 ± 0.31** | 3.63 ± 1.72 | 1.75 ± 1.72 |
| Cured | 6 | 3.03 ± 1.39* | 68.29 ± 35.90* | 24.78 ± 6.93 | 5.30 ± 3.83* | 0.80 ± 0.16 | 2.15 ± 0.44 | 0.91 ± 0.41 |
| Oral + Lyo LGG | ||||||||
| Tumor | 2 | 6.71 ± 1.55 | 102.56 ± 65.61 | 52.28 ± 41.2 | 13.38 ± 7.79 | 2.72 ± 1.00 | 2.57 ± 0.50 | 1.20 ± 1.12 |
| Cured | 5 | 5.16 ± 1.01 | 46.17 ± 24.39* | 32.91 ± 8.85* | 7.99 ± 3.78* | 4.09 ± 1.80* | 2.36 ± 0.64 | 1.81 ± 1.29 |
*P < 0.05 compared with control tumor. **P < 0.05 with respect to healthy bladder. †50 μg of bladder tissue homogenates were analyzed by ELISA and the results are expressed as the mean ± SD.
Analysis of immune cell populations in the spleens and lymph nodes. To monitor mobilization of the immune system, mice treated with LGG or PBS were compared regardless of their status (tumor bearing or cured). There was a decrease in CD3CD8+ cells in the spleen after Lyo LGG instillation (15.99 ± 3.30, P < 0.05) and O + I LGG (16.04 ± 2.47) compared with mice given PBS (20.94 ± 4.52). There was also a significant reduction in pan‐NK+ and Mac 3+ cells in the O + I LGG group (3.23 ± 0.91 and 3.55 ± 1.98, P < 0.05) compared with the control (5.51 ± 2.51 and 33.52 ± 31.21).
When mice within each group were segregated based on their tumor status, only Lyo LGG‐treated mice showed significant differences. In Lyo LGG‐treated mice that were cured as opposed to mice still bearing tumors, there were significantly lower levels of splenic Mac3+ cells (13.26 ± 10.74 and 40.56 ± 23.37, respectively) and in the draining lymph nodes lower Ly6G+ (40.05 ± 19.7 and 60.30 ± 26.4, respectively) and CD19+ (7.67 ± 8.71 and 38.90 ± 22.18, respectively) cells.
Immunohistochemical analysis of the bladder after two instillations of LGG. To study immune cell recruitment in tumor‐bearing bladders, it was necessary to terminate the experiment after a few instillations as by 6 weeks the majority of mice were cured. Therefore, mice were killed after the second Lyo LGG instillation. In LGG‐treated mice (n = 5) there was increased Mac3+ cells infiltrating the tumors as well as increased accumulation of these cells in the periphery of the tumors. This was reduced in untreated mice (n = 5) (Fig. 2). Only one in five control animals had more than 50% neurophil infiltration compared with three in five LGG‐treated animals (Fig. 2).
Figure 2.
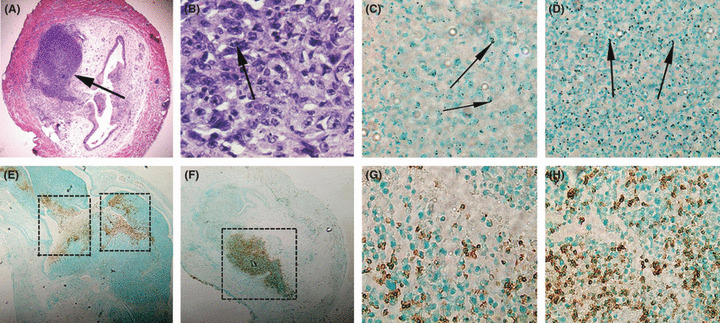
Bladder tumor sections were examined by immunohistochemistry. The photos show (A) a mouse bladder with a tumor indicated by the arrow (20x magnification), (B) infiltrating immune cells after lyophilized (Lyo) Lactobacillus rhamnosus GG (LGG) treatment (600× magnification), the arrow indicates bi‐lobed neutrophils. Mac‐3+ cells (stained brown) in (C) control tumors and (D) lyo LGG‐treated tumors (400× magnification) with arrows indicating the macrophage cells. Neutrophils (stained brown) in (E,G) control and (F,H) Lyo LGG‐treated tumors at (E,F) low magnification (40× magnification) and (G,H) high magnification (400× magnification of boxed regions in E,F). There were consistently more neutrophils within tumors of Lyo LGG mice than control mice when samples were examined under 400× magnification.
Comparison of the therapeutic ability of intravesical Lyo LGG and BCG. Lyo LGG was compared to lyophilized BCG (BCG). Tumor‐bearing mice were treated with BCG (1 × 107 cfu), Lyo LGG (1 × 108 cfu), or PBS weekly for a period of 6 weeks. Both Lyo LGG and BCG induced a significant and comparable increase in the number of cured mice (Table 3). BCG but not Lyo LGG instillation resulted in severe inflammation in some mice, which caused urethral stricture, and so for 4 of 15 mice one of the weekly instillations had to be skipped. Of the 15 mice treated with BCG, two were not evaluable as their bladders were not available for PSA analysis.
Table 3.
Comparison of the efficacy of Lactobacillus rhamnosus GG (LGG) and Mycobacterium bovis, Bacillus Calmette Guerin therapy in tumor‐bearing mice
| Group | n† | Cured | OR | 95% CI | P‐value | |
|---|---|---|---|---|---|---|
| n | % | |||||
| Control | 10 | 2 | 20 | 1.0 | — | — |
| Immucyst | 13 | 10 | 77 | 0.075 | 0.010–0.563 | 0.012* |
| Lyo LGG | 9 | 8 | 89 | 0.031 | 0.002–0.418 | 0.009* |
*Statistical significance at P < 0.05. †n = number of mice for which prostate‐specific antigen analysis could be carried out.
Comparison of the ability of LGG and BCG to stimulate neutrophils. Both LGG and BCG intravesical instillations resulted in recruitment of neutrophils to the tumour site. Therefore, the ability of both microbes to activate neutrophils were compared. Bone marrow‐derived neutrophils were incubated with the microbes (1:5 ratio) for 2 h and then the external bacteria were killed with gentamicin and the cells incubated for a further 18 h, and TNF‐α and IL10 levels were monitored. In a second set the neutrophils were incubated with the microbes for the full 18 h. LGG induced more TNF‐α than IL10 (ratio was 796:49) whereas BCG induced much less TNF‐α so the ratio was 146:33, after a 2‐h exposure to the microbes (Fig. 3). But after 18 h co‐incubation the TNF‐α:IL10 ratio for LGG was 774:134 and for BCG it was 487:69. Thus after a short 2‐h exposure to LGG, neutrophils responded with greater TNF‐α production rather than after a longer incubation, whereas the reverse was true for BCG. The number of LGG internalized by the neutrophils at 2 h was approximately 80 ± 32 cfu.
Figure 3.
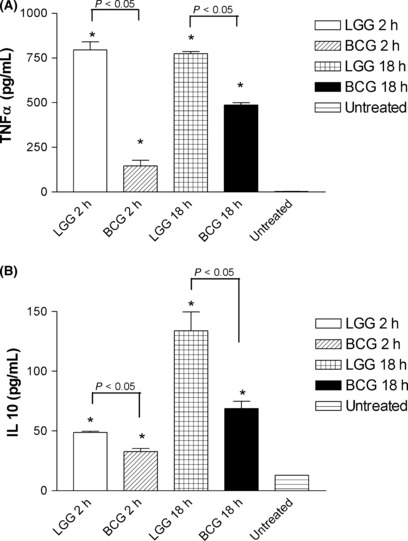
Cytokine production by neutrophils cultured with Lactobacillus rhamnosus GG (LGG) and Mycobacterium bovis, Bacillus Calmette Guerin (BCG). The production of (A) tumor necrosis factor (TNFα) and (B) interleukin (IL‐10) by neutrophils after 2 and 18 h of exposure to LGG or BCG, at a bacteria to neutrophil ratio of 5:1. The supernatant of the 2‐h treatment group was collected 18 h after killing off the extracellular bacteria and replacement with fresh media and this was therefore comparable to the 18‐h treatment group. Data are presented as the mean ± SEM. *Statistical significance (P < 0.05) from untreated control.
Discussion
Bladder cancer patients receive BCG immunotherapy after transurethral resection of the bladder tumor;( 2 ) therefore, in our study therapy was initiated at day 4 after tumor implantation when the tumor burden was small.( 14 ) Both LGG and BCG were left in the bladder for 2 h as is the procedure for patients receiving BCG immunotherapy. Only mice with measurable urinary PSA at day 11 were included in the study so mice not producing PSA at the end of the experiment are cured mice. Although Lyo LGG induced more cytokine production than live LGG in vitro, in vivo all LGG preparations were able to cure mice with a similar efficacy (Table 1). The lack of correlation between in vitro and in vivo data could be because the timeframe of the in vitro experiments, which were carried out over a period of 6–24 h, whereas intravesical instillations were only for 2 h and splenocytes are not representative of the tumor microenvironment.
ELISA analysis of bladder proteins revealed that LGG therapy decreased E‐selectin (highly expressed by MB49‐PSA cells) and OPN, whereas XCL1 levels was increased, VEGF‐D varied slightly, VEGFR and TNF‐α showed no difference, and Pro‐MMP9 decreased with Lyo LGG therapy. XCL1 is the C subgroup chemokine produced by activated CD8+ and γδ T cells, NK, and mast cells. It acts as a chemoattractant for T and NK cells and is able to induce tumor regression.( 16 , 17 ) Neutrophils also express the XCL1 receptor and therefore may be modulated by this chemokine.( 18 ) In our study, healthy bladders had high XCL1 levels, which were reduced after tumor implantation. However, LGG therapy increased XCL1 in the bladders, which could have induced tumor regression by recruiting immune cells into the bladder. MB49‐PSA cells express Pro‐MMP9 and OPN just like human bladder cancers.( 19 , 20 ) Pro‐MMP9 has a role in cancer metastasis and tumor growth, and is elevated in human bladder cancer.( 21 ) Therefore, reduction of Pro‐MMP9 would slow the growth of the tumor cells. Pro‐MMP9 is regulated by cytokines( 22 ) as well as OPN( 23 ) and therefore reduction of OPN or changes in the local cytokine environment after therapy may have decreased expression of this protein. Bladder TNFα levels were not significantly elevated but this may be because of the time of tissue harvest, which in this instance was 24 h after the last instillation. Even in patients receiving BCG therapy cytokine production following therapy rapidly declines to basal level by 24 h.( 24 )
Analysis of immune populations revealed that tumor implantation in the bladder resulted in increased Mac3+ cells in the spleen and in cured mice this was reduced to normal levels. There was decreased CD3+CD8+ cells and NK cells in the spleen of mice treated with LGG and this may be due to recruitment of these cells to the bladder. From the experiments with healthy mice it is clear that Lyo LGG instillation results in dendritic cell recruitment and activation. Immunohistochemistry of the tumor mass did reveal recruitment of more immune cells to the tumor in the presence of LGG, namely Mac3+ cells and neutrophils. The neutrophil migration may have been aided by the presence of XCL1 as well. Neutrophils are believed to mediate leukocyte chemotaxis to the bladder as an early inflammatory response. Neutrophil depletion completely abrogates BCG antitumor response( 25 , 26 ) as there are less chemokines produced( 26 , 27 ) and thus impairment in CD4+ T‐cell recruitment.( 26 ) Neutrophils can damage and cause the shedding of urothelial cells via the release of granules and enzymes.( 28 ) Comparing the ability of LGG and BCG to induce neutrophils at a 5:1 ratio (bacteria:neutrophils),( 29 ) we found that LGG induced more TNF‐α production by neutrophils after a short 2‐h exposure in contrast to BCG. In that 2‐h period neutrophils internalized some bacteria, and it is probably this that triggers cytokine production as the external bacteria were killed with gentamycin and removed by washing. Tanigawa et al. demonstrated the importance of increased TNF‐α and low IL10 by showing that draining lymph node cells treated with TNF‐α induced greater antitumor responses in tumor‐bearing mice when administered with anti‐IL10 therapy.( 30 ) We have previously shown that expression of TNF‐α in the bladder can induce tumor regression as well.( 31 )
Although there is evidence that MAC3+ cells may be associated with tumor‐induced immune dysfunction,( 32 ) there are also reports of antitumor effects. Boonman et al. reported that implantation of tumor cells in the eye resulted in spontaneous cures in 3–4 weeks but subconjunctival macrophage depletion caused progressive tumor growth, thus indicating an anti‐tumor role for macrophages.( 33 ) Reprogramming of tumor‐associated macrophages by expression of IRF‐3 and IRF‐7 ( 34 ) or blocking of NF‐κB activation( 35 ) or activation with anti‐CD40 and TLR9( 36 ) have all been shown to have anti‐tumor effects. It is possible that the macrophages are reprogrammed by LGG to be anti‐tumor macrophages. The infiltration of neutrophils and macrophages were similarly observed by Takahashi et al. when they instilled LcS in bladder tumor‐bearing C3H/He mice.( 10 )
However, Takahashi et al. followed a different treatment schedule; tumor‐bearing mice received daily LcS or BCG instillations for a period of 10 days.( 10 ) In contrast, we used the clinical schedule for BCG immunotherapy (1 instillation/week for 6 weeks) and found that LGG like BCG could induce tumor regression. For most mice, PSA levels started to drop after the second instillation and thus it may be possible that fewer instillations of Lyo LGG could also be efficacious.
The safety of LGG is shown by the lack of increase in the incidence of bacteremia in Finland where LGG has been used as a probiotic since 1999.( 37 ) When LGG bacteremia does occur (only in very ill patients) it responds to antimicrobial therapy.( 38 ) A recent study by Naito et al. has shown that oral LcS plus epirubicin is better than epirubucin alone for bladder cancer therapy, further confirming the efficacy of probiotics.( 39 ) Thus, probiotics may be a safer and efficacious alternative for bladder cancer immunotherapy.
Abbreviations
- Axl
axl receptor tyrosine kinase
- BCG
Mycobacterium bovis, Bacillus Calmette Guerin
- bFGF
basic fibroblast growth factor
- BLC
B lymphocyte chemoattractant
- cfu
colony forming units
- CRG
cytokine responsive gene
- CTACK
cutaneous T cell‐attracting chemokine
- CXCL
chemokine (CXC) motif ligand
- DPPIV
dipeptidyl peptidase IV
- Dtk
developmental receptor tyrosine kinase
- Flt
VEGF receptor type
- GCSF
granulocte colony stimulating factor
- GITR
glucocorticoid‐induced tumor necrosis factor receptor
- GMCSF
granuloctye macrophage colony stimulating factor
- HGFR
hepatocyte growth factor receptor
- ICAM
inter‐cellular adhesion molecule 1
- IFN
interferon
- IGF
insulin‐like growth factors
- IGFBP
insulin‐like growth factor binding proteins
- IL
interleukin
- IRF
inteferon regulatory factor
- I‐TAC
interferon‐inducible T cell alpha chemoattractant
- KC
keratinocyte‐derived chemokine
- LcS
lactobacillus casei Shirota
- LGG
lactobacillus rhamnosus GG
- LIX
LPS‐induced (C‐X‐C) chemokine
- Lyo
lyophilized
- MB49‐PSA
MB49 bladder cancer cells secreting human prostate‐specific antigen
- MCP
monocyte chemotactic protein
- MCSF
macrophage‐colony stimulating factor
- MDC
macrophage‐derived chemokine
- MIG
monokine induced by interferon‐gamma
- MIP
macrophage inflammatory protein
- MRS
de Man, Rogosa, Sharpe
- NF
nuclear factor
- NK
natural killer cells
- OPN
osteopontin
- PE
phycoerythrin
- PF
platelet factor
- PSA
prostate‐specific antigen
- RANTE
regulated upon activation, normal T cell expressed and secreted
- SCF
stem‐cell factor
- SDF
stroma‐derived factor
- Shh
sonic hedgehog
- TARC
thymus activation‐regulated chemokine
- TCA
T‐cell activation
- TECK
thymus‐expressed chemokine
- TIMP
tissue inhibitor of metalloproteinase
- TLR
toll like receptor
- TNF
tumor necrosis factor
- TPO
thrombopoietin
- TRANCE
TNF‐related activation‐induced cytokine
- TROY
tumor necrosis factor receptor superfamily, member 19
- TSLP
thymic stromal lymphopoietin
- VCAM
vascular cell adhesion molecule
- VEGF
vascular endothelial growth factor
Acknowledgements
This work was funded by a National Medical Research Council of Singapore grant (NMRC/0811/2003) to Associate Professor Lee Yuan Kun. We would like to thank Mrs Ng Geok Lan, Department of Anatomy, National University of Singapore, for technical assistance.
References
- 1. Nseyo UO, Lamm DL. Immunotherapy of bladder cancer. Semin Surg Oncol 1997; 13: 342–9. [DOI] [PubMed] [Google Scholar]
- 2. Alexandroff AB, Jackson AM, O’Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet 1999; 353: 1689–94. [DOI] [PubMed] [Google Scholar]
- 3. Amling CL. Diagnosis and management of superficial bladder cancer. Curr Probl Cancer 2001; 25: 219–78. [DOI] [PubMed] [Google Scholar]
- 4. Brandau S, Suttmann H. Thirty years of BCG immunotherapy for non‐muscle invasive bladder cancer: a success story with room for improvement. Biomed Pharmacother 2007; 61: 299–305. [DOI] [PubMed] [Google Scholar]
- 5. Aso Y, Akaza H, Kotake T, Tsukamoto T, Imai K, Naito S. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double‐blind trial. Eur Urol 1995; 27: 104–9. [DOI] [PubMed] [Google Scholar]
- 6. Aso Y, Akasa H. Prophylactic effects of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer. Urol Int 1992; 49: 125–9. [DOI] [PubMed] [Google Scholar]
- 7. Balansky R, Gyosheva B, Ganchev G, Mircheva Z, Minkova S, Georgiev G. Inhibitory effects of freeze‐dried milk fermented by selected Lactobacillus bulgaricus strains on carcinogenesis induced by 1,2‐dimethylhydrazine in rats and by diethylnitrosamine. Cancer Lett 1999; 147: 125–37. [DOI] [PubMed] [Google Scholar]
- 8. Lim BK, Mahendran R, Lee YK, Bay BH. Chemopreventive effect of Lactobacillus rhamnosus on growth of a subcutaneously implanted bladder cancer cell line in the mouse. Jpn J Cancer Res 2002; 93: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asano M, Karasawa E, Takayama T. Antitumor activity of Lactobacillus casei (LC 9018) against experimental mouse bladder tumor (MBT‐2). J Urol 1986; 136: 719–21. [DOI] [PubMed] [Google Scholar]
- 10. Takahashi T, Kushiro A, Nomoto K et al. Antitumor effects of the intravesical instillation of heat killed cells of the Lactobacillus casei strain Shirota on the murine orthotopic bladder tumor MBT‐2. J Urol 2001; 166: 2506–11. [PubMed] [Google Scholar]
- 11. Gorbach SL. Probiotics and gastrointestinal health. Am J Gastroenterol 2000; 95: S2–4. [DOI] [PubMed] [Google Scholar]
- 12. Seow SW, Rahmat JN, Mohamed AA, Mahendran R, Lee YK, Bay BH. Lactobacillus species is more cytotoxic to human bladder cancer cells than Mycobacterium bovis (bacillus Calmette–Guerin). J Urol 2002; 168: 2236–9. [DOI] [PubMed] [Google Scholar]
- 13. Seow SW, Rahmat JN, Bay BH, Lee YK, Mahendran R. Expression of chemokine/cytokine genes and immune cell recruitment following the instillation of Mycobacterium bovis, bacillus Calmette–Guérin or Lactobacillus rhamnosus strain GG in the healthy murine bladder. Immunology 2008; 124: 419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu Q, Esuvaranathan K, Mahendran R. Monitoring the response of orthotopic bladder tumors to granulocyte macrophage colony‐stimulating factor therapy using the prostate‐specific antigen gene as a reporter. Clin Cancer Res 2004; 10: 6977–84. [DOI] [PubMed] [Google Scholar]
- 15. Ninalga C, Loskog A, Klevenfeldt M, Essand M, Tötterman TH. CpG oligonucleotide therapy cures subcutaneous and orthotopic tumours and evokes protective immunity in murine bladder cancer. J Immunother 2005; 28: 20–7. [DOI] [PubMed] [Google Scholar]
- 16. Hedrick JA, Saylor V, Figueroa D et al. Lymphotactin is produced by NK cells and attracts both NK cells and T cells in vivo. J Immunol 1997; 158: 1533–40. [PubMed] [Google Scholar]
- 17. Cairns CM, Gordon JR, Li F, Baca‐Estrada ME, Moyana T, Xiang J. Lymphotactin expression by engineered myeloma cells drives tumor regression: mediation by CD4+ and CD8+ T cells and neutrophils expressing XCR1 receptor. J Immunol 2001; 167: 57–65. [DOI] [PubMed] [Google Scholar]
- 18. Huang H, Li F, Cairns CM, Gordon JR, Xiang J. Neutrophils and B cells express XCR1 receptor and chemotactically respond to lymphotactin. Biochem Biophys Res Commun 2001; 281: 378–82. [DOI] [PubMed] [Google Scholar]
- 19. Nutt JE, Durkan GC, Mellon JK, Lunec J. Matrix metalloproteinases (MMPs) in bladder cancer: the induction of MMP9 by epidermal growth factor and its detection in urine. BJU Int 2003; 91: 99–104. [DOI] [PubMed] [Google Scholar]
- 20. Coppola D, Szabo M, Boulware D et al. Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin Cancer Res 2004; 10 (1 Pt 1): 184–90. [DOI] [PubMed] [Google Scholar]
- 21. Van Kempen LC, Coussens LM. MMP9 potentiates pulmonary metastasis formation. Cancer Cell 2002; 2: 251–2. [DOI] [PubMed] [Google Scholar]
- 22. Langowski JL, Zhang X, Wu L et al. IL‐23 promotes tumour incidence and growth. Nature 2006; 442: 461–5. [DOI] [PubMed] [Google Scholar]
- 23. Lai CF, Seshadri V, Huang K et al. An osteopontin–NADPH oxidase signaling cascade promotes pro‐matrix metalloproteinase 9 activation in aortic mesenchymal cells. Circ Res 2006; 98: 1479–89. [DOI] [PubMed] [Google Scholar]
- 24. Saint F, Patard JJ, Maille P et al. T helper 1/2 lymphocyte urinary cytokine profiles in responding and nonresponding patients after 1 and 2 courses of bacillus Calmette–Guerin for superficial bladder cancer. J Urol 2001; 166: 2142–7. [PubMed] [Google Scholar]
- 25. Saban MR, Simpson C, Davis C et al. Discriminators of mouse bladder response to intravesical Bacillus Calmette–Guerin (BCG). BMC Immunol 2007; 8: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suttmann H, Riemensberger J, Bentien G et al. Neutrophil granulocytes are required for effective Bacillus Calmette–Guérin immunotherapy of bladder cancer and orchestrate local immune responses. Cancer Res 2006; 66: 8250–7. [DOI] [PubMed] [Google Scholar]
- 27. Suttmann H, Lehan N, Böhle A, Brandau S. Stimulation of neutrophil granulocytes with Mycobacterium bovis bacillus Calmette–Guérin induces changes in phenotype and gene expression and inhibits spontaneous apoptosis. Infect Immun 2003; 71: 4647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siracusano S, Vita F, Abbate R et al. The role of granulocytes following intravesical BCG prophylaxis. Eur Urol 2007; 51: 1589–97. [DOI] [PubMed] [Google Scholar]
- 29. Morel C, Badell E, Abadie V et al. Mycobacterium bovis BCG‐infected neutrophils and dendritic cells cooperate to induce specific T cell responses in humans and mice. Eur J Immunol 2008; 38 (2): 437–47. [DOI] [PubMed] [Google Scholar]
- 30. Tanigawa K, Craig RA, Stoolman LM, Chang AE. Effects of tumor necrosis factor‐alpha on the in vitro maturation of tumor‐reactive effector T cells. J Immunother 2000; 23: 528–35. [DOI] [PubMed] [Google Scholar]
- 31. Zang Z, Mahendran R, Wu Q, Yong T, Esuvaranathan K. Non‐viral tumor necrosis factor‐alpha gene transfer decreases the incidence of orthotopic bladder tumors. Int J Mol Med 2004; 14: 713–7. [PubMed] [Google Scholar]
- 32. Garner RE, Malick AP, Yurochko AD, Elgert KD. Shifts in macrophage (M phi) surface phenotypes during tumor growth: association of Mac‐2+ and Mac‐3+ M phi with immunosuppressive activity. Cell Immunol 1987; 108: 255–68. [DOI] [PubMed] [Google Scholar]
- 33. Boonman ZF, Schurmans LR, Van Rooijen N, Melief CJ, Toes RE, Jager MJ. Macrophages are vital in spontaneous intraocular tumor eradication. Invest Ophthalmol Vis Sci 2006; 47: 2959–65. [DOI] [PubMed] [Google Scholar]
- 34. Goubau D, Romieu‐Mourez R, Solis M et al. Transcriptional re‐programming of primary macrophages reveals distinct apoptotic and anti‐tumoral functions of IRF‐3 and IRF‐7. Eur J Immunol 2009; 39: 527–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hagemann T, Lawrence T, McNeish I et al. “Re‐educating” tumor‐associated macrophages by targeting NF‐κB. J Exp Med 2008; 205: 1261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu QL, Buhtoiarov IN, Sondel PM, Rakhmilevich AL, Ranheim EA. Tumoricidal effects of activated macrophages in a mouse model of chronic lymphocytic leukemia. J Immunol 2009; 182: 6771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Salminen MK, Tynkkynen S, Rautelin H et al. Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in Finland. Clin Infect Dis 2002; 35: 1155–60. [DOI] [PubMed] [Google Scholar]
- 38. Salminen MK, Rautelin H, Tynkkynen S et al. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L. rhamnosus GG. Clin Infect Dis 2004; 38: 62–9. [DOI] [PubMed] [Google Scholar]
- 39. Naito S, Koga H, Yamaguchi A et al. Prevention of recurrence with epirubicin and Lactobacillus casei after transurethral resection of bladder cancer. J Urol 2008; 179: 485–90. [DOI] [PubMed] [Google Scholar]


